A New Lichenized Fungus, Lendemeriella luteoaurantia, with a Key to the Species of Lendemeriella
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological and Chemical Analyses
2.2. Isolation, DNA Extraction, Amplification, and Sequencing
2.3. Phylogenetic Analyses
3. Results and Discussion
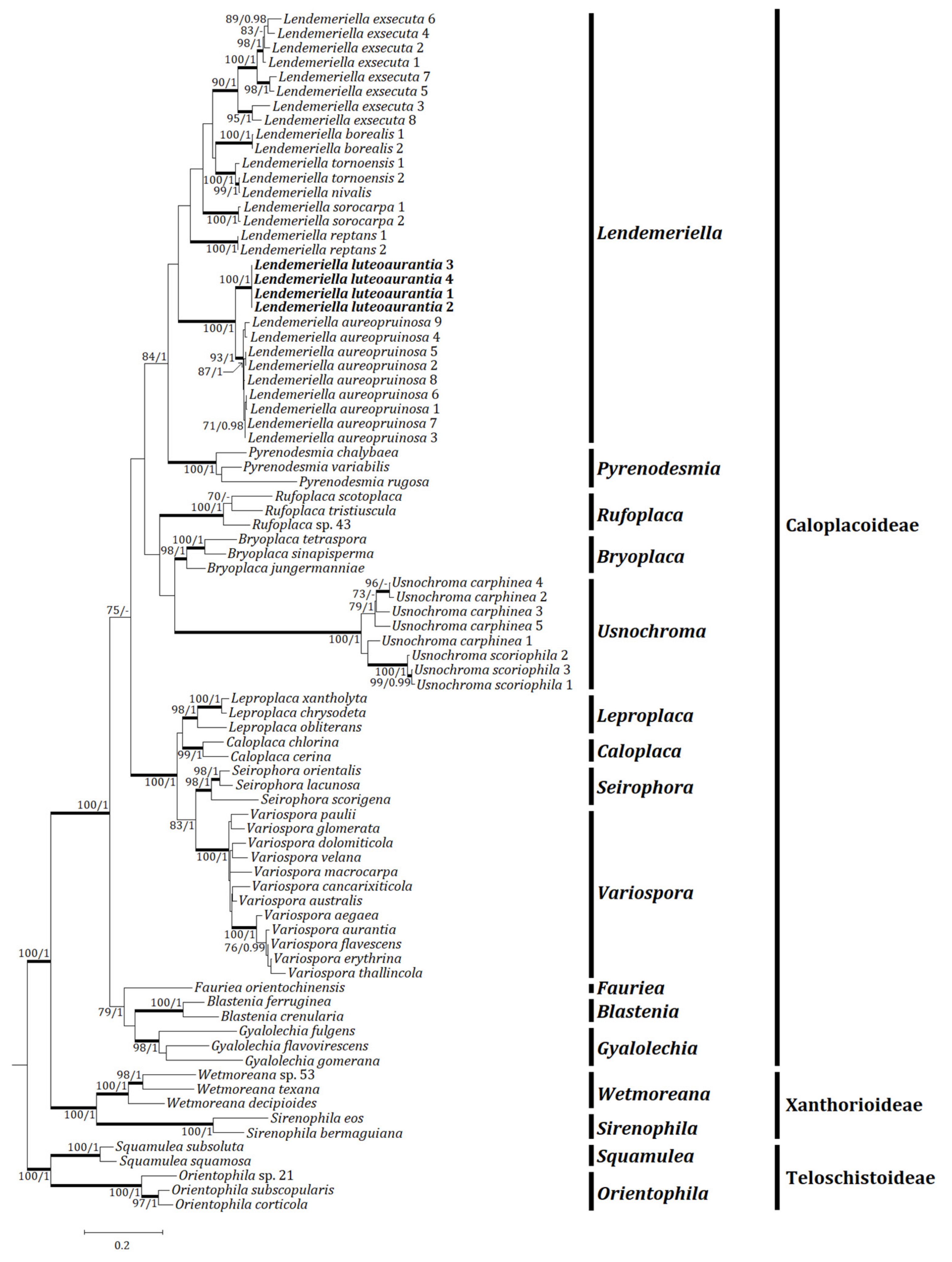
3.1. Phylogenetic Analyses
3.2. Taxonomy
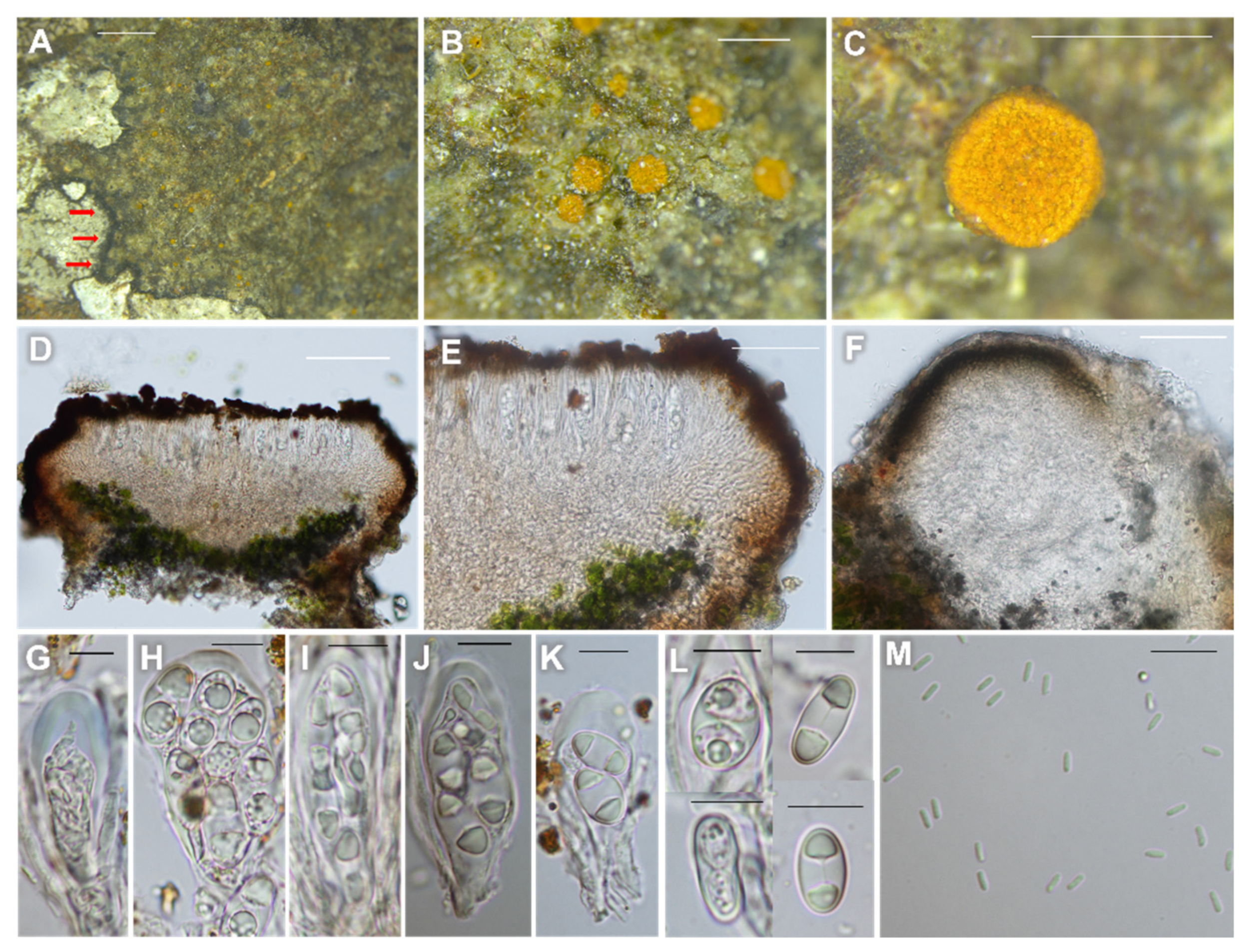
3.2.1. Lendemeriella luteoaurantia B.G. Lee sp. nov. (Figure 3)
| Species | Lendemeriella luteoaurantia | Lendemeriella aureopruinosa | Lendemeriella exsecuta | Lendemeriella reptans |
|---|---|---|---|---|
| Soredia | not observed | not observed | not observed | present |
| Apothecia color | yellow-orange to light orange | dark orange to orange-red | brown-yellow, orange-brown, or black | red-brown |
| Apothecia (mm in diam.) | 0.1–0.4 | 0.3–0.6 | 0.2–0.7 | immature |
| Thalline margin | absent | absent | absent | present (gray) |
| Hypothecium color | colorless to slightly yellowish | yellowish (upper), colorless (lower) | yellow to brown | – |
| Paraphysial tip width (μm) | 1–2 | 3–4 | 1.5–4 | – |
| Pigment in true exciple | not pigmented | Cinereorufa-green | Cinereorufa-green | – |
| Ascospores (μm) | 11–18 × 5–9.5 | 11.5–15 × 6–7 | 12–16.5 × 6–7.5 | – |
| Substance | emodin, parietin, parietinic acid, fallacinal | emodin, fallacinal parietin, parietinic acid, teloschistin | 7-chloroemodin, emodin, fallacinal, fragilin, parietin, parietinic acid, teloschistin | no substance |
| Reference | KBA-L-0004040 (isotype), KBA-L-0004041 (isotype), KBA-L-0004045 (holotype), KBA-L-0004046 (isotype) | [3] | [20] | [21] |
3.2.2. Key to the Species of Lendemeriella (Table 3)
| 1. | On rock or moss on rock | 2 |
| – | On bark | 7 |
| 2. | Directly on rock | 3 |
| – | On moss | 6 |
