Abstract
Parasitic moths are common in social wasp (Hymenoptera) nests, attacking many species of Polistinae and a few species of Vespinae. In the Republic of Korea, two moth species are known to parasitize the brood of Polistes rothneyi koreanus: Pyralis regalis (Pyralidae) and Anatrachyntis japonica (Cosmopterigidae). Although previously reported elsewhere, a novel case of parasitization was recently documented in the Republic of Korea, in which Hypsopygia mauritialis (Pyralidae) was identified in the nests of social wasps. Pyralis regalis is the most common parasitic moth in the Republic of Korea, feeding on the nests of 11 species of social wasps, mostly the Korean Vespa species. To that list of hosts, we add a species of Dolichovespula and two species of Polistes. Parasitism of Vespa velutina nigrithorax, an invasive alien hornet, by both P. regalis and H. mauritialis, was observed for the first time. However, their potential to control invasive alien hornets is expected to be low. This study provides new insights into the diversity of nest-parasitic moths in social wasp nests and their hosts in the Republic of Korea, and highlights the potential for these moths to impact pest populations.
1. Introduction
Vespidae harbor numerous parasites, including endoparasites and parasitoids. Adults wasps are parasitized by twisted-winged insects (Stylopidae: Strepsiptera) and nematode worms (Mermithidae: Nematoda) [1], and colonies are attacked by the larvae of various species of moths, including Pyralis sp. (Pyralidae), Hypsopygia spp. (Pyralidae), Aphomia sp. (Pyralidae), Chalcoela spp. (Crambidae), Anatrachyntis spp. (Cosmopterigidae), Tinea spp. (Tineidae), Antipolistes sp. (Tineidae), Taeniodictys sp. (Lyonetiidae), and Athrips sp. (Gelechiidae) [2,3,4,5,6,7,8,9,10,11,12]. Most of these moths parasitize Polistinae (Polistes spp. and Belonogaster spp.), but some species of Pyralidae occur in the nests of Vespinae as well [1]. Notably, Pyralis regalis (Denis and Schiffermüller, 1775) and Hypsopygia mauritialis (Boisduval, 1833) are known to parasitize Vespa spp. [1,13], while Aphomia sociella (Linnaeus, 1758) is parasitic in the nests of both Vespula spp. and Dolichovespula spp. [14,15].
Some parasitic moths facultatively feed on their host’s embryos. Others burrow through nest fibers, feeding on the developing wasp larvae and pupae in the cells, ultimately killing them. In addition, moth larvae obstruct the use of the nest by filling each cell with silk-like webbing, making it challenging for adult wasps to navigate the nest. Moth overwintering is typically accomplished in the abandoned nest, after the social wasp colony has completed its life cycle, with emergence usually taking place in early spring of the following year [6]. These moths are conspicuous natural enemies, weakening or disrupting wasp colonies [1,13,16]. Despite the commonness and importance of these moth-wasp interactions, studies on parasitoid moth hosts, development, and ecology are scarce.
Thirty species of social wasps have been recorded in the Republic of Korea [17], including the recently introduced hornet, Vespa velutina nigrithorax (du Buysson, 1905), which is now spreading [18], causing not only sting accidents in rural, forest, and urban areas, but also disrupting honeybees in apiaries, which has a significant economic impact [19,20,21]. Therefore, interest in biological control through natural enemies, as well as physico-chemical control, is increasing [22]; unfortunately, there is limited previous research on these topics. In the Republic of Korea, the only known nest-parasitic moths from social wasp nests are P. regalis and Anatrachyntis japonica (Kuroko, 1982), both which attack the nests of Polistes jadwigae (Dalla Torre, 1904) (=P. rothneyi, Cameron, 1900) [23]. In this study we identified moth species using morphological characters and DNA barcodes of the Hypsopygia species, which were newly recorded in the nests of social wasps. Our results are based on an eight-year study of parasitoid moths in Korea.
2. Materials and Methods
2.1. Sample Collection
We collected social wasp nests from 2014 to 2023. The nests underwent scrutiny, and any cells exhibiting evidence of silk bundles attributable to parasitic moths, or those harboring moth larvae and adults, during either indoor or outdoor storage or during nest dissection were recorded separately. For nests with severe infections, a breeding net (60 × 38 × 40 cm) (Manchun, Seoul, Republic of Korea) was utilized, and monitoring was conducted until all moths emerged. Pertinent information such as the host wasp species, collection site, date of nest collection, date of moth emergence, and the total count of moths was meticulously documented for each nest (Table 1 and Supplementary Table S1).

Table 1.
Parasitic moths developed in the nests of social wasps (host) from 2014 to 2023.
2.2. Identification
Based on a list of moths previously recorded by Jeong et al. [23], the moths in this study were identified based on studies by Lee and Bae [24], and Yoon and Byun [25], and the larvae were identified based on Heo [26].
The Hypsopygia sp., an undiscovered moth in the Republic of Korea, was identified based on its external morphology, genitalia, and DNA barcodes. Larvae were inferred based on the adult species in the nest and identified using a description by Yamane et al. [16].
