1. Introduction
Diatoms (Bacillariophyta) are perhaps best known for the possession of characteristic external siliceous cell walls (frustules), on which their identification and classification have traditionally been based [
1]. The frustule is an essentially bipartite structure, comprising two valves, each with a series of linking bands (cingulum). The addition of new girdle bands (copulae) to the younger half frustule during vegetative growth allows cell expansion until the next mitosis and cell division. While much attention has been given to the shape and structure of the valves—usually the larger, more elaborate components of the frustule—the cingulum and its copulae have been given less attention. However, not only are they critical to the integrity of the frustule, but the variation in their structure also both provides additional taxonomic characters and reveals the different ways in which diatoms potentially “solve” engineering challenges.
Two recent papers [
2,
3] described several new species of the diatom genus
Proschkinia Karayeva, discussing their phylogenetic relationships, but with little detail on the structure of their unusual girdle bands. Both papers show essentially the same phylogenetic trees, in which, within the raphid naviculoid diatoms,
Proschkinia groups with
Fistulifera Lange-Bertalot in a larger clade including
Craticula Grunow;
Stauroneis Ehrenberg and
Parlibellus harffianus Witkowski, Ch. Li and X. Yu (other
Parlibellus E.J. Cox species were part of another clade with
Astartiella Witkowski, Lange-Bertalot and Metzeltin and
Schizostauron Grunow). Stating that it required much further study, Round et al. [
1] placed
Proschkinia in its own family, the Proschkiniaceae, with
Craticula and
Stauroneis in their (preceding) family Stauroneidaceae (both within the Naviculineae, which also included the Naviculaceae and Pleurosigmataceae). They [
1] commented that
Proschkinia shows “superficial similarities to
Craticula and
Stauroneis” but questioned a close relationship due to differences in the raphe and girdle structure. On the other hand, the relationship between
Proschkinia and
Fistulifera was first shown in a mitochondria-based phylogeny [
4] and then in a study of
Fistulifera by Zgrundo et al. [
5] that included a phylogenetic analysis based on 18S rDNA. Considering their contrasting valve and girdle morphology, these were surprising results, but both genera share the presence of a fistula, an unusual, isolated pore near the central raphe endings, with a hymenate internal occlusion. Majewska et al. [
2] commented that a potential homology between a fistula and the internally occluded stigma of
Didymosphenia M. Schmidt, based on the mitochondrial tree [
4], was not supported by their expanded dataset [
2].
In terms of the cingulum structure of
Proschkinia, Majewska et al. [
2] noted the single row of hymenate areolae on the pars interior of the U-shaped bands, but none of their SEMs were at a high magnification. The cingulum structure in raphid diatoms is often relatively simple, comprising relatively few (in most cases), similar, band-like, split rings, which may or may not contain rows of areolae. Thus, in some genera (e.g.,
Navicula Bory
sensu stricto,
Gyrosigma Hassall,
Pleurosigma W. Smith,
Sellaphora Kützing) the girdle bands lack pores, whereas in others, one or two rows of open (e.g.,
Cymbella C.A. Agardh,
Gomphonema Ehrenberg,
Oricymba Jüttner et al.,
Cymbopleura (Krammer) Krammer), hymenate (e.g.,
Berkeleya Greville,
Parlibellus,
Dickieia Berkeley ex Kützing,
Amphipleura Kützing) or cribrate (e.g.,
Achnanthes Bory,
Craspedostauros E.J. Cox) pores are present. More complex, chambered valvocopulae are found in
Mastogloia Thwaites and
Aneumastus D.G. Mann and A.J. Stickle, associated with mucilage secretion in
Mastogloia [
1,
6,
7,
8,
9,
10]. Hollow tubular girdle bands are a distinctive characteristic of
Undatella Paddock and Sims and were used to define and discriminate this genus from
Auricula Castracane [
11]. However, despite the interest in cingulum structure shown by some authors [
12,
13,
14,
15,
16,
17,
18], most studies of raphid diatoms have focussed on the valve structure, and the girdle band details have been poorly documented. The only study that attempted to understand the cingulum of
Proschkinia [
17] showed that most bands were U-shaped in cross-section, with a plain external face, a perforate internal face and an external abvalvar ridge abutting the adjacent band. However, their interpretation was limited by the quality of their micrographs, and more recent observations [
19,
20] have provided more detail on these intriguing cingula.
