Abstract
The Fern Cave System, developed in the western escarpment of the Southern Cumberland Plateau of the Interior Low Plateau karst region in Northeastern Alabama, USA, is a global hotspot of cave-limited biodiversity as well as home to the largest winter hibernaculum for the federally endangered Gray Bat (Myotis grisescens). We combined the existing literature, museum accessions, and database occurrences with new observations from bioinventory efforts conducted in 2018–2022 to generate an updated list of troglobiotic and stygobiotic species for the Fern Cave System. Our list of cave-limited fauna totals twenty-seven species, including nineteen troglobionts and eight stygobionts. Two pseudoscorpions are endemic to the Fern Cave System: Tyrannochthonius torodei and Alabamocreagris mortis. The exceptional diversity at Fern Cave is likely associated with several factors, such as the high dispersal potential of cave fauna associated with expansive karst exposures along the Southern Cumberland Plateau, high surface productivity, organic input from a large bat colony, favorable climate throughout the Pleistocene, and location within a larger regional hotspot of subterranean biodiversity. Nine species are of conservation concern, including the recently discovered Alabama cave shrimp Palaemonias alabamae, because of their small range sizes, few occurrences, and several potential threats.
1. Introduction
The Fern Cave System in Jackson County, Northeastern Alabama, USA, is the most extensive cave system in the state of Alabama, with over 25 km (15.6 miles) of mapped passage [1], including 163 m (536 ft) of vertical extent and five entrances (Figure 1). The cave system is managed by the U.S. Fish and Wildlife Service (USFWS) and Southeastern Cave Conservancy, Inc. (SCCi). The largest colony of Gray Bats (Myotis grisescens) hibernates in sections of Fern Cave [2]. The 80.5 hectare Fern Cave National Wildlife Refuge was established in 1981 to protect this federally endangered species. Five entrances are known, with four located on Fern Cave National Wildlife Refuge and another entrance owned and managed by SCCi on the 32.4 hectare Kay Hill Deen Fern Cave Preserve. Fern Cave National Wildlife Refuge is managed as part of the Wheeler National Wildlife Refuge Complex.
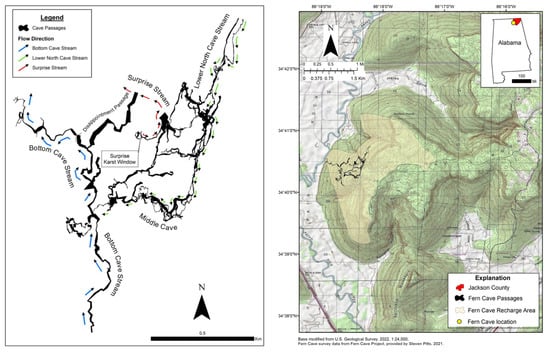
Figure 1.
Line map of the Fern Cave System (left) and location of the cave system on Nat Mountain, Jackson County, Alabama, USA (right).
Fern Cave is developed along the western margin of Nat Mountain in the Paint Rock River Valley of Jackson County, Alabama. Nat Mountain is a highly dissected lobe of the Southern Cumberland Plateau that is bounded to the north and west by the Paint Rock River and to the south by Yellow Branch in Peter Cove [3]. The geology of Nat Mountain was mapped and described recently by Osbourne et al. [4] and is briefly described herein. The vertical relief of Nat Mountain is 305 m from 180 to 488 m in elevation. Nat Mountain is capped by the Lower Pennsylvanian-aged Pottsville Formation (61 m thick quartzose sandstone with occasional conglomeratic interbeds of shale). Beneath the Pottsville Formation is the Upper Mississippian-aged Pennington Formation (91 m thick), which consists of interbeds of sandstone, limestones, chert, dolomites, and shales. The presence of carbonate interbeds within the Pennington Formation creates karstified intervals, which result in springs discharging from the base of the formation at the contact with the underlying Upper Mississippian-aged Bangor Limestone (91 m thick), which contains interbeds of chert in the upper part and shale in the lower part. The Upper Mississippian-aged Hartselle Sandstone underlies the Bangor Limestone but is absent or so thin in the study area that it is typically unmapped. However, there does appear to be some hydrologic control from the Hartselle Sandstone at locations along the flanks of Nat Mountain where its presence and limited vertical permeability may create small springs that issue from the upper contact of the formation and sink underground a short distance from the point of issuance [3]. The Upper Mississippian-aged Monteagle Limestone (55–67 mm thick) underlies the Hartselle Sandstone and is extensively karstified throughout the study area. Below the Monteagle Limestone is the Middle Mississippian-aged Tuscumbia Limestone (6–12 m thick) that forms the base of Nat Mountain and the valley floor of Hales Cove.
