Distribution Patterns of Large Jellyfish and Their Effects on the Zooplankton Community in the Northern Chinese Coastal Seas during the Summer of 2021
Abstract
1. Introduction
2. Materials and Methods
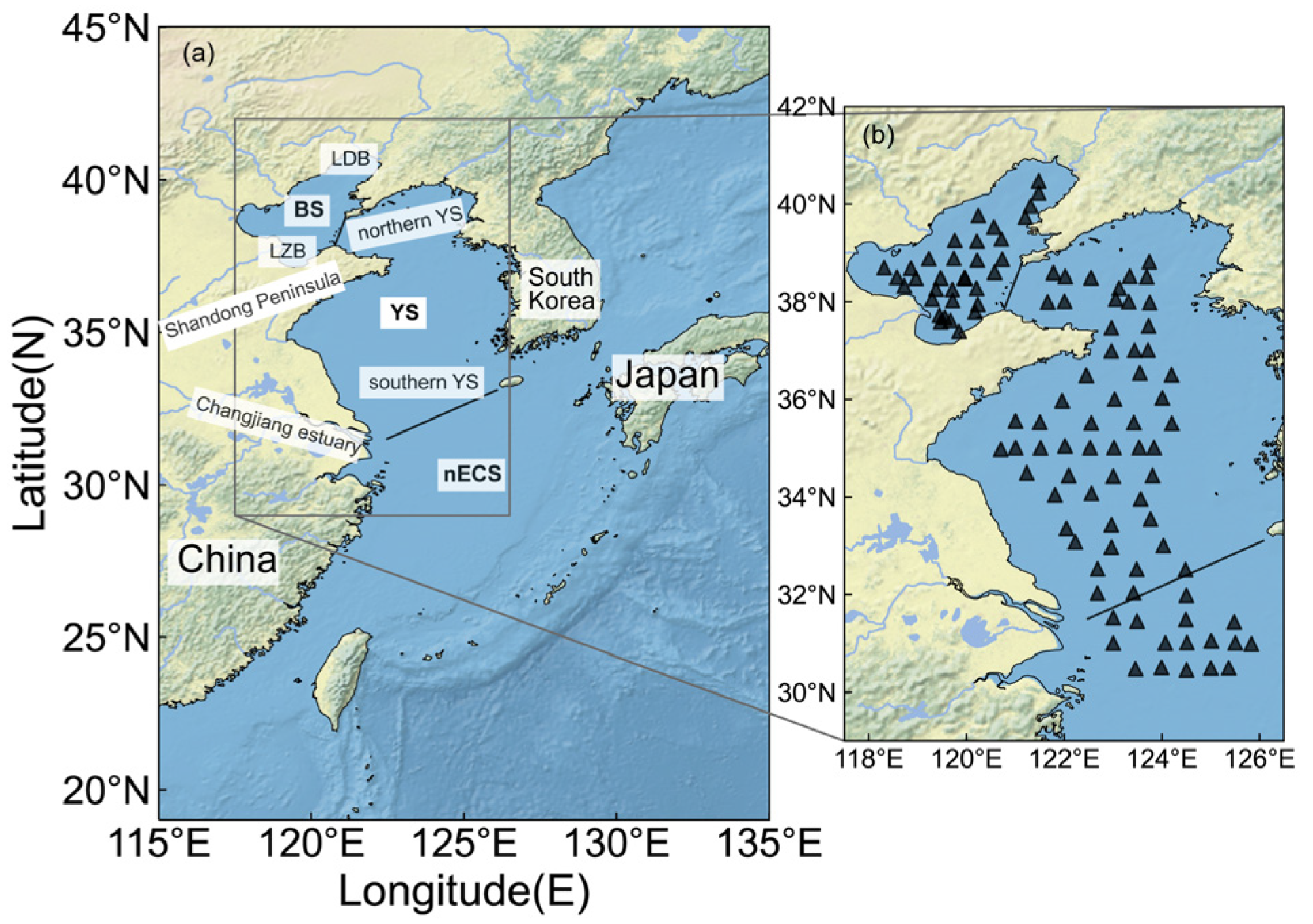
2.1. Study Area
2.2. Sampling
2.3. Data Analysis
2.3.1. Realized Niches of Large Jellyfish
2.3.2. The Feeding Pressure of Large Jellyfish on Zooplankton
2.3.3. Statistical Analyses
3. Results
3.1. Spatial Variation in Environmental Variables
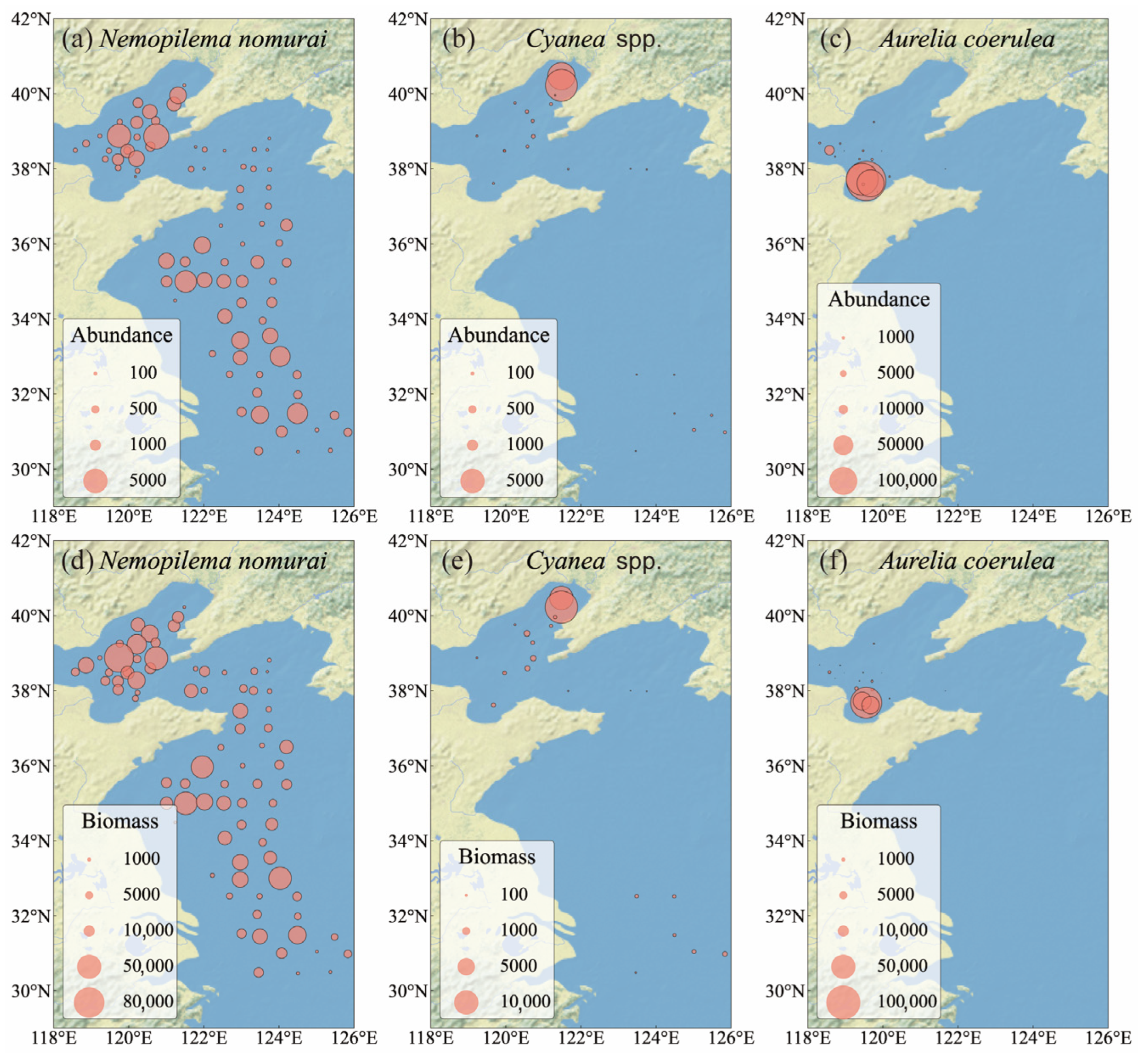
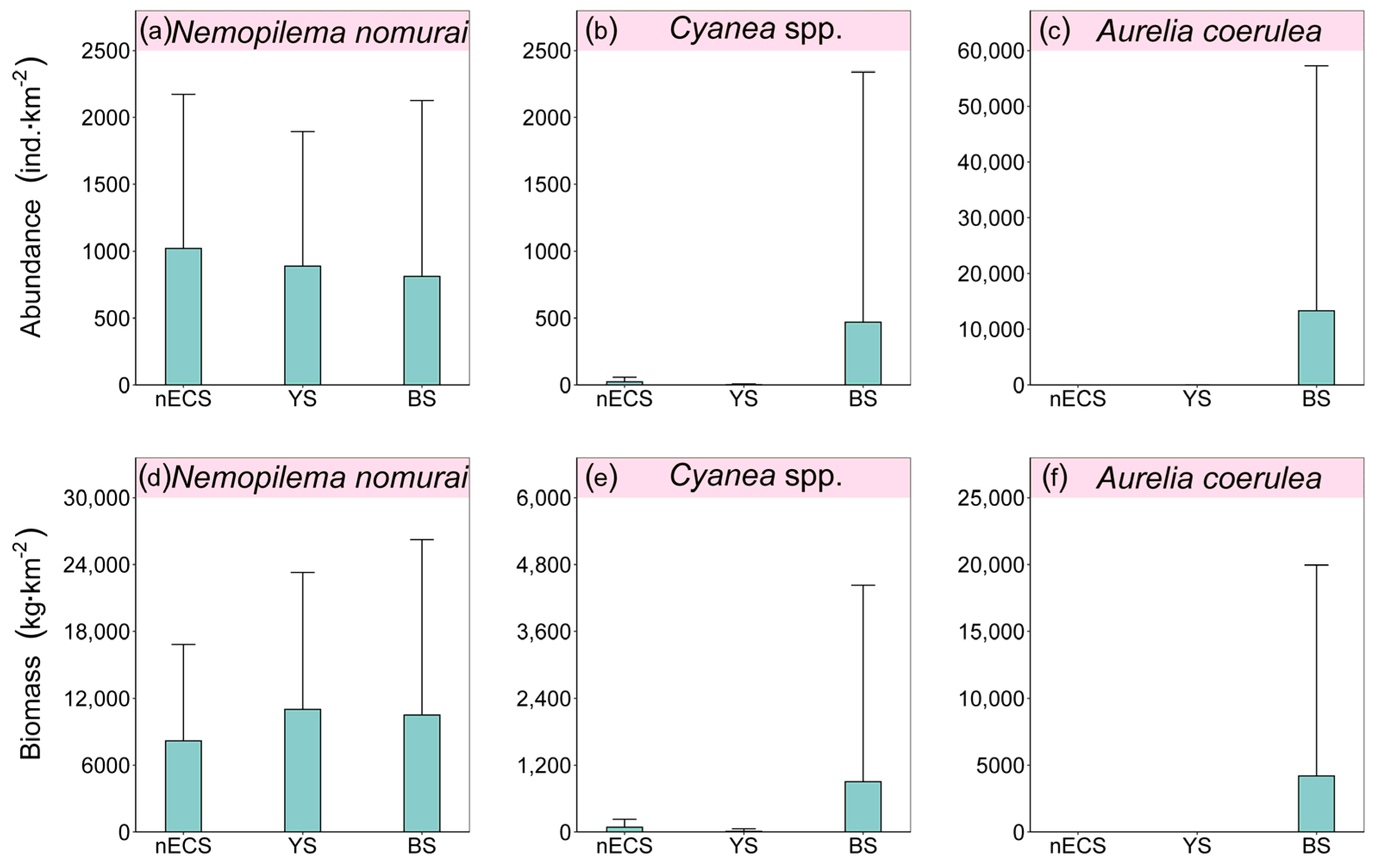
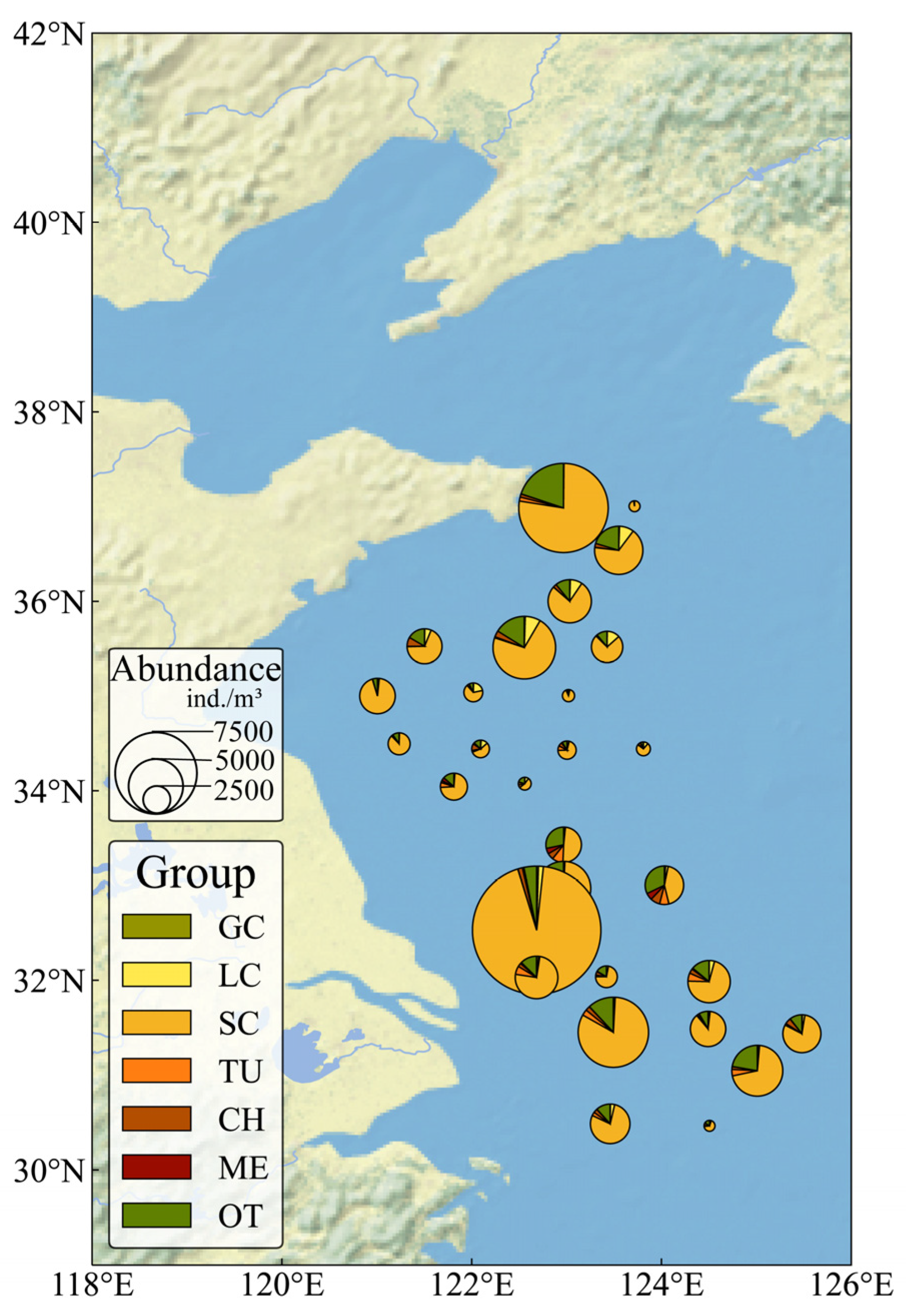
3.2. Distribution of Large Jellyfish Abundance and Biomass
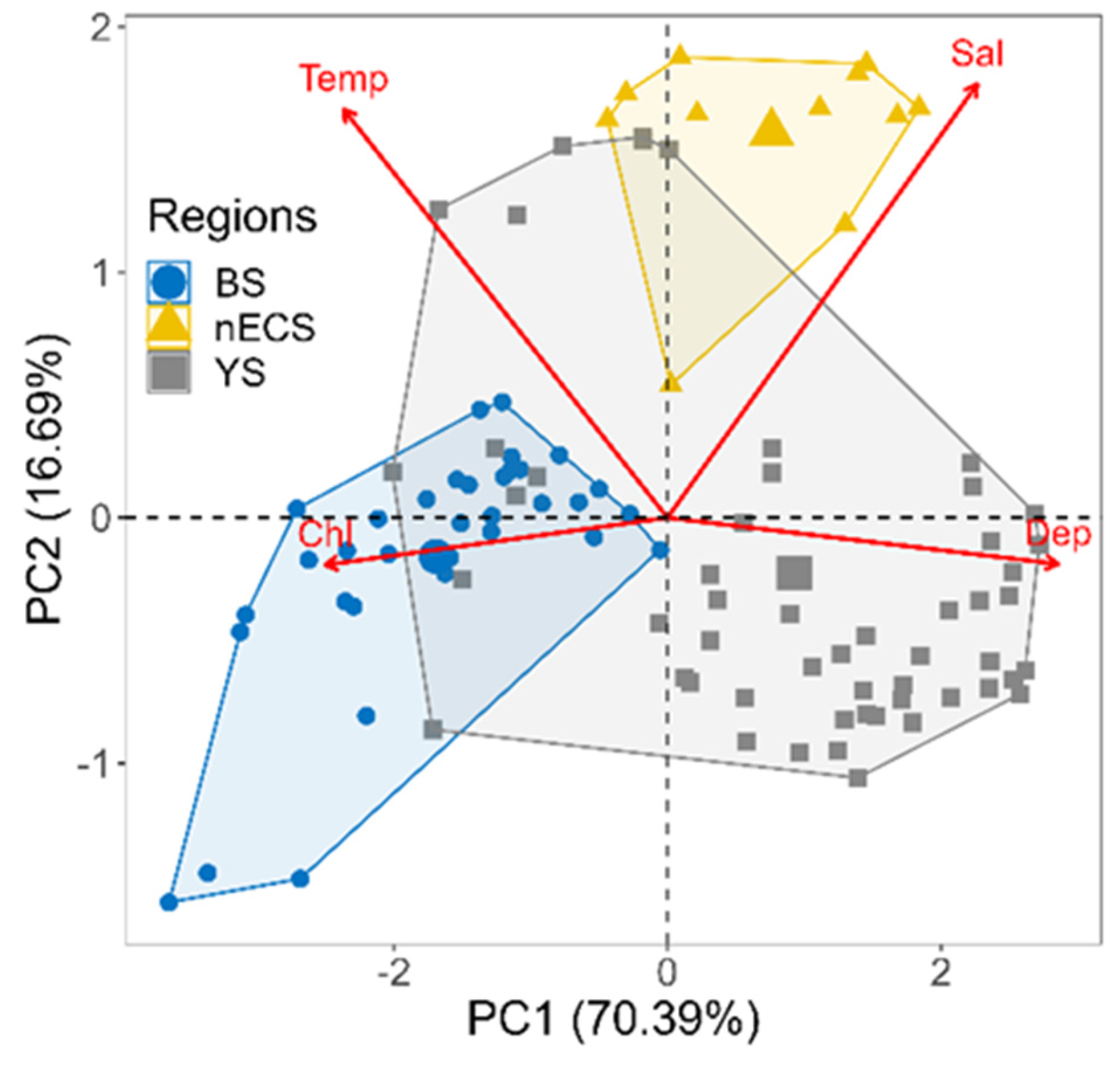
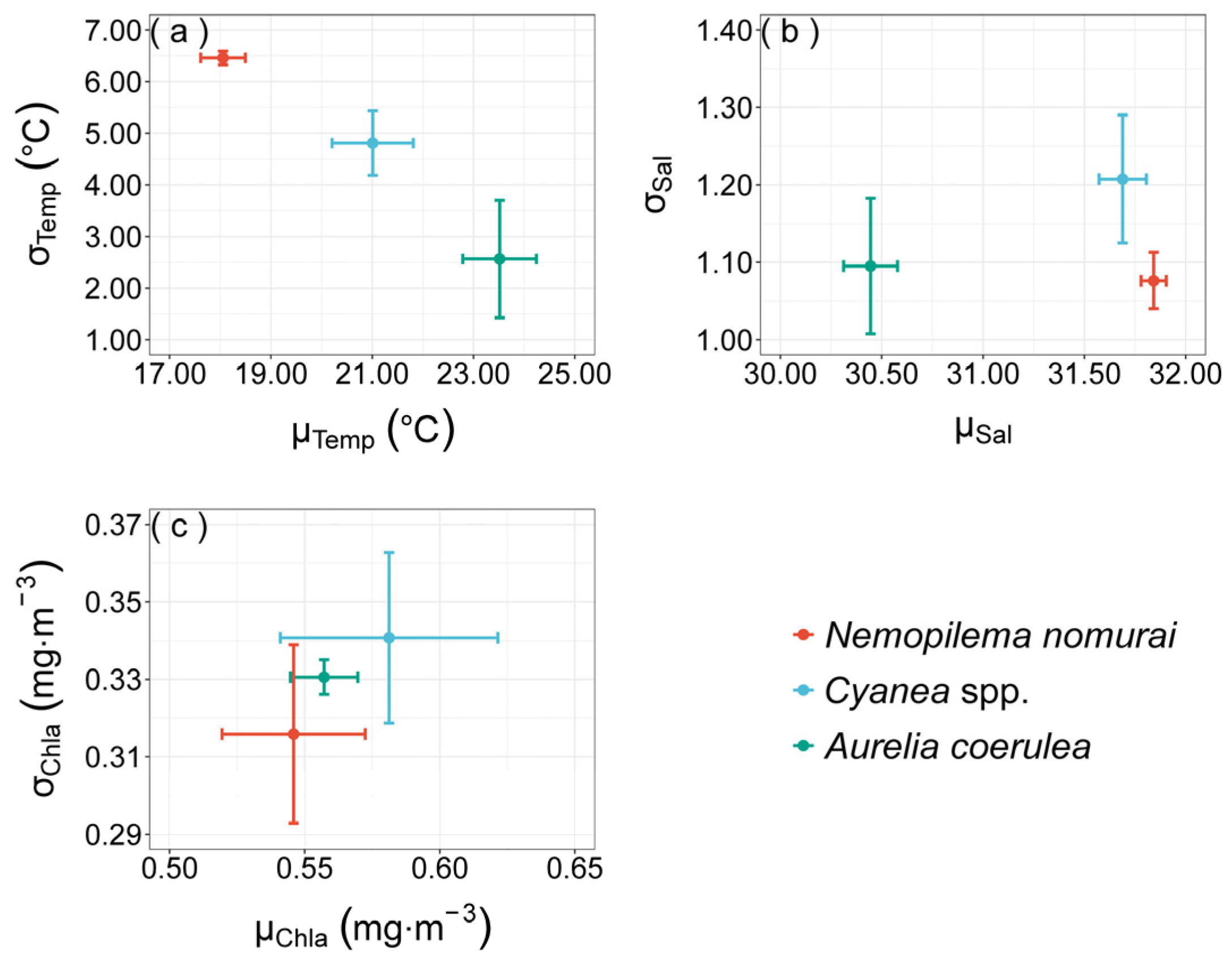
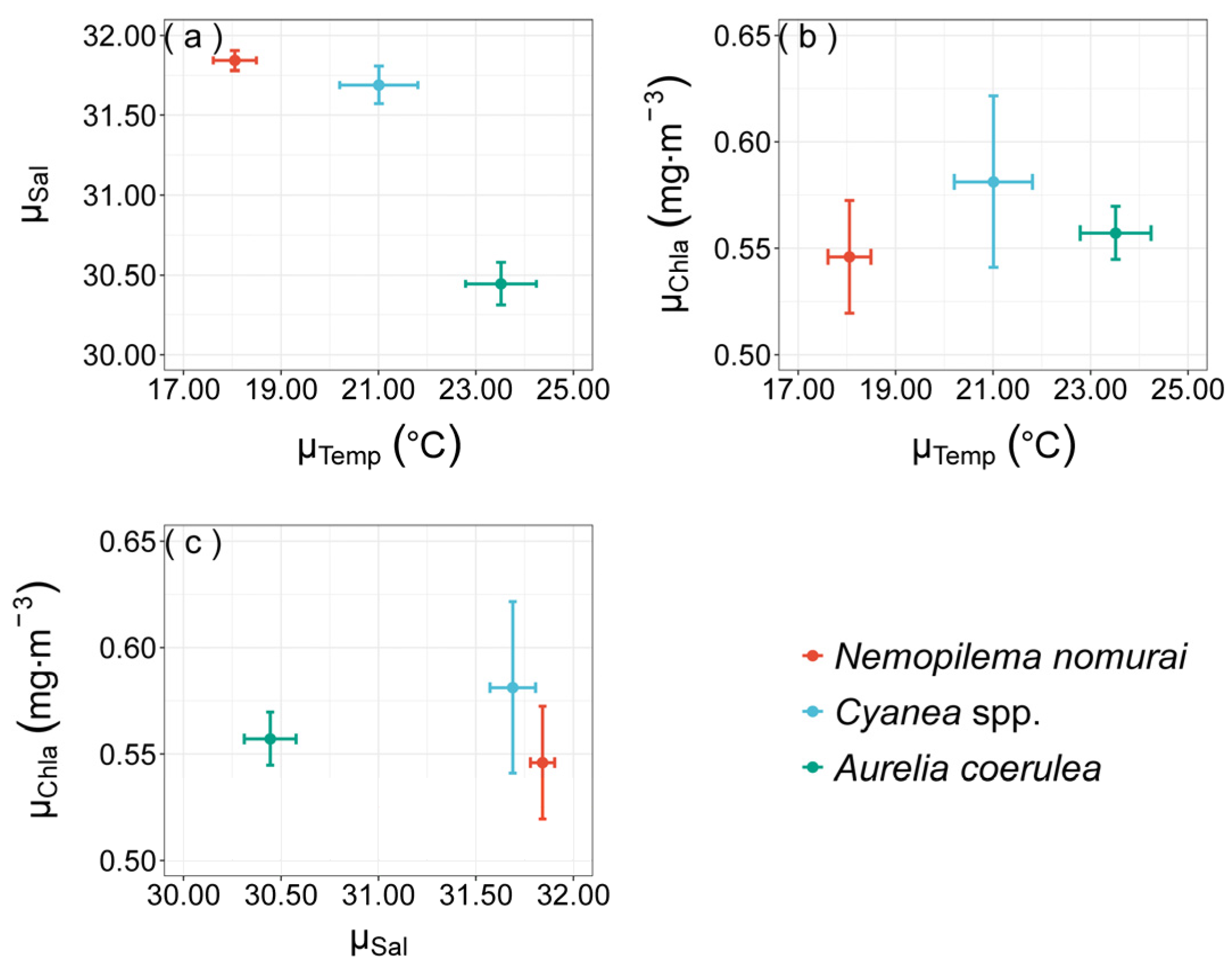
3.3. Realized Niches of Large Jellyfish
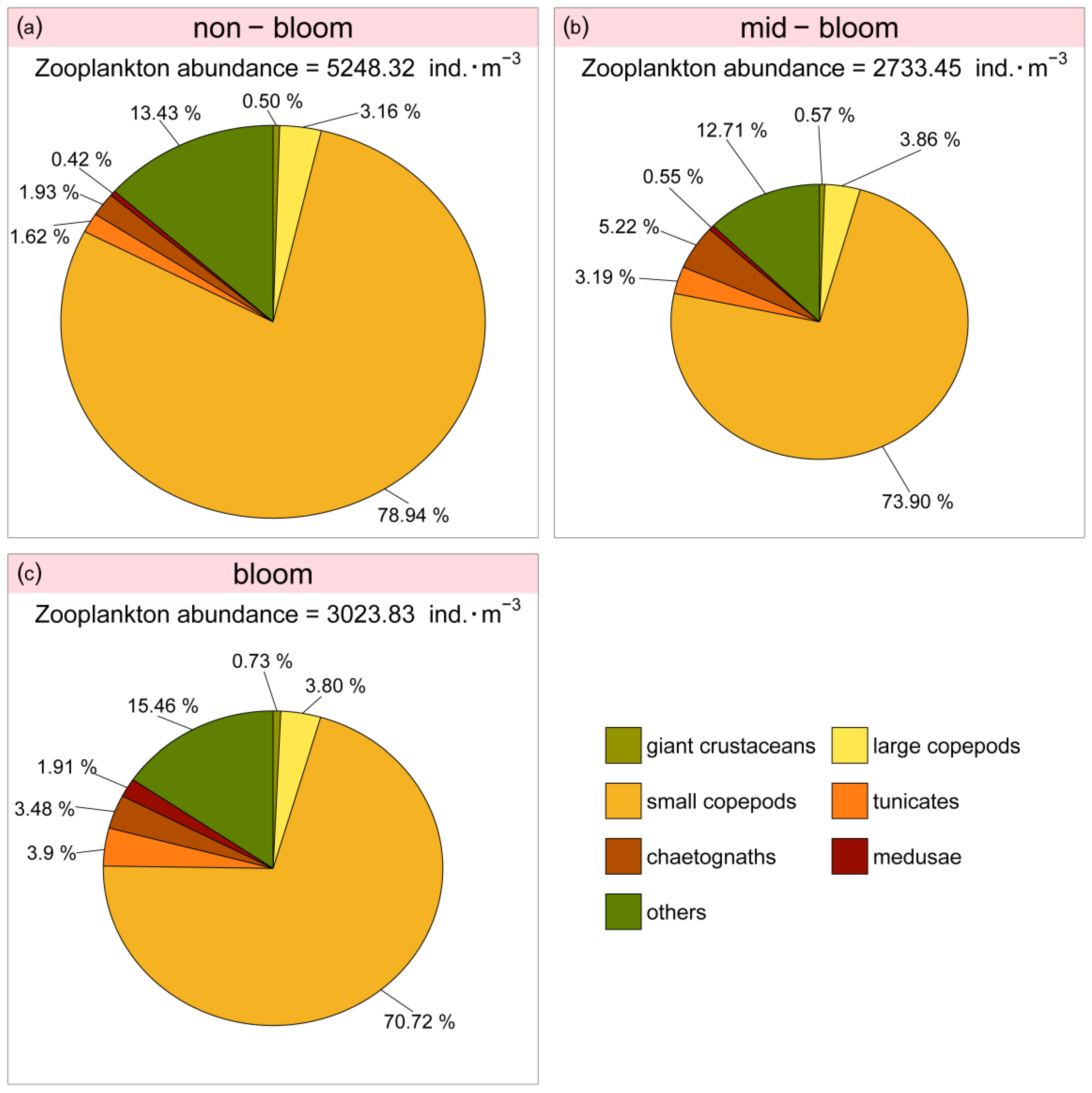
3.4. Relationship between Large Jellyfish and the Zooplankton Community
4. Discussion
4.1. Distribution of Large Jellyfish Abundance and Biomass
4.2. Relationship between Large Jellyfish and the Zooplankton Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lynam, C.P.; Gibbons, M.J.; Axelsen, B.E.; Sparks, C.A.J.; Coetzee, J.; Heywood, B.G.; Brierley, A.S. Jellyfish Overtake Fish in a Heavily Fished Ecosystem. Curr. Biol. 2006, 16, R492–R493. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, R.D.; Suchman, C.L.; Reese, D.C.; Miller, T.W.; Daly, E.A. Spatial Overlap and Trophic Interactions between Pelagic Fish and Large Jellyfish in the Northern California Current. Mar. Biol. 2008, 154, 649–659. [Google Scholar] [CrossRef]
- Chiaverano, L.M.; Robinson, K.L.; Tam, J.; Ruzicka, J.J.; Quiñones, J.; Aleksa, K.T.; Hernandez, F.J.; Brodeur, R.D.; Leaf, R.; Uye, S.-I.; et al. Evaluating the Role of Large Jellyfish and Forage Fishes as Energy Pathways, and Their Interplay with Fisheries, in the Northern Humboldt Current System. Prog. Oceanogr. 2018, 164, 28–36. [Google Scholar] [CrossRef]
- Bosch-Belmar, M.; Milisenda, G.; Basso, L.; Doyle, T.K.; Leone, A.; Piraino, S. Jellyfish Impacts on Marine Aquaculture and Fisheries. Rev. Fish. Sci. Aquac. 2020, 29, 242–259. [Google Scholar] [CrossRef]
- Lynam, C.P.; Hay, S.J.; Brierley, A.S. Interannual Variability in Abundance of North Sea Jellyfish and Links to the North Atlantic Oscillation. Limnol. Oceanogr. 2004, 49, 637–643. [Google Scholar] [CrossRef]