The male and female genitalia were dissected and mounted with an euparal solution, as described previously [27]. Images were captured using a DFC 495 digital camera (Leica, Wetzlar, Germany) attached to a LEICA M205C (Leica) and a Dhyana 95 scientific CMOS camera (Tucsen, Fuzhou, China) attached to a Leica DM 3000 LED optical microscope (Leica).
Genomic DNA from 13 Korean specimens of Hypsopygia sp., 6 specimens from Gurye, and 7 specimens from Jeonju were extracted using a Genomic Cell/Tissue Spin Mini Kit (Qiagen, Inc., Hilden, Germany) according to the manufacturer’s protocol. The DNA barcodes were amplified using the primers LepF1, LepR1 [28], MLepF1, MLepR1 [29]. The PCR conditions for amplification followed the manufacturer’s protocol (Platinum Taq, Invitrogen, Carlsbad City, CA, USA). The amplicons were purified using the QIAquick® PCR purification kit (QIAGEN, Inc., Hilden, Germany) and directly sequenced at Macrogen (Seoul, Republic of Korea). The contigs were assembled using Geneious 11 (Biomatters, Auckland, New Zealand).
We also used the data from 45 COI sequences of 11 species registered in BOLD SYSTEMS and NCBI. In total, 11 species with 58 COI sequences, including outgroups, were used for data analysis. Sequence alignments were performed using MEGA 11 [30], and uncertain anterior and posterior regions were removed. Neighbor-joining analysis was performed, and pairwise distances were calculated using MEGA 11 [30] based on the Kimura-2-Parameter (K2P) model. Bootstrap support values for each node were evaluated using 1000 replicates. As an outgroup, we selected P. regalis, which belongs to the sister group [31] of the Tribe Pyralini.
The specimens used in this study have been deposited in the KNAE (Entomological Collection, Korea National Arboretum, Pocheon). The abbreviations used in this study are as follows: TD, type depository; TL, type locality; Synonym, (=); BMNH, Natural History Museum, London, UK.
3. Results
3.1. Systematic Accounts
- Family Pyralidae Latreille, 1809 [32]
- Subfamily Pyralinae Latreille, 1809 [32]
- Genus Hypsopygia Hübner, 1825 [33]
- Hypsopygia mauritialis (Boisduval, 1833) [34]
- Asopia mauritialis Boisduval, 1833:119. TL: Mauritius; TD: BMNH. [34]
- Pyralis lucillalis Walker, 1859: 268 [35]
- Pyralis regalis Walker, 1866: 1241 [36]
- Pyralis ducalis Walker, 1866: 1242 [36]
- Endotricha crobulus Lucas, 1891: 305 [37]
- Hypsopygia laticilialis Ragonot, 1891: 28 [38]
- Hypsopygia sanguinalis Warren, 1897: 125 [39]
- Hypsopygia atralis Caradja, 1932: 121 [40]
- Hypsopygia pfeifferi Amsel, 1954: 310 [41]
Adult (Figure 1A,B): Wingspan 14–21 mm, head yellow on frons, vertex with yellow scales. Male antenna 4/5 of forewing length, yellow mixed with reddish purple, and ciliate. Female antenna broken. Proboscis well-developed. Thorax reddish purple. Abdomen reddish purple, with lightly scattered black scales. Forewing ground color pale reddish purple, lightly scattered with blackish purple scales; costal and outer margin blackish purple. Antemedial line arising from a yellow costal patch, pale yellow, and curved. Postmedial line narrow and rather indistinct. Costa between antemedial and postmedial line with several (4–6) yellow minute spots and blackish purple discocellular spots. Cilia bright yellow. Hindwing ground color concolorous with forewing; outer margin blackish-purple. Antemedial and post-medial yellow lines quite indistinct and sinuate, surrounded by dark purple scales. Cilia as bright yellow as the forewing.
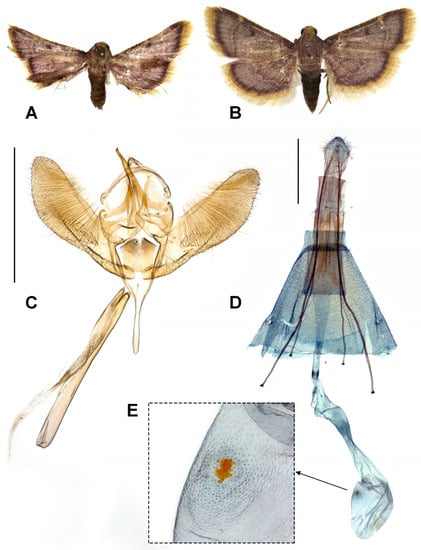
Figure 1.
Hypsopygia mauritialis. (A) Adult male; (B) adult female; (C) male genitalia (slide no. KNAEYM181); (D) female genitalia (slide no. KNAEYM182); (E) detail of signum. <Scale bar: 1 mm>.
