Abstract
Genetic conservation is key to maintaining forests for the future; however, these face several threats. Mexico is an example of the degradation of forest genetic resources during the past three decades due to its deforestation rates. This country is considered a center of pine diversity but its genetic conservation efforts are not enough. To define genetic conservation units (GCUs) and propose measures for the conservation and sustainable use of 18 Mexican pine species, we analyzed the distribution of the species at the national level and in germplasm transfer zones, and evaluated the species with a set of minimum requirements for conservation and indicators from the EUFORGEN program. We determined that 13 to 15 genetic zones harbored the target species, in which Pinus teocote, Pinus cembroides, Pinus devoniana, Pinus maximinoi, Pinus douglasiana, and Pinus leiophylla were the most widely distributed. We defined 173 areas for establishing GCUs for the total of the species studied; 50% of them were selected from areas with genetic information, 5% were selected from seed stands, and 45% from natural forests. We detected that most of the forest reproductive material used is collected from seed stands, and the use of seeds from breeding trials is scarce.
1. Introduction
Facing continuous environmental changes, genetic conservation is a key to maintaining forest resources for the future. The main objective of genetic conservation is to preserve the genetic integrity and natural levels of genetic diversity [1]. However, conservation activities are limited by the financial, temporal, and technical resources available [2]. For this reason, activities must be selected by keeping in mind the species with ecological, economic, and social potential uses by country based on current conservation status, potential economic use, threat of genetic erosion, genetic distinction, ecogeographic distinction, national or conservation agency priorities, biological importance, cultural importance, relative cost of conservation, conservation sustainability, and ethical and aesthetic considerations [3].
Forest genetic resources have been used for testing of tree species and provenance for different uses and under different environmental conditions [4]. The purpose of a provenance test is to establish the most healthy and fastest growing population among many others of the same tree species, in order to select such a population as a seed source for planting at appropriate sites [5]. This is a tool for improving forest genetics. In common garden experiments, also known as provenance trials, it is possible to test which provenances have adapted to expected climate conditions [6] because tree species possess numerous local adaptations which represent valuable genetic resources for the long-term survival of the species and for coping with climate change [7].
Forest genetic resources face several threats, such as habitat destruction, fragmentation, pollution, poor silvicultural practices, and the use of low-quality or poorly adapted forest reproductive material [8]. Mexico is an example of the degradation of forest genetic resources for the past three decades due to its deforestation rates which have varied from 260,000 to 1,600,000 ha/year [9]. This country possesses from 43 to 51 pine species (depending on the author) and is considered a center of pine diversity; it also accounts for almost half of the total number of pine species in the world [10]. On the other hand, Mexican pines are very important because they supply 85% of the national timber production and are the main support for the forest industry [11].
For Mexico, some research has been carried out to define the genetic variation of the genus Pinus. In the past, provenance and progeny tests have been the most widely used [12,13,14], but recently, molecular characterization has been the main objective of several studies to define genetic diversity [15]. Although there is genetic information for most Pinus species, their conservation efforts are limited, and the proposals to conserve them are few. For Pinus arizonica (Engelm.) Shaw, Pinus ayacahuite Ehrenb. ex Schltdl., P. cembroides Zucc., Pinus chiapensis (Martínez) Andresen, P. douglasiana Martínez, Pinus durangensis Martínez, Pinus engelmannii Carrière, Pinus greggii Engelm. ex Parl., Pinus hartwegii Lindl., P. leiophylla Schiede ex Schltdl. et Cham., Pinus montezumae Lamb., Pinus oocarpa Schiede ex Schltdl., Pinus patula Schiede ex Schltdl. & Cham., Pinus pseudostrobus Lindl., and Pinus strobiformis Engelm., some research analyzes genetic diversity [16,17,18,19,20,21,22,23,24,25,26,27,28,29] and discusses options for in situ or ex situ conservation [24,25,26,30]; however, these studies did not define any zone for genetic conservation, except for P. douglasiana.
In this sense, the authors of [31] prioritized the genetic conservation of four pine species (P. greggii Engelm. ex Parl., P. oocarpa Schiede ex Schltdl., P. patula Schiede ex Schltdl. & Cham., and P. pseudostrobus Lindl.) based on establishing a network with genetic conservation units (GCUs), but no other work has been recorded. The gene conservation network is a tool to evaluate the plasticity, adaptation, and migration potential of tree species that face environmental changes [8]. For this reason, it is imperative to define more GCU for other pine species in order to start their genetic conservation. On the other hand, Mexican legislation establishes: “Conserve and protect the country’s forest genetic resources”, but the way to implement it has not yet been defined.
Based on the above and due to the need to establish a genetic conservation network for pine species, the objectives of this work were to define genetic conservation units and propose measures for the conservation and sustainable use of 18 Mexican pines. The distribution of the species was defined at the national level and in the germplasm transfer zones, the gene conservation units were determined based on a set of minimum requirements, and finally, the use and importance of genetic resources were analyzed.
2. Materials and Methods
2.1. Species and Distribution
Eighteen Mexican pine species were chosen for this study: P. arizonica (Engelm.) Shaw, P. ayacahuite Ehrenb. ex Schltdl., P. cembroides Zucc., P. chiapensis (Martínez) Andresen, P. devoniana Lindl., P. douglasiana Martínez, P. durangensis Martínez, P. engelmannii Carrière, P. hartwegii Lindl., Pinus jeffreyi Balf., Pinus lawsonii Roezl ex Gordon, P. leiophylla Schiede ex Schltdl. et Cham., Pinus maximartinezii Rzed., P. maximinoi H. E. Moore, P. montezumae Lamb., Pinus quadrifolia Parl. ex Sudw., P. strobiformis Engelm., and Pinus teocote Schiede ex Schltdl. et Cham. These species are distributed across the country, and some of them are found in more than one of the temperate mountain ranges (Sierra Madre Occidental, Sierra Made Oriental, Eje Neovolcánico Transversal, Sierra Madre del Sur, and Sierra Madre Centroamericana and Altos de Chiapas) which occupy diverse habitats [32,33](Supplementary Materials Table S1).
The species distributions were determined based on geographic data (latitude and longitude) using the plots of the National Forest and Land Inventory (NFLI, 2004 to 2007 [34]), which covers Mexico in a systematic stratified random sampling with a grid of 5 km (Supplementary Materials Figure S1). Plots which considered forest plantations were removed. The geographic data were presented using QGIS software 3.28.2. [35].
2.2. Genetic Zones
The germplasm transfer zones (equivalent to seed zones) were used as a proxy because genetic zones are still undefined for the country. Seed zones are homogeneous areas in terms of climate and latitudinal or longitudinal distribution [36] for regulating seeds, fruits, and vegetative material. These seed transfer zones were previously defined by the Mexican National Forest Commission (CONAFOR by its Spanish acronym), and are available at https://www.gob.mx/conafor (accessed on 8 August 2022). The genetic zones were obtained for the 18 Mexican pines species by overlapping their distribution with the germplasm transfer zones. All genetic zones with less than 20 trees for each species reported in the NFLI were excluded from subsequent analysis. For the geographic data, QGIS software 3.28.2. was used [35].
2.3. Conservation Units
Genetic conservation units (GCU) were defined as an in situ conservation strategy. It was followed a set of minimum requirements for defining the units based on population size, management, monitoring, and ownership [37] (Supplementary Materials Table S2). We selected at least one GCU per genetic zone and species, but in fragmented distribution, one unit was selected from each patch. During the selection, it was prioritized extensive and centered populations in the genetic zone or patch. For each genetic zone, first, we chose the populations characterized in previous genetic studies or provenance or progeny trials [12,17,18,22,23,24,25,26,27,28,29,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89] (Table S3), which were selected for possessing representative trees within the zones. The previous studies and trials were obtained from an exhaustive documentary review from printed and digital scientific journals. Secondly, they were chosen populations located in seed stands defined by the Mexican National Forest Commission (CONAFOR, information available for 2021 at https://www.gob.mx/conafor, accessed on 5 September 2022). Thirdly, we selected populations from protected areas for biodiversity conservation established by the Mexican Commission for Biodiversity (CONABIO, information for 2016 available at http://www.gob.mx/conabio, accessed on 7 September 2022), instead private ownership. Finally, we selected populations situated in private forests. The geographic data were represented using QGIS software 3.28.2 [35].
