Abstract
Volcanic lava cave habitats are extreme environments. We carried out field investigations for five years and reported the results of bryophyte diversity in eight volcanic lava caves of Jingpo Lake World Geopark, where the largest underground lava caves in China are preserved. The results are as follows: (1) A total of 230 quadrats were set up, and 2041 bryophyte specimens were collected. The specimens belong to 272 species of 107 genera in 47 families, including 26 liverworts (13 genera, 11 families) and 246 mosses (94 genera, 36 families). (2) The α diversity of bryophytes in Underground Lava Fall Cave was the highest, while that in Foggy Cave was the lowest. (3) The dominant families included Mniaceae, etc, accounting for 55.9% of the total species. The dominant genera included Plagiomnium, etc, accounting for 24.3% of the total species. The dominant species included Sanionia uncinata (Hedw.) Loeske etc. (4) There are no shared species among all eight lava caves, and each cave has a unique species composition. (5) Compared with that in other habitats in our previous studies, the similarity of bryophyte species between lava caves and underground forests of craters was high (113 species, 40.07%), while it was low between lava caves and lava platforms (9 species, 4.65%). Our study revealed that the lava caves have a high potential for bryophyte diversity, and such ancient ecological disaster sites are now rare refuges for bryophytes. Mosses are more adapted to cave habitats than liverworts. Bryophytes in this special eco-environment need to be considered and protected in order to preserve high-quality gene resources for humans, which is of great significance for the maintenance and development of biodiversity.
1. Introduction
Biodiversity is a global research hotspot of common concern [1]. Bryophytes are the second largest group in the plant kingdom, with a species diversity second only to that of angiosperms, and play an important role in biodiversity [2]. Bryophytes widely colonize terrestrial ecosystems. To date, a large number of studies on bryophyte diversity in conventional habitats have been carried out in countries worldwide [3]. It is noteworthy that the secondary metabolites of plants in extreme habitats can facilitate resistance to a harsh external environment. Undoubtedly, bryophytes have proven to be a considerable repository of resources [4]. According to previous studies, some bryophytes in special eco-environments possess antitumour activities against different cancer cell lines [5].
Caves are a special type of landform harbouring highly distinct ecosystems. Cave habitats are characterized by weak light, small temperature fluctuations, high air humidity, limited organic matter, etc., and most organisms cannot survive and reproduce in such harsh habitats. Therefore, the special eco-environments in caves are defined as extreme habitats [6]. However, caves contain high levels of biodiversity, and cave organisms are an important component of biodiversity [7]. Organisms in cave habitats face high-intensity environmental stress, which stimulates the organisms to produce the most effective and best-adapted secondary metabolites to resist extreme eco-environments [8]. Therefore, because of their unique and rare natural properties, cave habitats have become an ideal natural laboratory for research on biodiversity and attract global scholars, who carry out field investigations.
Research on cave organisms has a history of more than 100 years. The darkness in caves cannot sustain plants because plants in cave habitats have more specific eco-environmental requirements for light, temperature, water, heat, etc. Accordingly, research on biodiversity in cave habitats has focused on animals [9] and microbes [10]. However, the plant communities in caves are the most important feature of the lava biosphere. Some shade- and moisture-tolerant plants that can adapt to cave habitats have been neglected for a long time. The earliest records of cave plant diversity were collected in the late 18th century [11]. Fossils discovered in recent years show a rich plant flora of recent centuries in the Makauwahi Cave habitats [12]. As the earliest terrestrial plants and the most primitive higher plants, bryophytes originated in the Cambrian approximately 500 million years ago [13]. Unlike other higher plants, bryophytes prefer dark and damp eco-environments and can adapt to cave habitats and establish populations successfully. Bryophytes are found to be pioneer plants in areas with strong environmental pressure [14]. It can be inferred that cave habitats preserve a high diversity of bryophytes, which is worth further exploration.
The diversity of bryophytes, a distinctive plant group in the extreme habitats of caves, has aroused great interest from researchers. In the early 20th century, for example, Ammons et al. reported a list of bryophytes in McKinney Cave [15]. Later, Thatcher et al. classified bryophytes in artificially illuminated caves in Crystal, Wisconsin, USA [16]. Next, Mason-Williams et al. reported the floristic composition of bryophytes in caves in South Wales, UK [17]. In the late 20th century, Zhang et al. conducted detailed studies on bryophytes in karst caves in China [18,19]. Monro et al. discovered a high diversity of vascular plants in cave habitats in Southwest China and mentioned that the bryophytes in caves flourished, yet they did not identify bryophyte species [20]. Internationally, there have been relatively few reports on the diversity of bryophytes in lava caves, especially in China.