The Fern Cave System is developed in four major limestone layers—Pennington Formation, Bangor Limestone, Monteagle Limestone, and Tuscumbia Limestone—and is known for two primary entrances: a 133 m (437 ft) deep, voluminous pit (Surprise Pit) popular with recreational cavers [1] and the largest known federally endangered Gray Bat (Myotis grisescens) hibernaculum in the world, with over 1.3 million bats [2]; [T. Inebnit, unpub. data]. The cave is characterized by a diversity of passage morphologies, providing a range of subterranean habitats. The Fern Cave System consists of a complex network of vadose and phreatic passages with more than 12 horizontal levels of passages representing distinct stages of development associated with past periods of water table stability and intervening periods of downcutting of the Paint Rock River through the resistant caprock into the soluble limestone layers below. The hydrology of the cave system was recently described by Miller and Tobin [3]. While much of the cave is dry, at least three distinct subterranean streams occur within the cave systems: Lower North Cave Stream, Surprise Stream, and the Bottom Cave Stream. These cave streams originate as surface streams at different locations atop Nat Mountain that sink into Fern Cave and ultimately issue into springs along the eastern bank of the Paint Rock River. Many of the upper-level passages in Fern Cave have floors with steep gradients; however, the lowest level—Bottom Cave—is characterized by a relatively flat floor with associated stream and flood debris indicative of back-flooding from the Paint Rock River. A recent dye tracing study by Miller and Tobin [3] delineated a recharge area of 6.7 km2 (2.6 mi2). The Fern Cave System is fed largely by recharge through a combination of surface-water runoff from atop Nat Mountain and discharge of groundwater in the overlying Pennington Formation sinking into the Monteagle Limestone. The recharge area for the Fern Cave System lies primarily along the western escarpment of Nat Mountain and drains to multiple springs along the Paint Rock River. The recharge area is bounded to the southeast by the Kennamer Cave System. There is evidence of some hydrological connection between the Fern and Kennamer cave systems. On the north side, the Fern Cave recharge area is bounded by recharge areas for Roadside Spring and Big Spring that discharge into Hales Cove. Current land use within the recharge area of the cave system is predominantly mixed deciduous forest with <1% shrub/scrub and pasture [3].
Unlike other hotspot caves in North America with a long history of biological studies (e.g., Mammoth Cave in Kentucky, reviewed in [5]; San Marcos artesian well in Texas, reviewed in [6]; and Shelta Cave in Alabama, ref. [7]), much of our knowledge of the biodiversity of the Fern Cave System is derived from a recent two year biological inventory presented herein. However, early knowledge of the Fern Cave fauna is based on visits and studies by biologists from the 1950s to the 1990s. Peck [8,9] summarized the terrestrial cave life of Alabama caves and reported on eight species from the Fern Cave System, including six troglobionts. Additional significant publications on the fauna of the Fern Cave System include Muchmore [10,11], Carpenter [12], Fleming [13], Holt [14], Peck [15,16], Hart and Hart [17], Hobbs et al. [18], Kenk [19], Hubbell and Norton [20], Ferguson [21], Gertsch [22], Lewis [23], McGregor et al. [24], Martin [2], Niemiller et al. [25], and Hedin and Milne [26].
Herein we present the first comprehensive list of terrestrial and aquatic cave obligate fauna (i.e., troglobionts and stygobionts, respectively) of the Fern Cave System based on the results of a recent two-year bioinventory of the cave system in 2018–2020 and a thorough search of the scientific literature and museum records. In addition to the species list, we include a comprehensive bibliography on the cave obligate fauna of Fern Cave, discuss factors associated with its exceptional biodiversity, and comment on the conservation status of the exceptional biodiversity of this North American and global hotspot of subterranean biodiversity.
2. Materials and Methods
2.1. Ecological Classification of Troglobionts and Stygobionts
We follow past authors in recognizing troglobionts (i.e., troglobites) as species that are permanent inhabitants of subterranean habitats [27,28,29,30] and cannot complete their life cycle outside of such habitats [30]. From a metapopulation perspective, troglobionts have source populations in subterranean habitats but may have sink populations in surface habitats [28]. While morphology alone cannot be used to definitively classify species ecologically [29], we used the presence of traits often observed in troglobiotic fauna, i.e., troglomorphisms such as reduced eyes, pigmentation, and hypertrophy of nonvisual sensory structures, but not found in presumed surface relatives, as evidence for isolation in subterranean habitats. We use the terms troglobiont and stygobiont in reference to species that occur in terrestrial and aquatic habitats, respectively.