Male genitalia (Figure 1C): Uncus dorsally stumpy; gnathos weakly sclerotized, slightly hooked distally; valva rather long and pilose; saccus long and tapered; juxta weakly sclerotized; phallus weakly sclerotized, rather long, approximately as long as valva; cornutus absent.
Female genitalia (Figure 1D,E): Apophyses thin and long, posterior apophyses 1.5 times longer than the anterior apophyses. Antrum membranous, long, and cylindrical with sclerotized colliculum. Ductus bursae long with ductus seminalis. Middle part wide and weakly sclerotized. Corpus bursae ovate with small and sclerotized signum.
Material examined: 1♂1♀ (genitalia slide no. KNAEYM281, 282), 7♀ (DNA barcode), 7 ♀ 16♀ (identification by external characters); Jeonju, Jeollabuk-do, 1. I. 2022. Coll. H.S. Lee; 3♂ (genitalia slide no. KNAEYM283), 1♂5♀ (DNA barcode), 3♂1♀ (identification by external characters), Gurye, Jeollanam-do, 23. I. 2022. Coll. M.B. Choi; 4♂1♀ (identification by external characters), Seongju, Gyeongsangbuk-do, 16. I. 2023. Coll. M.B. Choi; 6♂5♀ (identification by external characters), Jinju, Gyeongsangnam-do, 30. i—18. iii 2021. Coll. J.H. Kim; 5♂1♀ (identification by external characters), Jinju, Gyeongsangnam-do, 22 viii 2022. Coll. J.H. Kim.
Distribution: the Republic of Korea (new record), Japan, Taiwan, China, Iran, Nepal, India, Australia, Hawaii, Africa, Madagascar, and Mauritius.
3.2. DNA Barcode
The sequence was uploaded to NCBI (accession numbers: OQ579040–OQ579052). The DNA barcode obtained in this study indicated 100% genetic similarity to that of H. mauritialis (accession no. KX860326) (Figure 2). Molecular analyses revealed that all species were strongly supported with high bootstrap values as a single lineage on neighbor-joining trees. The maximum intraspecific variation in H. mauritialis was 0.008%, and the interspecific pairwise K2P distances were 0.069–0.058% from the most similar species, H. aflavmaculata (Shaffer, Nielsen and Horak, 1996) in the COI region (Figure 2).
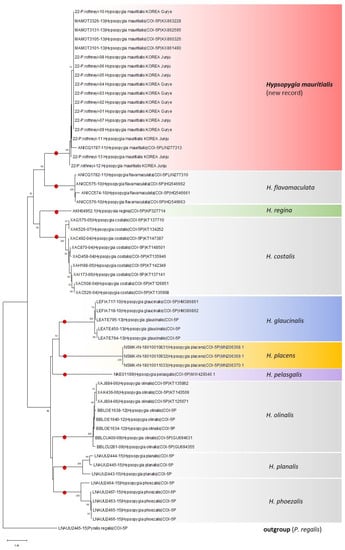
Figure 2.
Neighbor-joining tree of the genus Hypsopygia. The divergence was calculated using the Kimura 2-parameter model on the basis of 280–658 bp COI sequences. Bootstrap values below 50 are not shown. The sequences of the P. regalis, which includes Pyralini, were used as an outgroup.
3.3. New Host Records for Nest-Parasitic Moths
Nest-parasitic moths occurring in the nests of social wasps in the Republic of Korea consist of three species within three genera and two families (Table 1). Eleven wasp species are known as the hosts of P. regalis; of them, seven species are in Vespa (V. crabro flavofasciata (Cameron, 1903), V. simillima simillima (Smith, 1868) (Figure 3B), V. velutina nigrithorax, V. ducalis (Jordan, 1922), V. analis parallela (André, 1884) (Figure 3A), V. dybowskii (André, 1884) (Figure 3D), and V. mandarinia (Smith, 1852) (Figure 3C), one species is in Dolichovespula (D. kuami Kim et Yoon, 1996), and three species are in Polistes (P. rothneyi koreanus (van der Vecht, 1968) (Figure 4A–C), P. yokahamae (Rodoszkowski, 1887), and P. nipponensis (Pérez, 1905).
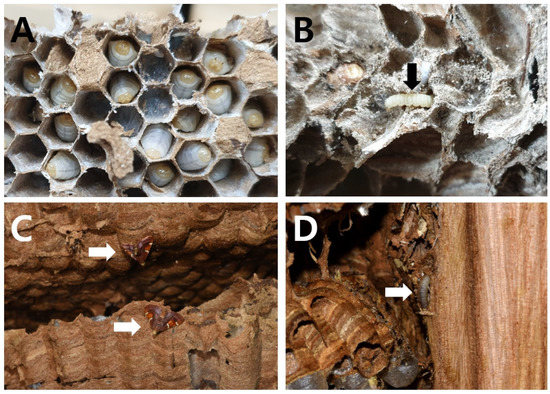
Figure 3.