2.4. Use of Genetic Resources
For each genetic zone, the presence of germplasm production units (seed stands or seed orchards) were used in order to define the use and importance of genetic resources. We assumed that the effort and investment during the establishment and maintenance of those units was an economic indicator of the species in the area. Then, genetic trials established by different institutions, that is, provenance and progeny trials, were used, as they report information about populations identified for choosing forest reproductive material with well-known genetic backgrounds.
2.5. Importance of Genetic Zones for Conservation and Sustainable Use
Based on the previous information, six species indicators were collected in all genetic zones: the number of trees sampled by the NFLI (ni), the number of populations with molecular data (nmk), the number of populations with seed stands (seed stand plus seed area) (nst), the number of individuals selected for progeny tests (nis), the number of populations present in provenance tests (npt), and the number of seed orchards (nso). Finally, we estimated the number of GCUs (ng). For each species, their status was calculated by applying indicators assigned and adapted from the EUFORGEN program [90] (Table 1).

Table 1.
Indicators of the genetic zones in conservation and management of genetic resources.
The importance of the genetic zones was calculated using the previous information published by studies [91,92,93], which defined their reforestation and restoration potential, and timber production by genetic zone. In these studies, a proportional assignment to each genetic zone was made using as a basis the distribution of the 18 species and those harbored in genetic zones. We also determined a value for conservation and a value for breeding for the different genetic zones of the species in a subjective scale from 1 to 4 [31], with 4 being the highest priority. For this, the expert knowledge and the recommendations established by the papers or reports previously analyzed were considered.
3. Results
3.1. Species in Genetic Zones
Twenty-three genetic zones were detected and harbored at least one population of the studied species. Particularly, eight zones included 11–13 species, 5 zones included 1–3 species, and 10 zones included 5–9 species (Figure 1). Pinus teocote, P. cembroides, P. devoniana, P. maximinoi, P. douglasiana, and P. leiophylla were the most widely distributed (18, 16, 16, 13, 12, and 12 genetic zones, respectively), P. arizonica var. cooperi, P. quadrifolia, and P. jeffreyi had a more restricted distribution (4, 4, and 1 genetic zones, respectively), and P. durangensis, P. engelmannii, P. hartwegii, P. montezumae, P. arizonica, P. ayacahuite, P. chiapensis, P. lawsonii, P. maximartinezii, and P. strobiformis had a medium distribution (9, 9, 9, 9, 7, 7, 7, 7, 7, and 7 genetic zones, respectively).
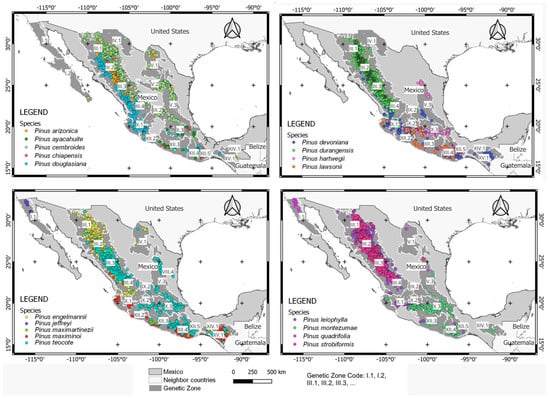
Figure 1.
Natural populations of target species distributed in the genetic zones from Mexico.
3.2. Genetic Conservation Units
For the studied species, 173 areas for established GCUs were detected (Figure 2 and Supplementary Materials Table S4). Specifically, 13–20 areas for P. leiophylla, Pinus teocote, P. cembroides (with two subspecies: bicolor and cembroides), P. devoniana, and P. douglasiana; 7–12 for P. maximinoi, P. engelmannii, P. montezumae, P. durangensis, P. strobiformis, P. hartwegii, P. arizonica, and P. lawsonii, and 1–2 for P. arizonica var. cooperi, P. ayacahuite var. ayacahuite, P. cembroides subsp. cembroides, P. ayacahuite var. veitchii, P. cembroides subsp. bicolor, P. jeffreyi, P. quadrifolia, P. cembroides subsp. lagunae, P. cembroides subsp. orizabensis, P. chiapensis, and P. maximartinezii. With respect to the selection criteria, 50% of the GCU were selected from areas with genetic information reported in previous research. In the absence of genetic information, 5% of GCU were selected from seed stands defined by CONAFOR and the other 45% in natural forests recorded for NFLI. Additionally, 38% of the GCU were chosen from protected areas.
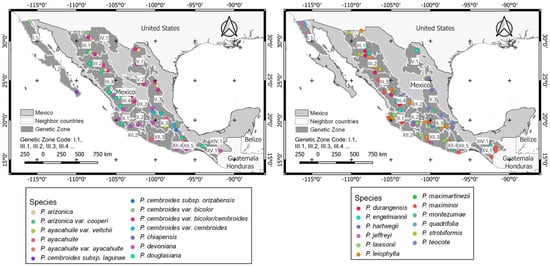
Figure 2.
Conservation units proposed by genetic zone for the target species from Mexico.
3.3. Use of Genetic Resources
For the target species, the conservation efforts and use of genetic resources by genetic zone were quite contrasting (Supplementary Materials Tables S5–S9). The genetic characterization was null or narrow for some species and regions, that is, P. jeffreyi, P. lawsonii, P. maximartinezii, P. quadrifolia, and P. cembroides subsp. orizabensis, but broader for others, that is, P. arizonica. The efforts to use forest reproductive material were reduced and concentrated on selecting seed stands (Table S7), for example, P. arizonica and P. durangensis had more seed stands than other species (26 and 20, respectively), but few or no seed orchards (2 and 0, respectively) or provenance/progeny trials (0 for both species). We detected many gaps in different regions for all of the species, but some zones had no efforts to use forest reproductive material; that is, I.2, IV.1, V.2, VIII.1, VIII.3, VIII.4, IX.2, XII.1, XII.2, XII.5, XIII.1, XIV.1, XIV.2, and XIV.3 did not record use of any genetic resources.
3.4. Conservation and Management of Genetic Resources
The conservation efforts, characterization, and use of forest genetic resources for the 18 species were incomplete (Table 2). Most of the genetic zones had gaps for the characterization of forest genetic resources or even seed production. Molecular studies were null for P. jeffreyi, P. lawsonii, P. maximartinezii, P. quadrifolia, and low for P. devoniana; the rest of the species ranged from 100 to 22.22% where P. strobiformis and P. ayacahuite possessed the higher values (100 and 85.71%, respectively). P. arizonica and P. leiophylla were the only species that had seen seed orchards established (I6 > 0). Provenance and progeny trials had been stablished for a few species, in which their efforts were limited (I3 and I4 > 0). P. arizonica and P. durangensis had more forest reproductive units, covered several states, and were used for reforestation and restoration of degraded lands and timber production.
3.5. Importance of Genetic Zones for Conservation and Use of Genetic Resources
For reforestation and restoration areas, XII.4, XII.5, X.3, X.2, XII.1, V.3, XII.2, and XV.1 zones were the most important due to having the potential of seedling production, germplasm production units, and seed conservation banks (see [91]); whereas for timber production, X.3, V.3, XII.5, XII.3, X.1, XII.2, III.1, and III.2 were the best zones due to their amount of timber harvested and used for the industry (see Table 1 and Table 2 from [93]). Based on this information, it was defined that V.3, X.2, X.3, XII.1, XII.2, XII.4, XII.5, and XV.1 were the most suitable areas of germplasm for the target species studied (Figure 3), which presented the highest potential for conservation and breeding. Although they were not the genetic zones with the greatest number of trees inventoried, they were the most diverse in the number of species and the ones where the actions dedicated to the conservation and use of genetic resources were more intensive (Table 3).
