Abstract
The independent origins of middle ears in testudinates, lepidosaurs, and archosaurs in the Triassic led to lineage-specific developments in their auditory epithelia. In comparison to the inferred ancestral state, little changed in testudinates, but archosaurs and most lepidosaurs evolved longer, differentiated auditory papillae. In archosaurs, sensory hair cells specialized across and along the papillae, resulting in mainly sensory tall hair cells and mainly mechanically active short hair cells. Crocodilians have 5 mm-long papillae, but relatively low upper frequency limits at around 4 kHz. Avian papillae are mostly 3 to 5 mm in length, being shorter in small species; in owls they exceptionally reach almost 12 mm and have an upper limit of above 10 kHz. Lepidosaurs retained the ancestral papilla as a low-frequency responsive area with one type of hair cell, but most added newly evolved areas (of max. 2 mm length) consisting of oppositely oriented hair cells responding to frequencies above 1 kHz. Initially, these areas flanked both ends of the ancestral area and were redundant in their responses to sound. The evolution of specific configurations in most families eliminated this redundancy, but the paths taken resulted in diverse anatomies that show high degrees of family specificity. Despite these differences, the high-frequency hearing limit of lepidosaurs rarely exceeds 5 kHz.
Keywords:
Testudinata; Lepidosauria; Archosauria; hearing; audition; basilar papilla; evolution of hearing 1. Introduction
The Permian–Triassic geological periods saw the diversification of stem amniotes into a mammal-like lineage (Synapsida), Testudinata, Archosauria, and Lepidosauria at a time when comparative information suggests that the inner-ear hearing organ—the basilar papilla—was a small (~1 mm) and simple epithelium that only responded to frequencies below ~1 kHz [1]. ‘Simple’ in this regard also conveys that the sensory cells were not specialized into different types. The further evolution of middle- and auditory inner-ear epithelia has been previously reviewed (for refs see [2]).
The independent evolution of tympanic middle-ear systems in Testudinata, Archosauria and Lepidosauria in the Triassic period (225 Ma [3]) was a remarkable phenomenon, since during the same period, mammalian lineages were also independently evolving three-ossicle tympanic middle-ears [4]. In mammals, however, these systems arose at a different position in the head: lower down and outside the caudal end of the lower jaw. The underlying selective pressures behind this remarkable parallel series of developments are still not known. Tympanic middle ears in Testudinata, Archosauria, and Lepidosauria arose in the tissue regime of the ancient spiracle, therefore mostly—and unlike in mammals—with widely open connections to the buccal cavity. In all lineages, tympanic middle ears enabled a great improvement in hearing sensitivity, and this initiated a major selection force increasing the importance of hearing as a sensory system. It also resulted in the ability to transfer higher frequencies than previously possible to the inner ear, resulting in an elongation of the basilar papilla in most groups, and, because of their independence, a lineage-specific configuration of hearing organs (Figure 1). These phenomena have been the subject of various reviews [2,5]. These elongations of the hearing organs were accompanied by a (sometimes very great) increase in the number of sensory cells and, presumably, innervating afferent nerve fibers and, parallel to this, an increase in the size of the brain nuclei processing the incoming auditory information.
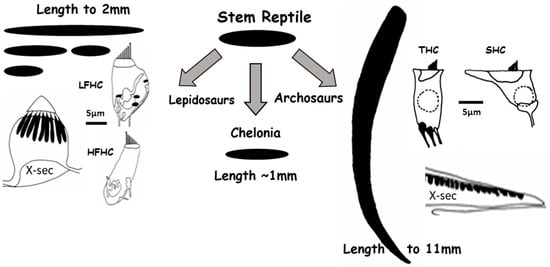
Figure 1.
Independent development of the auditory basilar papilla led to the diversity of hearing organs in reptilians. The black areas represent auditory papillae seen from above. For lepidosaurs and archosaurs, typical cross-sections are shown, together with enlarged sketches of the two types of hair cells found in each case. Turtles only have one simple type of hair cell, not shown here. X-sec—Cross section; LFHC—low frequency type of hair cell; HFHC—high-frequency type of hair cell; THC—tall hair cell; SHC—short hair cell, the latter visible in the X-sec. Modified after [1] and used with permission of SpringerNature.