- Robinson, K.L.; Graham, W.M. Long-Term Change in the Abundances of Northern Gulf of Mexico Scyphomedusae Chrysaora sp. and Aurelia spp. with Links to Climate Variability. Limnol. Oceanogr. 2013, 58, 235–253. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, J.S.; Kim, K.Y.; Molinero, J.C. Contrasting Effects of Regional and Local Climate on the Interannual Variability and Phenology of the Scyphozoan, Aurelia coerulea and Nemopilema nomurai in the Korean Peninsula. Diversity 2021, 13, 214. [Google Scholar] [CrossRef]
- Richardson, A.J.; Bakun, A.; Hays, G.C.; Gibbons, M.J. The Jellyfish Joyride: Causes, Consequences and Management Responses to a More Gelatinous Future. Trends Ecol. Evol. 2009, 24, 312–322. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; p. 2391. [Google Scholar] [CrossRef]
- Dong, Z.J.; Liu, D.Y.; Keesing, J.K. Jellyfish Blooms in China: Dominant Species, Causes and Consequences. Mar. Pollut. Bull. 2010, 60, 954–963. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, S.; Jin, X.S.; Li, C.L. Associations of Large Jellyfish Distributions with Temperature and Salinity in the Yellow Sea and East China Sea. Hydrobiologia 2012, 690, 81–96. [Google Scholar] [CrossRef]
- Feng, S.; Sun, S.; Li, C.L.; Zhang, F. Controls of Aurelia coerulea and Nemopilema nomurai (Cnidaria: Scyphozoa) Blooms in the Coastal Sea of China: Strategies and Measures. Front. Mar. Sci. 2022, 9, 1929. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, F.; Li, C.L.; Wang, S.W.; Wang, M.X.; Tao, Z.C.; Wang, Y.T.; Zhang, G.T.; Sun, X.X. Breeding Places, Population Dynamics, and Distribution of the Giant Jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) in the Yellow Sea and the East China Sea. Hydrobiologia 2015, 754, 59–74. [Google Scholar] [CrossRef]
- Dong, J.; Wang, B.; Duan, Y.; Yoon, W.D.; Wang, A.Y.; Liu, X.Z.; Li, Y.L.; Sun, M.; Chai, Y. Initial Occurrence, Ontogenic Distribution-Shifts and Advection of Nemopilema nomurai (Scyphozoa: Rhizostomeae) in Liaodong Bay, China, from 2005–2015. Mar. Ecol. Prog. Ser. 2018, 591, 185–197. [Google Scholar] [CrossRef]
- Uye, S.-I. Human Forcing of the Copepod–Fish–Jellyfish Triangular Trophic Relationship. Hydrobiologia 2010, 666, 71–83. [Google Scholar] [CrossRef]
- Kitajima, S.; Hasegawa, T.; Nishiuchi, K.; Kiyomoto, Y.; Taneda, T.; Yamada, H. Temporal Fluctuations in Abundance and Size of the Giant Jellyfish Nemopilema nomurai Medusae in the Northern East China Sea, 2006–2017. Mar. Biol. 2020, 167, 75. [Google Scholar] [CrossRef]
