Abstract
In order to document the diversity and distribution of mosquitoes inhabiting the Querétaro State of México, collection trips were conducted in all physiographic regions and sub-regions of the state (Sierra Madre Oriental, Central Plateau, and Neo-Volcanic Axis). In addition, mosquito specimens collected in Querétaro and deposited in the Collection of Arthropods of Medical Importance (CAIM) were re-examined. A total of 2718 specimens (570 larvae, 384 larval exuviae, 537 pupal exuviae, 30 pupae, 807 females, 368 males, and 22 male genitalia) were analyzed. In total, 2 subfamilies, namely Anophelinae and Culicinae, 5 tribes, 12 genera, 20 subgenera, and 50 species were found. Of these, 3 tribes, 8 genera, 11 subgenera, and 33 species are new records for the mosquito fauna of Querétaro. Two undescribed species were found, and one of them, Shannoniana huasteca Ortega n. sp., is described here using morphology and Cytochrome oxidase subunit 1 (COI) DNA barcoding. Taxonomic notes, new distribution limits, comments about the medical importance of species, and a key to identify adult females of Shannoniana species are provided.
1. Introduction
Mexico is divided into 32 political states of which only nine have been systematically studied in terms of the taxonomy, ecology, and distribution of mosquito species: Tlaxcala (26 spp.) [1], Quintana Roo (88 spp.) [2,3,4,5,6,7,8,9], Veracruz (141 spp.) [10,11,12], Tamaulipas (82 spp.) [13], Hidalgo (57 spp.) [14,15], Nuevo León (67 spp.) [16,17,18], Tabasco (107 spp.) [19,20,21,22], Mexico City (28 spp.) [23,24], and Mexico State (51 spp.) [25]. In Querétaro state, 17 mosquito species had been previously reported. However, most records are based on collections made in urban and sub-urban regions, not on collections from conserved forest and jungle regions, primarily in the north of the state. In this study, all physiographical regions of Querétaro (Sierra Madre Oriental, Central Plateau, and Neo-Volcanic Axis) were sampled with special emphasis on conserved forest regions and other sylvan regions of the state during the dry and rainy seasons.
A current checklist of the mosquito species that inhabit Querétaro state is provided in this study. Moreover, biological notes and medical importance are provided for the newly reported species and for species that reach their distributional limits within the state. Two undescribed species were found during the field collections, one of which is described here: Shannoniana huasteca Ortega n. sp. using morphological characters of adult stages and the analysis of DNA-barcodes. The second species, belonging to the genus Culiseta, subgenus Culiseta, is left undescribed until more material becomes available. Querétaro is the tenth state of Mexico to have the list of mosquito species updated. At present, 50 species are currently known (Table 1). Specimens collected and examined during this study were deposited in the Culicidae Collection of the Parasitology Department of the Autonomous Agrarian University Antonio Narro, Laguna unit, Torreón, Coahuila, Mexico.

Table 1.
Checklist of the mosquito species that occur in Querétaro state. VM-P: [26]. V: [27]. D-NV: [28]. IBMC: [29]. STEA: [30]. WEEA [31] OMEA a: [32]. OMEA b: [33]. F.R.: First Record. NSR: New State Record (in bold). NS: New Species (in bold). 1 Reported as Aedes atropalpus (Coquillett). 2 Reported as Culex peus Speiser.
2. Materials and Methods
2.1. Study Area
Querétaro state is located in the north-central part of Mexico, between 21º40′12″ and 20°00′54″ north latitude and the meridians 99°02′35″ and 100°35′48″ west longitude. The state has an area of 11,699 km2. It is bordered to the north by the state of San Luis Potosí; to the west by the state of Guanajuato; to the east by the state of Hidalgo; to the southeast by the state of Mexico; and to the southwest by the state of Michoacán. The state is divided into three physiographic regions and four subregions (Figure 1): Sierra Madre Oriental (Carso Huasteco); Central Plateau (Mountains and Plains of Northern Guanajuato); and Neo-Volcanic Axis (Plains and Mountains of Querétaro and Hidalgo, Thousand Peaks, and Lakes and Volcanoes of Anáhuac) [34]. A description of the regions and subregions of Querétaro and a list of the municipalities sampled are given in Table 2.
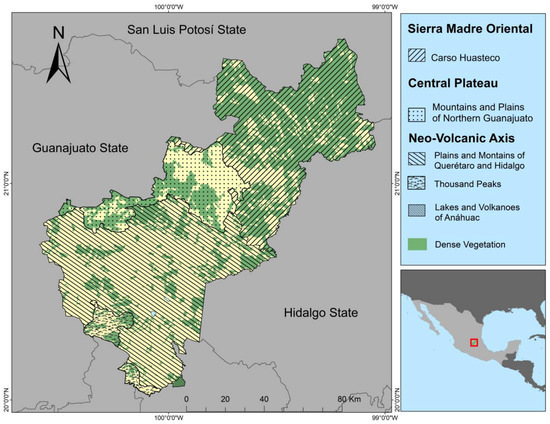
Figure 1.
Physiography of Querétaro state. Red bow shows the study area.

Table 2.
Description of the physiography of Querétaro state and list of municipalities sampled.
2.2. Mosquito Collection
Immature stages and adult mosquitoes were collected in specific locations in the four physiographic regions of Querétaro (Table 2). The collections were conducted in both the dry and rainy seasons from 2012 to 2021. Immature stages were collected from all bodies of water found in the study area. Larvae and pupae were placed alive in cups with water from the aquatic habitat and transported to the Laboratorio de Biología Molecular of the Parasitology Department of the Universidad Autónoma Agraria Antonio Narro unidad laguna (LBM-UAAAN-UL). A portion of fourth-instar larvae from each collection was mounted on microscope slides using Euparal, whereas the rest of the live larvae were placed in individual emergence tubes to obtain adults with associated larval and pupal exuviae. Male genitalia was dissected to assist identification when required. Adults were collected in the field using CDC light traps, Shannon traps, and/or biting/landing on humans, and they were killed using triethylamine vapors and later mounted on insect pins. Mosquitoes mounted on insect pins were identified using a stereomicroscope Zeiss Discovery V8, while immature stages and exuviae were identified using a microscope Zeiss Primostar. The morphological terminology proposed by Harbach and Knight [35] for mosquito taxonomy was followed in this study.
2.3. Review of Entomological Collections
The Collection of Arthropods of Medical Importance (CAIM) deposited in the Diagnostic and Epidemiologic Reference Institute (InDRE) was reviewed for additional records of mosquitoes of Querétaro. The species found in the CAIM collection that were not collected by us are Anopheles eiseni Coquillett, An. albimanus Wiedemann, Haemagogus equinus Theobald, and Culex salinarius Coquillett. The traditional classification of Culicidae [36] was followed in large part, except that we consider only two subfamilies, incorporating Toxorhynchites from the tribe Toxorhynchitini into Culicinae, and we followed [37] the arrangement of Aedini taxa that was incorporated into the online classification of the Walter Reed Biosystematics Unit (WRBU) [38]. Generic and subgeneric abbreviations of Culicidae names also followed the WRBU [38].