2. Testudinata
Testudinates, mainly because of their rather insensitive middle-ear systems, did not evolve any great improvements in hearing sensitivity, and today, have auditory thresholds that are at least 20 dB less sensitive than those of archosaurs and lepidosaurs [6]. Their basilar papillae remained small and simple, with only one kind of sensory cell and responses to only low sound frequencies (<1 kHz)—a situation that is likely to resemble the ancestral state for all descendants of this cell patch in all extant “reptiles” [7]. The dominant frequency tuning mechanism of the sensory hair cells of modern testudinates, which can also be identified as ancestral, consists of the so-called electrical tuning that acts in addition to a basic micromechanical tuning involving, for example, the masses and stiffnesses of the various structures of the papilla (Figure 2). In electrical tuning, the stimulation of a hair cell leads to a depolarization, partly due to an influx of calcium ions from the overlying endolymph fluid through voltage-sensitive membrane channels [8]. The calcium ions attach to and influence calcium-dependent potassium channels in the cell membrane, which then rapidly open and allow an efflux of potassium ions, thus repolarizing the hair cell membrane. A continued stimulus opens the calcium channels again, with the rate of opening being determined by the number of channels and their specific dynamic characteristics. This cycle of membrane depolarization and repolarization continues at the frequency of the sound stimulus as long as the stimulus is present.
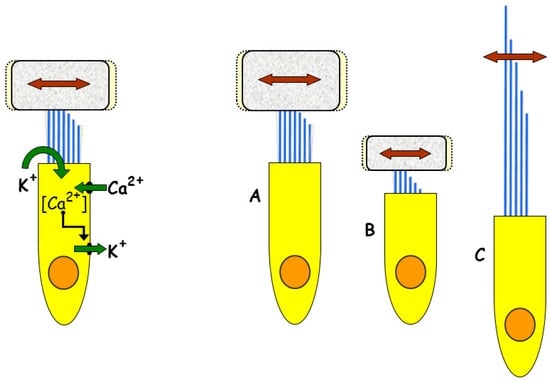
Figure 2.
Frequency tuning mechanisms in different reptilian groups. The main ancestral tuning mechanism (left) is electrical tuning, which depends on dynamic interactions between hair cell ion channels (see text) and is still prominent in chelonians and archosaurs. Micromechanical tuning (right) depends on the stiffness of the hair cell bundles and the mass of any tectorial structure (with a tectorial structure: A, low frequency; B, high frequency; C, without a tectorial structure and low frequency). Micromechanical tuning is prominent in high-frequency regions of lepidosaur papillae. Modified from [9]; Copyright (2000) National Academy of Sciences.
Each hair cell has a specific configuration of the numbers and response rates of the types of channels, such that each hair cell has a preferred frequency—called the best or characteristic frequency—at which the amplitudes of the voltage oscillations are largest. These frequencies are systematically arranged along the basilar papilla, forming a tonotopic organization that is typical for all land vertebrate hearing organs, even though the frequency limits and the underlying frequency selectivity mechanisms can differ widely between lineages. This mechanism is temperature-sensitive, so that in ectothermic species, the characteristic frequency responses rise rapidly with increasing body temperature.
3. Archosauria
In archosaurs, the basilar papilla is elongated: in Crocodilia, up to ~5 mm, and in some modern birds (owls) to ~12 mm (Figure 3 and Figure 4; [10]). These longer papillae are accompanied by an increase in the upper auditory frequency responses to ~5 kHz in Crocodilia and in some avian species to above 10 kHz. In archosaurs as well, the frequency selectivity of the hair cells is dominated by electrical tuning. Experimental and modeling studies indicate that at normal avian body temperatures, electrical tuning should be able to operate up to the average upper frequency range of birds (~5 kHz) [11], but it is not known whether specializations make it possible, as in barn owls, to respond to even higher frequencies.