- Kawahara, M.; Ohtsu, K.; Uye, S.-I. Bloom or Non-Bloom in the Giant Jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae): Roles of Dormant Podocysts. J. Plankton Res. 2013, 35, 213–217. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, F.; Sun, S.; Wang, S.W.; Li, C.L. Effects of Duration at Low Temperature on Asexual Reproduction in Polyps of the Scyphozoan Nemopilema nomurai (Scyphozoa: Rhizostomeae). Hydrobiologia 2015, 754, 97–111. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, G.T.; Sun, S.; Zhang, F.; Wang, S.W.; Liu, M.T. Effects of Temperature Regime and Food Supply on Asexual Reproduction in Cyanea Nozakii and Nemopilema nomurai. Hydrobiologia 2015, 754, 201–214. [Google Scholar] [CrossRef]
- Wang, P.P.; Zhang, F.; Liu, M.T.; Sun, S.; Xian, H.C. Isotopic Evidence for Size-Based Dietary Shifts in the Jellyfish Cyanea nozakii in the Northern East China Sea. J. Plankton Res. 2020, 42, 689–701. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Sun, S.; Zhang, G.T.; Wang, S.W.; Li, C.L. Distribution Pattern of Zooplankton Functional Groups in the Yellow Sea in June: A Possible Cause for Geographical Separation of Giant Jellyfish Species. Hydrobiologia 2015, 754, 43–58. [Google Scholar] [CrossRef]
- Yoon, W.D.; Yang, J.Y.; Shim, M.B.; Kang, H.K. Physical Processes Influencing the Occurrence of the Giant Jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) around Jeju Island, Korea. J. Plankton Res. 2008, 30, 251–260. [Google Scholar] [CrossRef]
- Purcell, J.E.; Brown, E.D.; Stokesbury, K.D.E.; Haldorson, L.H.; Shirley, T.C. Aggregations of the Jellyfish Aurelia labiata: Abundance, Distribution, Association with Age-0 Walleye Pollock, and Behaviors Promoting Aggregation in Prince William Sound, Alaska, USA. Mar. Ecol. Prog. Ser. 2000, 195, 145–158. [Google Scholar] [CrossRef]
- Condon, R.H.; Duarte, C.M.; Pitt, K.A.; Robinson, K.L.; Lucas, C.H.; Sutherland, K.R.; Mianzan, H.W.; Bogeberg, M.; Purcell, J.E.; Decker, M.B.; et al. Recurrent Jellyfish Blooms Are a Consequence of Global Oscillations. Proc. Natl. Acad. Sci. USA 2013, 110, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Batten, S.; Sasaoka, K.; Sasai, Y.; Sugisaki, H. Influence of the Pacific Decadal Oscillation on Phytoplankton Phenology and Community Structure in the Western North Pacific. Geophys. Res. Lett. 2012, 39, 1–6. [Google Scholar] [CrossRef]