2.4. DNA Extraction and COI Amplification
For DNA extraction, a modified Hotshot technique [39,40,41] was employed. Two legs were placed directly into 50 µL of alkaline lysis buffer in micro vials, which were then sonicated in a water bath for 20 min. Micro vials were subsequently incubated in a thermocycler for 30 min at 94 °C and cooled for 5 min. at 4 °C, after which 50 µL of the neutralizing buffer was added to each vial. PCR amplification of the full-length COI barcode region [42,43] was performed using Folmer primers (LCO1490 and HCO2198) and a Qiagen PCR system with the following reaction mix with a final volume of 50 µL: 2 µL of the DNA template, 25 µL of H2O, 5 µL of NH4, 5 µL of dNTPs (2 mM/µL), 2.5 µL of MgCl2 (25 mM/µL), 0.1 µL of Bioline Taq Polymerase (Bioline Reagents Ltd., London, UK), 5 µL of each primer (each at 10 pmol/µL), and 0.38 µL of bovine serum albumin (20 mg/mL) [39,40,41]. The thermal profile consisted of the following: An initial denaturation step at 94 °C for 1 min., 5 cycles of preamplification of 94 °C for 1 min., 45 °C for 1.5 min., 72 °C for 1.5 min., followed by 35 cycles of amplification of 94 °C for 1 min., 57 °C for 1.5 min., and 72 °C for 1 min., followed by a final elongation step of 72 °C for 5 min. All PCR products were visualized with a 1.5% agarose gel, and samples showing bands of the correct size were bidirectionally sequenced using the ABI PRISM® BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, MA, USA) at the Sequencing Unit, APHA.
2.5. Sequence Analysis
DNA sequences generated in both directions were edited manually using BioEdit sequence alignment Editor version 7.0.5.3 [44], and a consensus sequence was generated using ClustalW [45]. Full details for each specimen and sequence information can be found in the Barcode of Life Database (BOLD) within the “Human Pathogens and Zoonoses Initiative”, Working Group 1.4. The Digital Object Identifier (DOI) for the publicly available project in BOLD is dx.doi.org/requested. Accession numbers for the sequences of Shannoniana huasteca n. sp. were obtained from NCBI (accession numbers requested). For certain species, we used public COI barcode sequences publicly available in BOLD: Shannoniana fluviatilis (Grench Guiana) (FGMOS1099-16, FGMOS816-16), Sh. shcedocyclia (French Guiana) (FGMOS817-16, FGMOS946-16, FGMOS947-16, FGMOS1126-16, FGMOS1134-16). We also compared published sequences of Sh. moralesi (Mexico) (MOSQV056-18, MQCCHP015-16, MQCHP016-16, MQCHP017-16, MQCHP018-16, MQCHP019-16, XNSLC054-18, XNSLC055-18), and Trichoprosopon digitatum (Mexico) (MQCHP064-16, MQCHP080-16, MQCHP082-16). The dataset was analyzed in MEGA v.6 [41]. The Maximum Likelihood (ML) analysis was performed using the Kimura 2-Parameter distance metric to determine their distribution pattern, and the tree was rooted to Tr. digitatum. The tree robustness was measured by the bootstrap approach using 1000 pseudoreplicates [46].
2.6. Nomenclatural Acts
The electronic edition of this article conforms to the requirements of the amended International Code of the Zoological Nomenclature (ICZN), and hence the new name contained herein is available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/ (access date on 15 January 2023). The LSID for this publication is 4DD7EB32-56DD-41B6-AACC-877947FE26D4. The electronic edition of this work was published in a journal with an ISSN and has been archived and is available from the MDPI digital repositories.
3. Results
3.1. Mosquito Identification
A total of 2718 specimens from 203 collections were studied. Among the specimens were 570 fourth-instar larvae, 384 larval exuviae, 537 pupal exuviae, 30 pupae, 807 adult females, 368 adult males, and 22 dissected male genitalia. The mosquito fauna of Querétaro state consists of 50 species representing the subfamilies Anophelinae and Culicinae, 5 tribes of the subfamily Culicinae, 12 genera, and 20 subgenera (Table 1). Three tribes (Culisetini, Sabethini, and Toxorhynchitini), eight genera (Psorophora, Lutzia, Culiseta, Limatus, Sabethes, Shannoniana, Wyeomyia, and Toxorhynchites), 11 subgenera (Grabhamia, Anoedioporpa, Melanoconion, Neoculex, Phenacomyia, Lutzia, Culiseta, Sabethoides, Triamyia, Wyeomyia, and Lynchiella), and 33 species (Anopheles apicimacula, An. eiseni, An. franciscanus, Aedes quadrivittatus, Ae. angustivittatus, Ae. euplocamus, Ae. amabilis, Ae. schicki, Psorophora signipennis, Culex restrictor, Cx. coronator, Cx. declarator, Cx. erythrothorax, Cx. restuans, Cx. salinarius, Cx. tarsalis, Cx. thriambus, Cx. erraticus, Cx. peccator, Cx. apicalis, Cx. arizonensis, Cx. lactator, Lutzia bigoti, Culiseta inornata, Cs. particeps, Cs. n. sp., Limatus durhamii, Sabethes chloropterus, Shannoniana huasteca, Wyeomyia aporonoma, Wy. adelpha/guatemala, Wy. mitchellii, and Toxorhynchites moctezuma) are new records for the mosquito fauna of Querétaro. Finally, two new species were discovered, one of which (Sh. huasteca) is described herein. The species accumulation curve of 46 of the 50 mosquito species collected is shown in Figure 2.
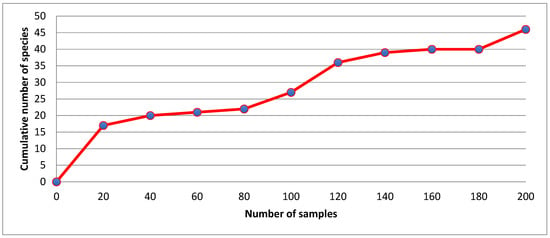
Figure 2.
Species accumulation curve for 46 of the 50 mosquito species (203 collections) collected in Querétaro during 2012–2021.
3.2. Biological and Ecological Notes for New State Records
Biological and ecological notes for each species group are reported here. Specific notes including the collection site, date of collection, larval habitat, aquatic parameters, and associated species are shown in Table 3.

Table 3.
Collection records of the newly reported mosquito species found in Querétaro, Mexico. The position (geographic coordinates in scale of degrees, minutes, and seconds); elevation (meters above sea level); type of aquatic habitat of the immature stages or environmental condition where adults were collected; environmental parameters of the aquatic habitat (pH, temperature, and dissolved salts in scale of parts per million); and associated species for each collection.
3.2.1. Genus Anopheles
Both the subgenera Anopheles and Nyssorhynchus had been previously reported in Querétaro. However, three species within the subgenus Anopheles are first recorded in the state: Anopheles apicimacula, An. eiseni, and An. franciscanus. One female of An. apicimacula was collected, while immature stages of An. franciscanus were collected from swamps, ponds, and stream margins in several locations. One record of An. eiseni was obtained from the CAIM collection; this species was not found during our collection trips.
3.2.2. Genus Aedes
Of the 16 species of Aedes known from Querétaro, five are reported for the first time in the state: Aedes quadrivittatus, Ae. angustivittatus, Ae. euplocamus, Ae. amabilis, and Ae. schicki. Aedes quadrivittatus was one of the most common species within the genus in the forested and conserved regions of the northern part of state; females of this species were collected approaching humans, while immature stages were collected from water in bromeliad axils. Adult females of Ae. angustivittatus were collected approaching humans at a single location while immature stages of Ae. euplocamus were collected from aquatic habitats at ground level in two sites. Aedes amabilis was very common in the conserved regions of the state, and this species frequently approached humans. Only one larvae of Ae. schicki was collected from a tree hole, making it the rarest species within the genus in Querétaro.
3.2.3. Genus Psorophora
In Mexico, the genus Psorophora is a common group of mosquitoes during the rainy season; however, this is the first record of this genus, subgenus Grabhamia, and Ps. signipennis in Querétaro. Immature stages of this species were collected from ponds during the rainy season. Psorophora signipennis is the only species within the genus Psorophora known for the state.