2.2. Cave Biosurveys
We conducted faunal bioinventories in several areas throughout the Fern Cave System as well as three additional caves that are hydrologically connected to Fern Cave located on Fern Cave National Wildlife Refuge between June 2018 and December 2020. Bioinventories primarily consisted of time-constrained visual encounter surveys for cave life in terrestrial, riparian, and aquatic habitats, including entrance areas and the twilight zone starting at the drip line, walls and ceilings, ledges, mud banks, rimstone pools, streams, and talus slopes. The search effort included examining and overturning rocks, detritus, organic debris, and other cover, as well as searching through stream cobble. Surveys were conducted by two to seven researchers per cave visit. In the West Passage, we supplemented visual encounter surveys with baited traps in December 2020.
We field-identified common vertebrate and invertebrate species. In other cases, we collected invertebrate specimens and identified them in the laboratory using available taxonomic keys and the literature. We outsourced identification to experts for taxa for which we had insufficient taxonomic knowledge when possible. For many vertebrates, we field-identified taxa by direct observation without capture or through taxonomically reliable indirect observations, such as visual identification of mammal scat or footprints left in mud. Where possible, we took voucher photographs of invertebrate and vertebrate taxa. For some salamanders and decapods, we collected tissue samples and voucher specimens.
2.3. Literature and Museum Searches
We conducted a search of the scientific literature to compile an updated list of troglobiont and stygobiont species for the Fern Cave System. Scientific literature sources included journal articles, book chapters, books, conference proceedings, theses and dissertations, and government reports. Searches of literature sources included keyword queries on ISI Web of Science, Google Scholar, and Zoological Record. In addition, we also searched biodiversity databases, including the Global Biodiversity Information Facility (GBIF; https://gbif.org; accessed on 24 June 2022), VertNet (http://www.vertnet.org; accessed on 24 June 2022), Symbiota Collections of Arthropods Network (SCAN; https://scan-bugs.org/portal/; accessed on 24 June 2022), and InvertEBase (http://www.invertebase.org/portal/; accessed on 24 June 2022). The list of cave-obligate fauna includes the scientific name, authority, and conservation status of each species. Taxonomic nomenclature followed primarily the Integrated Taxonomic Information System (ITIS; http://itis.gov; accessed on 15 September 2022). For conservation status, we include the International Union for Conservation of Nature (IUCN) Red List of Threatened Species (http://www.iucnredlist.org; accessed 28 March 2023) and NatureServe (http://www.natureserve.org; accessed 28 March 2023) conservation statuses when available. The status of a species according to the United States list of threatened and endangered species under the U.S. Endangered Species Act is included (http://www.fws.gov/endangered; accessed on 28 March 2023), as is its conservation status in the state of Alabama [31].
3. Results
Our list of cave-limited fauna documented within the Fern Cave System includes twenty-seven species, including nineteen troglobionts and eight stygobionts (Table 1; Figure 2 and Figure 3). Fern Cave is the type locality for two cave-limited species, and three species are endemic to the Fern Cave System (Table 1). The cave-limited fauna represents four phyla, ten classes, eighteen orders, and twenty-six families.

Table 1.
Troglobionts and stygobionts of the Fern Cave System, Jackson County, Alabama, USA. NatureServe conservation ranks include Secure (G5), Apparently Secure (G4), Vulnerable (G3), Imperiled (G2), Critically Imperiled (G1), Possibly Extinct (GH), Presumed Extinct (GX), Unranked (GNR), and Unrankable (GU). IUCN Red List categories include Least Concern (LE), Near Threatened (NT), Vulnerable (VU), Endangered (EN), Critically Endangered (CR), Extinct in the Wild (EW), and Extinct (EX). Federal conservation status under the U.S. Endangered Species Act includes Listed Endangered (LE) and Listed Threatened (LT). Alabama Department of Conservation and Natural Resources statuses include Highest Conservation Concern (Priority 1), High Conservation Concern (Priority 2), Moderate Conservation Concern (Priority 3), Low Conservation Concern (Priority 4), and Lowest Conservation Concern (Priority 5). Abbreviations: na—conservation status is not available for the species; X—species has been reported historically or during biosurveys conducted during the current study.
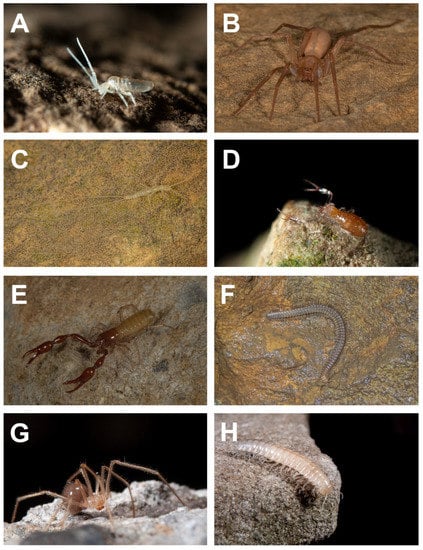
Figure 2.