China is a country with numerous caves. Generally, natural caves are divided into volcanic, karst, glacier, Danxia, and gypsum caves, among others. Volcanic lava caves form unique and fragile yet biodiverse ecosystems. Jingpo Lake World Geopark is one of 35 priority biodiversity conservation areas in China. Approximately 12,000 years ago in the Holocene, when the volcanic cluster in Jingpo Lake erupted on a large scale, the outer layer of liquid magma solidified, and the inner layer emptied, forming unique underground lava caves (also known as lava tunnels). Although there are many volcanoes in China, not all volcanic eruptions can form lava caves. Surprisingly, Jingpo Lake World Geopark is home to the largest preserved underground lava caves in China, with intact structures, rich geomorphic types and clear shapes, which are globally rare and have high scientific research value. In 2006, the geopark was rated as a world natural heritage site by UNESCO. Among all volcanic geological relics in the park, underground lava caves are important representative landscapes and key protected areas. Lava caves of Jingpo Lake World Geopark have received increasing attention owing to their unusual geologic processes [21]. Bryophytes are the main plant groups in the primary succession stage of volcanic ecosystems. Nevertheless, the diversity of bryophytes in lava caves of volcanic ecosystems in China has not been surveyed thus far. Our previous finding of numerous bryophytes in the underground forests in craters and lava platforms of Jingpo Lake World Geopark [22,23] prompted this detailed study of eight volcanic lava caves. Consequently, it is critical to carry out field investigations on bryophytes in ancient ecological disaster sites in lava caves.
We addressed the following questions: (1) How many and which species of bryophytes can be found in the lava caves of Jingpo Lake World Geopark? (2) Which cave has the highest bryophyte α diversity index? (3) What are the dominant groups of bryophytes? (4) How similar are the bryophytes among the eight caves?
2. Materials and Methods
2.1. Study Sites
Jingpo Lake World Geopark is located in southeastern Heilongjiang Province, China (44°02′–44°20′ N, 128°27′–128°55′ E), with a total area of 1200 km2 (Figure 1). The climate is temperate continental monsoon. Specifically, the annual average temperature is 3.6 °C, the average temperature in the coldest month (January) is −20 °C, the average temperature in the hottest month (July) is 22 °C, the annual temperature difference is 38–48 °C, the annual average precipitation is 589.6 mm, the annual evaporation is 1022.9 mm, the relative humidity is 70.9%, and the frost-free period lasts approximately 150 days. The soil is dark brown soil. The vegetation is temperate coniferous and broad-leaved mixed forest, and the dominant species include Pinus koraiensis Siebold & Zuccarini, Larix olgensis A. Henry, Tilia amurensis Rupr., Picea jezoensis (Siebold & Zucc.) Carrière, Fraxinus mandshurica Rupr., and Phellodendron amurense Rupr. The park has large-scale volcanic landforms, and lava caves were formed by volcanic eruptions. The matrix of the lava tunnels consists of basanites, alkali olivine basalts, and tephrites [24]. Under long-term effective protection, the bryophytes in the lava cave habitats remain primitive.
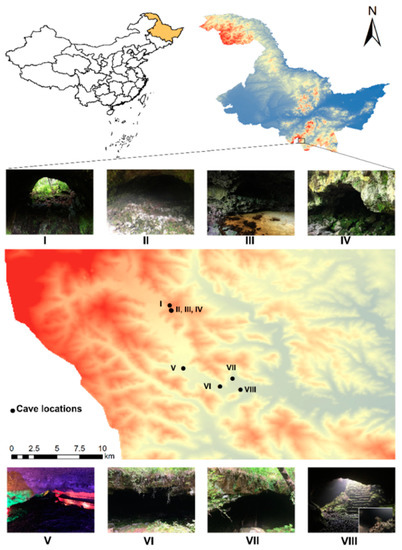
Figure 1.
Locations of eight volcanic lava caves in Jingpo Lake World Geopark. (The numbers correspond to the caves reported in Table 1).
2.2. Field Investigations
From September 2017 to September 2021, we visited eight volcanic lava caves in Jingpo Lake World Geopark five times and conducted field investigations on bryophytes using typical investigation methods. First, quadrats were established in representative areas where bryophytes were abundant. Second, a quadrat sieve (40 cm × 40 cm) was placed on the bryophyte community, and the cover of each species of bryophyte was recorded. A total of 230 quadrats were set up. Third, bryophytes were collected from the quadrat and placed into separate sealing plastic bags. We recorded the investigation information, including plot number, investigation time, name of the lava cave, longitude, latitude, elevation, habitats, cover, name of the investigator, etc. A total of 2041 bryophyte specimens were collected. Information on the lava caves, number of quadrats, and number of specimens is shown in Table 1.