- McGinty, N.; Barton, A.D.; Record, N.R.; Finkel, Z.V.; Irwin, A.J. Traits Structure Copepod Niches in the North Atlantic and Southern Ocean. Mar. Ecol. Prog. Ser. 2018, 601, 109–126. [Google Scholar] [CrossRef]
- Irwin, A.J.; Nelles, A.M.; Finkel, Z.V. Phytoplankton Niches Estimated from Field Data. Limnol. Oceanogr. 2012, 57, 787–797. [Google Scholar] [CrossRef]
- Xiao, W.X.; Wang, L.; Laws, E.A.; Xie, Y.Y.; Chen, J.X.; Liu, X.; Chen, B.Z.; Huang, B.Q. Realized Niches Explain Spatial Gradients in Seasonal Abundance of Phytoplankton Groups in the South China Sea. Prog. Oceanogr. 2018, 162, 223–239. [Google Scholar] [CrossRef]
- McGinty, N.; Barton, A.D.; Finkel, Z.V.; Johns, D.G.; Irwin, A.J. Niche Conservation in Copepods between Ocean Basins. Ecography 2021, 44, 1653–1664. [Google Scholar] [CrossRef]
- Marchessaux, G.; Luskow, F.; Sara, G.; Pakhomov, E.A. Predicting the Current and Future Global Distribution of the Invasive Freshwater Hydrozoan Craspedacusta sowerbii. Sci. Rep. 2021, 11, 23099. [Google Scholar] [CrossRef]
- Condon, R.H.; Lucas, C.H.; Pitt, K.A.; Uye, S.-I. Jellyfish Blooms and Ecological Interactions. Mar. Ecol. Prog. Ser. 2014, 510, 109–110. [Google Scholar] [CrossRef]
- Lüskow, F.; Galbraith, M.D.; Hunt, B.P.V.; Perry, R.I.; Boersma, M.; Pakhomov, E.A. Gelatinous and Soft-Bodied Zooplankton in the Northeast Pacific Ocean: Phosphorus Content and Potential Resilience to Phosphorus Limitation. Hydrobiologia 2021, 849, 1543–1557. [Google Scholar] [CrossRef]
- Ruzicka, J.J.; Brodeur, R.D.; Emmett, R.L.; Steele, J.H.; Zamon, J.E.; Morgan, C.A.; Thomas, A.C.; Wainwright, T.C. Interannual Variability in the Northern California Current Food Web Structure: Changes in Energy Flow Pathways and the Role of Forage Fish, Euphausiids, and Jellyfish. Prog. Oceanogr. 2012, 102, 19–41. [Google Scholar] [CrossRef]
- Purcell, J.E. Predation on Zooplankton by Large Jellyfish, Aurelia labiata, Cyanea capillata and Aequorea aequorea, in Prince William Sound, Alaska. Mar. Ecol. Prog. Ser. 2003, 246, 137–152. [Google Scholar] [CrossRef]