A nest-parasitic moth, P. regalis (arrow), appeared in the nests of the Vespa species in the Republic of Korea. (A) Traces of silk bundles generated by P. regalis in the nest of V. analis parallela; (B) larvae of P. regalis developed from the nest of V. simillima simillima; (C) P. regalis adults emerged from the nest of V. mandarinia; (D) larvae of P. regalis developed in the nest of V. dybowskii.
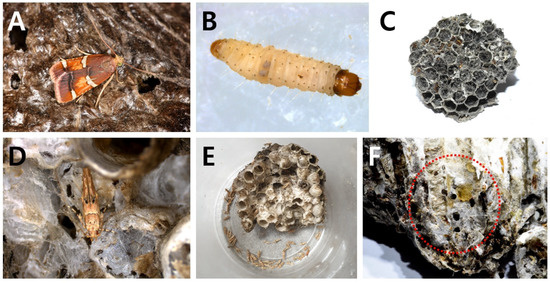
Figure 4.
Nest-parasitic moths from the nests of P. rothneyi koreanus. (A,B) Adult (A) and larvae (B) of P. regalis occurred in the nests; (C) excreta filled in most cells of the nest by P. regalis larvae feeding inside (28 September, Nest 24); (D) adult of A. japonica emerging from nest; (E) entire nest infected by A. japonica; (F) holes (red circle) made by A. japonica larvae feeding through the nest.
The hosts of A. japonica were two species in two genera: V. crabro flavofasciata and P. rothneyi koreanus (Figure 4D–F). In some cases, P. regalis and A. japonica coexisted in one nest of P. rothneyi koreanus (Nest 22 (=29) in Table 1). H. mauritialis, first recorded in the Republic of Korea, appeared to have two host species within two genera (P. rothneyi koreanus (Figure 5) and V. velutina nigrithorax (Figure 6)).
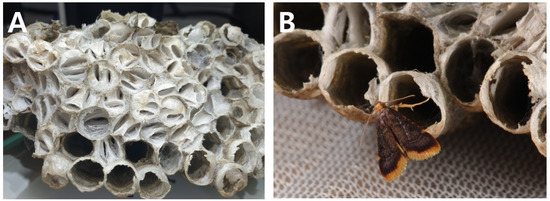
Figure 5.
H. mauritialis from nests of P. rothneyi koreanus. (A) Entire nest infested by H. mauritialis; (B) H. mauritialis adults newly emerged from a nest.
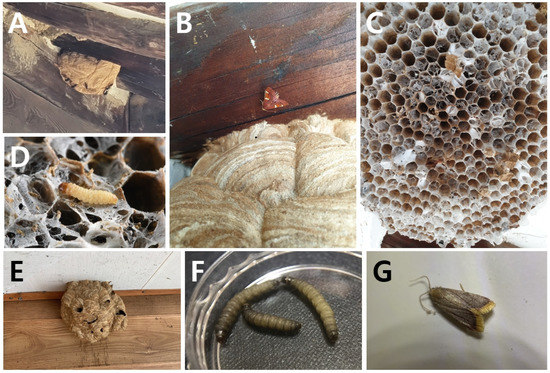
Figure 6.
Nest-parasitic moths from the nests of the invasive alien hornet, V. velutina nigrithorax. (A) The primary nest of V. velutina nigrithorax infected by P. regalis (Andong, Nest 11), with wasp workers remaining until early August; (B) adult P. regalis from Nest 11; (C) Nest 11, where most of the comb was parasitized by P. regalis; (D) P. regalis larvae occurred from Nest 10, of which most of the cells were covered by a silk web of the larvae; (E) the primary nest parasitized by H. mauritialis (Jinju, Nest 38), there were no workers in the nest in early August; (F,G) H. mauritialis larvae and adults found in Nest 37 (found dead in nests kept indoors). See Table 1 for the nest numbers.
3.4. Parasitic Moths from Invasive Social Wasp Nests
P. regalis and H. mauritialis occurred from the nests of the invasive alien hornet, V. velutina nigrithorax. The larvae of P. regalis were discovered in six mature nests after November. These nests were kept indoors during winter, and the adults emerged in February–March, after overwintering. However, in the Noha-dong and Andong nests, seven adult moths (Figure 6B) were collected from both the inside and outside of the nest on August 3rd, and 36 larvae (Figure 6D) were also found. A high percentage (91.6%) of the cells (2 combs, total cells 797, infected cells 730) were heavily covered with a silk web spun by the moth larvae (Figure 6C), while only 13 wasp workers still remained on the nests (Figure 6A). H. mauritialis was found in two nests (Figure 6E). In the first nest, 11 adults (Figure 6G) and four larvae (Figure 6F) were collected from the discarded nests between January and March, and in the second nest, six adults and 8 larvae were collected in August. At this time, both nests had three combs, with most of the first two combs being infected, and the third comb was not infected at all. Unfortunately, the total number of cells could not be measured because of the damage on the nests.