Table 1.
Information on eight volcanic lava caves and the numbers of quadrats and specimens in Jingpo Lake World Geopark.
2.3. Species Identification
The bryophyte samples were brought back to the laboratory and dried under a ventilated shade. The bryophytes were placed into kraft specimen bags with the same specification (15 cm × 10 cm), and then the collected information was transcribed to the cover of the specimen bags. According to bryoflora taxonomic keys, by using a stereoscopic microscope (manufacturer: Olympus Corporation; model: SZ2-ILST) and an optical microscope (manufacturer: Nikon Corporation; model: Eclipse Ci-L), the species were identified morphologically. Then, we organized the scientific names of the bryophyte species. All voucher specimens were deposited in the bryophyte herbarium of Jiangxi Normal University (JXNU).
2.4. Data Analyses
In quantitative ecology, the importance value reflects the importance of species in communities [25]. In this study, the dominance of bryophyte species was quantified by using the importance value. After converting the number of individuals to cover, we calculated the ecological importance value of each bryophyte species according to the relative frequency and relative cover [26]. α diversity indices were employed to quantitatively describe the diversity characteristics of bryophytes [27]. The data analyses and graphic visualization were performed in R 4.3.0. The calculation formulas are as follows:
where F is frequency; Fr is relative frequency; Cr is relative cover; and IV is the species importance value.
where H′ is the Shannon–Wiener index; D is the Simpson index; E is the Pielou index; pi is the importance value of a species; and S is the total number of bryophyte species in each quadrat.
where Si is the Sørensen similarity coefficient; a is the total number of species in habitat a; b is the total number of species in habitat b; and c is the total number of shared species between habitats a and b [28].
F = (number of quadrats with a certain bryophyte/total number of quadrats) × 100%
Fr = (frequency of a certain bryophyte/sum of frequencies of all bryophytes) × 100%
Cr = (average cover of a certain bryophytes/sum of average covers of all bryophytes) × 100%
IV = (Fr + Cr)/2
3. Results
3.1. Species Richness and Species Composition of Bryophytes in Lava Caves of Jingpo Lake World Geopark
In total, 272 species of bryophytes belonging to 107 genera and 47 families were identified in the lava caves. Among them, there were 26 liverworts belonging to 13 genera and 11 families and 246 mosses belonging to 94 genera and 36 families. The numbers of families, genera, and species of bryophytes in each cave are shown in Figure 2.
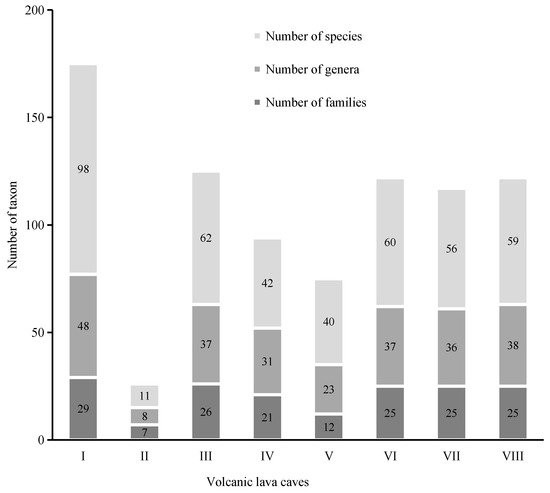
Figure 2.
Family, genera, and species numbers of bryophytes in eight volcanic lava caves of Jingpo Lake World Geopark. (The names of caves are listed in Table 1).
3.2. Evaluation of the α Diversity of Bryophytes in Lava Caves of Jingpo Lake World Geopark
The Shannon–Wiener and Simpson indices showed more diverse bryophyte communities within cave I than in the other seven caves, while the lowest diversity was observed in cave II. The maximum and minimum values of the Pielou evenness index were observed in caves III and VII, respectively (Table 2).

Table 2.
Alpha diversity indices of bryophytes in eight lava caves of Jingpo Lake World Geopark.
3.3. Dominant Groups of Bryophytes in Lava Caves of Jingpo Lake World Geopark
3.3.1. Dominant Families
The dominant families (species ≥ 10) of bryophytes in the lava caves of Jingpo Lake World Geopark were Mniaceae, Brachytheciaceae, Pottiaceae, Hypnaceae, Entodontaceae, Dicranaceae, Bryaceae and Plagiotheciaceae, accounting for 37.4% of the total genera and 55.9% of the total species (Table 3).