3.2.4. Genus Culex
The genus Culex is the most diverse group of mosquitoes in Querétaro, including fifteen species in the state, of which two had previously been reported. The subgenera Anoedioporpa, Melanoconion, Neoculex, and Phenacomyia are recorded for the first time in Querétaro. Immature stages of Cx. restrictor were collected from two locations, in a tree hole and a discarded tire; this type of larval habitat is common for this species in Mexico. The new records within the subgenus Culex are Cx. coronator, Cx. declarator, Cx. erythrothorax, Cx. restuans, Cx. salinarius, Cx. tarsalis, and Cx. thriambus. The Coronator complex includes five recognized species: Cx. camposi Dyar, Cx. coronator, Cx. ousqua Dyar, Cx. ousquatissimus Dyar, and Cx. usquatus Dyar [47]. Based on male genitalia morphology, Cx. coronator s.s. was identified from material collected in Querétaro; this is a common species whose immature stages were collected from a variety of aquatic habitats, mostly natural habitats at ground level. Although Cx. declarator is a common species in the central-northern region of Mexico, immature stages of this species were collected from an artificial container in only a single location. Culex erythrothorax, Cx. restuans, Cx. tarsalis, and Cx. thriambus are common species that were found frequently and collected from a variety of aquatic habitats such as natural and artificial containers. The record of Cx. salinarius was obtained from the CAIM collection. The subgenus Melanoconion in Querétaro includes Cx. erraticus and Cx. peccator; immature stages of both species were collected from natural habitats at ground level in two locations. Within the subgenus Neoculex, the species found in Querétaro are Cx. apicalis and Cx. arizonensis. The subgenus Phenacomyia and Cx. lactator are recorded for the first time in Querétaro. Adult females of this species were collected approaching humans during the day at a single location.
3.2.5. Genus Lutzia
The genus Lutzia, subgenus Lutzia, and Lt. bigoti are recorded for the first time in Querétaro. Immature stages of this species were collected from one spring and an artificial container with clear water, predating larvae of Ae. albopictus and Cx. spp., respectively.
3.2.6. Genus Culiseta
The tribe Culisetini, genus Culiseta, and subgenera Culiseta, Culiseta inornata, and Cs. particeps are recorded for the first time in Querétaro. Immature stages of Cs. inornata were collected from an irrigation gutter in one location, while immature stages of Cs. particeps were collected from a variety of aquatic habitats, such as natural ponds and swamps, artificial containers, and discarded tires. Immature stages of one undescribed species within this genus were discovered in discarded tires. These specimens are in a poor condition; hence, this species will be formally described when more specimens are obtained.
3.2.7. Genus Limatus
The tribe Sabethini, genera Limatus, Sabethes, Shannoniana, and Wyeomyia; subgenera Sabethoides, Triamyia, and Wyeomyia; species Li. durhammi, Sa. chloropterus, Sh. huasteca, Wy. apronoma, Wy. adelpha/guatemala, and Wy. mitchellii are recorded for the first time in Querétaro. Immature stages of Li. durhamii were collected from an artificial container with clear water in one location with no associated species.
3.2.8. Genus Sabethes
In Mexico, the genus Sabethes is divided into two subgenera: Sabethes and Sabethoides. The latter is reported for the first time in Querétaro and is represented by Sa. chloropterus. Adult females of this species were collected approaching humans during the day.
3.2.9. Genus Shannoniana
In Mexico, three species of the genus Shannoniana had been previously recorded: Sh. fluviatilis (Theobald), Sh. moralesi (Dyar and Knab), and Sh. schedocyclia (Dyar and Knab). In the present study, we discovered a fourth species within the genus. Adult females of Sh. huasteca n. sp. were collected approaching humans and males were collected resting in vegetation and approaching humans together with the females. Adults of both sexes are described herein.
3.2.10. Genus Wyeomyia
Three species of the genus Wyeomyia are reported for the first time in Querétaro: Adult females of Wy. aporonoma were collected approaching humans in one location in association with Ae. allotecnon, Cx. lactator, Sa. chloropterus, Sh. huasteca, and Wy. mitchellii. Since Wy. guatemala is possibly a synonymy of Wy. adelpha [48], and both species are treated here as a single taxon. Adult females of Wy. adelpha/guatemala were collected approaching humans during the day in one location, while immature stages of Wy. mitchelli were collected from bromeliad axils and adult females were collected approaching humans in several locations of Querétaro.
3.2.11. Genus Toxorhynchites
The tribe Toxorhynchitini, genus Toxothynchites, and subgenera Lynchiella and Tx. moctezuma are recorded for the first time in Querétaro. Immature stages of Tx. moctezuma were collected from discarded tires and one tree hole, always with clear water and predating on larvae of Ae. sp. and Cx. thriambus in tropical and conserved regions of the state.
3.3. Molecular Analysis
In total, we analyzed 25 DNA barcodes for five species within the genus Shannoniana (four taxa) and Trichoprosopon (one taxa) (Table 4). In general, all specimens of the same species clustered together (Figure 3), although there was a deep split in Sh. fluviatilis (BOLD:ACZ4319, BOLD:ACZ4320) and Sh. schedocyclia (BOLD:ACZ3895, BOLD:ACZ:3896), where two BINs were found in each taxon. All specimens identified as Sh. huasteca n. sp. were grouped closely with Sh. moralesi, although both groups are well separated with high support bootstrap values (Figure 3). The average genetic divergence was 0.08%; the intra-specific genetic divergence varied from 0.04% in Sh. huasteca n. sp., Sh. moralesi (0.55%), Sh. schedocyclia (1.31%), and Tr. digitatum (0.20%). In Sh. fluviatilis, the genetic divergence was above 2% (3.96%). Interspecific genetic divergence varied from 4.70% to 13.13%; the pair Sh. moralesi/Sh. fluviatilis were the more divergent species (13.13%), while the pair Sh. huasteca n. sp./Sh. moralesi were less divergent (4.70%).

Table 4.
Percentage of interspecific (between groups) pairwise K2P genetic divergence of unique DNA barcodes (658 bp), representing five species of Sabethini.
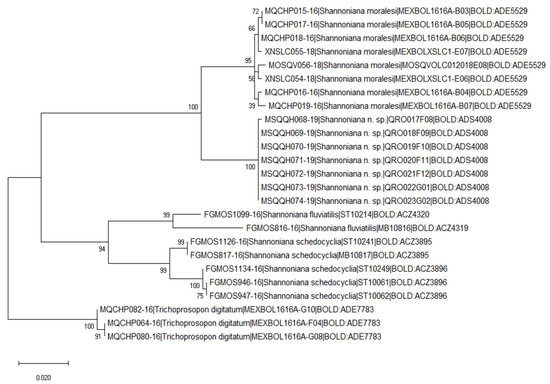
Figure 3.
Maximum likelihood tree base on the Kimura 2-parameter of the COI DNA barcodes (658 bp) for species of Shannoniana (n = 4) and Trichoprosopon (n = 1). A divergence of >2% may be indicative of separate operational taxonomic units.
3.4. Description of New Species
Shannoniana huasteca Ortega n. sp. 4DD7EB32-56DD-41B6-AACC-877947FE26D4. Type specimens: Holotype: adult female (A♀) without associated larval and pupal exuviae [CC-UL, 04240918-CN], Camino a Neblinas, Landa de Matamoros, Querétaro, Mexico (21°15′29.3″ N–99°4′11.12″ W) (Figure 4), elevation 1010 m, 24 Sep 2018, 17:00–18:00, human biting at day, tropical cloud forest with oaks and conserved vegetation (Figure 5), col. A.I. Ortega-Morales. Paratypes: 10A♀, (same data as holotype); [CAIM]. Allotypes: 3♂ with dissected genitalia, (same data as holotype); [CC-UL] (Table 3).