Representative terrestrial cave-limited fauna from the Fern Cave System, Alabama, USA: (A) Pseudosinella hirsuta (photo by Michael E. Slay); (B) Liocranoides unicolor (photo by Matthew L. Niemiller); (C) Litocampa valentinei (photo by Matthew L. Niemiller); (D) Tyrannochthonius torodei (photo by Michael E. Slay); (E) Alabamocreagris mortis (photo by Matthew L. Niemiller); (F) Tetracion jonesi (photo by Matthew L. Niemiller); (G) Nesticus barri (photo by Michael E. Slay); and (H) Gyalostethus sp. nov. (photo by Michael E. Slay).
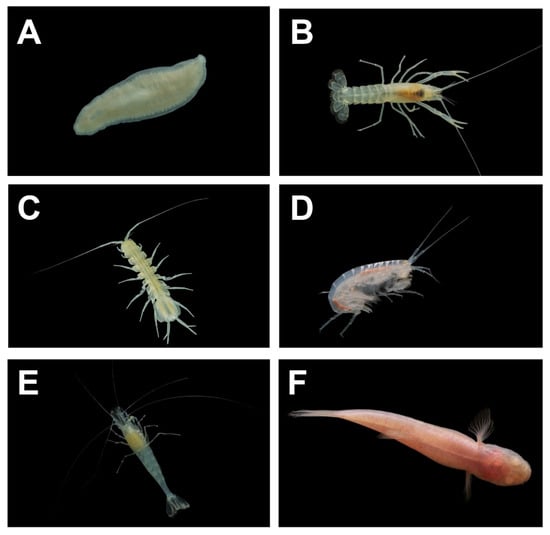
Figure 3.
Representative aquatic cave-limited fauna from the Fern Cave System, Alabama, USA: (A) Sphalloplana percoeca (photo by Matthew L. Niemiller); (B) Orconectes australis (photo by Matthew L. Niemiller); (C) Caecidotea bicrenata (photo by Matthew L. Niemiller); (D) Stygobromus sp. (photo by Michael E. Slay); (E) Palaemonias alabamae (photo by Matthew L. Niemiller); and (F) Typhlichthys subterraneus (photo by Matthew L. Niemiller).
3.1. Terrestrial Fauna
Troglobiotic spiders documented in the Fern Cave System include one linyphiid, one nesticid, and one zoropsid. Phanetta subterranea has one of the largest distributions of any troglobiont in North America [32,33]. Nesticus barri is one of the most common troglobionts in dozens if not hundreds of caves along the escarpments of the Southern Cumberland Plateau in South-Central Tennessee and Northeastern Alabama, reaching its southwestern range limit in the Fern Cave area [22,26,34,35]. Liocranoides unicolor has a broad distribution throughout much of the Interior Low Plateau, including several caves in Northeastern Alabama [8]. This species is pale in coloration but does not possess other troglomorphic characters [36,37].
Three troglobiotic pseudoscorpions occur in the Fern Cave System. Hesperochernes mirabilis is a widely distributed species most abundant near entrances, where it is associated with bat guano, rodent nests, and other mammal scat [38,39,40]. The other two species are typically associated with deep cave habitats. Tyrannochthonius torodei was described from Fern Cave and named after William Torode, who first collected specimens in 1968 [11]. Alabamocreagris mortis is the largest of three troglobiotic pseudoscorpions in the Fern Cave System. It was described from specimens collected near the Morgue Entrance by Muchmore [10] and is the most widely distributed pseudoscorpion within the Fern Cave System.
At least one unidentified troglobiotic rhagidiid mite is known from the Fern Cave System, which was observed from several sections of the cave. This mite may be a species in the genus Rhagidia, which has been reported from caves in Northwestern Georgia [38,40].
Four troglobiotic millipedes have been documented in the Fern Cave System, including one callipodidan, two chordeumatids, and one polydesmid. Tetracion jonesi is the most widely distributed and frequently observed millipede in the Fern Cave System. This large cave-limited millipede has been reported from more than 85 caves along the Cumberland Plateau in Northeastern Alabama [8]. Pseudotremia is a diverse genus with several described troglobionts, including three from Alabama [41]. Troglobiotic individuals have been observed in multiple locations within the Fern Cave System and may represent one of the three described species in Alabama or an undescribed taxon. Millipedes of the genus Scoterpes have been observed throughout the Fern Cave System, often found in moist habitats with organic matter (rotting wood, debris, and cricket guano). The distributions of these two species—S. syntheticus and S. stewartpecki—overlap in the area, and two species are known to co-occur at nearby Crossings Cave [42]. Preliminary genetic analyses indicate that both forms likely occur in the Fern Cave System, but additional morphological analyses are required for confirmation. An undescribed troglobiotic species of Gyalostethus was discovered in cracks and under rocks on mud banks near the sump in the Bottom Cave section. This new species appears to be a relative of Gyalostethus monitcolens, the lone species currently described in this genus, which ranges from Southwestern Virginia to Northern Georgia and Northeastern Alabama, including Jackson and Morgan counties [43].