The lack of interest in the girdle structure derives not only from the traditional focus on the valve structure for diatom identification and classification but also from the fact that the majority of diatoms are seen in valve view with both LM and SEM. This is particularly the case for raphid diatoms, which tend to lie in valve view even when entire frustules are present. The frustules of
Proschkinia are unusual in having very deep girdle regions and narrowly lanceolate valves, with frustules usually lying in the girdle view in cleaned preparations. Even then, it is difficult to understand the 3D structure of their cingula unless cross-sectional breaks are found, as shown for
Mastogloia [
7]. However, the use of an embedding and back-etching technique [
16,
19] makes it possible to see how the gutter-shaped bands fit together with each other and the valve. This paper will present more detailed images of the
Proschkinia cingula and discuss the potential significance of their structure.
3. Results
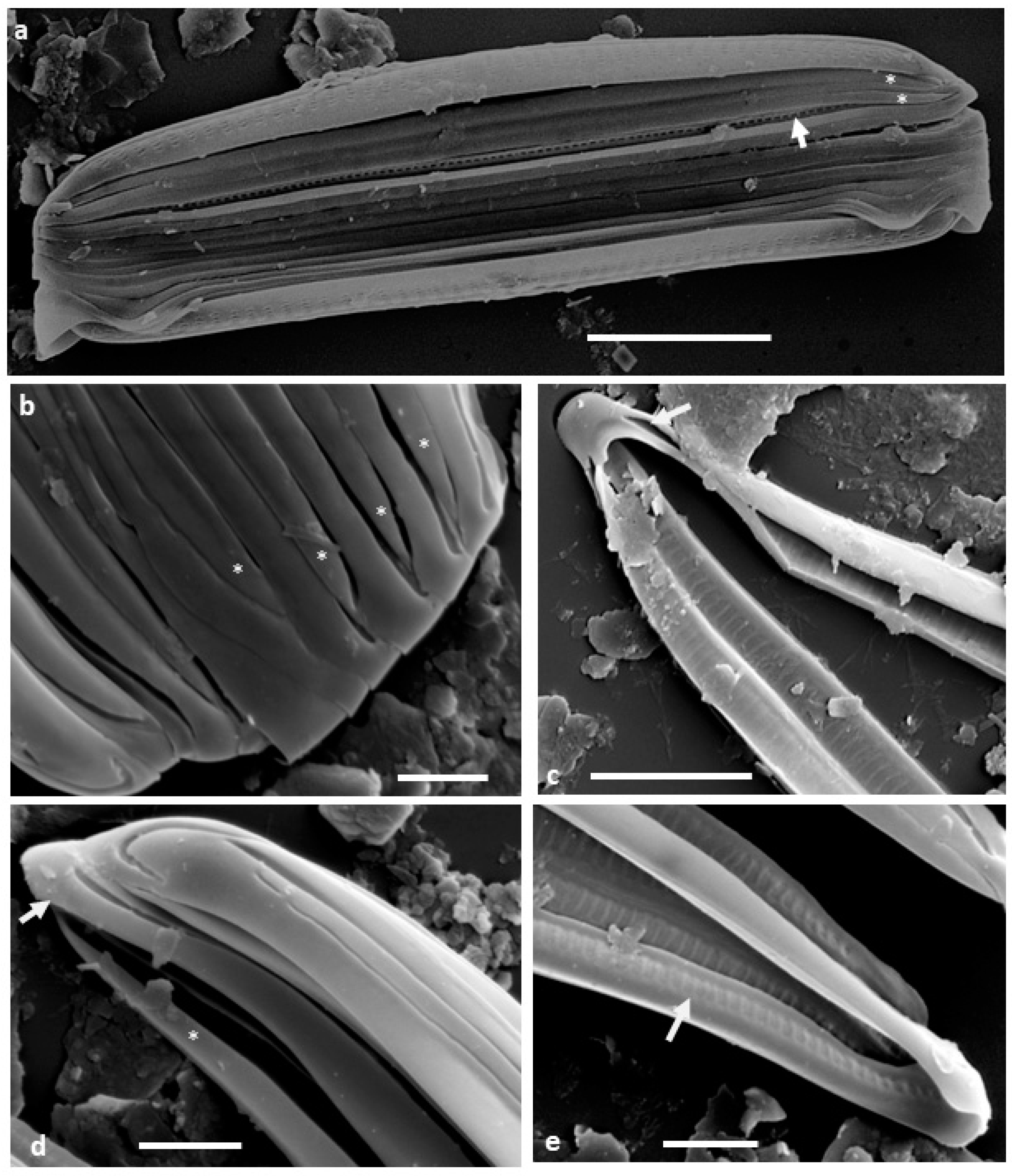
When the specimens are dried directly on stubs for SEM, they are usually observed in girdle view (
Figure 1a) with the valve apices curving down so that the frustule is slightly biconvex, narrower at the apices than the middle. The cingulum appears to comprise narrow, unperforated bands (copulae), sometimes separating from each other, revealing longitudinal grooves particularly in the mid-region (
Figure 1a). The individual copulae are split rings, with the tapered open ends alternating and ending slightly short of the frustule apices (
Figure 1a,b,d and
Figure 2a). At their closed ends, the copulae are simple band-like structures around the frustule apices, slightly broader than the depth of the channel, with the sides of the channel converging as the channel reduces in depth and width (
Figure 1c–e and
Figure 2a). The tapered open ends of the copulae fit in against the narrowed ends of the channel of the adjacent copula (
Figure 1a,b). In isolated copulae, the perforate inner wall of the channel can also be observed (
Figure 1c,e).