- Kamiyama, T. Planktonic Ciliates as a Food Source for the Scyphozoan Aurelia aurita (S.L.): Feeding Activity and Assimilation of the Polyp Stage. J. Exp. Mar. Biol. Ecol. 2011, 407, 207–215. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, S.; Li, C.L. Estimation on food requirement by large jellyfish Nemopilema nomurai in summer. Oceanol. Limnol. Sin. 2017, 48, 1355–1361, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Ding, F.Y.; Cheng, J.H. The analysis on fish stock characteristics in the distribution areas of large jellyfish during summer and autumn in the East China Sea region. Mar. Fish. 2005, 27, 120–128, (In Chinese with English abstract). [Google Scholar]
- Brodeur, R.D.; Sugisaki, H.; Hunt, G.L. Increases in Jellyfish Biomass in the Bering Sea: Implications for the Ecosystem. Mar. Ecol. Prog. Ser. 2002, 233, 89–103. [Google Scholar] [CrossRef]
- Moller, L.F.; Riisgard, H.U. Population Dynamics, Growth and Predation Impact of the Common Jellyfish Aurelia aurita and Two Hydromedusae, Sarsia tubulosa, and Aequorea vitrina in Limfjorden (Denmark). Mar. Ecol. Prog. Ser. 2007, 346, 153–165. [Google Scholar] [CrossRef]
- Uye, S.-I. Blooms of the Giant Jellyfish Nemopilema nomurai: A Threat to the Fisheries Sustainability of the East Asian Marginal Seas. Plankton Benthos Res. 2008, 3, 125–131. [Google Scholar] [CrossRef]
- Sun, X.H.; Sun, X.Y.; Zhu, L.X.; Li, X.; Sun, S. Seasonal and Spatial Variation in Abundance of the Copepod Calanus sinicus: Effects of Decreasing Dissolved Oxygen and Small Jellyfish Bloom in Northern Yellow Sea, China, Nearshore Waters. Mar. Pollut. Bull. 2020, 161, 111653. [Google Scholar] [CrossRef]
- Gorsky, G.; Ohman, M.D.; Picheral, M.; Gasparini, S.; Stemmann, L.; Romagnan, J.B.; Cawood, A.; Pesant, S.; Garcia-Comas, C.; Prejger, F. Digital Zooplankton Image Analysis Using the Zooscan Integrated System. J. Plankton Res. 2010, 32, 285–303. [Google Scholar] [CrossRef]
- Grosjean, P.; Picheral, M.; Warembourg, C.; Gorsky, G. Enumeration, Measurement, and Identification of Net Zooplankton Samples Using the Zooscan Digital Imaging System. ICES J. Mar. Sci. 2004, 61, 518–525. [Google Scholar] [CrossRef]
- Sun, S.; Huo, Y.Z.; Yang, B. Zooplankton Functional Groups on the Continental Shelf of the Yellow Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 1006–1016. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Duque-Lazo, J.; van Gils, H.; Groen, T.A.; Navarro-Cerrillo, R.M. Transferability of Species Distribution Models: The Case of Phytophthora cinnamomi in Southwest Spain and Southwest Australia. Ecol. Model. 2016, 320, 62–70. [Google Scholar] [CrossRef]