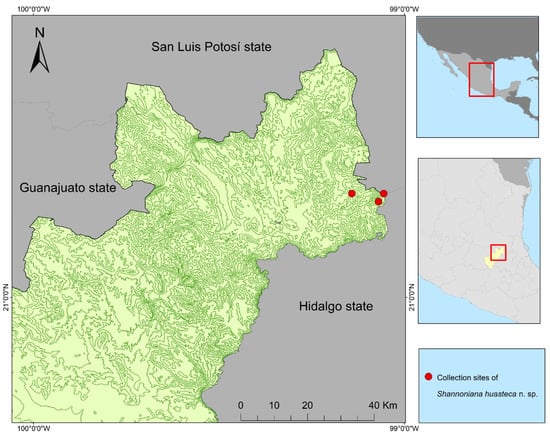
Figure 4.
Distribution of Sh. huasteca.

Figure 5.
Collection site of Shannoniana huasteca n. sp., showing the tropical and conserved types of vegetation. The site is near Neblinas road, Landa de Matamoros, Querétaro, Mexico.
Female. Head: Occiput and vertex covered with flat decumbent blue, green and silver scales, with purple and greenish reflections, with a row of erect scales (Figure 6A), interocular setae large, pedicel bare, yellow-brown. Antennae approximately 0.50–0.75 forefemur length. Clypeus bare, dark-brown. Maxillary palpus approximately 0.25 proboscis length, three-segmented, third palpomere longer than the first two, dark-scaled with purplish reflections. Proboscis as long as forefemur, sometimes slightly longer forefemur length of 1.10–1.20, with dark scales with purplish reflections. Thorax: Integument of scutum golden, covered with pale golden-brown decumbent narrow scales without iridescent reflections, acrostichal and dorsocentral setae absent (Figure 6B). Scutellum trilobed, with 7–10 setae on lateral lobes and 5–7 setae on mid lobe, all lobes covered with flat dark-blue scales with purplish reflections. Row of erect dark setae above the paratergite and the wing. Postpronotum covered with flat yellow-golden scales with golden reflections, without setae. Antepronotum lobe with silvery scales, with 4–5 setae. Integument of mesokatepisterum and mesanepimeron dark-golden, mostly covered with large patch of silvery flat scales (Figure 7A), mesanepimeron with 10–12 dark-brown setae. Wing: Approximately 1.30–1.50 mm, scales on veins flat and light-brown (Figure 7B). Halter: Dark-brown with blue scales. All trochanters with patches of silvery scales. All femora dark-scaled, with some iridescent scales bluish-greenish on dorsal line, with a small knee spot of pale scales, fore and midfemora predominantly dark-scaled, with bluish reflections, hindfemur with dorsal dark scaled line and ventral line withe-scaled. Hindtibia dark-scaled, with a complete ring of white-yellow scales apically. Foretarsus covered predominantly with dark scales, mid and hindtarsus with tarsomeres 1–4 dark-scaled, tarsomere 5 with dark scales on dorsal line and white scales on ventral line. Abdomen: All terga covered with dark scales with bluish reflections, apical corners of dark scales on terga extending into 0.50 of sternal segments, sterna covered with white scales.
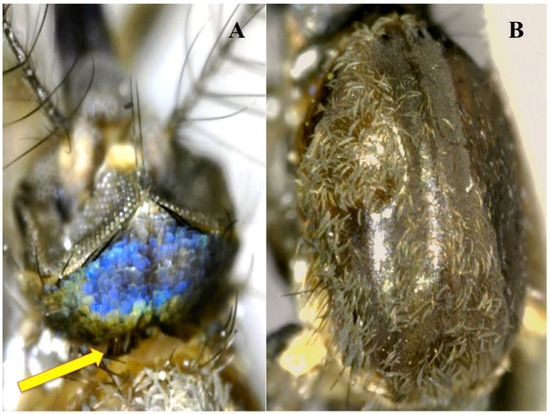
Figure 6.
Adult female of Shannoniana huasteca, holotype. (A) Occiput showing the decumbent green, blue, and silvery scales, arrow showing the row of erect scales; (B) scutum covered with pale golden-brown narrow scales.
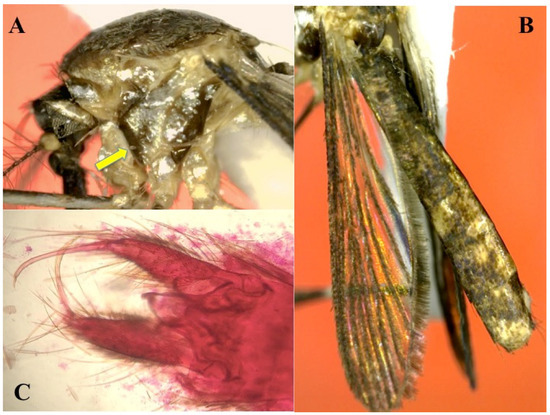
Figure 7.
Adult of Shannoniana huasteca n. sp. (A) General aspect of lateral view of thorax showing the integument golden, mesokaterpisternum, and mesanepimeron dark-golden (holotype), arrow shows the posprocoxal area; (B) wing covered with light-brown scales and dark scales on abdominal terga (holotype); (C) general aspect of male genitalia (allotype).
Male. In general, as in females except for sexual characteristics and plumose antennae.
Male genitalia (Figure 7C). Segment IX: Tergum with deep emargination between tergal lobes, the lobes slightly longer than broad, with 6–8 long, curved setae. Gonocoxite: Length approximately three times median width, basal tergomesal lobe well developed, with 5–7 long and strong setae. Gonostylus: Narrow, simple, and long, slightly curved apically. Aedeagus strongly sclerotized, simple and ovate.
Larva. Unknown.
Pupa. Unknown.
Systematics. Females of Sh. huasteca n. sp. are distinguished from all three previously described species within the genera Shannoniana (Sh. fluviatilis (Theobald), Sh. moralesi (Dyar and Knab), and Sh. schedocyclia (Dyar and Knab)) by having all legs covered with dark scales, except for tarsomere five of hind leg, which has white scales in a ventral line; silvery, decumbent scales on vertex not extending to the ocular line, but interrupted by a patch of dark scales with bluish and greenish reflections; and the absence of a patch of silvery scales on postprocoxal area. The males are readily distinguished by the structure of the male genitalia (Figure 7C), especially the narrow and long gonostyle, slightly curved apically.
Bionomics. Although the type locality was visited on numerous occasions to search for immature stages of Sh. huasteca n. sp., these were not found. Immature stages of other species were collected in different aquatic habitats such as containers and phytotelmata (e.g., axils of bracts of Xanthosoma spp. and bromeliads). In addition, ovitraps were displayed at different elevations from ground level, but all those collections failed to find immature stages of Sh. huasteca n. sp. Adult females were collected approaching humans probing to bite during the day in association with Aedes allotecnon, Ae. quadrivittatus, Culex lactator, Sabethes chloropterus, Wyeomyia aporonoma, and Wy. mitchellii. The medical importance of Sh. huasteca n. sp. is unknown, but since females can be persistent biters of humans, the species could be involved in the transmission of pathogens.
Distribution. Shannoniana huasteca n. sp. has been collected in the northern region of the state of Querétaro (Neblinas road, location of Landa de Matamoros County). Locations in which the species was collected belong to Huasteco Carso of the Sierra Madre Oriental. Shannoniana huasteca n. sp. may occur in the forested regions of the states adjacent to Querétaro such as the southeastern San Luis Potosí state and northwestern Hidalgo state, with both states sharing physiographical conditions belonging to the Carso Huasteco of the Sierra Madre Oriental.