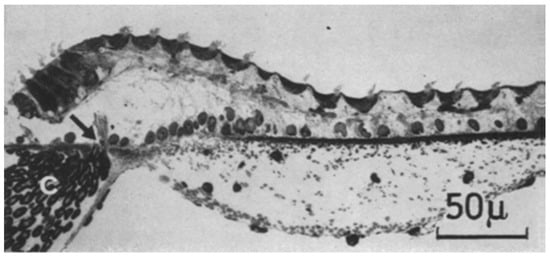
Figure 3.
A cross-section through part of the auditory papilla of Caiman crocodilus. On the top left are tall hair cells, with a sharp transition to short hair cells, that at this position cover most of the papilla. The arrow points to the habenula perforata, where nerve axons enter and leave the papilla. From Figure 2C in [12], used with the permission of SpringerNature.
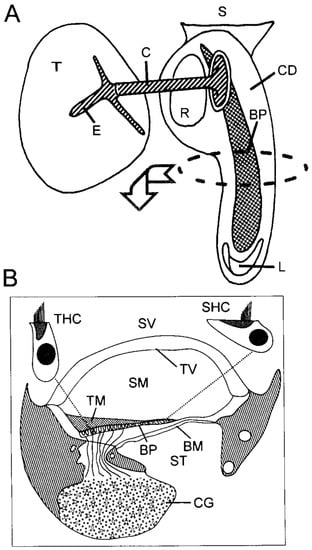
Figure 4.
(A) Schematic of an avian basilar papilla (BP) within the cochlear duct (CD), which is an outsacking of the sacculus (S). At its tip the CD also contains the lagenar macula (L). The middle ear is driven by the tympanum (T) via the extra-columella (E) and columella (C), and the footplate of the latter is in the oval window next to the round window (R). (B) Schematic of a cross-section of the basilar papilla in the mid-frequency region, showing the three scalae (vestibuli SV and media SM, which are separated by the tegmentum vasculosum TV, and media SM and tympani ST, which are separated by the basilar membrane BM). On the BM is the basilar papilla BP, which is covered by a tectorial membrane TM. Single, enlarged tall (THC) and short (SHC) hair cells are shown, and their innervations are gathered in the cochlear ganglion, whose volume is largely filled by the somata of the afferent neurons. The striped areas depict the cartilage support of the whole structure. From Figure 3.1 in [13], used with the permission of SpringerNature.
In both groups of archosaurs, Crocodilia and Aves, the sensory hair cells both across and along the epithelium evolved morphological specializations into two extreme forms. Named because of their general shapes as seen in transverse sections of the organ, tall hair cells are found on the neural and apical areas (note: ‘apical’ is low frequency), and short hair cells on the abneural side and basal end of the epithelium (Figure 1 and Figure 3). Some authors defined other intermediate types, without, however, any notion as to possible differences in function [10,13]. A study by Fischer [14] brought a breakthrough in understanding. Fischer showed that hair cells over areas of the abneural papilla whose sizes were species-specific received no afferent innervation. In other words, those hair cells sent no information to the brain, although efferent innervation indicated that the brain could influence their response activity. This revealed that the actual shape of an individual hair cell was less important than whether or not it was afferently innervated. The general situation suggested a parallel to the mammalian cochlea in terms of a division of labor between hair cell types. Most of the hair cells of mammals (that is, the outer hair cells, which make up ~90% of the total) are only very sparsely afferently innervated compared to the dense afferents to the inner hair cells [15]. In mammals, the outer hair cells are known to act as motor elements, increasing the amplitude of weak sound stimuli that then are encoded by the inner hair cells [16]. There are some functional indications—apart from the obvious anatomical similarity—that the auditory organ in birds may also use such an interaction between hair cell groups to amplify weak stimuli [13,17].
In most cases, the auditory papillae of different birds show little diversity—they are simply larger or smaller versions of the same structure with the same hair cell patterns, with few exceptions (Figure 5). Smaller passerines have shorter papillae whose hair cell patterns reflect higher-frequency regions of the larger papillae, as seen, for example, in the emu, in pigeons, and in galliform birds [13]. The barn owl is a major exception, as may be some other owls; this bird has a much longer auditory papilla (more than 11 mm in the living animal [18]) and hears higher frequencies than most birds. The barn owl’s auditory papilla is organized into a highly nonlinear representation of frequency. Its papilla devotes fully half of the length to the highest octave, from 5 to 10 kHz [19].