- Schneider, G. The Common Jellyfish Aurelia aurita: Standing Stock, Excretion and Nutrient Regeneration in the Kiel Bight, Western Baltic. Mar. Biol. 1989, 100, 507–514. [Google Scholar] [CrossRef]
- Wiebe, P.H.; Boyd, S.; Cox, J.L. Relationships between Zooplankton Displacement Volume, Wet Weight, Dry Weight and Carbon. Fish. Bull. 1975, 73, 777–786. [Google Scholar]
- Omori, M. Weight and Chemical Composition of Some Important Oceanic Zooplankton in the North Pacific Ocean. Mar. Biol. 1969, 3, 4–10. [Google Scholar] [CrossRef]
- Huo, Y.Z.; Sun, S.; Zhang, F.; Wang, M.X.; Li, C.L.; Yang, B. Biomass and Estimated Production Properties of Size-Fractionated Zooplankton in the Yellow Sea, China. J. Mar. Syst. 2012, 94, 1–8. [Google Scholar] [CrossRef]
- Ikeda, T. Metabolic Rates of Epipelagic Marine Zooplankton as a Function of Body Mass and Temperature. Mar. Biol. 1985, 85, 1–11. [Google Scholar] [CrossRef]
- Runge, J.A.; Roff, J.C. The Measurement of Growth and Reproductive Rates. In ICES Zooplankton Methodology Manual; Harris, R.P., Wiebe, P., Lenz, J., Huntley, M., Skjoldal, H.R., Eds.; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar] [CrossRef]
- Ikeda, T.; Motoda, S. Estimated Zooplankton Production and Their Ammonia Excretion in the Kuroshio and Adjacent Seas. Fish. Bull. 1978, 76, 357–366. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wang, J.J.; Li, C.L.; Yang, G.; Tao, Z.C.; Wang, Y.Q.; Xian, H.C. Dietary Preferences and Potential Ecological Impact on the Zooplankton Community of Nemopilema nomurai Based on Stable Isotope and Fatty Acid Analyses. J. Oceanol. Limnol. 2022, 40, 1085–1096. [Google Scholar] [CrossRef]
- Wang, P.P.; Zhang, F.; Sun, S.; Yang, T. Distribution of giant jellyfish in the Bohai Sea in June 2018. J. Oceanol. Limnol. 2020, 51, 85–94, (In Chinese with English abstract). [Google Scholar]
- Wang, B.; Li, Y.L.; Shen, H.; Li, T.P.; Wang, W.B.; Sun, M.; Dong, J. Quantity distribution of Cyanea nozakii in inshore waters of northern Liaodong Bay, Bohai Sea in 200–2013. Mar. Fish. 2014, 36, 146–154. [Google Scholar] [CrossRef]
- Zuo, T.; Wu, Q.; Wang, J.; Li, Z.Y. Annual survey of the species dicersity and assemblage dynamics of medusae in Laizhou Bay, Bohai Sea. Acta Ecol. Sin. 2016, 36, 5646–5656, (In Chinese with English abstract). [Google Scholar]