4. Discussion
4.1. Occurrence of Moths in Social Wasp Nests
P. regalis appeared to be the most widespread moth species in Korean social wasp nests, parasitizing the nests of 11 different host species within three genera, namely Vespa, Dolichovepula, and Polistes. With the exception of V. binghami (du Buysson, 1905), P. regalis was found in almost all Vespa species (eight in total, excluding two subspecies) found in the Republic of Korea [17]. The nest of V. binghami, being a rare nocturnal wasp, has never been observed in the Republic of Korea, and the parasitism status of the moth on this particular wasp is still unknown. The subspecies V. simillima xanthoptera (Cameron, 1903) is restricted to Jeju Island and has not been adequately studied. Although P. regalis reportedly infests V. simillima [42], it is highly likely that this moth also infests V. simillima xanthoptera nests. Thus, P. regalis is capable of parasitizing the nests of most Vespa species. Moreover, the moth occurs in the Polistes species as well, as recorded in P. yokahamae, P. nipponensis, and P. rothneyi koreanus [23]. Notably, the nest parasitization of P. regalis has been observed for the first time in the genus Dolichovespula, namely D. kuami, a species endemic to the Republic of Korea in the present study. However, P. regalis has never been found in the nests of any Vespula. While moths have been found in the nests of V. crabro flavofasciata, V. mandarinia, and V. ducalis, which also have underground nests like Vespula, at present, it is unclear why the moth does not occur in the nests of the Vespula species.
A. japonica occurred in P. rothneyi koreanus nests in the Republic of Korea [23] and P. chinensis antennalis (Perez, 1905) nests in Japan [8]. In our study, A. japonica was found to infest the nest of V. crabro flavofasciata for the first time; therefore, this moth can parasitize Vespa, as well as Polistes, much like other moth species. However, ecological and biological information on these species is still lacking, and further sample and host investigations are required.
Two species in Hypsopygia, namely H. mauritialis and H. postflava (Hampson, 1893), were previously recorded, occurring in the nests of social wasps in Japan, and their identification is challenging due to their similar appearance [16]. The Hypsopygia species are mostly known to parasitize the nests of Polistinae wasps [5,6,43], with an exception of H. mauritialis, which has also been found in the nest of V. affinis (Linnaeus, 1764) in Japan [13]. Although these two Hypsopygia species were not previously reported from the Republic of Korea, and their morphology closely resembles H. regina (Butler, 1879) [24,42], we confirmed their identity as H. mauritialis based on their external morphology, genitalia structure, and DNA barcoding. In the Republic of Korea, H. mauritialis was newly discovered in Jinju in 2021 and subsequently found in Jeonju, Gurye, and Seongju in the southern region of the country, suggesting its wide distribution in the region. Given its occurrence in Vespa and Polistes, it appears to have a similar host preference to P. regalis.
4.2. First Occurrence in Invasive Alien Social Wasp Nests
The invasive alien hornet, V. velutina nigrithorax, which was introduced to the Republic of Korea from China in 2003, has spread throughout the Republic of Korea [18,44] and Europe, causing negative impacts [45]. Only few natural enemies of this hornet were ever documented in Europe and the Republic of Korea, where the conopid fly [46], nematode [47], and marten [48] have been studied as predators. Therefore, the occurrence of P. regalis and H. mauritialis is the very first record of nest-parasitic moths of V. velutina nigrithorax in an invaded country, the Republic of Korea.
When bees and ants (other social insects) are infected by parasites such as viruses, mites, and fungi, their colonies often collapse [49,50,51,52]. However, colony collapse due to parasitism is rare in social wasps, indicating that their ability to control such parasites is significant [46,47,53]. Compared with other parasites, these parasitic moths do not affect the brood of wasp hosts directly, not as an obligatory parasite, but rather disrupt the development of nests in feeding, webbing, and occasional predation on larvae or pupae in social wasp nests [5,6,8,13,16] (see also [1]).
In general, for Polistes nests with small colonies, if the moth infection rate is high, colonies often collapse (Figure 4C,E) due to the feeding activity of the moth larvae (Figure 4F) [11,54,55]. However, large colonies such as the Vespa species are known to have little impact [13].
For P. regalis development within V. velutina nigrithorax nests between November and December, some larvae were found in the uppermost combs of the discarded mature nests, which appeared to give little negative impact on the colonies. However, when the nests were discovered in August, during periods of rapid expansion, such as Nests 11 and 38 (Table 1), few combs were almost entirely infested (Figure 6C), and there were limited numbers of hornet larvae, pupae, and adults remaining (Figure 6A). As this is the time when V. velutina nigrithorax relocates from the primary to the secondary nest [56], it seems that the nest gradually emptied due to the movement of V. velutina nigrithorax, rather than the colony collapsing, allowing the moths to occupy the entire nest. Therefore, these nest-feeding moths do not appear to significantly affect the maintenance of V. velutina nigrithorax colonies.