At least three species of cave-limited collembolans (i.e., springtails) have been documented from the Fern Cave System. Both Pseudosinella hirsuta and P. spinosa are broadly distributed in the Interior Low Plateau and were observed in several locations throughout the cave system. Pseudosinella hirsuta was reported previously from Fern Cave [9]. Troglomorphic individuals of the genus Pygmarrhopalites were collected from several locations in the Fern Cave System. These individuals have extremely reduced pigment and a troglomorphic foot complex. No troglobiotic Pygmarrhopalites have been reported from Alabama to date, but several species have been described from the Interior Low Plateau and Appalachians Karst regions in the Eastern United States [44,45,46,47]. The troglophile Pygmarrhopalites pygmaeus, a cosmopolitan species, was also observed and has been reported from several caves in Jackson, Madison, and Marshall counties [9].
A single troglobiotic dipluran occurs in the Fern Cave System. Litocampa valentinei is known from several caves in Northeastern Alabama and South-Central Tennessee along the escarpments of the Cumberland Plateau [21]. This cave dipluran has been reported previously from the Fern Cave System [9,21].
The troglobiotic beetle fauna of the Fern Cave System includes one carabid, one leiodid, and one staphylinid. Five carabids of the troglobiotic genus Pseudanophthalmus were collected from the Fern Cave System. These specimens may be P. profundus, which is known from nearby Crossings, Nat, and Paint Rock caves, and/or an undescribed species, which is known from nearby Pig Pen Cave [9]. The round fungus beetle Ptomaphagus hatchi was observed throughout the Fern Cave System, often in great abundance. This species was previously reported by Peck [9,15,16]. An unidentified cave ant beetle (subfamily Pselaphinae) was collected from the West Passage and likely belongs to either the genus Batrisodes or Speleochus. Several species of both genera are known from caves in Alabama [9,48,49,50].
The only other troglobiotic insect documented from the Fern Cave System is the dipteran Spelobia tenebrarum, which has been reported from many caves in the Eastern United States [9,40,51,52,53], where it is associated with scat. This species has reduced eyes and is the only known troglobiotic fly in the United States [51,52].
3.2. Aquatic Fauna
One cave flatworm—Sphalloplana percoeca—has been reported previously from the Fern Cave System [12,19]. Sphalloplana percoeca occurs primarily in epikarst-fed drip pools in upper-level passages, often in great numbers, but has also been observed in lower-level passages. A medium-sized branchiobdellid (2.5 mm), Cambarincola sheltensis, is an ectosymbiont of the stygobiotic crayfish Orconectes australis. This species has been confirmed from the type locality of Shelta Cave in Madison County, but identifications also include specimens taken from O. australis from Fern Cave collected by John E. and Martha R. Cooper [14].
The cave-limited crustacean fauna includes one shrimp, one crayfish, one isopod, one amphipod, and one ostracod. The federally endangered stygobiotic shrimp Palaemonias alabamae was discovered in August 2018 when four individuals were observed in an isolated pool near the Davison Entrance in the Bottom Cave section. Two cave shrimp were observed in July 2019: one in the same isolated pool and a second shrimp in a pool in the main stream. A single cave shrimp was again observed in the isolated pool in September 2020. Cave shrimp have yet to be observed upstream in Bottom Cave. Morphological and genetic analyses confirmed that this population is P. alabamae [25]. Preliminary environmental DNA analyses detected P. alabamae eDNA from water samples collected from Haley Spring Cave in addition to the water samples collected from pools near the Davison Entrance. This discovery represents the first new occurrence for this federally endangered species in 14 years and just the fifth population discovered to date, extending the geographic range into the Paint Rock River watershed [25]. The stygobiotic crayfish Orconectes australis is common in streams in both upper- and lower-level passages of the Fern Cave System. McGregor et al. [24] observed 34 individuals, including a female with eggs, in the Davison section of Bottom Cave in September 1993. This cave crayfish has also been reported from the Fern Cave System by Holt [14] and Hobbs et al. [18].