| 3. | Soredia present; apothecia immature; thalline margin gray; no substance | L. reptans |
| – | Soredia absent; apothecia developed; proper margin present only; emodin, fallacinal, parietin, parietinic acid present or other substance present | 4 |
| 4. | Apothecia yellow-orange to light orange, less than 0.5 mm diam.; hypothecium entirely colorless; tips of paraphyses little swollen; ascospores 11–18 × 5–9.5 μm; Cinereorufa-green pigment absent; teloschistin absent | L. luteoaurantia |
| – | Apothecia brown-yellow, red-brown to black, 0.5–0.6 mm diam.; hypothecium yellowish to brownish; tips of paraphyses swollen; ascospores 12–17 × 6–7.5 μm; Cinereorufa-green pigment present in true exciple; teloschistin present | 5 |
| 5. | Apothecia brownish or entirely black; 7-chloroemodin and fragilin present; arctic-alpine | L. exsecuta |
| – | Apothecia reddish; 7-chloroemodin and fragilin absent; boreal-montane | L. aureopruinosa |
| 6. | On Racomitrium; thalline margin pale to dark gray; ascospores 25–30 × 3–5 μm; distributed in mid to high latitudes | L. nivalis |
| – | On Andreaea or Grimmia; thalline margin absent; ascospores 17–20 × 6–7.5 μm; limited to high latitudes | L. tornoensis |
| 7. | Soredia present; apothecia extremely rare or absent | 8 |
| – | Soredia absent; apothecia generally present | 9 |
| 8. | Soralia K+ purple | L. lucifuga |
| – | Soralia K–, C+ pale yellow | L. sorocarpa |
| 9. | On deciduous trees (Alnus, Salix or Sorbus); thallus whitish, smooth to wrinkled; prothallus black; apothecia yellow, orange to reddish, 0.2–0.5 mm diam.; thalline margin absent; epihymenium K+ red; emodin, fallacinal, parietin, parietinic acid, and teloschistin present | L. borealis |