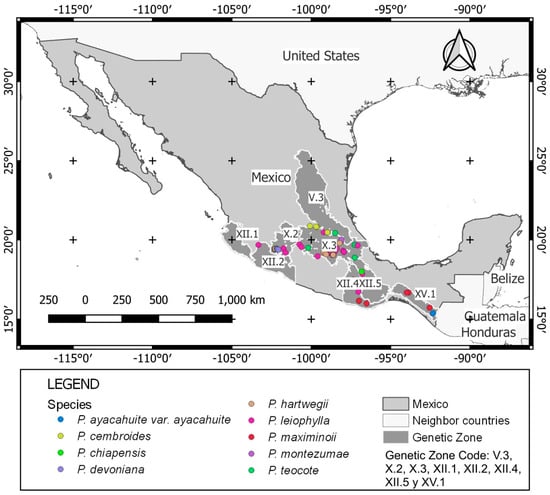
Figure 3.
Populations considered as the most suitable source for supplying forest reproductive material for nine species from Mexico.

Table 3.
Importance for conservation and use of forest genetic resources of pines by genetic zone.

Table 2.
Indicators of the conservation and management of genetic resources.
Table 2.
Indicators of the conservation and management of genetic resources.
| Code | Indicator 1 | AR 2 | AY | CE | CH | DE | DO | DU | EN | HA | JE | LA | LE | MM | MA | MO | QU | ST | TE | Media |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I1 | Number of genetic zones | 8 | 7 | 16 | 7 | 16 | 12 | 9 | 9 | 9 | 1 | 7 | 12 | 7 | 13 | 9 | 4 | 7 | 18 | 10 |
| I2 | Molecular characterization effort 3 | 27.27 | 85.71 | 50.00 | 71.43 | 6.25 | 33.33 | 44.44 | 44.44 | 66.67 | 0.00 | 0.00 | 66.67 | 0.00 | 53.85 | 44.44 | 0.00 | 100.00 | 22.22 | 39.82 |
| I3 | Provenance characterization effort | 0.00 | 14.29 | 12.50 | 0.00 | 0.00 | 0.00 | 0.00 | 11.11 | 11.11 | 0.00 | 0.00 | 8.33 | 0.00 | 15.38 | 11.11 | 0.00 | 0.00 | 5.56 | 4.97 |
| I4 | Progeny characterization effort | 0.00 | 14.29 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 8.33 | 0.00 | 7.69 | 0.00 | 0.00 | 0.00 | 5.56 | 1.99 |
| I5 | Seed stands index | 27.27 | 14.29 | 0.00 | 14.29 | 25.00 | 25.00 | 33.33 | 44.44 | 22.22 | 100.00 | 14.29 | 16.67 | 0.00 | 15.38 | 33.33 | 25.00 | 0.00 | 11.11 | 23.42 |
| I6 | Seed orchard index | 18.18 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 8.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.47 |
| I7 | Genetic conservation index | 54.55 | 57.14 | 75.00 | 14.29 | 68.75 | 83.33 | 55.56 | 77.78 | 66.67 | 100.00 | 100.00 | 91.67 | 14.29 | 84.62 | 88.89 | 25.00 | 85.71 | 66.67 | 67.22 |
1 I2, I3, I4, and I6 indicators used data from previous studies recorded in scientific journals, and the I5 indicator was estimated from CONAFOR information. 2 AR—Pinus arizonica, AY—P. ayacahuite, CE—P. cembroides, CH—P. chiapensis, DE—P. devoniana, DO—P. douglasiana, DU—P. durangensis, EN—P. engelmannii, HA—P. hartwegii, JE—P. jeffreyi, LA—P. lawsonii, LE—P. leiophylla, MM—P. maximartinezii, MA—P. maximinoi, MO—P. montezumae, QU—P. quadrifolia, ST—P. strobiformis, TE—P. teocote. 3 Based on 1 sample by stand/population.
4. Discussion
The priority zones were defined to help the conservation and the use of forest genetic resources in 18 Mexican pine species. Based on the germplasm transfer zones [36] as a proxy for genetic zones, 173 areas have been identified for establishing conservation units into 22 genetic zones. Additionally, it was considered that eight genetic zones could supply the forest reproductive material in order to be used for the species’ afforestation, restoration, and for establishing plantations.
We detected that some species were distributed more than others, and have high levels of genetic variation due to their biological characteristics; for example, P. cembroides is one pine that harbors more than one temperate mountain range, and P. teocote is the most abundant and widespread in Mexico, both having a high level of genetic diversity [15,51]. It is possible that the species’ distributions are influenced by the environment and their ecological factors. This is in line with the authors of [39], who determined that elevation is an important factor that drives the geographical location and genetic diversity of the species. Additionally, they pointed out that P. cembroides possesses the ability to adapt to its growth conditions. On the other hand, some species have a more restricted distribution but possess high genetic variation as P. arizonica [15,52], which is according to the authors of [22] who studied forests in Chihuahua state. Based on the results of study [38], this is possible due to their populations having enough individuals and sufficient outcrossing to prevent genetic drift.
Most of the species have potential for restoration of land; consequently, they are used during reforestation or conservation programs, for example, seedlings of P. cembroides and P. devoniana are the most produced in the nurseries for the reforestation of areas across the country [91], while P. douglasiana, P. maximinoi, and P. teocote are species most harvested and used in the forest industry [93]. P. cembroides is a tree to tolerate drought and is able to grow in sites in poor soils [94], while P. devoniana is a pine to resist drought [95]. These characteristics have been attractive for nurserymen during seedling production and for planters in the restoration of semi-arid regions and dry zones. However, sometimes hard conditions in the sites selected (hydric stress and low mineral nutrition) can strongly reduce their survival [96]. For conservation, P. cembroides has been suggested to promote the natural functions of forest dynamics in reforested areas [97].
Although there are efforts to conserve Mexican pine species, the preservation of their genetic resources is limited because the current legislation only points out the use of forest genetic resources [98,99] but does not define conservation strategies. The little conservation actions made for governmental agencies depend on their budget, which is more scarce each year. These conditions demand some activities during forest management proposed by the authors of [100] that help to maintain the populations of target species as (i) limiting livestock grazing, (ii) controlling forest fires, (iii) eliminating competing vegetation, (iv) expanding the present populations by reforestation outside the priority populations’ boundaries using local reproductive material, (v) finding new and potentially suitable locations and establishing new populations that are sufficiently large to be genetically viable, (vi) establishing provenance trials and traditional progeny tests as genetic archives, and (vii) storing seeds in gene banks.
The results showed that the information about genetic characterization for most species is not enough; however, the results can help start a national conservation program for the species most used in forest management. Some of the target species have been more characterized than others which could be due to their economic importance, for example, P. douglasiana or P. arizonica, which produce an average of 56 554 and 13 410 m3 year–1 timber, respectively [93]. The conservation of genetic diversity must be a priority because it is the basis for the eventual adaptation and resilience of tree species studied regarding environmental stress and change [101]. On the other hand, although there is a Mexican network of Natural Protected Areas (NPA) that harbor some of the tree species and could be used as genetic diversity reserves, this is insufficient for future conservation needs. According to the authors of [102], the protection of pine species is inadequate because they possess a small percentage of the area of the total species, therefore, they suggest directing this strategy (NPA) to species with limited distribution such as P. cembroides subsp. cembroides.
The purpose of defining genetic conservation units is to generate a network in the country in order to maintain the Mexican pines which are facing current environmental changes in other parts of the world; for example, in Europe, the establishing of genetic conservation units is a tool to assess, monitor, and support the conservation of forest genetic resources [90,103]. This action can allow the future use of pines from Mexico in three programs: breeding, production, and conservation.
The target species have high genetic diversity. According to the authors of [104], the Mexican pines possess high levels of genetic variation and relatively little genetic differentiation among populations. However, there are other species with low levels of diversity such as P. greggii Engelm. ex Parl., which is used in national or international breeding programs [12,80]. P. ayacahuite, P. leiophylla, and P. teocote are some pines that have provenance trials to define their potential for commercial plantations, for example, P. ayacahuite is one of the species most used for Christmas tree producers [105] while P. leiophylla and P. teocote are managed in natural forests for timber production [93]. Facing climatic changes, adaptive trials have been tested in the studied species: P. ayacahuite, P. arizonica (water availability and water stress) [106,107]; P. arizonica, P. leiphylla, and P. teocote (fire damage) [108]; P. arizonica, P. ayacahuite, P. cembroides, P. chiapensis, P. devoniana, P. durangensis, P. engelmannii, P. hartwegii, P. jeffreyi, P. lawsonii, P. maximartinezii, P. maximinoi, P. montezumae, P. strobiformis, P. teocote (frost resistance) [109], and Pinus cembroides (salt tolerance) [110].