Table 3.
Analysis of dominant families of bryophytes in volcanic lava caves of Jingpo Lake World Geopark.
3.3.2. Dominant Genera
The dominant genera (species ≥ 9) were Plagiomnium, Entodon, Brachythecium, Dicranum and Bryum, accounting for 24.3% of the total species (Table 4).

Table 4.
Analysis of the dominant genera of bryophytes in volcanic lava caves of Jingpo Lake World Geopark.
3.3.3. Dominant Species
In terms of the importance value, the dominant species were Sanionia uncinata (Hedw.) Loeske, Myuroclada maximowiczii (G. G. Borshch.) Steere & W. B. Schofield, and Plagiomnium cuspidatum T. J. Kop. (Table 5).

Table 5.
Analysis of the dominant species of bryophytes in volcanic lava caves of Jingpo Lake World Geopark.
3.4. Shared Species of Bryophytes among Volcanic Lava Caves in Jingpo Lake World Geopark
The Venn diagram showed that there were no species of bryophytes shared among all eight volcanic lava caves (Figure 3A). Underground Lava Fall (I) had the largest number of unique species (46), followed by III, VII, VI, VIII, V and IV, while Foggy Cave (II) had the lowest number (3). In the pairwise comparisons, there were more shared species (8) in III and IV and fewer than or equal to six shared species between the other lava caves (Figure 3B).
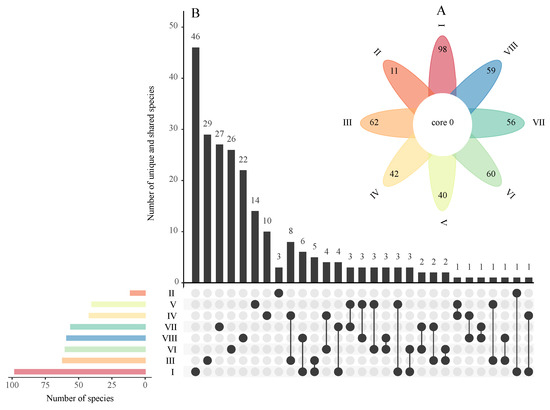
Figure 3.
Unique and shared species of bryophytes among eight volcanic lava caves of Jingpo Lake World Geopark. (A). Flower diagram of the shared bryophytes among eight volcanic lava caves; (B). UpSet intersection diagram of unique and shared bryophytes among eight volcanic lava caves).
3.5. Similarities in Bryophyte Species among Lava Cave Habitats and Other Volcanic Habitats in Jingpo Lake World Geopark
We analysed the shared species of bryophytes and the similarity coefficient among three habitats: underground forests of craters, lava platforms, and lava caves in Jingpo Lake World Geopark. The results showed that there were 36 shared bryophyte species in all three habitats. The species similarity between lava caves and underground forests of craters was high (113 species, 40.07%), while it was low between lava caves and lava platforms (9 species, 4.65%) (Figure 4, Table 6).
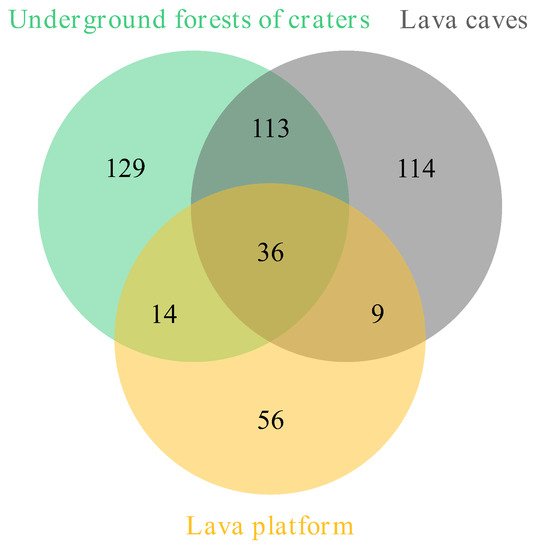
Figure 4.
Number of shared species of bryophytes among lava caves and other volcanic geomorphic relics in Jingpo Lake World Geopark.

Table 6.
Similarity coefficients of bryophytes among lava caves and other volcanic geomorphic relic habitats in Jingpo Lake World Geopark.