| – | On conifers; thallus gray-brown, subsquamulose, margin uplifted and lobed; prothallus absent; apothecia brown 0.5–0.8 mm diam.; thalline margin present; epihymenium K–; no substance | L. dakotensis |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vondrák, J.; Frolov, I.; Davydov, E.A.; Yakovchenko, L.; Malíček, J.; Svoboda, S.; Kubásek, J. The lichen family Teloschistaceae in the Altai-Sayan region (Central Asia). Phytotaxa 2019, 396, 1–66. [Google Scholar] [CrossRef]
- Kondratyuk, S.Y.; Lőkös, L.; Farkas, E.; Kärnefelt, I.; Thell, A.; Yamamoto, Y.; Hur, J.S. Three new genera of the Teloschistaceae proved by three gene phylogeny. Acta Bot. Hung. 2020, 62, 109–136. [Google Scholar] [CrossRef]
- Frolov, I.V.; Vondrák, J.; Konoreva, L.A.; Chesnokov, S.V.; Himelbrant, D.E.; Arup, U.; Stepanchikova, I.S.; Prokopiev, I.A.; Yakovchenko, L.S.; Davydov, E.A. Three new species of crustose Teloschistaceae in Siberia and the Far East. Lichenologist 2021, 53, 233–243. [Google Scholar] [CrossRef]
- Arup, U.; Søchting, U.; Frödén, P. A new taxonomy of the family Teloschistaceae. Nord. J. Bot. 2013, 31, 016–083. [Google Scholar] [CrossRef]
- Orange, A.; James, P.W.; White, F.J. Microchemical Methods for the Identification of Lichens; The British Lichen Society: London, UK, 2001; pp. 1–101. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Sninsky, J., White, T., Eds.; Academic Press: Cambridge, MA, USA, 1990; Volume 1, pp. 315–322. [Google Scholar]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Ekman, S. Molecular phylogeny of the Bacidiaceae (Lecanorales, lichenized Ascomycota). Mycol. Res. 2001, 105, 783–797. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.R.; Drummond, A.J. bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol. Biol. 2017, 17, e42. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v1.4.2. Edinburgh: University of Edinburgh. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 28 February 2022).