Some of the areas selected could be affected by illegal extraction of timber [111] or other factors that limit genetic conservation, for example, land use change for agriculture [112]. However, the establishing of GCUs in protected areas can help with maintaining them in the long term because the rate of forest loss is lower [113]. In these areas, the financial resources, human capacity, and appropriate equipment are the main aspects that have the greatest effect on forest land loss [114]. For this reason, administration and finance must be continually evaluated during the management of the protected areas in order to preserve the UCGs selected.
On the other hand, the diversity of pines from Mexico across the mountain regions promotes habitats that harbor local wildlife. This is an element to add more importance to forest conservation. This is in line with the authors of [115], who defined that natural forests dominated by P. montezumae and P. pseudostrobus conform to the best habitat to comprise the highest bird species richness from Northeast Michoacán, Mexico, for example, Hylocharis leucotis, Archilochus alexandri, Selasphorus platycercus, Trogon mexicanus, and Melanerpes formicivorus.
Finally, we suggest incorporating these GCUs into a national management program based on three steps. Firstly, the legislation must promote and pay for the conservation of the forest resources to the forest owners as this action will motivate the society to protect the GCUs against fires, illegal extraction, grazing, or changes in land use. Secondly, there must be education provided for the communities regarding banning the extraction of forest resources near and inside of GCUs, for example, wood, land, and resin. Thirdly, government institutions, universities, and research institutes must work together to develop a plan to establish the UCGs defined in this work, install common garden experiments in order to evaluate the future effects of climatic changes and analyze adaptation strategies, and promote the conservation ex situ of germplasm in seed banks, for example, in the National Center for Genetic Resources (CNRG by its Spanish acronym). This plan must be accompanied mainly by silvicultural guidelines, activities for the prevention and control of pests and diseases, use of local seed sources for stand renewal, and control of the germplasm movement.
5. Conclusions
In this study, 173 genetic conservation units were defined for 18 pine species from Mexico in their natural range of distribution and some actions determined for their conservation and sustainable use. P. leiophylla, Pinus teocote, P. cembroides (with two subspecies: bicolor and cembroides), P. devoniana, and P. douglasiana were the species with more genetic conservation units due to their broad distribution. These harbored similar or different genetic zones due to their adaptation to different ecological requirements (temperature, rainwater, soil). For the number of areas detected for genetic conservation units, half of them contain genetic information which was reported in journals, and almost 40% of them were proposed in protected areas based on our selection. We found that most of the forest reproductive material used currently is collected from seed stands, and the use of seeds from breeding trials is scarce. The genetic characterization of the target species has gaps in the genetic zones. Therefore, this demands, in the short term, future studies regarding those species most used in the forest industry, breeding programs, and restoration of degraded lands.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15060735/s1, Table S1: climatic and edaphic patterns of target species [32,33] are cited here, Table S2: minimum requirements for genetic conservation units, Table S3: previous genetic studies or provenance or progeny trials reviewed [12,17,18,21,22,23,24,25,26,27,28,29,32,33,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89] are cited here, Table S4: proposed conservation units for the target species, Table S5: inventoried trees by genetic zone and species, Table S6. genetic characterization by genetic zone and species, Table S7: germplasm production units by genetic zone and species, Table S8: germplasm orchards by genetic zone and species, Table S9: conservation units by genetic zone and species, Figure S1: Species distribution in Mexico using data from the NFLI.
Author Contributions
A.F. conceived and design the research; T.P.O. contributed materials; A.F. and E.B.R. analyzed the data; A.F., T.P.O., E.F.A. and J.M.-G. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
A.F. is supported by Project No 10283335082 (from National Institute for Forestry, Agriculture and Livestock Research) established in the frame of the research “Definition of areas for the conservation and use of forest genetic resources for species of the genus Pinus”. No other external funding was received.
Acknowledgments
The authors are grateful to the National Institute of Forestry, Agriculture, and Livestock Research of Mexico for funding all stages of this study through Project No 10283335082.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsor had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Rajora, O.P.; Mosseler, A. Challenges and opportunities for conservation of forest genetic resources. Euphytica 2001, 118, 197–212. [Google Scholar] [CrossRef]
- Maxted, N. In situ, ex situ conservation. Encycl. Biodivers. 2013, 4, 313–323. [Google Scholar] [CrossRef]
- Maxted, N.; Hawkes, J.G.; Guarino, L.; Sawkins, M. Towards the selection of taxa for plant genetic conservation. Genet. Resour. Crop Evol. 1997, 44, 337–348. [Google Scholar] [CrossRef]
- Koskela, J.; Vinceti, B.; Dvorak, W.; Bush, D.; Dawson, I.K.; Loo, J.; Kjaer, E.D.; Navarro, C.; Padolina, C.; Bordács, S.; et al. Utilization and transfer of forest genetic resources: A global review. For. Ecol. Manage. 2014, 333, 22–34. [Google Scholar] [CrossRef]
- Hosius, B.; Leinemann, L.; Konnert, M.; Bergmann, F. genetic aspects of forestry in the Central Europe. Eur. J. For. Res. 2006, 125, 407–417. [Google Scholar] [CrossRef]
- Wang, H.; Sun, J.; Duan, A.; Zhu, A.; Wu, H.; Zhang, J. Dendroclimatological analysis of chinese fir using a long-term provenance trial in Southern China. Forests 2022, 13, 1348. [Google Scholar] [CrossRef]
- Schueler, S.; Falk, W.; Koskela, J.; Lefèvre, F.; Bozzano, M.; Hubert, J.; Kraigher, H.; Longauer, R.; Olrik, D.C.; Ev, O.I.S.L.E.F. Vulnerability of dynamic genetic conservation units of forest trees in Europe to climate change. Glob. Chang. Biol. 2014, 20, 1498–1511. [Google Scholar] [CrossRef]
- Koskela, J.; Buck, A.; Teissier du Cros, E. Climate Change and Forest Genetic Diversity: Implications for Sustainable Forest Management in Europe; Bioversity International: Rome, Italy, 2007; ISBN 978-92-9043-749-9. [Google Scholar]