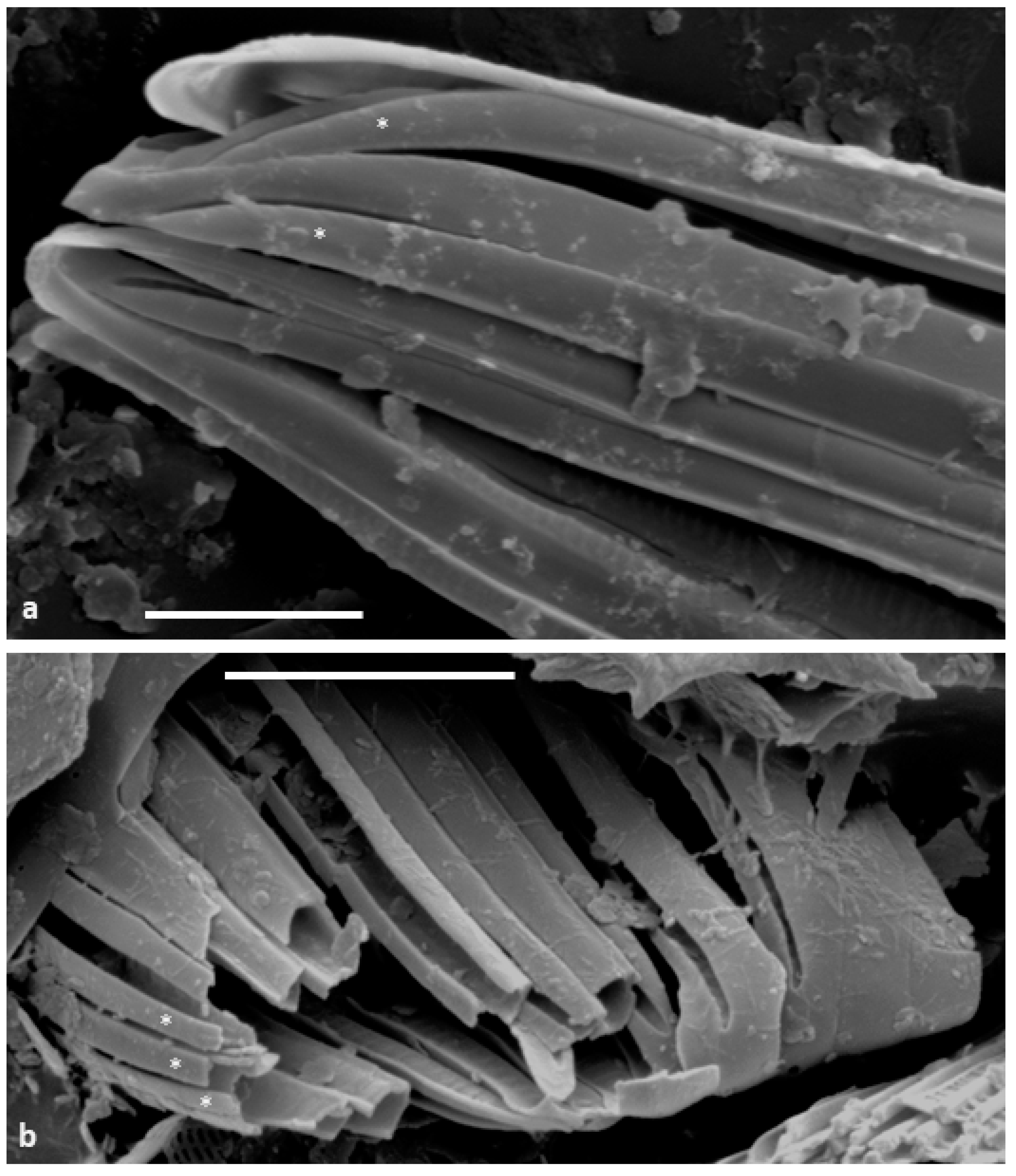
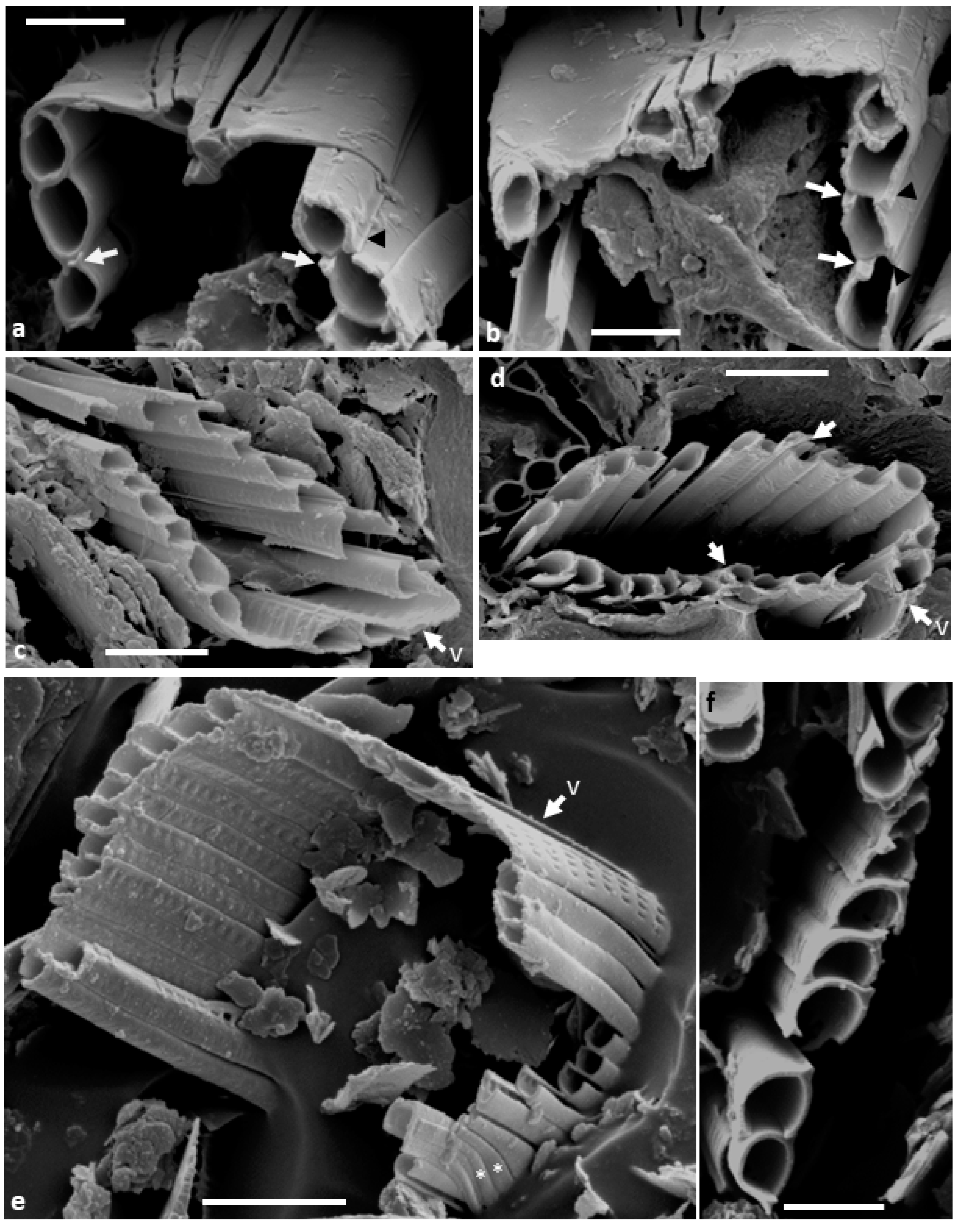
The embedded and back-etched material confirms that the copulae are U-shaped (
Figure 2a,b and
Figure 3a–f); the outer face and base of the U are plain and unperforated (
Figure 2b and
Figure 3b,e), while the inner face contains square to elongate, hymenate areolae (
Figure 2b and
Figure 3c,e,f). The copulae open towards their respective valve (
Figure 3a–e), with an abvalvar flange on the outer portion overlapping the outer side of the adjacent abvalvar copula (
Figure 3a,b,f) and a flat cup-like thickening on the advalvar internal margin abutting the bottom of its advalvar neighbour (
Figure 3a,b,f,g). Thus, thanks to these abutments, the intact cingulum appears to comprise hollow tube-like structures (
Figure 3a,c–e). The images of the embedded specimens show that the valve overlaps the valvocopula (
Figure 3a,b,e). A few simpler, unchannelled pleurae form the most abvalvar bands (
Figure 2b and
Figure 3e).