4. Discussion
Species richness, that is, the total number of species, is a basic indicator for measuring species diversity in an ecological environment. At present, the species richness of bryophytes reported in lava caves internationally is as follows: 136 bryophytes in 28 caves in Sicily [29] and only six bryophytes in Selarong Cave, Indonesia [30]. Several bryophytes have been recorded in Azorean lava tube caves [31]. We took systematic samples in eight lava caves in Jingpo Lake World Geopark, representing the first report of bryophyte species diversity in lava cave habitats in China. We found that there were abundant bryophyte species (272 species) in the lava caves of Jingpo Lake World Geopark. Thus, they are a hitherto hidden cradle of bryophyte diversity. Meanwhile, the species richness of mosses was nine times higher than that of liverworts. This result is the same as that reported for karst caves in China [18,19], suggesting that mosses are more adapted to wet and dark cave habitats than liverworts are. Furthermore, each cave had a unique species composition. One possibility is that, although the eight lava caves were formed simultaneously when the volcano erupted, due to their different shapes and sizes of caves in the later stage, the availability of internal habitats was different [32]. Bryophytes are sensitive to the microenvironment, and habitat heterogeneity filters out species that cannot adapt to the extreme conditions. Therefore, the differences in habitats contributed to the unique species compositions of the eight caves.
α diversity indices are indicators used in quantitative ecology to assess species diversity. The α diversity indices of the eight lava caves further confirmed that cave I (Underground Lava Fall Cave) had the most bryophytes, while cave II (Foggy Cave) had the least. The reason for this may be that light has the greatest impact on bryophyte diversity among many ecological factors [33]. Bryophytes can use light efficiently in low-light environments [34]. It is speculated that the differences in the diversity indices of bryophytes among the caves could be related to differences in light among the caves. In our research, the highest light intensity was observed in Underground Lava Fall Cave, which also had the highest diversity index of bryophytes. Comparatively, Foggy Cave is 200 m long with high air humidity; theoretically, such a large sampling area and humid habitats are suitable for bryophytes. However, we found in the field survey that only areas with light at the entrance had bryophytes, and there was no growth in the dark inside the caves. Thus, the bryophyte diversity index of Foggy Cave was the lowest. Additionally, we found that bryophytes in cave habitats exhibited a phototropic distribution. The size and scale of bryophyte populations in the same cave decreased to zero with decreasing light. Population size and species diversity tended to be limited due to the small size of the lava cave habitats. Light not only affects the species diversity of bryophytes in caves but also affects their distribution patterns.
The relationship between species and the environment is bidirectional. Bryophytes are indicators of the environment, with the dominant groups reflecting particular habitat conditions [26]. Specifically, in the eight volcanic lava caves of Jingpo Lake World Geopark, the dominant families were mainly large families with a wide ecological range, such as Mniaceae, Brachytheciaceae, and Pottiaceae. The dominant genera were mainly the largest genera in the dominant families, such as Plagiomnium, Entodon and Brachythecium, which are resistant to humidity, drought and cold, respectively [23]. The dominant species was Sanionia uncinata (Hedw.) Loeske, which is resistant to shade, cold, humidity and poor nutrition, occupies an important niche and has a wide ecological range. The species is distributed widely in most of the lava caves. It has been reported that this species survives in oligotrophic and low-temperature eco-environments in polar regions [35]. This dominant species may be involved in the deposition of stalactites and the formation of soil. The abundance of this species indicates that the lava cave habitats of Jingpo Lake are similar to those of polar regions. Extreme cave habitats can accommodate most dominant families and dominant genera with broad niches. Simultaneously, they also have complex and diverse local niches and a polar-like microhabitat to support dominant species.
The study of shared species is of great significance for revealing the degree of association among different habitats. The superset intersection graph demonstrated that there were no shared species among all eight lava caves. That is, the internal habitats of the eight caves differ from each other, which may be related to the shape of the caves and the way in which they connect with the ground [36]. The number of unique species indicates the independence of the geographical unit, i.e., its difference from other geographical units. In terms of unique species, Underground Lava Fall (I) had the largest number, while Foggy Cave (II) had the smallest number. The ranking of unique species was essentially consistent with the total species in each cave. In the pairwise comparisons, caves III and IV had the largest number of shared species. This may be due to the close geographical locations of the two caves, which are conducive to the spread, diffusion and colonization of bryophyte spores. Thus, bryophytes in the eight caves are both closely related and independent of each other, and their similarity and independence may depend on factors such as the geographical location, altitude, terrain, size, light, and vegetation of the caves [37].
Compared with our previous results [22], the similarity value of bryophytes between volcanic lava caves and underground forest habitats in Jingpo Lake World Geopark was the highest. The reason for this may be that both sites have relatively closed internal habitats, which are less disturbed by the outside world. Lava platforms are exposed to sunlight, which is quite different from the relatively hidden internal habitats. Therefore, the similarity coefficient of bryophytes between the lava caves and platforms was the lowest. The extremely different ecological environment makes the lava platform less similar to the other two. Therefore, similar ecological environments can support similar species.