- Zang, W.X.; Zhang, F.; Chi, X.P.; Sun, S. Relationship between Asexual Reproduction of Aurelia coerulea Polyps and Jellyfish Blooms under the Influence of Temperature Dynamics in Winter and Spring. Front. Mar. Sci. 2022, 9, 948. [Google Scholar] [CrossRef]
- Henschke, N.; Stock, C.A.; Sarmiento, J.L. Modeling Population Dynamics of Scyphozoan Jellyfish (Aurelia spp.) in the Gulf of Mexico. Mar. Ecol. Prog. Ser. 2018, 591, 167–183. [Google Scholar] [CrossRef]
- Wang, L.; Xu, K.D. Spatiotemporal Distribution of Protozooplankton and Copepod Nauplii in Relation to the Occurrence of Giant Jellyfish in the Yellow Sea. Chin. J. Oceanol. Limnol. 2013, 31, 1226–1240. [Google Scholar] [CrossRef]
- Xiao, W.P.; Zeng, Y.; Liu, X.; Huang, X.G.; Chiang, K.P.; Mi, T.Z.; Zhang, F.; Li, C.L.; Wei, H.; Yao, Q.Z.; et al. The Impact of Giant Jellyfish Nemopilema nomurai Blooms on Plankton Communities in a Temperate Marginal Sea. Mar. Pollut. Bull. 2019, 149, 110507. [Google Scholar] [CrossRef]
- Jin, X.S.; Zhao, X.Y.; Meng, T.X.; Cui, Y. Biological Resources and Habitat of Yellow and Bohai Seas; Science Press: Beijing, China, 2005. [Google Scholar]
- Schneider, G.; Behrends, G. Top-Down Control in a Neritic Plankton System by Aurelia aurita Medusae—A Summary. Ophelia 1998, 48, 71–82. [Google Scholar] [CrossRef]
- Wang, W.C. Long-Term Changes of Zooplankton Functional Groups in Jiaozhou Bay. Doctoral Thesis, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China, 2017. (In Chinese with English abstract). [Google Scholar]
- Sun, S.; Zhou, K.; Yang, B.; Zhang, Y.S.; Ji, P. Ecology of zooplankton in the Jiaozhou Bay I. species composition. China J. Oceanol. Limnol. 2008, 39, 1–7+2. [Google Scholar]
- Eriksen, E.; Skjoldal, H.R.; Dolgov, A.V.; Strand, E.; Keulder-Stenevik, F.; Prokopchuk, I.P.; Prokhorova, T.A.; Prozorkevich, D.; Benzik, A.N. Diet and Trophic Structure of Fishes in the Barents Sea: Seasonal and Spatial Variations. Prog. Oceanogr. 2021, 197, 102663. [Google Scholar] [CrossRef]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. The Key Role of Zooplankton in Ecosystem Services: A Perspective of Interaction between Zooplankton and Fish Recruitment. Ecol. Indic. 2021, 129, 107867. [Google Scholar] [CrossRef]
- Vinogradov, M.E.; Shushkina, E.A.; Bulgakova, Y.V.; Serobaba, I.I. The Consumption of Zooplankton by Comb Jelly Mnemiopsis leidyi and Pelagic Fishes in the Black Sea. Okeanologiya 1996, 35, 523–527. [Google Scholar]