4. Discussion
Brugan and Rosowski [
17] concluded that the copulae of
Proschkinia (such as
Navicula complanatoides Hustedt) were “hollow, forming a semi-tube the length of the frustule”, with unornamented external walls, although they suggested that the internal walls had regularly spaced slits and comb-like edges [
7] (p. 267). Most of the above observations confirm many of Brugan and Rosowski’s [
17] conclusions but provide more detail on the way in which the copulae interlink, although they contradict their interpretation of copulae having comb-like edges to their pars interior and the lack of overlap between succeeding elements. These interpretations were probably due to artefacts created by the eroded nature of their material [
17] (Figure 15) and the way in which the cingulum elements were opening as frustules dried on stubs [
17] (Figures 22 and 23). However, even in better prepared material, if seen obliquely [
2] (Figure 11), the perforate structure of the pars interior of the valvocopulae can create this impression, although the internal margins of the copulae are always intact and do not abut the valve.
As discussed previously [
16,
17], the development of more complex copulae in diatoms with deep cingula may be an adaptation to provide strengthening in dynamic littoral environments [
11,
16,
17]. Thus, although only a few genera with deep cingula have channelled, tubular or loculate copulae, others (e.g.,
Tabellaria Ehrenberg,
Grammatophora Ehrenberg,
Striatella C.A. Agardh,
Pseudostriatella Sato et al.,
Hyalosira Kützing,
Hanicella Lobban and Ashworth) have septa on one or more copulae [
1,
21,
22,
23], which would also reduce the likelihood of cell distortion. These taxa usually form chains of some kind, straight or zig–zag, often attached to a substratum as well as to each other, and are thus susceptible to wave and water movement. Whereas some centric taxa with deep cingula (
Urosolenia Round and Crawford,
Rhizosolenia Brightwell) have multiple, imbricate scale-like copulae [
1] (pp. 319, 325), these are usually planktonic and therefore unlikely to be subject to the same stresses.
As a benthic motile naviculoid genus,
Proschkinia is unusual in having such a deep cingulum. (
Parlibellus cells also have numerous copulae, but their cells are usually only about twice as deep in girdle view as in valve view, with proportionally wider valves [
24]). Fresh samples show that
Proschkinia cells are highly motile, but, whereas most motile taxa remain in valve view when observed under the light microscope,
Proschkinia cells do not and often seem to “fall over” into girdle view (pers. obs.). This may not, however, be a disadvantage in soft sediments, where they can still move between particles. Their occurrence as epibionts shows that they can also live within biofilms on moving hosts [
2] where motility may be less important.
Although Paddock and Sims [
11] do not discuss its habit or habitat, as another deep-girdled raphid diatom (with a keeled, sometimes fibulate raphe),
Undatella is presumably motile and, similar to
Proschkinia, would be found on benthic sediments. (Original material of
Undatella quadrata (Brébisson ex Kützing) Paddock and Sims was collected from oyster beds, and Round et al. [
1] indicate its habitat as marine, epipelic.) The numerous hollow copulae of
Undatella have external advalvar and abvalvar flanges that overlap the adjacent copulae [
11] (Figures 22, 23 and 28), thereby linking the elements of the cingulum. Osada [
18] (Figures 18, 19, 22, 23, 25 and 26) provides more detailed micrographs of
Undatella copulae, showing multiple rows of areolae on the inner side of the copulae and plain external surfaces. Short struts from the recurved advalvar margin on the outer surface link across to the pars interior to create the tubular form of the copulae [
18] (Figures 17 and 18) but without closing them completely. Paddock and Sims [
11] (p. 173) suggest that intermittent linkage between copulae across the cell lumen via short struts (seen as bright dots in LM) [
11] (Figures 11 and 47–50) could offer additional strengthening to the frustule, although they do not discuss whether hollow bands could provide strength.