Figure 5.
The basilar papillae of diverse avian species, seen as if flattened and from above the hair cells. For each species, the length of the papilla in the fixed, dried state (add about 25% for the living state) and the total number of sensory hair cells is shown. Modified from Figure 3.2 in [13], used with the permission of SpringerNature.
Studies in crocodilians are relatively rare, and the functional significance of the relatively sudden transition between hair cell types in them—as their hair cell forms show almost no intermediates—when compared to birds is not understood (Figure 3). Nonetheless, it is fair to say that one glance at the anatomy of these hearing organs immediately places them in the archosaurs and then distinguishes them as crocodilians.
4. Lepidosauria
Fossil evidence suggests that the clade including the Tuatara Sphenodon (the only remaining representative of the group Rhynchocephalia), was once a much larger group that existed parallel to the earliest lizards [20]. It is possible that the simple auditory papilla in modern Tuatara represents the ancestral papilla that never achieved the complexity of the papillae of most modern lizards. It responds only to low sound frequencies (below 1 kHz, discussion in [5]). In contrast, all other lepidosaurs evolved a fundamentally more complex type of papilla that followed the evolution of an improved tympanic middle ear.
The most interesting variations from the point of view of reptilian systematics are found in the auditory organs of modern lizards, within which generally family- and even subfamily-specific morphologies, including forms with sub-papillae, can be recognized (Figure 1 and Figure 6). This complexity was first identified by Shute and deBellairs [21] and Hamilton [22], but greatly expanded upon by the extensive morphological work of Wever [23] and Miller [7,24]. One classical case of a clear basis for a classification assignment was discussed by Miller regarding Xantusiiden. While some systematic studies had assigned xantusids to the skink group, Miller correctly insisted that they were clearly related to geckos, which have a unique kind of auditory papilla. This was later confirmed by mitochondrial studies of familial relationships. Nonetheless there are clearly cases in which very similar papillae are found in only remotely related groups (e.g., iguanid-agamid papillae resemble anguid papillae).
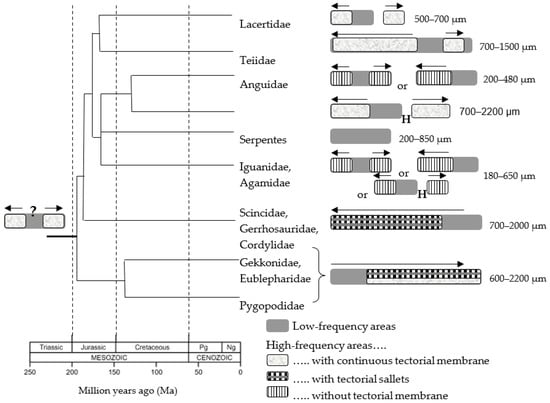
Figure 6.
Origin and evolution of the diversity of basilar papillar configurations in lepidosaurs. On the left, the putative ancestral three-part papilla is shown, with a central, low-frequency hair cell area flanked apically (right end) or basally (left end) by two mirror-image high-frequency hair cell areas, probably all covered by a tectorial membrane. As shown in the drawings on the right side, during the diversification of their lineages over geological time (time scale at lower left), different lizard families either kept this pattern of hair cell areas, lost one or the other of the high-frequency areas during papillar elongation, or interrupted the hair cell array with a hair-cell-free hiatus (H). These evolutionary developments were accompanied by specializations of the tectorial structures as shown (see pattern code on the bottom right). For each family, the typical size range is shown. Arrows show the direction of the increases in response frequency along the high-frequency hair cell areas. From [25], used with the permission of Elsevier.