- Opdal, A.F.; Brodeur, R.D.; Cieciel, K.; Daskalov, G.M.; Mihneva, V.; Ruzicka, J.J.; Verheye, H.M.; Aksnes, D.L. Unclear Associations between Small Pelagic Fish and Jellyfish in Several Major Marine Ecosystems. Sci. Rep. 2019, 9, 2997. [Google Scholar] [CrossRef]

| Area | Number | Depth (m) | Temperature (°C) | Salinity | Chlorophyll a (mg·m−3) |
|---|---|---|---|---|---|
| nECS | 11 | 46.63 ± 14.79 | 24.36 ± 2.24 | 32.85 ± 2.21 | 0.37 ± 0.18 |
| YS | 50 | 52.88 ± 18.90 | 14.22 ± 5.94 | 32.11 ± 0.74 | 0.52 ± 0.47 |
| BS | 34 | 23.03 ± 9.21 | 23.24 ± 2.66 | 29.46 ± 4.78 | 0.79 ± 0.38 |
| Species | AUC | Variable | Percent Contribution (%) |
|---|---|---|---|
| Nemopilema nomurai | 0.655 | Temp | 23.7 |
| Sal | 57.2 | ||
| Chl | 19.1 | ||
| Cyanea spp. | 0.819 | Temp | 70.9 |
| Sal | 13.7 | ||
| Chl | 15.4 | ||
| Aurelia coerulea | 0.912 | Temp | 27.3 |
| Sal | 67.9 | ||
| Chl | 4.7 |
| Capture Rate | Mean | Range | |
|---|---|---|---|
| Standing stock of zooplankton | - | 453.37 | 51.09–1267.85 |
| Production rate of zooplankton | - | 59.38 | 9.54–121.04 |
| Feeding rate | 0.1 | 21.05 | 0.00–62.94 |
| 0.4 | 5.26 | 0.00–15.74 | |
| Feeding pressure on standing stock | 0.1 | 7.70 | 0.00–25.51 |
| 0.4 | 1.92 | 0.00–6.38 | |
| Feeding pressure on production rate | 0.1 | 55.16 | 0.00–183.45 |
| 0.4 | 13.79 | 0.00–45.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, D.; Zhang, F.; Wang, P.; Sun, S. Distribution Patterns of Large Jellyfish and Their Effects on the Zooplankton Community in the Northern Chinese Coastal Seas during the Summer of 2021. Diversity 2023, 15, 729. https://doi.org/10.3390/d15060729
Guo D, Zhang F, Wang P, Sun S. Distribution Patterns of Large Jellyfish and Their Effects on the Zooplankton Community in the Northern Chinese Coastal Seas during the Summer of 2021. Diversity. 2023; 15(6):729. https://doi.org/10.3390/d15060729
Chicago/Turabian StyleGuo, Dongjie, Fang Zhang, Pengpeng Wang, and Song Sun. 2023. "Distribution Patterns of Large Jellyfish and Their Effects on the Zooplankton Community in the Northern Chinese Coastal Seas during the Summer of 2021" Diversity 15, no. 6: 729. https://doi.org/10.3390/d15060729
APA StyleGuo, D., Zhang, F., Wang, P., & Sun, S. (2023). Distribution Patterns of Large Jellyfish and Their Effects on the Zooplankton Community in the Northern Chinese Coastal Seas during the Summer of 2021. Diversity, 15(6), 729. https://doi.org/10.3390/d15060729