The isopod Caecidotea bicrenata was found throughout the Fern Cave System in several habitats, including stream riffles and pools, rimstone pools, and drip pools. Caecidotea bicrenata is widely distributed throughout the Interior Low Plateau [23] but may represent a cryptic species complex. This stygobiotic asellid was previously reported from the Fern Cave System by Fleming [13] and Lewis [23]. Cave amphipods of the genus Stygobromus were collected from isolated drip pools in the Morgue Pit area, West Passage, and Bottom Cave sections of the Fern Cave System. These specimens may be S. vitreus or S. dicksoni, which are known from Jackson and Madison counties [54], or an undescribed species. The ostracod Sagittocythere barri is an ectocommensal of the stygobiotic crayfish Orconectes australis and was reported from the Fern Cave System by Hart and Hart [17].
The only cave-limited vertebrate known from the Fern Cave System is the amblyopsid cavefish, Typhlichthys subterraneus. This cavefish was abundant in the stream in Bottom Cave and is considered a top aquatic predator. Typhlichthys subterraneus is a cryptic species complex [55] with two lineages contacting in Western Madison/Eastern Jackson counties. McGregor et al. [24] reported this cavefish previously.
4. Discussion
The Fern Cave obligate cave fauna is exceptionally rich with 27 troglobionts and stygobionts, making it one of the most diverse cave systems in North America. The terrestrial fauna is particularly diverse, with 19 species trailing only the Mammoth Cave System in North America (49 species overall, 32 troglobionts; ref. [5]). The stygofauna of the Fern Cave System is diverse (eight species) but not exceptional compared to other hotspot subterranean communities in North America, such as the San Marcos Artesian Well in Texas (55 species; ref. [6]), the Mammoth Cave System in Kentucky (17 species; ref. [5]), and Shelta Cave in Alabama (12 species; refs. [7,56]).
The exceptional cave-limited diversity within the Fern Cave System may be explained by several factors that operate in concert to influence patterns of subterranean biodiversity and endemism in the region. First, Fern Cave lies along the escarpments of the Southern Cumberland Plateau, which is highly dissected with numerous karst exposures and cave systems. The expansive karst and higher cave density in the region are expected to support greater species richness [57,58], but they may also offer greater dispersal opportunities [57]. Greater cave density may also provide increased opportunities for colonization of subterranean habitats [58]. The Fern Cave System lies within a hypothesized mid-latitude biodiversity ridge for terrestrial subterranean fauna, which is associated with long-term higher surface productivity and a favorable climate, particularly during the Pleistocene [58]. Within this region, cave systems likely have greater energy inputs from allochthonous sources to support more species, larger populations, and consequently lower extinct rates relative to other karst regions [57,58,59].
Of the 18 cave-limited species with a NatureServe conservation rank, half of the species (five troglobionts and four stygobionts) are of conservation concern (i.e., G1–G3 NatureServe conservation rank, federal or state status), highlighted by the federally endangered Alabama Cave Shrimp Palaemonias alabamae discovered in 2018 [25]. Most of these species are at an elevated risk of extinction due to their limited distributions and/or their few known occurrences. In particular, the cave pseudoscorpions Tyrannochthonius torodei and Alabamocreagris mortis are known only from the Fern Cave System. Cave-limited fauna face many threats, such as habitat loss and degradation, groundwater overexploitation and contamination, and climate change [60,61]. Although much of the Fern Cave System (and all five entrances) lies within the boundaries of the Fern Cave National Wildlife Refuge and the SCCi Kay Hill Deen Fern Cave Preserve, the cave system is not entirely immune to potential direct and indirect threats to its biodiversity. Nearly all (99%) of the 6.7 km2 recharge area of the Fern Cave System is composed of mixed deciduous forest, which suggests a minimal risk of groundwater pollution [3]. However, future land use modifications, such as possible logging, could impact groundwater quality, although the risk is low at present. The Paint Rock River is known to backflood into the lowest level of the Fern Cave System [3]. Consequently, water quality in this section may be influenced by the water quality and flood stage of the Paint Rock River. Fortunately, the Paint Rock River has escaped most of the adverse anthropogenic impacts of other major tributaries of the Tennessee River; however, non-point source pollution of low to moderate magnitude primarily caused by nutrient enrichment has been identified within the Paint Rock watershed [62].