Two other benthic marine diatoms with broad cingula that are usually observed in girdle view (
Entomoneis Ehrenberg and
Auricula) have keeled, fibulate and laterally compressed valves. However, unlike
Proschkinia, their valves have deeper mantles, and therefore, their copulae only contribute approximately one third of their total girdle depth, presumably not requiring additional strengthening to retain their integrity [
1] (pp. 632–625), [
11] (Figures 8–12).
The chambered valvocopula of
Mastogloia is involved with the secretion of mucilage around the cell [
6,
10], and although the valvocopula of
Aneumastus also has small chambers that open to the exterior [
1], there is currently no evidence that it is also associated with mucilage secretion. In both genera, the valvocopulae have small pores opening to the exterior. On the other hand, the pars exterior of
Proschkinia copulae are plain, without pores, which makes a mucilage secretion function less likely, unless the channels in the copulae widen advalvarly. If this were the case, it could facilitate the growth of epibiontic taxa in biofilms on turtles and other marine animals [
2].
The mechanical properties of some chain-forming planktonic diatoms have been explored experimentally [
25], and there have been some studies investigating the impact of grazing and nutrients on chain length [
26,
27]. However, although there has been speculation on the significance of the structural features of the frustules themselves, these derive from structural engineering concepts and have not been tested for microscopic organisms. Thus, it is assumed that the septa on copulae in genera with deep cingula in the Striatellaceae and Grammatophoraceae (both sensu Lobban and Ashworth [
21]) act in a stabilising manner, maintaining the valve outline through the cingulum, keeping the sides of the more abvalvar copulae from bulging outwards. Similarly, potentially stabilising septa are present on the copulae at the more inflated head pole of
Licmophora C.A. Agardh and across (transapical) the cell lumen of some particularly elongated cells with relatively shallow cingula (e.g.,
Climacosphenia Ehrenberg,
Climaconeis Grunow) [
1].
Although the cingulum structure remains relatively poorly understood across the diatoms as a whole, the occurrence of loculate, channelled or tubular copulae is limited to a few genera, whose phylogenetic relationships have barely been investigated. Although they fall in the same clade in phylogenetic studies,
Fistulifera lack a deep complex cingulum but share the possession of a fistula with
Proschkinia. Górecka et al. [
28] showed a shallowly U-shaped valvocopula in the monoraphid
Schizostauron, which sits in a clade with
Astartiella, sister to the clade containing
Stauroneis,
Craticula,
Fistulifera and
Proschkinia [
3] (S4). However, whereas, similar to
Fistulifera and
Proschkinia,
Astartiella has a fistula,
Schizostauron does not.
Undatella with its tubular copulae has not yet been included in any molecular studies but was placed in a different family and order by Round et al. [
1]. Neither does current evidence suggest that
Mastogloia is phylogenetically close to
Proschkinia [
3] (S4). On present evidence, the cingulum structure of
Proschkinia is unique within the diatoms (albeit with some similarities to that of
Undatella), whilst the shared possession of a fistula, across otherwise morphologically dissimilar taxa, is intriguingly supported by the molecular phylogenies.
Although genera within some orders, e.g., Cymbellales, Naviculales, share the same copula structure, it is clear that we still have little understanding of the diversity of cingulum structure in the diatoms or of how that variety contributes to frustule stability, let alone any functional role. Comparing
Proschkinia and
Undatella, we can see convergent structural developments across major diatom groups in the same environment. However, other genera with similar cell dimensions, also living on marine sediments, have simpler, unchambered bands, e.g.,
Auricula,
Entomoneis and
Thalassiophysa Conger, suggesting that it is not simply an adaptation for frustule stability. The continued focus on valve structure and cleaned specimens means that we are still missing much information, not only on structural features but also on how the cells are living and interacting in their environment. Brugan and Rosowski [
17] asked two interesting questions: “Was the evolution of deep girdles via selection for that trait or the result of selection on a correlated trait?” and “If deep girdles were selected for, what is their adaptive value?”. It seems that over 30 years later, those questions remain unanswered, and we still need to look more closely at all the structural elements of diatoms and consider them in both a phylogenetic context and ecologically.