- Couturier, S.; Manuel, J.; Kolb, M. Measuring tropical deforestation with error margins: A method for REDD monitoring in South-Eastern Mexico. In Tropical Forests; Sudarshana, P., Nageswara-Rao, M., Soneji, J.R., Eds.; InTech: London, UK, 2012; pp. 269–296. [Google Scholar] [CrossRef]
- Ortiz-García, S.; Pinero, D. Pines: A mexican gift to the world. Voices Mex. 2018, 55, 99–103. [Google Scholar]
- Moctezuma López, G.; Flores, A. Economic importance of pine (Pinus spp.) as a natural resource in Mexico. Rev. Mex. Ciencias For. 2020, 11, 161–185. [Google Scholar] [CrossRef]
- Darrow, W.K.; Coetzee, H. Potentially valuable Mexican pines for the summer rainfall region of southern Africa. South Afr. For. J. 1983, 124, 23–35. [Google Scholar] [CrossRef]
- Cambrón-Sandoval, V.H.; Sánchez-Vargas, N.M.; Sáenz-Romero, C.; Vargas-Hernández, J.J.; España-Boquera, M.L.; Herrerías-Diego, Y. Genetic parameters for seedling growth in Pinus pseudostrobus families under different competitive environments. New For. 2013, 44, 219–232. [Google Scholar] [CrossRef]
- Fabián-Plesníková, I.; Sáenz-Romero, C.; de León, J.C.; Martínez-Trujillo, M.; Sánchez-Vargas, N.M. Growth trait genetic parameters in a progeny trial of Pinus oocarpa. Madera Bosques 2020, 26, 1–14. [Google Scholar] [CrossRef]
- Wehenkel, C.; Mariscal-Lucero, S.D.R.; Jaramillo-Correa, J.P.; López-Sánchez, C.A.; Vargas-Hernández, J.J.; Sáenz-Romero, C. Genetic diversity and conservation of Mexican forest trees. In Biodiversity and Conservation of Woody Plants; Ahuja, M., Jain, S., Eds.; Sustainable Development and Biodiversity; Springer International Publishing: Cham, Switzerland, 2017; pp. 37–67. ISBN 9783642209246. [Google Scholar]
- Parraguirre Lezama, C.; Vargas-Hernández, J.J.; Ramírez Vallejo, P.; Azpíroz Rivero, H.S.; Jasso Mata, J. Estructura de la diversidad genética en poblaciones naturales de Pinus greggii Engelm. Rev. Fitotec. Mex. 2002, 25, 279–287. [Google Scholar] [CrossRef]
- Hernández-Velasco, M.R.; de Almeida-Souza, M.; Hernández-Díaz, J.C.; Escobar-Flores, J.G.; López-Sánchez, C.A.; Wehenkel, C.A. Diversidad genética en catorce poblaciones de Pinus arizonica y su relación con variables ambientales. Rev. Mex. Agrosistemas 2019, 6, 67–77. [Google Scholar]
- Delgado, P.; Piñero, D. Sistemática filogeográfica y sus aplicaciones a la evolución y conservación de los bosques de coníferas en México: El caso de Pinus montezumae y P. pseudostrobus. Acta Univ. 2003, 13, 47–52. [Google Scholar] [CrossRef]
- Dvorak, W.S.; Potter, K.M.; Hipkins, V.D.; Hodge, G.R. Genetic diversity and gene exchange in Pinus oocarpa, a mesoamerican pine with resistance to the pitch canker fungus (Fusarium circinatum). Int. J. Plant Sci. 2009, 170, 609–626. [Google Scholar] [CrossRef]
- Alfonso-Corrado, C.; Campos-Contreras, J.; Sánchez-García, G.; Monsalvo-Reyes, A.; Clark-Tapia, R. Manejo forestal y diversidad genética de Pinus patula Schiede ex Schltdl, & Cham, en Sierra Juárez, Oaxaca. Madera Bosques 2014, 20, 11–22. [Google Scholar]
- Castro-Félix, P.; Sierra, J.S.; Pérez-de la Rosa, J.A.; Aguirre-Gutiérrez, J.; Barragán, D.M.; Villalobos-Arámbula, A.R. Genetic diversity and structure of morphologically characterized populations Pinus ayacahuite and Pinus strobiformis through the analysis of neutral nuclear markers. e-CUCBA 2019, 6, 34–45. [Google Scholar] [CrossRef]
- Morales-Nieto, C.R.; Siqueiros-Candia, M.; Álvarez-Holguín, A.; Gil-Vega, K.D.C.; Corrales-Lerma, R.; Martínez-Salvador, M. Diversidad, estructura genética e hibridación en poblaciones de Pinus arizonica y P. durangensis. Madera y Bosques 2021, 27, 1–14. [Google Scholar] [CrossRef]
- Castro-Félix, P.; Ramos Navarro, C.; Pérez de la Rosa, J.A.; Vargas Amado, G.; Villalobos-Arámbula, A.R. Diversidad genética de Pinus ayacahuite utilizando marcadores RAPDs en genoma diploide y haploide. Scentia-CUCBA 2006, 8, 193–202. [Google Scholar]
- Ledig, F.T.; Capó-Arteaga, M.A.; Hodgskiss, P.D.; Sbay, H.; Flores-López, C.; Conkle, M.T.; Bermejo-Velázquez, B. Genetic diversity and the mating system of a rare Mexican piñon, Pinus pinceana, and a comparison with Pinus maximartinezii (Pinaceae). Am. J. Bot. 2001, 88, 1977–1987. [Google Scholar] [CrossRef]
- Molina-Freaner, F.; Delgado, P.; Piñero, D.; Perez-Nasser, N.; Alvarez-Buylla, E. Do rare pines need different conservation strategies? evidence from three Mexican species. Can. J. Bot. 2001, 79, 131–138. [Google Scholar] [CrossRef]
- Ramírez Enríquez, E.; Delgado Valerio, P.; García Magaña, J.J.; Molina Sánchez, A. Diversidad genética y conservación de pinos nativos de la cuenca del Río Cupatitzio, en Michoacán. Rev. Mex. Ciencias For. 2019, 10, 4–32. [Google Scholar] [CrossRef]
- Morales-Nieto, C.R.; Siqueiros-Candia, M.; Álvarez-Holguín, A.; Corrales-Lerma, R.; Alarcón-Bustamante, M.; Martínez-Salvador, M. Estructura y diversidad genética en poblaciones de Pinus engelmannii Carr. en Chihuahua, México. Rev. Fitotec. Mex. 2020, 43, 197–204. [Google Scholar] [CrossRef]
- Heredia-Bobadilla, R.L.; Gutiérrez-González, G.; Arzate-Fernández, A.-M.; Franco-Maass, S. Genetic variability of mountain pine (Pinus hartwegii Lindl) in the protection of flora and fauna area Nevado de Toluca. In Genetic Diversity in Plant Species: Characterization and Conservation; El-Esawi, M., Ed.; IntechOpen: London, UK, 2018; pp. 71–85. [Google Scholar]
- Rodríguez-Banderas, A.; Vargas-Mendoza, C.F.; Buonamici, A.; Vendramin, G.G. Genetic Diversity and phylogeographic analysis of Pinus leiophylla: A post-glacial range expansion. J. Biogeogr. 2009, 36, 1807–1820. [Google Scholar] [CrossRef]
- Ramírez-Herrera, C.; Vargas-Hernández, J.J.; López-Upton, J. Distribución y conservación de las poblaciones naturales de Pinus greggii. Acta Botánica Mex. 2005, 72, 1–16. [Google Scholar] [CrossRef]
- Flores, A.; López-Upton, J.; Rullán-Silva, C.D.; Olthoff, A.E.; Alía, R.; Sáenz-Romero, C.; Garcia del Barrio, J.M. Priorities for conservation and sustainable use of forest genetic resources in four Mexican pines. Forests 2019, 10, 675. [Google Scholar] [CrossRef]
- Farjon, A. A handbook of the world’s conifers, 2nd ed.; Koninklijke Brill: Leiden, The Netherlands, 2017; ISBN 9781119130536. [Google Scholar]
- Perry, J.P. The pines of Mexico and Central America; Timber Press, Inc.: Portland, OR, USA, 1991; ISBN 9781604691108. [Google Scholar]
- CONAFOR. Inventario Nacional Forestal y de Suelos. Informe 2004-2009. Available online: http://www.ccmss.org.mx/descargas/Inventario_nacional_forestal_y_de_suelos_informe_2004_-_2009_pdf (accessed on 18 January 2018).
- QGIS Development Team. QGIS Geographic Information System; v 3.28.2; Open Source Geospatial Foundation Project. 2022. Available online: https://qgis.org/en/site/ (accessed on 1 February 2023).