Etymology. This species is named huasteca because of the type locality in the Carso Huasteco sub-region. “Huasteco” is a word derived from the huasteco language, which means someone from an Amerindian tribe of the Mayan family that lives in the Mexican states of Tamaulipas, San Luis Potosí, Querétaro, and Veracruz.
3.5. Keys to Species of Adult Female of Shannoniana
1. Hind tarsi with basal rings of white scales on segments I–IV (Figure 9A)…………………………Sh. schedocyclia Distr.: Bolivia, Brazil, French Guiana, Guatemala, Mexico, Nicaragua, Panama, Venezuela [50] (Distr. Mex.: Chiapas [27,28,51,52,53], Oaxaca [27,28], Veracruz [10,28,52]).
- Hind tarsi with segments I–IV covered only with dark scales (Figure 9B).…….……2
2(1) Tarsomere V of hind leg covered completely with dark scales (Figure 9C)…………….………….Sh. fluviatilis
Distr.: Argentina, Bolivia, Brazil, Colombia, Ecuador, French Guiana, Guatemala, Guyana, Mexico, Nicaragua, Panama [50] (Distr. Mex.: Chiapas [28,52,54], Veracruz [10,28,52,55], Oaxaca [28], Quintana Roo [2,7]).
- Tarsomere V of hind leg with white scales (Figure 9D)………………………………3
3(2) Silver scales on occiput extending to the ocular line and reaching the inner corner of the eye, mostly with silver reflections (Figure 10); postprocoxal area with a patch of silvery scales….………………….Sh. moralesi
Distr.: Belize, Guatemala, Mexico, Panama [50] (Distr. Mex.: Chiapas [27,28,51,52,53,55,56], Veracruz [10,12,27,28,52], Oaxaca [27,28], Tabasco [20]).
Silver scales on occiput are restricted to the occiput and do not extend to the eyes, the rest of scales on ocular line are dark, with purplish and greenish reflections (Figure 6A); postprocoxal area without a patch of silvery scales (Figure 7A). Sh. huasteca n. sp. Ortega
Distr.: Mexico (Distr. Mex.: Querétaro).
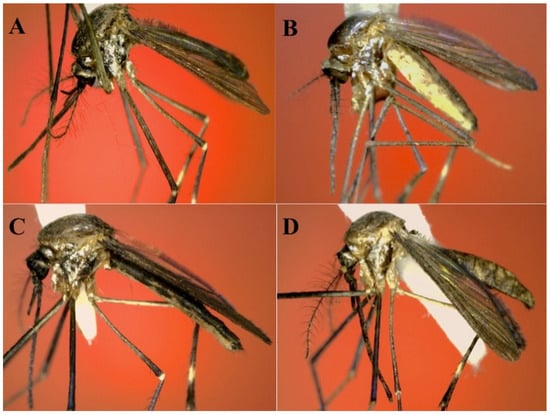
Figure 8.
General aspect of adult female of Shannoniana spp. (A) Sh. schedocyclia (Chiapas, Mexico); (B) Sh. fluviatilis (Quintana Roo, Mexico, MX-QROO-19); (C) Sh. moralesi (Chiapas, Mexico, 01010818-EU); (D) Sh. huasteca n. sp. (holotype).
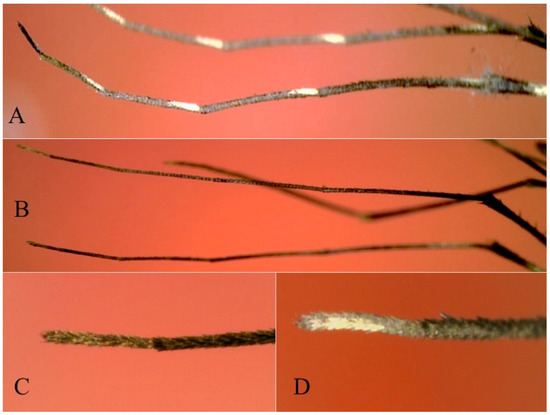
Figure 9.
Hind tarsi of adult female of Shannoniana spp. (A) Sh. schedocyclia (Chiapas, Mexico); (B,C) Sh. fluviatilis (Quintana Roo, Mexico, MX-QROO-19); (D) Sh. huasteca n. sp.
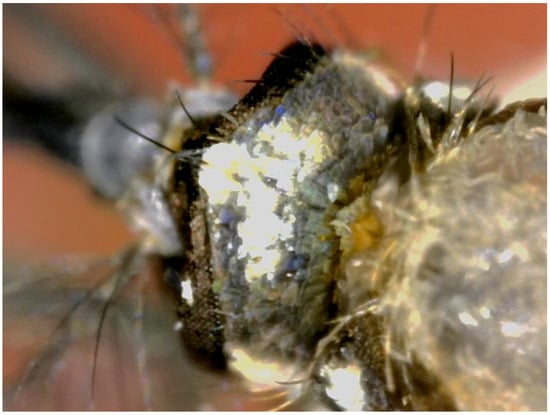
Figure 10.
Occuput of Sh. moralesi (Chiapas, Mexico, 01010818-EU) showing the decumbent scales mostly with silver reflections.
4. Discussion and Conclusions
4.1. Ecology and Distributional Groups of Species
Based on our collection records and the known distributions of the mosquito species collected in Querétaro state, three groups of species are recognized. The species of each group have similar geographical distributions, reaching their southern or northern distributional limits across the state. The immature stages of groups 1 and 2 develop in phytotelmata such as tree holes and bromeliad axils, while immature stages of group 3 develop in ponds and swamps (Table 3).
4.1.1. Group 1
Species that occur in the Nearctic Region and extend into northern Mexico where they reach their southern limit of distribution in Querétaro include Aedes brelandi, Ae. triseriatus and the endemic species Ae. schicki. Immature stages of those species develop in tree holes filled with rainwater; the presence of species in this group is restricted to the forested and conserved areas of the northern part of the state, extending from the Nearctic Region into the Huasteco Carso of the Sierra Madre Oriental in Querétaro.
4.1.2. Group 2
Species of this group occur in tropical forests in the Neotropical Region and extend into Querétaro where they reach their northern limit. Only one species is reported in this group, Wyeomyia apronoma, whose immature stages occur in bamboo internodes, tree holes, and coconut shells. This species has been previously reported in several states of southeastern Mexico, but the distribution is restricted to the north region by the Huasteco Carso of the Sierra Madre Oriental in Querétaro state.
4.1.3. Group 3
Ground pool inhabiting species that extend from the Neotropical Region into Middle Mexico in Querétaro state but no farther north are Aedes euplocamus, which has been previously reported in tropical regions of southeastern Mexico where it is a common species during the rainy season, and the Mexican endemic species Ae. shannoni, which has been previously reported in the states of Michoacán, Morelos, Querétaro, and Mexico State, but reaches its northernmost distributional limit in Querétaro.