Although China has strongly called for the protection of cave habitats, this protection is mainly aimed at karst caves [38]. In fact, although the scale of lava caves is smaller than that of karst caves, the species richness of bryophytes in volcanic lava caves is 2~14 times greater than that in currently known karst caves [18,19,37]. Our study revealed that the extreme habitats in lava caves are an important refuge for bryophytes. Similar to our findings, Fraser et al. found that volcanoes might have acted as glacial microrefugia for a wide range of species, including bryophytes [39]. Rich and luxuriant bryophytes in lava caves also provide a habitat similar to the paleoclimate for relict animals [40]. Notably, the relative growth rate of bryophytes is low in extreme cave environments. Such rich bryophytes survive in the volcanic lava caves of Jingpo Lake World Geopark, which may be the result of millions of years of evolution, mutual adaptation to extreme habitats and competition between individuals [41]. Lava caves are threatened biodiversity hotspots, and they receive little governmental attention or appropriate management action [7]. Hence, we argue that volcanic lava caves should be listed as priority and key protected areas of biodiversity. Otherwise, the lack of conservation of cave plant diversity will restrict the discovery, development, and utilization of resources.
5. Conclusions
This is the first extensive collection of specimens undertaken in lava cave habitats in China, and diverse bryophyte flora were observed. Although habitats in volcanic lava caves are poor, extreme habitats, these ancient ecological disaster sites are now rare refuges for bryophytes, with 272 species (107 genera in 47 families). We predict that such a pattern can occur elsewhere in lava caves around the world. Among the eight lava caves, the Underground Lava Fall Cave had the most abundant bryophytes, the highest diversity index and the most unique species, and thus needs key protection. We speculate that the α diversity of bryophytes is related to light. The dominant groups are mainly large families with a wide ecological range, large genera of dominant families, and species resistant to shade, humidity, and barrenness. The species richness of different caves is different, and the species composition of each cave is unique. The diversity of bryophytes in volcanic cave habitats is greater than that in underground forest and lava platform. Our findings support the idea that lava cave environments can nurture high bryophyte diversity. This study accumulates information on bryophyte diversity in lava cave habitats of Jingpo Lake World Geopark, and it yields a scientific basis for enriching the biodiversity in lava caves in China. Bryophytes are the dominant plants in lava cave habitats, and their existence increases the value of habitats. We call on the Government of China to strengthen large-scale research on bryophyte diversity in volcanic lava caves in order to preserve, develop and utilize more bryophyte resources.
Author Contributions
Writing—review and editing, M.C.; Data curation, T.Z.; Investigation, Y.L. and Y.W.; Software, W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31700168), Jiangxi Provincial Natural Science Foundation (20202BABL213044, 20212BAB203023) and Science and Technology Project of Educational Department in Jiangxi Province (GJJ200327). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The dataset used in this study is available from the first author on reasonable request.
Acknowledgments
We thank AJE (https://www.aje.cn/, accessed on 6 June 2023) for proofreading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saran, S.; Chaudhary, S.K.; Singh, P.; Tiwari, A.; Kumar, V. A comprehensive review on biodiversity information portals. Biodivers. Conserv. 2022, 31, 1445–1468. [Google Scholar] [CrossRef]