- CONAFOR. Manual Para el Establecimiento de Unidades Productoras de Germoplasma Forestal; CONAFOR: Zapopan, México, 2016. [Google Scholar]
- Koskela, J.; Lefèvre, F.; Schueler, S.; Kraigher, H.; Olrik, D.C.; Hubert, J.; Longauer, R.; Bozzano, M.; Yrjänä, L.; Alizoti, P.; et al. Translating conservation genetics into management: Pan-European minimum requirements for dynamic conservation units of forest tree genetic diversity. Biol. Conserv. 2013, 157, 39–49. [Google Scholar] [CrossRef]
- Friedrich, S.C.; Hernández-Díaz, J.C.; Leinemann, L.; Prieto-Ruíz, J.A.; Wehenkel, C. Spatial genetic structure in seed stands of Pinus arizonica Engelm. and Pinus cooperi Blanco in the State of Durango, Mexico. For. Sci. 2018, 64, 191–202. [Google Scholar] [CrossRef]
- Ramírez-Orozco, C.L.; Hernández-Díaz, J.C.; Carrillo-Parra, A.; Wehenkel, C.; Quiñones-Pérez, C.Z.; López-Sánchez, C.A.; Bailón-Soto, C.E. The centre–periphery model, a possible explanation for the distribution of some Pinus spp. in the Sierra Madre Occidental, Mexico. Forests 2022, 13, 215. [Google Scholar] [CrossRef]
- Simental-Rodríguez, S.L.; Pérez-Luna, A.; Hernández-Díaz, J.C.; Jaramillo-Correa, J.P.; López-Sánchez, C.A.; Flores-Rentería, L.; Carrillo-Parra, A.; Wehenkel, C. Modelling shifts and contraction of seed zones in two Mexican pine species by using molecular markers. Forests 2021, 12, 570. [Google Scholar] [CrossRef]
- Siqueiros Candia, I.E.M. Variabilidad Morfológica y Molecular en tres Especies Forestales de Importancia Económica en el Estado de Chihuahua. Master’s Thesis, Universidad Autónoma de Chihuahua, Chihuahua, México, 2017. [Google Scholar]
- Arvizu Franklin, L.E. Caracterización de Marcadores RAPDs en Pinus chiapensis y en el Complejo Pinus ayacahuite-P. strobiformis. Ph.D. Thesis, Universidad de Guadalajara, Jalisco, México, 2003. [Google Scholar]
- Castro-Félix, P.; Pérez de la Rosa, J.A.; Amado, G.V.; Magaña, S.V.; Santerre, A.; López-Dellamary Toral, F.; Villalobos-Arámbula, A.R. Genetic relationships among Mexican white pines (Pinus, Pinaceae) based on RAPD markers. Biochem. Syst. Ecol. 2008, 36, 523–530. [Google Scholar] [CrossRef]
- Hernández-León, S.; Little, D.P.; Acevedo-Sandoval, O.; Gernandt, D.S.; Rodríguez-Laguna, R.; Saucedo-García, M.; Arce-Cervantes, O.; Razo-Zárate, R.; Espitia-López, J. Plant core DNA barcode performance at a local scale: Identification of the conifers of the state of Hidalgo, Mexico. Syst. Biodivers. 2018, 16, 791–806. [Google Scholar] [CrossRef]
- Moreno-Letelier, A.; Piñero, D. Phylogeographic structure of Pinus strobiformis Engelm. across the Chihuahuan desert filter-barrier. J. Biogeogr. 2009, 36, 121–131. [Google Scholar] [CrossRef]
- Moreno-Letelier, A.; Barraclough, T.G. Mosaic genetic differentiation along environmental and geographic gradients indicate divergent selection in a white pine species complex. Evol. Ecol. 2015, 29, 733–748. [Google Scholar] [CrossRef]
- Ramos Navarro, C.L. Diversidad Genética de Pinus ayacahuite Ehrenberg Ex Schlechtenda: Reproductividad y Segregación de Marcadores RAPDs. Bachelor’s Thesis, Universidad de Guadalajara, Zapopan, México, 2005. [Google Scholar]
- Villalobos-Arámbula, A.R.; Pérez de la Rosa, J.A.; Arias, A.; Rajora, O.P. Cross-species transferability of eastern white pine (Pinus strobus) nuclear microsatellite markers to five Mexican white pines. Genet. Mol. Res. 2014, 13, 7571–7576. [Google Scholar] [CrossRef]
- Farfán Vázquez, E.d.G.; Jasso Mata, J.; López Upton, J.; Vargas Hernández, J.J.; Ramírez Herrera, C. Parámetros genéticos y eficiencia de la selección temprana en Pinus ayacahuite Ehren. var. ayacahuite. Rev. Fitotec. Mex. 2002, 25, 239–246. [Google Scholar] [CrossRef]
- Castilleja Sánchez, P. Éxito Reproductivo y Comportamiento de Caracteres Cuantitativos en dos Especies de pino Endémicas de México: Pinus rzedowskii Madrigal et Caballero y Pinus ayacahuite var. veitchii Shaw. Master’s Thesis, Universidad Michoacana de san Nicolás de Hidalgo, Michoacán, México, 2015. [Google Scholar]
- Flores-Rentería, L.; Wegier, A.; Ortega Del Vecchyo, D.; Ortíz-Medrano, A.; Piñero, D.; Whipple, A.V.; Molina-Freaner, F.; Domínguez, C.A. Genetic, morphological, geographical and ecological approaches reveal phylogenetic relationships in complex groups, an example of recently diverged pinyon pine species (Subsection Cembroides). Mol. Phylogenet. Evol. 2013, 69, 940–949. [Google Scholar] [CrossRef]
- Fuentes-Amaro, S.L.; Legaria-Solano, J.P.; Ramírez-Herrera, C. Estructura genética de poblaciones de Pinus cembroides de la región central de México. Rev. Fitotec. Mex. 2019, 42, 57–65. [Google Scholar] [CrossRef]
- García-Zubia, L.C.; Hernández-Velasco, J.; Hernández-Diáz, J.C.; Simental-Rodriguez, S.L.; López-Sánchez, C.A.; Quinõnes-Pérez, C.Z.; Carrillo-Parra, A.; Wehenkel, C. Spatial genetic structure in Pinus cembroides Zucc. at population and landscape levels in Central and Northern Mexico. PeerJ 2019, 7, e8002. [Google Scholar] [CrossRef]
- Hernández-Velasco, J.; Hernández-Díaz, J.C.; Fladung, M.; Cañadas-López, Á.; Prieto-Ruíz, J.Á.; Wehenkel, C. Spatial genetic structure in four pinus species in the Sierra Madre Occidental, Durango, Mexico. Can. J. For. Res. 2017, 47, 73–80. [Google Scholar] [CrossRef]
- Alva-Rodríguez, S.; López-Upton, J.; Vargas-Hernández, J.J.; del Mar Ruiz-Posadas, L. Biomasa y crecimiento de Pinus cembroides Zucc. y Pinus orizabensis D. K. Bailey & Hawksworth en respuesta al déficit hídrico. Rev. Chapingo Ser. Ciencias For. y del Ambient. 2020, 26, 71–83. [Google Scholar] [CrossRef]
- González Avalos, J.; García Moya, E.; Cetina Alcalá, V.M.; Vargas-Hernández, J.J.; Trinidad-Santos, A.; Romero-Manzanares, A. Variación morfológica e índice de calidad en plantas de Pinus cembroides var. cembroides Zucc. Cienc. For. en Mex. 2005, 30, 29–44. [Google Scholar]
- Núñez Álvarez, E. Crecimiento y Estructura de copa en tres Procedencias de Pinus cembroides Zucc. en Los Lirios, Arteaga, Coahuila. Bachelor’s Thesis, Universidad Autónoma Agraria Antonio Narro, Saltillo, México, 2016. [Google Scholar]
- Del Castillo, R.F.; Trujillo Argueta, S.; Sáenz-Romero, C. Pinus chiapensis, a keystone species: Genetics, ecology, and conservation. For. Ecol. Manage. 2009, 257, 2201–2208. [Google Scholar] [CrossRef]
- Newton, A.C.; Allnutt, T.R.; Dvorak, W.S.; Del Castillo, R.F.; Ennos, R.A. Patterns of genetic variation in Pinus chiapensis, a threatened Mexican pine, detected by RAPD and mitochondrial DNA RFLP markers. Heredity (Edinb) 2002, 89, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Acuña, D.; Sáenz-Romero, C.; Lindig-Cisneros, R.A.; Sánchez-Vargas, N.M.; Lobbit, P.; Montero-Castro, J.C. Variación altitudinal entre especies y procedencias de Pinus pseudostrobus, P. devoniana y P. leiophylla. Ensayo de vivero. Rev. Chapingo Ser. Ciencias For. y del Ambient. 2013, 19, 399–411. [Google Scholar] [CrossRef]