The list of cave-limited fauna within the Fern Cave System is likely incomplete, and there is great potential to discover new taxa. Much of the 25+ km of passage remains to be comprehensively bioinventoried, and some habitats, such as epikarst, are under-sampled and may harbor additional taxa. For example, an undescribed Anillinus beetle was collected from Magic City Cave on Fern Cave National Wildlife Refuge, which is likely hydrologically connected to the Fern Cave System. Most species in this genus are small (1–2 mm) litter or soil-dwelling inhabitants that are depigmented and lack eyes and wings, but several species are considered troglobionts, including some from Alabama [63]. In addition to the possibility of two Scoterpes millipedes co-occurring, it would be unsurprising if at least two species of Pseudanophthalmus cave beetles co-occur within the Fern Cave System, which is a common occurrence throughout much of the Interior Low Plateau and Appalachian Karst regions (e.g., refs. [64,65]). Several taxa are notably absent from the cave-limited fauna of Fern Cave, including terrestrial cavesnails, terrestrial woodlice, stygobiotic copepods, and stygobiotic salamanders, all of which may be discovered in the future. For example, three terrestrial cavesnails are known from Northern Alabama, including Helicodiscus barri, which has a broad distribution throughout the Interior Low Plateau karst region [66,67]. Twelve stygobiotic copepods occur in the Interior Low Plateau [68], but only one, Diacyclops alabamensis, has been reported from Alabama. Gyrinophilus palleucus is a top aquatic predator of many cave streams in Northern Alabama, including caves within the Paint Rock River watershed [69,70,71]. It is known to co-occur with Orconectes australis, Typhlichthys subterraneus, and Palaemonias alabamae [71], all of which occur within Fern Cave. Other taxonomic groups have not been particularly well studied in the Fern Cave System, including springtails and mites. More intensive biosurvey work on these groups may uncover additional taxa. Finally, few phylogenetic studies to date have incorporated specimens and samples from the Fern Cave System. Comprehensive sampling within the Fern Cave System has the potential to uncover cryptic diversity in some taxonomic groups, such as stygobiotic isopods and amphipods, which is an increasingly common discovery of phylogenetic studies in cave-limited taxa [55,72,73].
Author Contributions
Conceptualization: M.L.N.; methodology and analysis: M.L.N., M.E.S. and T.I.; data acquisition: M.L.N., M.E.S., T.I., B.M., B.T., B.C., A.H., B.D.J., N.M., K.D.K.N. and S.P.; original draft preparation: M.L.N., M.E.S. and T.I.; review and editing: M.L.N., M.E.S., T.I., B.M., B.T., B.C., A.H., B.D.J., N.M., K.D.K.N. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
The National Wildlife Refuge System (NWR) of the US Fish and Wildlife Service awarded funding for this project through the NWR’s Inventory and Monitoring Program (Agreement no. F18AC00681). M.L. Niemiller was supported in part by the National Science Foundation (award no. 2047939), the Cave Conservancy Foundation, and the Alabama Department of Conservation and Natural Resources.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this manuscript. Data sharing is not applicable for this manuscript.
Acknowledgments
We thank Pedro Ardapple, Reilly Blackwell, Forbes Boyle, Daniel Chapman, Kevin England, Mark Jones, Jacob Lieber, Hannah Lieffring, Pete Pattavina, Jennifer Pinkley, Dave Richardson, Mike Senn, Nick Sharp, Elliot Stahl, Matt Tomlinson, and Drew Westerman for assistance with fieldwork. We thank Marshal Hedin, Paul Marek, Marc Milne, Karen Ober, and Charles Stephen for identifying specimens. M.L. Niemiller and K.D.K. Niemiller thank E.R. Niemiller for his cooperation in utero during the study. The collection of specimens was authorized under ALDCNR scientific permit nos. 2018035450068680, 2018061776268680, 2018061777068680, 2019060225068680, 2019060224868680, 2020083527668680, and 2020083528068680. The views presented herein are those of the authors and do not necessarily represent those of the U.S. Fish and Wildlife Service or the U.S. Geological Survey.
Conflicts of Interest
Author S.P. was employed by the company Southeastern Cave Conservancy, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Pinkley, J.E. Fern Cave: The Discovery, Exploration, and History of Alabama’s Greatest Cave; Blue Bat Books: Knoxville, TN, USA, 2014. [Google Scholar]
- Martin, C.O. Assessment of the Population Status of the Gray Bat (Myotis grisescens); Report ERDC/EL TR-07-22; U.S. Army Corps of Engineers: Vicksburg, MI, USA, 2007.
- Miller, B.V.; Tobin, B. Mapping karst groundwater flow paths and delineating recharge areas for Fern Cave, Alabama through the use of dye tracing. U.S. Geological Survey Scientific Investigation Map; in press.
- Osborne, W.E.; Ward, W.E., II; Irvin, G.D. Geologic Map and Cross Sections of the Paint Rock 7.5-Minute Quadrangle, Jackson and Madison Counties, Alabama. Geological Survey of Alabama Quadrangle Series Map QS59, Scale 1:24,000. 2013. Available online: https://www.gsa.state.al.us/img/Geological/Quads/QS59/QS59_Plate.pdf (accessed on 27 April 2023).