As a result of the evolution of the tympanic middle ear, the early lizard papilla began to elongate, but the hair cells that were accrued at each end (apical and basal) of the papilla were different to those initially present. Apart from their slimmer forms, one prominent difference was that the hair cell bundles of cells in the “new” areas were placed in oppositely oriented patches and thus the slopes of the bundle “wedges” faced each other. The responses of these two hair cell populations to a given sine wave of sound would thus occur with a phase difference of 180°. Some physiological data suggest that this difference correlates with a change in the dominant form of frequency tuning of the hair cell areas such that at frequencies above 1 kHz, instead of electrical tuning, micromechanical tuning is very dominant [9]. Micromechanical tuning is determined by the physical, i.e., morphological, characteristics of the hair cells (Figure 2; the number of stereovilli and their height determine the stiffness, and the tectorial membrane and hair cell volumes within the fluid determine the mass). As discussed below, these morphological features show clear and appropriate gradients in lizard papillae.
Studies of recent species indicate that the hair cells of the “newly evolved” areas respond to frequencies higher than 1 kHz. Hair cells directly adjacent to the ancestral, low-frequency, hair cell center prefer 1 kHz, and the preferred response frequency increases (species-specifically up to ~8 kHz) the further from the center the hair cells lie. The most parsimonious explanation for the pattern of evolution of lizard papillae is that initially, these areas—here referred to as “high-frequency” areas—arose on both ends of the ancestral papilla and were mirror-images, both anatomically and functionally. One of the most interesting features of the later evolution and differentiation of lizard groups was that they—in most cases—eliminated the redundancy of possessing mirror-image areas (Figure 6). A few lizard families—notably many (but not all) iguanids and anguids—retained the mirror-image pattern, but most families reduced it by differentiating the two new areas or lost it by eliminating one or the other area completely.
The reduction of mirror-imagery apparently required that in development, these areas were somehow separated. This was achieved in varanids and lacertids, for example, by thinning the papilla between the hair cell areas and having a short stretch without hair cells. In some cases (lacertids form a good example, as shown in Figure 7, but there are isolated cases elsewhere, such as in the basilisk iguanids), the areas were physically divided into separated sub-papillae. Functionally, this apparently allowed the previously mirror-image areas to differentiate and respond to different frequencies. Thus, the originally identical frequency ranges were split into two complementary ranges that together covered the whole range. The advantage of this change is obvious—as a result of it, the entire high-frequency range was covered in a more differentiated way by the hair cells and nerve fibers, presumably resulting in a coding advantage for the sensory information [25].
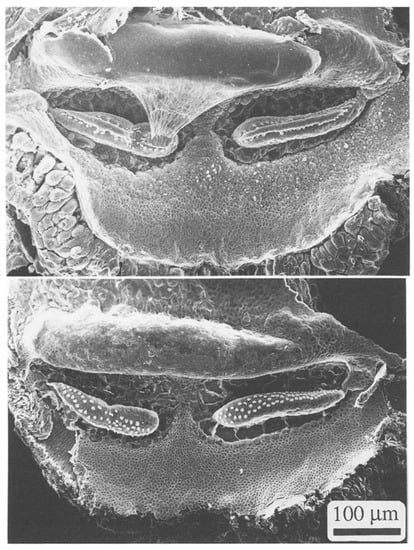
Figure 7.
Scanning electron micrographs of the auditory papilla of Podarcis muralis, the European Wall Lizard, with (top) and without (bottom) the tectorial membrane. White dots are individual hair cell bundles. From Figure 8.1. in [5], used with the permission of SpringerNature and courtesy Christine Köppl.
One of the most frequent changes during the evolution of lizard papillae is the complete loss of one or another area of “new” hair cells that form the high-frequency regions. This is perhaps the most radical way to eliminate the mirror-image redundancy. In the skink group, for example (Scincidae, Gerrhosauridae, Cordilidae), the entire area at the apical end of the papilla was lost so that the ancestral center patch of low-frequency hair cells then lay at the apex (Figure 6). Their present arrangement coincidentally corresponds to the “normal” tonotopy of amniote papillae, with the highest frequencies processed at the very base of the papilla. The gecko group, including pygopods, however, lost the other “new” hair cell patch at the basal end, so that the ancestral low-frequency area then lay at the base of the papilla (Figure 6). This reversed tonotopy is unique among amniotes and has been demonstrated physiologically [26].