- López-Reyes, A.; Pérez de la Rosa, J.; Ortiz, E.; Gernandt, D.S. Morphological, molecular, and ecological divergence in Pinus douglasiana and P. maximinoi. Syst. Bot. 2015, 40, 658–670. [Google Scholar] [CrossRef]
- Ávila-Flores, I.J.; Hernández-Díaz, J.C.; Gonzáez-Elizondo, M.S.; Prieto-Ruíz, J.Á.; Wehenkel, C. Degree of hybridization in seed stands of Pinus engelmannii Carr. in the Sierra Madre Occidental, Durango, Mexico. PLoS ONE 2016, 11, e0152651. [Google Scholar] [CrossRef]
- Rodríguez Laguna, R.; Vargas Hernández, J.J.; Cetina Alcalá, V.M.; Ramírez Herrera, C.; Escalante Estrada, J.A. Variación en el patrón de alargamiento del brote terminal en diferentes procedencias de Pinus engelmannii Carr. Rev. Cienc. For. en México 2000, 25, 77–103. [Google Scholar]
- Iglesias Andreu, L.G.; Tivo Fernández, Y. Polimorfismos proteico en Pinus hartwegii Lindl. del Cofre de Perote, Veracruz, México. Rev. Chapingo Ser. Ciencias For. y del Ambient. 2008, 14, 5–9. [Google Scholar]
- Obregón Molina, G. Estudio Filogeográfico de Pinus hartwegii Lindley (Pinaceae). Master’s Thesis, Instituto Politécnico Nacional, Distrito Federal, México, 2010. [Google Scholar]
- Solís Ramos, L.Y.; Iglesias Andreu, L.G. Variación en la composición isoenzimática en la población de Pinus hartwegii Lindl. del Pico de Orizaba, Veracruz. Cuad. Biodivers. 2001, 4–7. [Google Scholar] [CrossRef]
- Treviño Cuellar, K.L. Genómica del Paisaje para la Identificación de Hotspots Adaptativos en Coníferas de alta Montaña del Noreste de México. Master’s Thesis, Universidad de Nuevo León, Linares, México, 2021. [Google Scholar]
- Viveros-Viveros, H.; Tapia-Oivares, B.L.; Sáenz-Romero, C.; Vargas-HerNández, J.J.; López-Upton, J.; Santacruz-Valera, A.; Ramírez-Valverde, G. Variación isoenzimática de Pinus hartwegii Lindl. en un gradiente altitudinal en Michoacán, México. Agrociencia 2010, 44, 723–733. [Google Scholar]
- Viveros-Viveros, H.; Sáenz-Romero, C.; Vargas-Hernández, J.J.; Tapia-Olivares, B.L.; López-Upton, J.; Santacruz-Varela, A.; Beaulieu, J. Comparación de QST vs. FST en poblaciones naturales de Pinus hartwegii Lindl. Rev. Fitotec. Mex. 2014, 37, 117–127. [Google Scholar] [CrossRef]
- Ortega-Mata, A.; Mendizábal Hernández, L.; Alba-Landa, J.; Aparicio Rentería, A. Germinación y crecimiento inicial de Pinus hartwegii Lindl. de siete poblaciones del Estado de México. For. Veracruzana 2003, 5, 29–34. [Google Scholar]
- Sáenz-Romero, C.; Lamy, J.-B.; Loya-Rebollar, E.; Plaza-Aguilar, A.; Burlett, R.; Lobit, P.; Delzon, S. Genetic variation of drought-induced cavitation resistance among Pinus hartwegii populations from an altitudinal gradient. Acta Physiol. Plant. 2013, 35, 2905–2913. [Google Scholar] [CrossRef]
- Viveros-Viveros, H.; Sáenz-Romero, C.; López-Upton, J.; Vargas-Hernández, J.J. Growth and frost damage variation among Pinus pseudostrobus, P. montezumae and P. hartwegii tested in Michoacán, México. For. Ecol. Manage. 2007, 253, 81–88. [Google Scholar] [CrossRef]
- Castelán Muñoz, N. Fisiología de Plántulas de Pinus leiophylla Sometidas a estrés Hídrico. Master’s Thesis, Colegio de Postgraduados, Texcoco, México, 2014. Available online: http://colposdigital.colpos.mx:8080/xmlui/handle/10521/2429 (accessed on 1 March 2023).
- Dvorak, W.S.; Hodge, G.R.; Kietzka, J.E. Genetic variation in survival, growth, and stem form of Pinus leiophylla in Brazil and South Africa and provenance resistance to pitch canker. South. Hemisph. For. J. 2007, 69, 125–135. [Google Scholar] [CrossRef]
- Martínez-Trinidad, T.; Vargas-Hernández, J.J.; López-Upton, J.; Muñoz-Orozco, A. Respuesta al deficit hidrico en Pinus leiophylla: Acumulacion de biomasa, desarrollo de hojas secundarias y mortandad de plántulas. Terra 2002, 20, 291–301. [Google Scholar]
- Castelán-Muñoz, N.; Jiménez-Casas, M.; López-Delgado, H.A.; Campos-García, H.; Vargas-Hernández, J.J. Familial variation in Pinus leiophylla Schiede Ex Schltdl. & Cham. seedlings in response to drought: Water and osmotic potential. Rev. Chapingo Ser. Ciencias For. y del Ambient. 2015, XXI, 295–306. [Google Scholar] [CrossRef]
- Gapare, W.J.; Hodge, G.R.; Dvorak, W.S. Genetic parameters and provenance variation of Pinus maximinoi in Brazil, Colombia and South Africa. For. Genet. 2001, 8, 159–170. [Google Scholar]
- Hodge, G.R.; Dvorak, W.S. Differential responses of Central American and Mexican species and Pinus radiata to infection by the pitch canker fungus. New For. 2000, 19, 241–258. [Google Scholar] [CrossRef]
- Hodge, G.; Dvorak, W. Growth potential and genetic parameters of four mesoamerican pines planted in the Southern hemisphere. South. For. a J. For. Sci. 2012, 74, 27–49. [Google Scholar] [CrossRef]
- Lopez-Upton, J.; Donahue, J.K.; Plascencia-Escalante, F.O.; Ramírez-Herrera, C. Provenance variation in growth characters of four subtropical pine species planted in Mexico. New For. 2005, 29, 1–13. [Google Scholar] [CrossRef]
- Mendizábal-Hernández, L. del C.; Alba-Landa, J.; Márquez Ramírez, J.; Ramírez-García, E.O.; Cruz-Jiménez, H. Movimiento de especies. For. Veracruzana 2011, 13, 37–42. [Google Scholar]
- Salazar-García, J.G.; Vargas-Hernández, J.J.; Jasso-Mata, J.; Molina-Galán, J.D.; Ramírez-Herrera, C.; López-Upton, J. Variación en el patrón de crecimiento en altura de cuatro especies de Pinus en edades tempranas. Madera y Bosques 1999, 5, 19–34. [Google Scholar] [CrossRef]
- Barrera Marías, S. Diversidad Genética en Rodales Semilleros de Pinus montazumae Lamb., Distribuidos en México. Bachelor’s Thesis, Universidad de Michoacán de San Nicolás de Hidalgo, Uruapán, México, 2014. [Google Scholar]
- Delgado, P.; Salas-Lizana, R.; Vazquez-Lobo, A.; Wegier, A.; Anzidei, M.; Alvarez-Buylla, E.R.; Vendramin, G.G.; Piñero, D. Introgressive hybridization in Pinus montezumae Lamb and Pinus pseudostrobus Lindl. (Pinaceae): Morphological and molecular (CpSSR) evidence. Int. J. Plant Sci. 2007, 168, 861–875. [Google Scholar] [CrossRef]
- Delgado Valerio, P.; Nunez Medrano, J.; Rocha Granados, M.C.; Munoz Flores, H.J. Variación genética en dos áreas semilleras de pino establecidas en el estado de Michoacán. Rev. Mex. Ciencias For. 2013, 4, 104–115. [Google Scholar]
- Molina Sánchez, A. Fragmentación del Hábitat y su Efecto en la Estructura Genética de dos Linajes del Género Pinus Distribuidos en la Meseta Purépecha. Master’s Thesis, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, México, 2017. [Google Scholar]
- Barragán Reynaga, D.M. Diversidad y diferenciación genética con marcadores RAPDs en poblaciones naturales de Pinus strobiformis. Master’s Thesis, Universidad de Guadalajara, Zapopan, México, 2006. [Google Scholar]
- Simental-Rodríguez, S.L.; Quinones-Pérez, C.Z.; Moya, D.; Hernández-Tecles, E.; Lopez-Sanchez, C.A.; Wehenkel, C. The relationship between species diversity and genetic structure in the rare Picea chihuahuana tree species community, Mexico. PLoS ONE 2014, 9, e111623. [Google Scholar] [CrossRef] [PubMed]