5. Conclusions
This study identified three nest-parasitic moths from social wasp nests in the Republic of Korea, including the newly recognized H. mauritialis, along with P. regalis and A. japonica that were previously recorded. Additionally, over the course of a decade-long search, a total of 11 species within three genera of hosts were newly recorded. Notably, this study identified, for the first time, P. regalis and H. mauritialis extensively feeding in the nests of V. velutina nigrithorax, the invasive alien hornet in the Republic of Korea. While these parasitic moths may have potential as natural enemies of social wasps, they do not appear to have a significant controlling effect when a rapid response is required, such as in situations where social wasps are increasing in urban areas or when invasive social wasps are rapidly spreading.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15060789/s1, Table S1: Collected location of social wasp nests.
Author Contributions
Conceptualization, M.B.C. and C.-J.K.; methodology, Y.-M.S., I.-K.K. and M.B.C.; formal analysis, Y.-M.S. and M.B.C.; investigation, H.S.L. and M.B.C.; resources, M.B.C. and H.S.L.; supervision M.B.C. and C.-J.K.; visualization, Y.-M.S. and M.B.C.; data curation, M.B.C. and Y.-M.S.; writing—original draft preparation, M.B.C., Y.-M.S. and C.-J.K.; writing—review and editing, M.B.C. and I.-K.K.; project administration, M.B.C., I.-K.K. and C.-J.K.; funding acquisition, C.-J.K. and I.-K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Scientific Research [KNA1-1-25, 19-1] of the Korea National Arboretum.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data can be found within the manuscript.
Acknowledgments
We thank C. H. Ma for providing the host hornet nests. We also thank J. H. Kim for collecting H. mauritialis individuals from invasive hornet nests.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matsuura, M.; Yamane, S. Biology of the Vespine Wasps; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Nelson, J.M. Parasites and symbionts of nests of Polistes wasps12. Ann. Entomol. Soc. Am. 1968, 61, 1528–1539. [Google Scholar] [CrossRef]
- Makino, S. List of parasitoids of polistine wasps. Sphecos 1985, 10, 19–25. [Google Scholar]
- Hodges, A.C.; Hodges, G.S.; Espelie, K.E. Parasitoids and parasites of Polistes metricus Say (Hymenotera: Vespidae) in Northeast Georgia. Ann. Entomol. Soc. Am. 2003, 96, 61–64. [Google Scholar] [CrossRef]
- Kato, N.; Yamada, Y.Y.; Matsuura, M.; Tsukada, M. Mating, oviposition, and prey use by larvae of Hypsopygia postflava (Lepidoptera: Pyralidae), a moth parasitic on nests of the paper wasp, Polistes jokahamae. Jpn. J. Appl. Entomol. Zool. 2007, 51, 45–50. [Google Scholar] [CrossRef]
- Kato, N.; Yamada, Y.Y.; Matsuura, M.; Tsukada, M. Life cycle of Hypsopygia postflava (Lepidoptera: Pyralidae), a moth parasitic on nests of the paper wasp, Polistes jokahamae. Jpn. J. Appl. Entomol. Zool. 2007, 51, 115–120. [Google Scholar] [CrossRef]
- Starr, C.K.; Nelson, J.M. Differential nest parasitism in three sympatric social wasps (Hymenoptera: Vespidae: Polistes spp.) in the West Indies. Sociobiology 2015, 62, 604–606. [Google Scholar] [CrossRef]
- Miyano, S. Life tables of colonies and workers in a paper wasp, Polistes chinensis antennalis, in central Japan (Hymenoptera: Vespidae). Res. Popul. Ecol. 1980, 22, 69–88. [Google Scholar] [CrossRef]
- Makino, S. Paper wasps as hosts of parasitoids. Kotaigun Seitaigakkai Kaihô (Bull. Soc. Popul. Ecol.). 1983, 37, 53–66. (In Japanese) [Google Scholar]
- Inoue, H. Pyralidae. In Moths of Japan 1; Inoue, H., Sugi, S., Kuroko, H., Moriuti, S., Kawabe, A., Owada, M., Eds.; Kodansha: Tokyo, Japan, 1982; pp. 307–404. (In Japanese) [Google Scholar]
- Strassmann, J.E. Evolutionary implications of early male and satellite nest production in Polistes exclamans colony cycles. Behav. Ecol. Sociobiol. 1981, 8, 55–64. [Google Scholar] [CrossRef]
- Strassmann, J.E. Parasitoids, predators, and group size in the paper wasp, Polistes exclamans. Ecology 1981, 62, 1225–1233. [Google Scholar] [CrossRef]