4.2. Species from Adjacent Regions That May Occur in Querétaro
Some species of mosquitoes that have not yet been reported from Querétaro occur in adjacent states and may occur within the state. Included among these are 20 species that have been previously recorded in the state of Hidalgo [14]: Anopheles aztecus Hoffmann, An. crucians Wiedemann, An. parapunctipennis Martini, An. punctimacula Dyar and Knab, An. argyritarsis Robineau-Desvoidy, Aedeomyia sqamipennis Lynch-Arribálzaga, Aedes muelleri Dyar, Psorophora ferox (von Humboldt), Culex bidens Dyar, Cx. interrogator Dyar and Knab, Cx. nigripalpus Theobald, Cx. pinarocampa Dyar and Knab, Cx. pseudostigmatosoma Strickman, Cx. stenolepis Dyar and Knab, Cx. rejector Dyar and Knab, Cx. territans Walker, Cx. corniger Theobald, Sabethes gymnothorax Harbach and Petersen, Uranotaenia coatzacoalcos Dyar and Knab, and Ur. sapphirina (Osten Sacken); and 12 species that have been previously recorded in the state of México [25]: Ae. ramirezi Vargas and Downs, Ae. guerrero Berlin, Ae. lorraineae Berlin, Ae. chionotum Zavortink, Ae. gabriel Schick, Ae. idanus Schick, Ae. kompi Vargas and Downs, Ae. vargasi Schick, Ae. zoosophus Dyar and Knab, Haemagogus mesodentatus Komp and Kumm, Culiseta incidens (Thomson), and Ur. geometrica Theobald.
4.3. Medical Importance of Mosquitoes of Querétaro
Some of the species reported in Querétaro are of medical and veterinary importance because they are vectors of pathogens causing diseases. In Table 5 the most important public health species that occur in Querétaro are listed.

Table 5.
Medical importance and pathogens of mosquito vector species collected in Querétaro state, Mexico. Mal: Malaria. DI: Dirofilaria immitis. DENV: Dengue virus. ZIKV: Zika virus. CHIKV: Chikungunya virus. YF: Yellow fever virus. SLE: St. Louis encephalitis virus. WNV: West Nile virus. VEEV: Venezuelan equine encephalitis virus. EEEV: Eastern equine encephalitis virus. WEEV: Western equine encephalitis virus. LCV: La Crosse virus.
4.4. Molecular Analysis
The DNA barcode sequences of specimens belong to five Sabethini species of mosquitoes we analyzed in this study grouped together, although a discrepancy in BINs were found in Sh. fluviatilis and Sh. schedocyclia. This agrees with Talaga [57] in their analysis of the Culicidae DNA barcodes from French Guiana. As we have not been able to examine the voucher specimens from where the DNA barcodes sequences were obtained, we cannot make further comments with regards to the taxonomic status of these two BINs. The specimen we identified as Sh. huasteca n. sp. separate with strong support values from those identified as Sh. moralesi, which supports our hypothesis that they represent a new species. The latter finding is also supported by the different morphological traits found in the adult female general coloration and the male genitalia.
4.5. Mosquitoes Diversity in Querétaro and Mexico
With the addition of the new mosquito records found in Querétaro reported here, there are currently 50 species known in the state. The state ranks eighth in species richness of the ten Mexican states that have been systematically inventoried for mosquito species. With the addition of Shannoniana huasteca n. sp. to the list of mosquito species in Mexico, there are currently 247 known species in the country.
Author Contributions
A.I.O.-M. and L.M.H.-T.—study conceptualization; A.I.O.-M. and L.M.H.-T.—methodology; A.I.O.-M.—sampling; A.I.O.-M. and L.M.H.-T.—data curation; A.I.O.-M.—mosquito identification; L.M.H.-T. and Q.K.S.-R.—molecular assays; A.I.O.-M., L.M.H.-T., and Q.K.S.-R.—analyses; A.I.O.-M. and Q.K.S.-R.—figures conceptualization; A.I.O.-M.—writing—original draft preparation; L.M.H.-T. and Q.K.S.-R.—writing—review and editing; A.I.O.-M., L.M.H.-T. and Q.K.S.-R.—visualization and supervision; A.I.O.-M. and L.M.H.-T.—project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
Aldo I. Ortega-Morales was the recipient of a grant supported by the Consejo Nacional de Ciencia y Tecnología-Convocatoria Ciencia Básica (National Council for Science and Technology-Basic Science Call) CONACyT (Project: Catálogo Nomenclatural de los mosquitos (Diptera: Culicidae) presentes en el Eje Neovolcánico de algunos estados de México) [Nomenclatural Catalogue of the Mosquitoes (Diptera: Culicidae) from Neo-Volcanic Axis of some states of Mexico] (Grant 182216/QH1113).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All mosquitoes (mounted on pins and/or microscope slides) collected in Querétaro State were deposited in the Culicidae Collection (CC-UL), Parasitology Department, Universidad Autónoma Agraria Antonio Narro unidad laguna (Autonomous Agrarian University Antonio Narro laguna unit), Torreón, Coahuila, Mexico.
Acknowledgments
We are grateful to the mosquito collection staff Salvador Morales-Avitia, Adelfo Sánchez-Trinidad, Félix Ordóñez-Sánchez, Juan J. Castro-Xochitla, Oscar L. Galindo-Soto, Federico Ortega-Lozano, Isis J. Morales-Avitia, Guillermo Morillón-Borjón, and Antonio A. Sánchez-García for their valuable collaboration during our collection trips; Jorge A. Alvarado-Zapata and Imelda Correa-Tinajero from the Biological Station “Quin” for their hospitality in Arcila, Querétaro; Eva I. Conde-Sánchez from Secretaría de Salud del estado de Querétaro (Health Secretary of Querétaro State); Herón Huerta-Jiménez from Instituto de Diagnóstico y Referencia Epidemiológica (Institute of Diagnosis and Epidemiological Reference) for providing some mosquito records collected in Querétaro state, and Thomas J. Zavortink for the revision of the English language and suggestions, which have improved the quality of this paper. Aldo I. Ortega-Morales would like to thank the National Council of Science and Technology (CONACyT) for founding some collection trips, Project: “Nomenclatural Catalog of the mosquitoes (Diptera: Culicidae) present in the Neo-volcanic Axis of some States of Mexico” (Grant QH1113). Luis M. Hernández-Triana would like to thank the Department of Environment and Rural Affairs (DEFRA) and the Devolved Administrations of Scotland and Wales who co-founded Contract C, project SV3045.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Muñoz-Cabrera, L.O.; Ibáñez-Bernal, S.; Corona-Vargas, M.C. Los mosquitos (Diptera: Culicidae) de Tlaxcala, México. I: Lista comentada de especies. Folia Entomol. Mex. 2006, 45, 223–271. [Google Scholar]
- Ortega-Morales, A.I.; Mis-Ávila, P.C.; Elizondo-Quiroga, A.; Harbach, R.E.; Siller-Rodríguez, Q.K.; Fernández-Salas, I. The mosquitoes of Quintana Roo state, Mexico (Diptera: Culicidae). Acta Zool. Mex. 2010, 26, 33–46. [Google Scholar]