These morphological characteristics thus form useful tools for helping establish systematic relationships within lizards. There are, of course, pitfalls in this categorization, and the anatomy of the ear cannot alone serve a function in systematics—otherwise, the basilisk iguanids might be coupled to the lacertids, for example, since both have completely separated sub-papillae. Used together with the huge range of morphological features typically used in systematic studies, however, such superficial issues are not critical.
Among the lepidosaurs, snake and amphisbaenid basilar papillae have the simplest structures, and it is most likely that the papillae of these organisms lost complexity over time. The reason for these losses probably lies in evolutionary modifications of the middle ear involving loss of the eardrum and the outer region of the middle-ear ossicle, the columella, which was then embedded in nearby tissue. In snakes, one possible explanation for this is that their evolution from lizard ancestors was accompanied by large changes in feeding mechanisms. The ability to ingest relatively huge prey items, involving increased cranial kinesis, was incompatible with the maintenance of a tympanic middle ear, since the delicate tympanum lay precisely in the area of maximum stretch of the skin of the skull during prey ingestion. The great advantage initially gained through the evolution of a tympanic middle ear was thus largely lost, resulting in insensitive hearing that is comparable, for example, to that of some testudinates. In addition, this loss event likely occurred after ancestral lizards had elongated their papillae, and the elongated portion was lost, resulting in small papillae of ~1 mm length. The papillae thus bear resemblance to those of chelonians and sphenodontians, but their condition in snakes was arrived at secondarily.
Among the lepidosaurs, one group, the amphisbaenids, has a papilla that shows the putative ancestral amniote pattern. Like snakes, this is likely due to the reduction of the middle ear, which lacks a tympanum, and the embedding of the ossicles in tissue. These papillae have very few hair cells, matched only by some typically very small (but more complex) lizard papillae, such as those of some iguanids and agamids. Similar to what has been found in snakes and Sphenodon, the auditory sensitivity of amphisbaenids is best below 1 kHz [23]. These measurements, which were made using cochlear microphonics, are reliable, since the measured papillar types only contain hair cells of the same bundle orientation. As noted in earlier work [5], microphonic measurements from bidirectionally oriented hair cell areas (the higher-frequency areas of lizard papillae) show cancellation of voltages and are therefore not reliable. Wever [23], who included the amphisbaenids in his exhaustive study of lizard auditory papillar anatomy, gave a range for the number of hair cells in different species from 38 to 153.
5. Conclusions
The auditory papillae of different reptile groups show a high degree of diversity, with major pattern differences between the archosaurs and other groups. While chelonians, sphenodontians, and some lepidosaurs retain (or in the case of snakes, have reduced their papillae to) a small, ancestral papilla, most extant lizard families demonstrate the results of the independent evolution of papillae from the ancestral state. Diversity at the family level is very common, and many anatomical patterns are seen only in certain families, making the lizard papilla of great cladistic significance.
Funding
This review work received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks are owed to the author’s many colleagues and to the Deutsche Forschungsgemeinschaft for the research funding and cooperative work over the past 50 years.
Conflicts of Interest
The author declares no conflict of interest.