- Martin, S.J. Occurrence of the pyralid moth Hypsopygia mauritialis (Lepidoptera, Pyralidae) in the nests of Vespa affinis (Hymenoptera, Vespidae). Jpn. J. Entomol. 1992, 60, 267–270. [Google Scholar]
- Thomas, C.R. The European wasp (Vespula germanica Fabricius) in New Zealand. Inform. Ser. Dep. Sci. Ind. Res. N. Z. 1960, 27, 1–74. [Google Scholar]
- Gambino, P. Dolichovespula (Hymenoptera: Vespidae), Hosts of Aphomia sociella (L.) (Lepidoptera: Pyralidae). J. N. Y. Entomol. Soc. 1995, 103, 165–169. [Google Scholar]
- Yamane, S.; Fukuda, T.; Makino, S. Observations on a nest of a paper wasp (Polistes sp.) infested by a pyralid moth, Hypsopygia mauritialis (Boisduval, 1833). Lepid. Sci. 2022, 73, 27–32. [Google Scholar]
- Choi, M.B.; Kim, J.K.; Lee, J.W. Checklist and distribution of Korean Vespidae Revisited. Korean J. Appl. Entomol. 2013, 52, 85–91. [Google Scholar] [CrossRef]
- Choi, M.B.; Martin, S.J.; Lee, J.W. Distribution, spread, and impact of the invasive hornet Vespa velutina in South Korea. J. Asia Pac. Entomol. 2012, 15, 473–477. [Google Scholar] [CrossRef]
- Choi, M.B.; Kim, T.G.; Kwon, O. Recent trends in wasp nest removal and Hymenoptera stings in South Korea. J. Med. Entomol. 2019, 56, 254–260. [Google Scholar] [CrossRef]
- Choi, M.B. Defensive behavior of the invasive alien hornet Vespa velutina nigrithorax against potential human aggressors. Entomol. Res. 2021, 51, 186–195. [Google Scholar] [CrossRef]
- Choi, M.B. Foraging behavior of an invasive alien hornet (Vespa velutina) at Apis mellifera hives in Korea: Foraging duration and success rate. Entomol. Res. 2021, 51, 143–148. [Google Scholar] [CrossRef]
- Turchi, L.; Derijard, B. Options for the biological and physical control of Vespa velutina nigrithorax (Hym.: Vespidae) in Europe: A review. J. Appl. Entomol. 2018, 142, 553–562. [Google Scholar] [CrossRef]
- Jeong, G.J.; Lee, S.W.; Kim, J.K. A study on the colony development, parasite infestation rate and colony survival rate of the paper wasp, Polistes jadwigae Dalla Torre according to their nesting habitat (Hymenoptera: Vespidae). Nat. Sci. 1987, 3, 81–89. [Google Scholar]
- Lee, B.W.; Bae, Y.S. A review of the tribe Pyralini Latreille (Lepidoptera, Pryaralidae, Pyralinae) from Korea. Trans. Lepidopterol. Soc. Jpn. 2007, 58, 47–68. [Google Scholar]
- Yoon, H.K.; Byun, B.K. Taxonomic revision of the family Cosmopterigidae (Lepidoptera) in Korea. J. Asia Pac. Entomol. 2017, 20, 1032–1042. [Google Scholar] [CrossRef]
- Heo, U.H. Guidebook of Moth Larvae; Jayeongwasaengtae: Seoul, Republic of Korea, 2012. [Google Scholar]
- Holloway, J.D.; Bradley, J.D.; Carger, D.J. CIE Guides to Insects of Importance to Man, 1: Lepidoptera; CA B International: London, UK, 1987; p. 262. [Google Scholar]
- Hebert, P.D.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the Neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef]
- Hajibabaei, M.; Janzen, D.H.; Burns, J.M.; Hallwachs, W.; Hebert, P.D. DNA barcodes distinguish species of tropical Lepidoptera. Proc. Natl. Acad. Sci. USA 2006, 103, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Regier, J.C.; Mitter, C.; Solis, M.A.; Hayden, J.E.; Landry, B.; Nuss, M.; Simonsen, T.J.; Yen, S.H.; Zwick, A.; Cummings, M.P. A molecular phylogeny for the pyraloid moths (Lepidoptera: Pyraloidea) and its implications for higher-level classification. Syst. Entomol. 2012, 37, 635–656. [Google Scholar] [CrossRef]
- Latreille, P.A. Genera Crustaceorum et Insectorum Secundum Ordinem Naturalem in Familias Disposita, Iconibus Exemplisque Pluribus Explicate; Cornell University Library: Ithaca, NY, USA, 1809; p. 399. [Google Scholar]
- Hübner, J. Verzeichnis Bekannter Schmettlinge; Biodiversity Heritage Library: Augsburg, Germany, 1816; p. 432. [Google Scholar]
- Boisduval, J.B.A. Faune Entomologique de Madagascar, Bourbon et Maurice: Lépidoptéres; Librairie Encyclopédique de Roret: Paris, France, 1833; p. 122. [Google Scholar]
- Walker, F. List of the Specimens of Lepidopterous Insects in the Collection of the British Museum: Part XVIII. Pyralides 1859, 17, 256–508. [Google Scholar]
- Walker, F. List of the Specimens of Lepidopterous Insects in the Collection of the British Museum: Part XXXIV. Pyralides 1965, 34, 1121–1533. [Google Scholar]