- Ordóñez-Sánchez, F.; Sánchez-Trinidad, A.; Mis-Ávila, P.; Canul-Amaro, G.; Fernández-Salas, I.; Ortega-Morales, A.I. Nuevos registros de mosquitos (Diptera: Culicidae) en algunas localidades de Campeche y Quintana Roo. Entomol. Mex. 2013, 12, 850–854. [Google Scholar]
- Chan-Chablé, R.J.; Ortega-Morales, A.I.; Martínez-Arce, A. First record of Psorophora albipes in Quintana Roo, Mexico. J. Am. Mosq. Control Assoc. 2016, 32, 237–239. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Casas-Martínez, M.; Bond, G.; Harbach, R.E. First records of Psorophora cilipes and Culex theobaldi in Quintana Roo, Mexico. J. Am. Mosq. Control Assoc. 2018, 34, 124–127. [Google Scholar] [CrossRef]
- Chan-Chablé, R.J.; Martínez-Arce, A.; Mis-Ávila, P.C.; Ortega-Morales, A.I. DNA barcodes and evidence of cryptic diversity of anthropophagous mosquitoes in Quintana Roo, Mexico. Ecol. Evol. 2019, 9, 4692–4705. [Google Scholar] [CrossRef]
- Chan-Chablé, R.J.; Martínez-Arce, A.; Ortega-Morales, A.I.; Mis-Ávila, P.C. New records and updated checklist of mosquito species in Quintana Roo, Mexico, using DNA-Barcoding. J. Am. Mosq. Control Assoc. 2020, 36, 264–268. [Google Scholar] [CrossRef]
- Canto-Mis, K.L.; Chan-Chablé, R.J.; Gómez-Rivera, A.S.; López-Sosa, X.Y.; González-Acosta, C.; Correa-Morales, F.; Mis-Ávila, P.C. Nuevos registros de distribución para Uranotaenia sapphirina (Osten Sacken, 1868) (Diptera: Culicidae) en Quintana Roo, México. Rev. Chil. Entomol. 2021, 47, 613–617. [Google Scholar]
- Canto-Mis, K.L.; Gómez-Rivera, A.S.; Chan-Chablé, R.J.; González-Acosta, C.; Correa-Morales, F.; Mis-Ávila, P.C. Primer registro de distribución para Psorophora varipes (Coquillett, 1904) (Diptera: Culicidae) en Quintana Roo, México. Rev. Chil. Entomol. 2022, 48, 293–297. [Google Scholar]
- Ibáñez-Bernal, S.; Mendoza-Palmero, F.S.; Hernández-Xoliot, R.A. Mosquitos (Insecta: Diptera: Culicidae). In La Biodiversidad de Veracruz Estudio de Estado, Vol. II; Cruz-Angón, A., Lorca-Hernández, F.G., Hernández-Ortiz, V., Morales-Mavil., J.E., Eds.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad CONABIO: Mexico City, México, 2011; pp. 399–403, Appendix 5–8. [Google Scholar]
- Mendez-Andrade, A.; Rivera-García, K.D.; Ibáñez-Bernal, S. Notes on the Toxorhynchites of Mexico: Redescription of Tx. moctezuma (Dyar & Knab) and new record for Tx. grandiosus (Williston) in Veracruz (Diptera: Culicidae). Zootaxa 2019, 4576, 140–150. [Google Scholar]
- Rivera-García, K.D.; Méndez-Andrade, A.; Ibáñez-Bernal, S. Trichoprosopon mixtli sp. nov., a new sabethini species (Diptera: Culicidae) from a Mexican cloud forest, with an assessment of the genus and keys for the identification pk known species. Zootaxa 2023, 5254, 94–116. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Morales, A.I.; Zavortink, T.J.; Huerta-Jiménez, H.; Sánchez-Rámos, F.J.; Valdés-Perezgasga, M.T.; Reyes-Villanueva, F.; Siller-Rodríguez, Q.; Fernández-Salas, I. Mosquito records from Mexico: The mosquitoes (Diptera: Culicidae) of Tamaulipas state. J. Med. Entomol. 2015, 52, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Morales, A.I.; Zavortink, T.J.; Huerta-Jiménez, H.; Ibáñez-Bernal, S.; Siller-Rodríguez, Q.K. The mosquitoes (Diptera: Culicidae) of Hidalgo state, Mexico. Acta Trop. 2019, 189, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Morales, A.I.; Pérez-Paredes, M.G.; Siller-Rodríguez, Q.K.; Moreno-García, M.; González-Acosta, C.; Correa-Morales, F. First record of Aedes gabriel in Hidalgo state, Mexico. J. Am. Mosq. Control Assoc. 2019, 35, 51–54. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Zavortink, T.J.; Garza-Hernández, J.A.; Siller-Rodríguez, Q.K.; Fernández-Salas, I. The mosquitoes (Diptera: Culicidae) of Nuevo León, Mexico, with descriptions of two new species. PLoS ONE 2019, 14, e0217694. [Google Scholar] [CrossRef]
- Villegas-Ramírez, H.M.; Ortega-Morales, A.I.; Flores-Suárez, A.; Fernández-Salas, I.; Ponce-García, G. First record of Aedes podographicus in Nuevo León state, Mexico. J. Am. Mosq. Control Assoc. 2021, 37, 87–89. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Morillón-Borjón, G.; Morales-Avitia, I.J.; Sánchez-García, A.A.; Ayala-Sulca, Y.O.; Sánchez-Rámos, F.J. First record of Wyeomyia mitchellii in Nuevo León, Mexico. J. Am. Mosq. Control Assoc. 2022, 38, 216–218. [Google Scholar] [CrossRef]
- Torres-Chable, O.M.; Baak-Baak, C.M.; Cigarroa-Toledo, N.; Zaragoza-Vera, C.V.; Arjona-Jiménez, G.; Moreno-Perez, L.G.; Machain-Williams, C.; García-Rejon, J. Mosquito fauna un home environments of Tabasco, Mexico. Southwest. Entomol. 2017, 42, 969–982. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Méndez-López, R.; Garza-Hernández, J.A.; González-Álvarez, V.H.; Ruiz-Arrondo, I.; Huerta-Jiménez, H.; Rodríguez-Martínez, L.M.; Rodríguez-Pérez, M.A. The mosquitoes (Diptera: Culicidae) of Tabasco, Mexico. J. Vector Ecol. 2019, 44, 57–67. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, L.M.; Yzquierdo-Gómez, P.; González-Acosta, C.; Correa-Morales, F. First record of Aedes (Ochlerotatus) fulvus in Tabasco and distribution notes of other Aedes in Mexico. Southwest. Entomol. 2020, 45, 263–268. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Rodríguez-Martínez, L.M.; Méndez-Alvarado, W.; Garza-Hernández, J.A.; López-Hernández, I.; Medrano-Santillana, M.; González-Acosta, C.; Correa-Morales, F. The distribution of Uranotaenia sapphirina and Ur. socialis in Tabasco, southern Mexico. J. Am. Mosq. Control Assoc. 2022, 38, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Dampf, A. Distribución y ciclo annual de Uranotaenia syntheta Dyar & Shannon en México y descripción del hipopigio masculine (Insecta, Diptera). Rev. Soc. Mex. Hist. Nat. 1943, 4, 147–170. [Google Scholar]
- Dávalos-Becerril, E.; Correa-Morales, F.; González-Acosta, C.; Santos-Luna, R.; Peralta-Rodríguez, J.; Pérez-Rentería, C.; Ordóñez-Álvarez, R.; Huerta, H.; Carmona-Perez, M.; Díaz-Quiñonez, J.A.; et al. Urban and semi-urban mosquitoes of Mexico City: A risk for endemic mosquito borne-disease transmission. PLoS ONE 2019, 14, e0212987. [Google Scholar] [CrossRef] [PubMed]
- Adeniran, A.A.; Hernández-Triana, L.M.; Ortega-Morales, A.I.; Garza-Hernández, J.A.; de la Cruz-Ramos, J.; Chan-Chablé, R.J.; Vázquez-Marroquín, R.; Huerta-Jiménez, H.; Nikolova, N.I.; Fooks, A.R.; et al. Identification of mosquitoes (Diptera: Culicidae) from Mexico State, Mexico using morphology and COI DNA barcoding. Acta Trop. 2021, 213, 105730. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.; Martínez-Palacios, A. Anofelinos mexicanos, taxonomía y distribución. In Anofelinos Mexicanos Taxonomia y Distribución; Secretaría de Salud: Ciudad de México, Mexico, 1956; p. 181. [Google Scholar]