- Lee, B.G.; Hur, J.S. A new lichenized fungus, Lecanora baekdudaeganensis, from South Korea, with a taxonomic key for Korean Lecanora species. MycoKeys 2020, 70, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.W. American Arctic Lichens II: The Microlichens; University of Wisconsin Press: Madison, WI, USA, 1997; pp. 1–675. [Google Scholar]
- Wetmore, C.M. Notes on Caloplaca cerina (Teloschistaceae) in North and Central America. Bryologist 2007, 110, 798–807. [Google Scholar] [CrossRef]
- Stenroos, S.; Velmala, S.; Pykälä, J.; Ahti, T. Lichens of Finland; Botanical Museum, Finnish Museum of Natural History: Helsinki, Finland, 2016; pp. 1–896. [Google Scholar]
- Joshi, Y.; Andreev, M.; Hur, J.-S. Caloplaca lacinulata rediscovered for the lichen flora of South Korea. Feddes Repert. 2011, 122, 421–423. [Google Scholar] [CrossRef]
- Hue, A. Lichenes morphologice et anatomice disposuit (continuatio). Nouv. Arch. Mus. Hist. Nat. 1913, 5, 133–398. [Google Scholar]
- Nimis, P.L. ITALIC-The Information System on Italian Lichens. Version 7.0. Trieste: University of Trieste, Department of Biology. Available online: https://dryades.units.it/italic (accessed on 18 May 2023).
- Hodkinson, B.P.; Lendemer, C. Phylogeny and Taxonomy of an Enigmatic Sterile Lichen. Syst. Bot. 2012, 37, 835–844. [Google Scholar] [CrossRef]

| Species | nuITS | nuLSU | mtSSU | Voucher |
|---|---|---|---|---|
| Blastenia crenularia | KC179415 | KC179162 | KC179492 | Søchting 7523 |
| Blastenia ferruginea | KC179416 | KC179163 | KC179493 | Søchting 9996 |
| Bryoplaca jungermanniae | KC179420 | MT952895 | MT952925 | Søchting 10451 |
| Bryoplaca sinapisperma | KC179421 | MT952896 | KC179495 | Arup L08184 |
| Bryoplaca tetraspora | KC179422 | MT952897 | KC179496 | Søchting 7979 (ITS); Søchting 10,480 (LSU, SSU) |
| Caloplaca cerina | KC179425 | KC179168 | KC179499 | Elvebakk 03:084 |
| Caloplaca chlorina | KC179426 | KC179169 | KC179500 | Frödén 1876 (ITS); Søchting 7321 (LSU, SSU) |
| Fauriea orientochinensis | KX793097 | KX793100 | KX793103 | KoLRI 013957 |
| Gyalolechia fulgens | KC179440 | KC179199 | KC179533 | Poelt, Nimis and Tretiach 95/460 (ITS); Arup L06206 (LSU); Søchting 10,586 (SSU) |
| Gyalolechia gomerana | KC179441 | KC179200 | KC179534 | Søchting 9653 |
| Gyalolechia flavovirescens | AF353966 | KC179198 | KC179532 | Arup L97253 (ITS); Søchting 8648 (LSU, SSU) |
| Lendemeriella aureopruinosa 1 | MG954212 | - | - | Chesnokov_sn |
| Lendemeriella aureopruinosa 2 | MG954213 | - | - | Chesnokov 239 |
| Lendemeriella aureopruinosa 3 | MG954214 | - | - | Chesnokov230 |
| Lendemeriella aureopruinosa 4 | MN814228 | MW227504 | MW227332 | LE-L15207 |
| Lendemeriella aureopruinosa 5 | MN814229 | MW227511 | MW227333 | LE-L15209 |
| Lendemeriella aureopruinosa 6 | MN814230 | - | - | LE-L15210 |
| Lendemeriella aureopruinosa 7 | MN814231 | - | - | LE-L15212 |