References
- Manley, G.A.; Clack, J.A. An outline of the evolution of vertebrate hearing organs. In Evolution of the Vertebrate Auditory System; Manley, G.A., Popper, A., Fay, R.R., Eds.; Springer: New York, NY, USA, 2004; pp. 1–26. [Google Scholar]
- Manley, G.A. Comparative auditory neuroscience: Understanding the evolution and function of ears. J. Assoc. Res. Otolaryngol. 2016, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Clack, J.A. Patterns and in the Early Evolution of the Tetrapod Ear. J. Neurobiol. 2002, 53, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-X. Developmental Patterns in Mesozoic Evolution of Mammal Ears. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 355–380. [Google Scholar] [CrossRef]
- Manley, G.A. Peripheral Hearing Mechanisms in Reptiles and Birds; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Manley, G.A. Fundamentals of hearing in amniote vertebrates. In Perspectives on Auditory Research; Popper, A.N., Fay, R.R., Eds.; Springer: New York, NY, USA, 2014; pp. 321–341. [Google Scholar] [CrossRef]
- Miller, M.R. The reptilian cochlear duct. In Comparative Studies of Hearing in Vertebrates; Popper, A.N., Fay, R.R., Eds.; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 1980; pp. 169–204. [Google Scholar]
- Crawford, A.C.; Fettiplace, R. An electrical tuning mechanism in turtle cochlear hair cells. J. Physiol. 1981, 312, 377–412. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.A. Cochlear mechanisms from a phylogenetic viewpoint. Proc. Natl. Acad. Sci. USA 2000, 97, 11736–11743. [Google Scholar] [CrossRef] [PubMed]
- Gleich, O.; Fischer, F.P.; Köppl, C.; Manley, G.A. Hearing organ evolution and specialization: Archosaurs. In Evolution of the Vertebrate Auditory System; Manley, G.A., Popper, A., Fay, R.R., Eds.; Springer: New York, NY, USA, 2004; pp. 224–255. [Google Scholar]
- Wu, Y.-C.; Art, J.J.; Goodman, M.B.; Fettiplace, R. A kinetic description of the calcium activated potassium channel and its application to electrical tuning of hair cells. Prog. Biophys. Mol. Biol. 1995, 63, 131–158. [Google Scholar] [CrossRef] [PubMed]
- von Düring, M.; Karduck, A.; Richter, H.G. The fine structure of the inner ear in caiman crocodilus. Z. Fuer Anat. Und Entwickl. 1974, 145, 41–65. [Google Scholar] [CrossRef] [PubMed]
- Gleich, O.; Manley, G.A. The hearing organ of birds and crocodilia. In Comparative Hearing: Birds and Reptiles; Dooling, R.J., Fay, R.R., Popper, A.N., Eds.; Springer: New York, NY, USA, 2000; pp. 70–138. [Google Scholar]
- Fischer, F.P. Quantitative analysis of the innervation of the chicken basilar papilla. Hear. Res. 1992, 61, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Spoendlin, H. The afferent innervation of the cochlea. In Evoked Electrical Activity in the Auditory Nervous System; Naunton, R.F., Fernandez, C., Eds.; Academic Press: New York, NY, USA, 1978; pp. 3–19. [Google Scholar]
- Hudpeth, A.J. Making an Effort to Listen: Mechanical Amplification in the Ear. Neuron 2008, 59, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Fettiplace, R. Diverse Mechanisms of Sound Frequency Discrimination in the Vertebrate Cochlea. Trends Neurosci. 2020, 43, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.P.; Köppl, C.; Manley, G.A. The basilar papilla of the barn owl: A quantitative morphological SEM analysis. Hear. Res. 1988, 34, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Köppl, C.; Gleich, O.; Manley, G.A. An auditory fovea in the barn owl cochlea. J. Comp. Physiol. 1993, 171, 695–704. [Google Scholar] [CrossRef]
- Jones, E.H.; Anderson, C.L.; Hipsley, C.A.; Müller, J.; Evans, S.E.; Schoch, R.R. Integration of molecules and new fossils supports a Triassic origin for Lepidosauria (lizards, snakes, and tuatara). BMC Evol. Biol. 2013, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Shute, C.D.; Belairs, A.D. The cochlear apparatus of Gekkonidae and Pygopodidae and its bearing on the affinities of these groups of lizards. Proc. Zool. Soc. Lond. 1953, 123, 695–709. [Google Scholar] [CrossRef]
- Hamilton, D.W. Observations on the morphology of the inner ear in certain gekkonoid lizards. Univ. Kansas Sci. Bull. 1960, 41, 983–1024. [Google Scholar]
- Wever, E.G. The Reptile Ear; Princeton University Press: Princeton, NJ, USA, 1978. [Google Scholar]
- Miller, M.R. The evolutionary implications of the structural variations in the auditory papilla of lizards. In The Evolutionary Biology of Hearing; Fay, R.R., Popper, A.N., Webster, D.B., Eds.; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 1992; pp. 463–487. [Google Scholar]
- Manley, G.A. Lizard auditory papillae: An evolutionary kaleidoscope. Hear. Res. 2011, 273, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.A.; Köppl, C.; Sneary, M. Reversed tonotopic map of the basilar papilla in Gekko gecko. Hear. Res. 1999, 131, 107–116. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).