- Vargas, L. Especies y distribución de mosquitos mexicanos no Anofelinos (Insecta, Diptera). Rev. Inst. Salubr. Enf. Trop. 1956, 1, 19–36. [Google Scholar]
- Díaz-Nájera, A.; Vargas, L. Mosquitos mexicanos: Distribución geográfica actualizada. Rev. Inv. Salud Pública. 1973, 33, 111–125. [Google Scholar]
- Ibáñez-Bernal, S.; Martínez-Campos, C. Clave para la aidentificación de larvas de mosquitos comunes en las áreas urbanas y suburbanas de la República Mexicana (Diptera: Culicidae). Folia Entomol. Mex. 1994, 92, 43–73. [Google Scholar]
- Sánchez-Trinidad, A.; Ordóñez-Sánchez, F.; Valdez-Perezgasga, M.T.; Sánchez-Ramos, F.J.; Zavortink, T.J.; Cortés-Guzman, A.J.; Ortega-Morales, A.I. Geographical distribution of the Aedes Triseriatus group (Diptera: Culicidae) in Mexico. J. Vector Ecol. 2014, 39, 134–137. [Google Scholar] [CrossRef]
- Quezada-Yaguachi, W.E.; Alquisira-Domínguez, M.; Vázquez-Anzúres, M.J.; Rebollo-Salinas, D.; Rescalvo-Luna, L.D.; Medina-Castañeda, A.; González-Acosta, C.; Correa-Morales, F.; Viveros-Santos, V.; Moreno-García, M. Nuevo registro de Haemagogus (Haemagogus) equinus Theobald, 1903 y otros mosquitos (Diptera: Culicidae) recolectados con ovitrampas en Jalpan de Serra, Querétaro, México. Rev. Chil. Entomol. 2023, 49, 73–81. [Google Scholar]
- Ortega-Morales, A.I.; Moreno-García, M.; González-Acosta, C.; Correa-Morales, F. Mosquito surveillance in Mexico: The use of ovitraps for Aedes aegypti, Ae. albopictus, and non-target species. Fla. Entomol. 2018, 101, 623–626. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Pérez-rentería, C.; Ordóñez-Álvarez, J.; Salazar, J.A.; Dzul-Manzanilla, F.; Correa-Morales, F.; Huerta-Jiménez, H. Update on the dispersal of Aedes albopictus in Mexico: 1988–2021. Front. Trop. Dis. 2022, 2, 72. [Google Scholar] [CrossRef]
- INEGI. Instituto Nacional de Estadística y Geografía. 2018. Available online: https://www.inegi.org.mx/app/cuadroentidad/Qro/2018/01/1_4 (accessed on 15 February 2023).
- Harbach, R.E.; Knight, K.L. Taxonomist’s Glossary of Mosquito Anatomy; Plexus Publishing, Inc.: Marlton, NJ, USA, 1980; ISBN 9780937548004. [Google Scholar]
- Knight, K.L.; Stone, A. A Catalog of the Mosquitoes of the World (Diptera: Culicidae); Thomas Say Foundation: College Park, MD, USA, 1977. [Google Scholar]
- Wilkerson, R.C.; Linton, Y.M.; Fonseca, D.M.; Schultz, T.R.; Price, D.C.; Strickman, D.A. Making mosquito taxonomy useful: A stable classification of Tribe Aedini that balances utility with current knowledge of evolutionary relationships. PLoS ONE 2015, 10, e0133602. [Google Scholar] [CrossRef]
- WRBU. Walter Reed Biosystematics Unit, Smithsonian Institution. 2005. Available online: http://www.wrbu.org (accessed on 15 November 2022).
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, T.; Vrijenhoek, R. DNA Primers for amplification of mitochondrial Cytochrome c Oxidase Subunit I from diverse Metazoan Invertebrtes. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Prosser, W.J.S.; deWaard, J.R.; Miller, S.C.; Hebert, P.D.N. DNA barcodes from century-old type specimens using next generation sequencing. Mol. Ecol. Res. 2016, 16, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stoecher, G.; Peterson, N.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.N.D.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identification through DNA barcodes. Proc. R. Soc. Biol. Sci. 2003, 270, 213–321. [Google Scholar] [CrossRef]
- Hebert, P.N.D.; Ratnasingham, S.; deWaard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Biol. Sci. 2003, 270, 596–599. [Google Scholar] [CrossRef]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor analysis program for Windows 95/98/NT. Nucelic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Hernández-Triana, L.M.; Brugman, V.A.; Nikolova, N.I.; Ruiz-Arrondo, I.; Barrero, E.; Thorne, T.; de Marco, M.F.; Kruger, A.; Lumley, S.; Johnson, N.; et al. DNA barcoding of British mosquitoes (Diptera, Culicidae) to support species identification, discovery of cryptic genetic diversity and monitoring invasive species. Zookeys 2019, 832, 57–76. [Google Scholar] [CrossRef]
- Laurito, M.; Briscoe, A.G.; Almirón, W.R.; Harbach, R.E. Systematics of the Culex coronator complex (Diptera: Culicidae): Morphological and molecular assessment. Zool. J. Linn. Soc. 2018, 182, 735–757. [Google Scholar] [CrossRef]
- Clark-Gil, S.; Darsie, R.F. The mosquitoes of Guatemala, their identification, distribution and bionomics, with keys to adult female and larvae. Mosq. Syst. 1983, 15, 151–294. [Google Scholar]
- Lane, J.; Cerquiera, N.L. Os Sabetíneos de América (Diptera, Culicidae). Arq. Zool. Sáo Paulo 1942, 9, 473–849. [Google Scholar]
- Wilkerson, R.C.; Linton, T.-M.; Strickman, D. Mosquitoes of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2021; Volume 2. [Google Scholar]
- Vargas, L.; Martínez-Palacios, A. Descripción de Wyeomyia (Wyeomyia) stonei, n. sp., y notas sobre otros Sabethini de Mexico. Rev. Inst. Salubr. Enf. Trop. 1953, 4, 293–307. [Google Scholar]
- Díaz-Nájera, A. Mosquitos tropicales de Mexico. Rev. Invest. Salud Publ. 1966, 1, 57–63. [Google Scholar]
- Viveros-Santos, V.; Hernández-Triana, L.M.; Ibáñez-Bernal, S.; Ortega-Morales, A.I.; Nikolova, N.I.; Pairot, P.; Fooks, A.R.; Casas-Martínez, M. Integrated approaches for the identification of mosquitoes (Diptera: Culicidae) from the volcanoes of Central America Physiographic subprovence of the state of Chiapas, Mexico. Vector Borne Zoonotic Dis. 2022, 22, 120–137. [Google Scholar] [CrossRef]
- Vargas, L. Notas sobre mosquitos nuevos para Mexico. Rev. Inst. Salubr. Enf. Trop. 1939, 1, 101–104. [Google Scholar]
- Díaz-Nájera, A. Variaciones morfológicas en larvas de dos especies de Trichoprosopon y datos sobre su distribución. Rev. Inst. Salubr. Enf. Trop. 1961, 3–4, 201–219. [Google Scholar]
- Hernández-Triana, L.M.; Garza-Hernández, J.A.; Ortega Morales, A.I.; Prosser, S.W.; Hebert, P.D.; Nikolova, N.I.; Barrero, E.; de Luna-Santillana, E.D.J.; González-Alvarez, V.H.; Mendez-López, R.; et al. An integrated molecular approach to untangling host-vector-pathogen interactions in mosquitoes (Diptera: Culicidae) from sylvan communities in Mexico. Front. Vet. Sci. 2021, 7, 564791. [Google Scholar] [CrossRef]
- Talaga, S.; Leroy, C.; Guidez, A.; Dusfour, I.; Girod, R.; Dejean, A.; Murienne, J. DNA reference libraries of French Guianese mosquitoes for barcoding and metabarcoding. PLoS ONE 2017, 12, e0176993. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).