| Lendemeriella aureopruinosa 8 | MN814233 | - | - | Frolov 2232 |
| Lendemeriella aureopruinosa 9 | MN814234 | MW227505 | MW227331 | Frolov 2236 (ITS); Frolov 2473 (LSU, SSU) |
| Lendemeriella borealis 1 | KX216687 | - | - | IF 1184 |
| Lendemeriella borealis 2 | MW227317 | MW227512 | MW227334 | Frolov 2476 |
| Lendemeriella exsecuta 1 | MG954130 | - | - | Vondrák 11105 |
| Lendemeriella exsecuta 2 | MG954131 | - | - | Vondrák 11110 |
| Lendemeriella exsecuta 3 | MG954211 | - | - | Tønsberg 46194 |
| Lendemeriella exsecuta 4 | MG954223 | - | - | Zhdanov_sn |
| Lendemeriella exsecuta 5 | MG954224 | - | - | Spribille 39677 |
| Lendemeriella exsecuta 6 | MG954225 | - | - | Vondrák 7420 |
| Lendemeriella exsecuta 7 | MG954226 | - | - | Vondrák 6201 |
| Lendemeriella exsecuta 8 | MG954227 | - | - | Spribille 24441 |
| Lendemeriella luteoaurantia 1 | OQ981385 | OQ981381 | KBA-L-0004040 | |
| Lendemeriella luteoaurantia 2 | OQ981386 | OQ981382 | KBA-L-0004041 | |
| Lendemeriella luteoaurantia 3 | OQ981387 | OQ981383 | KBA-L-0004045 | |
| Lendemeriella luteoaurantia 4 | OQ981388 | OQ981384 | KBA-L-0004046 | |
| Lendemeriella nivalis | MG954222 | - | - | Spribille 29306 |
| Lendemeriella reptans 1 | JQ686192 | - | JQ686191 | Lendemer 11745 |
| Lendemeriella reptans 2 | MH104934 | MH100766 | MH100796 | Lendemer 48186 |
| Lendemeriella sorocarpa 1 | MG773658 | - | - | Vondrák 14274 |
| Lendemeriella sorocarpa 2 | MG954132 | - | - | Vondrák 12695 |
| Lendemeriella tornoensis 1 | MG954220 | - | - | Spribille 26816 |
| Lendemeriella tornoensis 2 | MG954221 | - | - | Spribille 29473 |
| Leproplaca chrysodeta | KC179448 | KC179206 | MT952933 | Arup L7107 (ITS, LSU); Arup L13261 (SSU) |
| Leproplaca obliterans | KC179449 | KC179207 | KC179541 | Arup L02331 (ITS, SSU); Arup L03472 (LSU) |
| Leproplaca xantholyta | KC179451 | KC179208 | KC179542 | Arup L97278 (ITS); Søchting 9675 (LSU, SSU) |
| Orientophila corticola | MN687909 | - | MN687910 | KBA-L-0000118 |
| Orientophila subscopularis | KC179375 | - | KC179546 | Frisch Jp171 |
| Orientophila sp. 21 | KC179372 | KC179210 | KC179544 | Frisch Jp99 |
| Pyrenodesmia chalybaea | KC179454 | MT952921 | KC179571 | Søchting 9351 |
| Pyrenodesmia rugosa | MW832828 | MW832804 | MW832825 | KBA-L-0001099 |
| Pyrenodesmia variabilis | AF353963 | KC179234 | KC179572 | Arup s.n. (ITS); Arup L03134 (LSU, SSU) |
| Rufoplaca scotoplaca | KC179457 | KC179235 | KC179573 | Arup L10032 |
| Rufoplaca sp. 43 | KC179458 | KC179236 | KC179574 | Arup L09201 |
| Rufoplaca tristiuscula | KC179460 | KC179237 | KC179575 | Arup L08171 |
| Seirophora lacunosa | KC179465 | KC179243 | KC179582 | Moberg & Nordin K18:04 |
| Seirophora orientalis | KJ021240 | - | - | KoLRI 011917 |
| Seirophora scorigena | KC179466 | KC179244 | KC179583 | S. & B Snogerup 17201 |
| Sirenophila bermaguiana | KC179299 | KC179245 | KC179584 | Kondratyuk 20487 |