- Maksimova, V.; Klavina, L.; Bikovens, O.; Zicmanis, A.; Purmalis, O. Structural characterization and chemical classification of some bryophytes found in Latvia. Chem. Biodivers. 2013, 10, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Stefańska-Krzaczek, E.; Swacha, G.; Żarnowiec, J.; Raduła, M.W.; Kącki, Z.; Staniaszek-Kik, M. Central European forest floor bryophytes: Richness, species composition, coexistence and diagnostic significance across environmental gradients of forest habitats. Ecol. Indic. 2022, 139, 108954. [Google Scholar] [CrossRef]
- Chandra, S.; Chandrd, D.; Barh, A.; Pankaj; Pandey, R.K.; Sharma, I.P. Bryophytes: Hoard of remedies, an ethno-medicinal review. J. Tradit. Complement. Med. 2017, 7, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Dziwak, M.; Wróblewska, K.; Szumny, A.; Galek, R. Modern use of bryophytes as a source of secondary metabolites. Agronomy 2022, 12, 1456. [Google Scholar] [CrossRef]
- Kosznik-Kwaśnicka, K.; Golec, P.; Jaroszewicz, W.; Lubomska, D.; Piechowicz, L. Into the unknown: Microbial communities in caves, their role, and potential use. Microorganisms 2022, 10, 222. [Google Scholar] [CrossRef]
- Medellin, R.A.; Wiederholt, R.; Lopez-Hoffman, L. Conservation relevance of bat caves for biodiversity and ecosystem services. Biol. Conserv. 2017, 211, 45–50. [Google Scholar] [CrossRef]
- Jaroszewicz, W.; Bielańska, P.; Lubomska, D.; Kosznik-Kwaśnicka, K.; Golec, P.; Grabowski, Ł.; Wieczerzak, E.; Dróżdż, W.; Gaffke, L.; Pierzynowska, K.; et al. Antibacterial, antifungal and anticancer activities of compounds produced by newly isolated streptomyces strains from the Szczelina Chochołowska Cave (Tatra Mountains, Poland). Antibiotics 2021, 10, 1212. [Google Scholar] [CrossRef]
- Oromí, P.; Socorro, S. Biodiversity in the Cueva del Viento lava tube system (Tenerife, Canary Islands). Diversity 2021, 13, 226. [Google Scholar] [CrossRef]
- Riquelme, C.; Hathaway, J.J.M.; Dapkevicius, M.D.N.E.; Miller, A.Z.; Kooser, A.; Northup, D.E.; Jurado, V.; Fernandez, O.; Saiz-Jimenez, Z.; Cheeptham, N. Actinobacterial diversity in volcanic caves and associated geomicrobiological interactions. Front. Microbiol. 2015, 6, 1342. [Google Scholar] [CrossRef]
- Scopoli, J.A. Plantae subterraneae descriptae et delineatae. Diss. Sci. Nat. 1772, 1, 84–120. [Google Scholar]
- Burney, D.A.; Burney, L.P. Monitoring results from a decade of native plant translocations at Makauwahi Cave Reserve, Kauái. Plant Ecol. 2016, 217, 139–153. [Google Scholar] [CrossRef]
- Morris, J.L.; Puttick, M.N.; Clark, J.W.; Edwards, D.; Kenrick, P.; Pressel, S.; Wellman, C.H.; Yang, Z.H.; Schneider, H.; Donoghue, P.C.J. The timescale of early land plant evolution. Proc. Natl. Acad. Sci. USA 2018, 115, E2274–E2283. [Google Scholar] [CrossRef]
- Ren, J.; Liu, F.; Luo, Y.; Zhu, J.; Luo, X.; Liu, R. The pioneering role of bryophytes in ecological restoration of manganese waste residue areas, southwestern China. J. Chem. 2021, 2021, 9969253. [Google Scholar] [CrossRef]
- Ammons, N. Bryophytes of McKinney’s cave. Bryologist 1933, 36, 16–19. [Google Scholar] [CrossRef]
- Thatcher, E.P. Bryophytes of an artificially illuminated cave. Bryologist 1949, 52, 212–214. [Google Scholar] [CrossRef]
- Mason-Williams, M.; Benson-Evans, K. Summary of results obtained during a preliminary investigation into the bacterial and botanical flora of caves in south Wales. Int. J. Speleol. 1967, 2, 397–402. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, T.; Li, X.; Zhao, C. A study on the bryophytes of karst cave threshhold at Kunming area in Yunnan province, P.R. China. Carsologica Sin. 2004, 23, 229–233. (In Chinese) [Google Scholar]
- Liu, R.; Zhang, Z.; Shen, J.; Wang, Z. Bryophyte diversity in karst sinkholes affected by different degrees of human disturbance. Acta Soc. Bot. Pol. 2019, 88, 3620. [Google Scholar] [CrossRef]
- Monro, A.K.; Bystriakova, N.; Fu, L.; Wen, F.; Wei, Y. Discovery of a diverse cave flora in China. PLoS ONE 2018, 13, e0190801. [Google Scholar] [CrossRef]
- Wei, F.; Pan, B.; Xu, J. Sr-Nd-Pb-Ca isotopes of Holocene basalts from Jingpohu, NE China: Implications for the origin of their enriched mantle signatures. Minerals 2021, 11, 790. [Google Scholar] [CrossRef]