- García Ramírez, E.O.; Landa-Alba, J.; Mendizabal-Hernández, L.C. Evaluación en vivero de un ensayo de procedencias/progenies de Pinus teocote Schl & Cham. For. Veracruzana 2001, 3, 27–35. [Google Scholar]
- Lefèvre, F.; Alia, R.; Fjellstad, K.B.; Graudal, L.; Oggioni, S.D.; Rusanen, M.; Vendramin, G.G.; Bozzano, M. Dynamic Conservation and Utilization of Forest Tree Genetic Resources: Indicators for In Situ and Ex Situ Genetic Conservation and Forest Reproductive Material; European Forest Genetic Resources Programme (EUFORGEN), European Forest Institute: Barcelona, Spain, 2020; ISBN 9789525980813. [Google Scholar]
- Flores, A.; Romero-Sánchez, M.E.; Pérez-Miranda, R.; Pineda-Ojeda, T.; Moreno-Sánchez, F. Potential of restoration of coniferous forests from germplasm transfer zones in Mexico. Rev. Mex. Ciencias For. 2021, 12, 4–27. [Google Scholar] [CrossRef]
- Flores, A.; Méndez-González, J.; Muñoz-Flores, H.J. Degraded forest lands and pine plantations in homogeneous ecological areas. Agro Product. 2021, 14, 49–59. [Google Scholar] [CrossRef]
- Flores, A.; Moctezuma-López, G. Harvest of timber from 20 coniferous in germplasm movement zones. Rev. Mex. Ciencias For. 2021, 12, 122–140. [Google Scholar] [CrossRef]
- Herrera-Soto, G.; González-Cásares, M.; Pompa-García, M.; Camarero, J.J.; Solís-Moreno, R. Growth of Pinus cembroides Zucc. in response to hydroclimatic variability in four sites forming the species latitudinal and longitudinal distribution limits. Forests 2018, 9, 440. [Google Scholar] [CrossRef]
- Castellanos-Acuña, D.; Mota-Narváez, L.A.; López-Mondragón, T.; Lindig-Cisneros, R.A.; Sáenz-Romero, C. Pinus devoniana likely avoids drought stress by delaying shoot elongation. Rev. Fitotec. Mex. 2022, 45, 135–143. [Google Scholar] [CrossRef]
- Sandoval-García, R.; Jiménez-Pérez, J.; Yerena-Yamallel, J.I.; Aguirre-Calderón, O.A.; Alanís-Rodríguez, E.; Gómez-Meza, M.V. Ecological restoration strategies associated with reforestations of Pinus cembroides Zucc., in the Parque Nacional Cumbres de Monterrey. Madera Bosques 2022, 28, e2822298. [Google Scholar] [CrossRef]
- Bruhns, W. Measuring Reforestation Sucess in the Sierra Gorda Guanajuato Biosphere Reserve, Mexico. Master’s Thesis, Northern Arizona University, Flagstaff, AZ, USA, 2013. [Google Scholar]
- Secretaría de Economía. Ley General de Desarrollo Forestal Sustentable. Available online: https://www.diputados.gob.mx/LeyesBiblio/pdf/LGDFS.pdf (accessed on 1 March 2023).
- Secretaría de Economía. Reglamento de la Ley General de Desarrollo Forestal Sustentable. Available online: https://www.diputados.gob.mx/LeyesBiblio/regley/Reg_LGDFS_091220.pdf (accessed on 1 March 2023).
- Sáenz-Romero, C.; Mendoza-Maya, E.; Gómez-Pineda, E.; Blanco-García, A.; Endara-Agramont, A.R.; Lindig-Cisneros, R.; López-Upton, J.; Trejo-Ramírez, O.; Wehenkel, C.; Cibrián-Tovar, D.; et al. Recent evidence of Mexican temperate forest decline and the need for ex situ conservation, assisted migration, and translocation of species ensembles as adaptive management to face projected climatic change impacts in a Megadiverse country. Can. J. For. Res. 2020, 50, 843–854. [Google Scholar] [CrossRef]
- Potter, K.M.; Jetton, R.M.; Bower, A.; Jacobs, D.F.; Man, G.; Hipkins, V.D.; Westwood, M. Banking on the future: Progress, challenges and opportunities for the genetic conservation of forest trees. New For. 2017, 48, 153–180. [Google Scholar] [CrossRef]
- Gutiérrez Aguirre, J.; Duivenvoorden, J.F. Can we expect to protect threatened species in protected areas? A case study of the genus Pinus in Mexico. Rev. Mex. Biodivers. 2010, 81, 875–882. [Google Scholar]
- Lefèvre, F.; Koskela, J.; Hubert, J.; Kraigher, H.; Longauer, R.; Olrik, D.C.; Schüler, S.; Bozzano, M.; Alizoti, P.; Bakys, R.; et al. Dynamic conservation of forest genetic resources in 33 European countries. Conserv. Biol. 2013, 27, 373–384. [Google Scholar] [CrossRef]
- Galicia, L.; Potvin, C.; Messier, C. Maintaining the high diversity of pine and oak species in Mexican temperate forests: A new management approach combining functional zoning and ecosystem adaptability. Can. J. For. Res. 2015, 45, 1358–1368. [Google Scholar] [CrossRef]
- Patiño Ayala, E.A. Mercado Potencial de Árboles de Navidad en Guadalajara, Jalisco. Master Thesis, Colegio de Postgraduados, Texcoco, Mexico, 2017. [Google Scholar]
- Esperón-Rodríguez, M.; Barradas, V.L. Ecophysiological vulnerability to climate change: Water stress responses in four tree species from the central mountain region of Veracruz, Mexico. Reg. Environ. Chang. 2015, 15, 93–108. [Google Scholar] [CrossRef]
- Kilgore, J.S.; Jacobsen, A.L.; Telewski, F.W. Hydraulics of Pinus (Subsection Ponderosae) populations across an elevation gradient in the Santa Catalina mountains of Southern Arizona. Madroño 2021, 67, 218–226. [Google Scholar] [CrossRef]
- De Ronde, C.; du Plessis, M. Determining the relative resistance of selected Pinus species to fire damage. In Forest Fire Research and Wildland Fire Safety; Millpress: Rotterdam, The Netherlands, 2002; pp. 1–9. [Google Scholar]
- Bannister, P.; Neuner, G. Frost resistance and the distribution of conifers. In Conifer Cold Hardiness; Bigras, F.J., Colombo, S.J., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 3–21. ISBN 9789048155873. [Google Scholar]
- Miyamoto, S.; Martinez, I.; Padilla, M.; Portillo, A.; Ornelas, D. Landscape plant lists for salt tolerance assessment. USDI Bureau Of Reclamation. Texas Agricultural Extension Station, El Paso. 2004. Available online: http://agrilife.org/elpaso/files/2011/10/Landscape-Plant-Lists-for-Salt-Tolerance-Assessment.pdf (accessed on 6 February 2023).
- García-Jiménez, C.I.; Vargas-Rodriguez, Y.L. Passive government, organized crime, and massive deforestation: The case of western Mexico. Conserv. Sci. Pract. 2021, 3, e562. [Google Scholar] [CrossRef]
- Barsimantov, J.; Navia Antezana, J. Forest cover change and land tenure change in Mexico’s avocado region: Is community forestry related to reduced deforestation for high value crops. Appl. Geogr. 2012, 32, 844–853. [Google Scholar] [CrossRef]
- Rayn, D.; Sutherland, W.J. Impact of nature reserve establishment on deforestation: A test. Biodivers. Conserv. 2011, 20, 1625–1633. [Google Scholar] [CrossRef]
- Powlen, K.A.; Gavin, M.C.; Jones, K.W. Management effectiveness positively influences forest conservation outcomes in protected areas. Biol. Conserv. 2021, 260, 109192. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Blanco-García, A.; Lindig-Cisneros, R. Bird community shifts related to different forest restoration efforts: A case study from a managed habitat matrix in Mexico. Ecol. Eng. 2010, 36, 1492–1496. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).