| Sirenophila eos | KC179300 | KC179246 | KC179585 | Kärnefelt 20044702 |
| Squamulea squamosa | KC179125 | KC179252 | KC179591 | Kärnefelt AM960105 |
| Squamulea subsoluta | AF353954 | KC179253 | KC179592 | Arup L97072 |
| Usnochroma carphinea 1 | EU639594 | - | - | A. Terron (LEB 4452) |
| Usnochroma carphinea 2 | EU639595 | - | - | E. Gaya 201, X. Llimona & M. De Caceres (BCN 13714) |
| Usnochroma carphinea 3 | KC179468 | KC179259 | KC179598 | 1998, Roux s.n. |
| Usnochroma carphinea 4 | MZ391142 | - | - | VAL_Lich 31793 |
| Usnochroma carphinea 5 | - | JQ301548 | JQ301482 | E. Gaya, S. Fernandez-Brime 542 & X. Llimona (BCN) |
| Usnochroma scoriophila 1 | EU639596 | - | - | N. Hladun & A. Gomez-Bolea (BCN) |
| Usnochroma scoriophila 2 | JQ301664 | JQ301560 | JQ301496 | P. & B. v.d. Boom 38386 |
| Usnochroma scoriophila 3 | KC179469 | KC179260 | KC179599 | 1995, Gomez-Bolea |
| Variospora aegaea | EU639597 | - | - | E. Gaya 248 & X. Llimona (BCN) |
| Variospora aurantia | KC179470 | KC179261 | KC179600 | 1998, Llimona (ITS, SSU); 2006, Lange (LSU) |
| Variospora australis | AY233223 | - | - | Gaya 239 |
| Variospora cancarixiticola | EU639608 | - | KT291482 | Llimona and Egea s.n. |
| Variospora dolomiticola | KC179471 | KC179262 | KC179601 | Thell SP0514 |
| Variospora erythrina | KC179472 | - | - | 1998, Lutzoni s.n. |
| Variospora flavescens | KC179473 | KC179263 | KC179602 | Søchting 9601 (ITS); Arup L03060 (LSU, SSU) |
| Variospora glomerata | KC179474 | KC179264 | KC179603 | Arup L03119 |
| Variospora macrocarpa | AF353956 | - | - | Arup L97306 |
| Variospora paulii | EU639606 | - | KT291503 | Gaya 183 |
| Variospora thallincola | KC179475 | JQ301563 | KC179604 | Søchting 7481 (ITS); Gaya et al. s.n. (LSU); Arup L92148 (SSU) |
| Variospora velana | KC179476 | KC179265 | KC179605 | Arup L07194 (ITS); Arup L07123 (LSU, SSU) |
| Wetmoreana decipioides | KC179333 | KC179269 | KC179608 | Thor 20768 |
| Wetmoreana texana | KC179337 | KC179273 | KC179612 | Søchting 9925 |
| Wetmoreana sp. 53 | KC179335 | KC179271 | KC179610 | Frödén 1519 |
| Overall | 82 | 49 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-G.; Hur, J.-S. A New Lichenized Fungus, Lendemeriella luteoaurantia, with a Key to the Species of Lendemeriella. Diversity 2023, 15, 845. https://doi.org/10.3390/d15070845
Lee B-G, Hur J-S. A New Lichenized Fungus, Lendemeriella luteoaurantia, with a Key to the Species of Lendemeriella. Diversity. 2023; 15(7):845. https://doi.org/10.3390/d15070845
Chicago/Turabian StyleLee, Beeyoung-Gun, and Jae-Seoun Hur. 2023. "A New Lichenized Fungus, Lendemeriella luteoaurantia, with a Key to the Species of Lendemeriella" Diversity 15, no. 7: 845. https://doi.org/10.3390/d15070845
APA StyleLee, B.-G., & Hur, J.-S. (2023). A New Lichenized Fungus, Lendemeriella luteoaurantia, with a Key to the Species of Lendemeriella. Diversity, 15(7), 845. https://doi.org/10.3390/d15070845