- Cong, M.; Li, Y.; Yang, W.; Chen, P. Bryophyte diversity of underground forests in craters of Jingpo Lake World Geopark. Bull. Bot. Res. 2023, 43, 361–369. (In Chinese) [Google Scholar]
- Cong, M.; Xu, Y.; Tang, L. Analysis on diversity of bryophytes in volcanic lava platform of Jingpo Lake World Geopark. J. Plant Resour. Environ. 2020, 29, 57–65. (In Chinese) [Google Scholar]
- Zhang, Z.; Feng, C.; Li, Z.; Li, S.; Xin, Y.; Li, Z.; Wang, X. Petrochemical study of the Jingpohu Holocene alkali basaltic rocks, northeastern China. Geochem. J. 2002, 36, 133–153. [Google Scholar] [CrossRef]
- Zhang, J.T. Quantitative Ecology, 3rd ed.; Science and Technology Press: Beijing, China, 2018; p. 79. (In Chinese) [Google Scholar]
- Printarakul, N.; Meeinkuirt, W. The bryophyte community as bioindicator of heavy metals in a waterfall outflow. Sci. Rep. 2022, 12, 6942. [Google Scholar] [CrossRef]
- Mario, S.; Bitetti, D. The distribution of grooming among female primates: Testing hypotheses with the Shannon-Wiener diversity index. Behaviour 2000, 137, 1517–1540. [Google Scholar]
- Sørensen, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analysis of the vegetation on Danish commons. Biol. Skr. 1948, 5, 1–34. [Google Scholar]
- Marta, P.; Maria, P.; Pietro, M.; Rosanna, C. Diversity and ecology of the bryophytes in the cave environment: A study on the volcanic and karstic caves of Sicily. Plant Biosyst. 2018, 153, 134–146. [Google Scholar]
- Salamah, Z.; Sasongko, H.; Zulianti, E. Diversity of bryophyte in the Selarong Cave Area, Bantul, Yogyakarta. Indones. J. Biol. Educ. 2019, 2, 35–39. [Google Scholar] [CrossRef]
- Gabriel, R.; Pereira, F.; Borges, P.A.V.; Constância, J.P. Indicators of conservation value of Azorean caves based on its bryophyte flora at the entrance. Proc. X XI XII Int. Symp. Vulcanospeleology 2008, 7, 114–118. [Google Scholar]
- Calderón-Gutiérrez, F.; Sánchez-Ortiz, C.A.; Huato-Soberanis, L. Ecological patterns in anchialine caves. PLoS ONE 2018, 13, e0202909. [Google Scholar] [CrossRef] [PubMed]
- Mulec, J.; Kubešová, S. Diversity of bryophytes in show caves in Slovenia and relation to light intensities. Acta Carsologica 2010, 39, 587–596. [Google Scholar] [CrossRef]
- Sheue, C.R.; Sarafis, V.; Kiew, R.; Liu, H.Y.; Salino, A.; Kuo-Huang, L.L.; Yang, Y.P.; Tsai, C.C.; Lin, C.H.; Yong, J.W.H.; et al. Bizonoplast, a unique chloroplast in the epidermal cells of microphylls in the shade plant Selaginella erythropus (Selaginellaceae). Am. J. Bot. 2007, 94, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Tojo, M.; West, P.N.; Hoshino, T.; Kida, K.; Fujii, H.; Hakoda, A.; Kawaguchi, Y.; Mühlhauser, H.A.; Van den Berg, A.H.; Kuepper, F.C.; et al. Pythium polare, a new heterothallic oomycete causing brown discolouration of Sanionia uncinata in the Arctic and Antarctic. Fungal Biol. 2012, 116, 756–768. [Google Scholar] [CrossRef]
- Mazina, S.E. Bryophytes and ferns as part of lamp flora caves. South Russ. Ecol. Dev. 2016, 11, 140–150. [Google Scholar] [CrossRef]
- Ren, H.; Wang, F.; Ye, W.; Zhang, Q.; Han, T.; Huang, Y.; Chu, G.; Hui, D.; Guo, Q. Bryophyte diversity is related to vascular plant diversity and microhabitat under disturbance in karst caves. Ecol. Indic. 2021, 120, 106947. [Google Scholar] [CrossRef]
- Duan, Y.F.; Li, M.; Xu, K.W.; Zhang, L.; Zhang, L.B. Protect China’s karst cave habitats. Science 2021, 374, 699. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.I.; Connell, L.; Lee, C.K.; Cary, C. Evidence of plant and animal communities at exposed and subglacial (cave) geothermal sites in Antarctica. Polar Biol. 2018, 41, 417–421. [Google Scholar] [CrossRef]
- Wynne, J.J.; William, W.A. A new millipede, Austrotyla awishoshola n. sp. (Diplopoda, Chordeumatida, Conotylidae) from New Mexico, USA, and the importance of cave moss gardens as refugial habitats. Zootaxa 2016, 4084, 285–292. [Google Scholar] [CrossRef]
- During, H.J.; Van Tooren, B.F. Bryophyte interactions with other plants. Bot. J. Linn. Soc. 1990, 104, 79–98. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).