- Lucas, T.P. On Queensland and other Australian Macro-Lepidoptera, with localities and descriptions of new species. Proc. Linn. Soc. N. S. W. Ser. 1891, 2, 277–306. [Google Scholar] [CrossRef]
- Ragnot, E.L. Essai sur une classification des Pyralites (suite). Ann. Soc. Entomol. Fr. 1891, 60, 15–114. [Google Scholar]
- Warren, W. New genera and species of moths from the Old-World regions in the Tring Museum. Novit. Zool. 1897, 4, 12–130. [Google Scholar] [CrossRef]
- Caradja, A.V. Dritter Beitrag zur Kleinfalterfauna Chinas nebst kurzer Zusammenfassung der bisherigen biogeographischen Ergebnisse. Acad. Roum. Bull. Sect. Sci. 1932, 15, 111–123. [Google Scholar]
- Amsel, H.G. Die Microlepidopteren der Brandt’schen Iran-Ausbeute. Teil Arkiv För Zoologi. 1954, 6, 255–326. [Google Scholar]
- Bae, Y.S. Family Pyraloidea: Pyraustinae & Pyralinae. Economic Insects of Korea 9. Insecta Koreana Suppl. 2001, 16, 251. [Google Scholar]
- Pham, P.H. Biology of Hypsopygia postflava (Lepidoptera: Pyralidae), a snout moth parasitic on the nest of the paper wasp Polistes olivaceus (Vespidae: Polistes). Biol. Forum Int. J. 2014, 6, 90–93. [Google Scholar]
- Kim, J.-K.; Choi, M.; Moon, T.-Y. Occurrence of Vespa velutina Lepeletier from Korea, and a revised key for Korean Vespa species (Hymenoptera: Vespidae). Entomol. Res. 2006, 36, 112–115. [Google Scholar] [CrossRef]
- Carisio, L.; Cerri, J.; Lioy, S.; Bianchi, E.; Bertolino, S.; Porporato, M. Impacts of the invasive hornet Vespa velutina on native wasp species: A first effort to understand population-level effects in an invaded area of Europe. J. Insect Conserv. 2022, 26, 663–671. [Google Scholar] [CrossRef]
- Darrouzet, E.; Gévar, J.; Dupont, S. A scientific note about a parasitoid that can parasitize the yellow-legged hornet, Vespa velutina nigrithorax, in Europe. Apidologie 2015, 46, 130–132. [Google Scholar] [CrossRef]
- Villemant, C.; Zuccon, D.; Rome, Q.; Muller, F.; Poinar, G.O., Jr.; Justine, J.L. Can parasites halt the invader? Mermithid nematodes parasitizing the yellow-legged Asian hornet in France. PeerJ 2015, 3, e947. [Google Scholar] [CrossRef]
- Kim, C.J.; Choi, M.B. First Discovery of Vespa velutina nigrithorax du Buysson (Hymenoptera: Vespidae), an Invasive Hornet in the Feces of the Yellow-Throated Marten in South Korea. Insects 2021, 12, 296. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Cui, L.; Ostiguy, N.; Cox-Foster, D. Intricate transmission routes and interactions between picorna-like viruses (Kashmir bee virus and sacbrood virus) with the honeybee host and the parasitic varroa mite. J. Gen. Virol. 2005, 86, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Kurze, C.; Routtu, J.; Moritz, R.F.A. Parasite resistance and tolerance in honeybees at the individual and social level. Zoology 2016, 119, 290–297. [Google Scholar] [CrossRef]
- Carreck, N.L.; Ball, B.V.; Martin, S.J. Honey bee colony collapse and changes in viral prevalence associated with Varroa destructor. J. Apic. Res. 2010, 49, 93–94. [Google Scholar] [CrossRef]
- Baty, J.W.; Bulgarella, M.; Dobelmann, J.; Felden, A.; Lester, P.J. Viruses and their effects in ants (Hymenoptera: Formicidae). Myrmecol. News 2020, 30, 213–228. [Google Scholar]
- Beggs, J.R.; Brockerhoff, E.G.; Corley, J.C.; Kenis, M.; Masciocchi, M.; Muller, F.; Rome, Q.; Villemant, C. Ecological effects and management of invasive alien Vespidae. BioControl 2011, 56, 505–526. [Google Scholar] [CrossRef]
- Miller, G.L.; Donnelly, C.R.; Gamboa, G.J. A ten-year comparative study of the population dynamics and parasitoidism in the native paper wasp Polistes fuscatus and the invasive P. dominulus. Insect. Soc. 2013, 60, 49–56. [Google Scholar] [CrossRef]
- Nacko, S.; Henderson, G. A preliminary investigation of the interactions between the brood parasite Chalcoela iphitalis and its Polistine wasp hosts. Insects 2017, 8, 89. [Google Scholar] [CrossRef]
- Diéguez-Antón, A.; Escuredo, O.; Seijo, M.C.; Rodríguez-Flores, M.S. Embryo, relocation and secondary nests of the invasive species Vespa velutina in Galicia (NW Spain). Animals 2022, 12, 2781. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).