- Niemiller, M.L.; Helf, K.; Toomey, R.S. Mammoth Cave: A hotspot of subterranean biodiversity in the United States. Diversity 2021, 13, 373. [Google Scholar] [CrossRef]
- Hutchins, B.T.; Gibson, J.R.; Diaz, P.H.; Schwartz, B.F. Stygobiont diversity in the San Marcos Artesian Well and Edwards Aquifer groundwater ecosystem, Texas, USA. Diversity 2021, 13, 234. [Google Scholar] [CrossRef]
- Culver, D.C.; Sket, B. Hotspots of subterranean biodiversity in caves and wells. J. Cave Karst Stud. 2000, 62, 11–17. [Google Scholar]
- Peck, S.B. The cave fauna of Alabama: Part I. The terrestrial invertebrates (excluding insects). Nat. Speleolog. Soc. Bull. 1989, 51, 11–33. [Google Scholar]
- Peck, S.B. The cave fauna of Alabama. Part II: The insects. Nat. Speleolog. Soc. Bull. 1995, 40, 39–63. [Google Scholar]
- Muchmore, W.B. New species and records of cavernicolous pseudoscorpions of the genus Microcreagris (Arachnida, Chelonethida, Neobisiidae, Ideobisiinae). Am. Mus. Novit. 1969, 2392, 1–21. [Google Scholar]
- Muchmore, W.B. The genus Tyrannochthonius in the eastern United States (Pseudoscorpionida: Chthoniidae). Part II. More recently described species. Insecta Mundi 1996, 10, 153–168. [Google Scholar]
- Carpenter, J.H. Systematics and Ecology of Cave Planarians of the United States. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 1970. [Google Scholar]
- Fleming, L.E. The evolution of the eastern North American isopods of the genus Asellus (Crustacea: Asellidae). Part I. Int. J. Speleol. 1972, 4, 221–256. [Google Scholar] [CrossRef]
- Holt, P.C. Branchiobdellids (Annelida: Clitellata) from some eastern North American caves, with descriptions of new species of the genus Cambarincola. Int. J. Speleol. 1973, 5, 219–256. [Google Scholar] [CrossRef]
- Peck, S.B. A systematic revision and evolutionary biology of the Ptomaphagus adelops. Bull. Mus. Comp. Zool. 1973, 145, 29–162. [Google Scholar]
- Peck, S.B. The distribution and evolution of cavernicolous Ptomaphagus beetles in the southeastern United States (Coleoptera; Leiodidae; Cholevinae) with new species and records. Can. J. Zool. 1984, 62, 730–740. [Google Scholar] [CrossRef]
- Hart, D.G.; Hart, C.W., Jr. The ostracod family Entocytheridae. Acad. Nat. Sci. Phila. Monogr. 1974, 18, 1–239. [Google Scholar]
- Hobbs, H.H., Jr.; Hobbs, H.H., III; Daniel, M.A. A review of the troglobitic decapod crustaceans of the Americas. Smithson. Contrib. Zool. 1977, 244, 1–83. [Google Scholar] [CrossRef][Green Version]
- Kenk, R. Freshwater triclads (Turbellaria) of North America, IX. The genus Sphalloplana. Smithson. Contrib. Zool. 1977, 246, 1–38. [Google Scholar] [CrossRef]
- Hubbell, T.H.; Norton, R.M. The systematics and Biology of the cave crickets of the North American tribe Hadenoecini (Orthoptera), Saltatoria, Ensifera, Rhaphidophoridae, Dolichopodinae. Misc. Publ. Mus. Zool. Univ. Mich. 1978, 156, 1–124. [Google Scholar]
- Ferguson, L.M. Systematics, Evolution, and Zoogeography of the Cavernicolous Campodeids of the Genus Litocampa (Diplura: Campodeidae) in the United States. Ph.D. Dissertation, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 1981. [Google Scholar]
- Gertsch, W.J. The spider family Nesticidae (Araneae) in North America, Central America, and the West Indies. Tex. Mem. Mus. Bull. 1984, 31, 1–91. [Google Scholar]
- Lewis, J.J. The Systematics, Zoogeography and Life History of the Troglobitic Isopods of the Interior Plateaus of the Eastern United States. Ph.D. Thesis, University of Louisville, Louisville, KY, USA, 1988. [Google Scholar]
- McGregor, S.W.; Rheams, K.F.; O’Neil, P.E.; Moser, P.H.; Blackwood, R. Biological, Geological, and Hydrological Investigations in Bobcat, Matthews, and Shelta Caves and Other Selected Caves in North Alabama; Open-File Report; Geological Survey of Alabama: Tuscaloosa, AL, USA, 1994. [Google Scholar]
- Niemiller, M.L.; Inebnit, T.; Hinkle, A.; Jones, B.D.; Jones, M.; Lamb, J.; Mann, N.; Miller, B.; Pinkley, J.; Pitts, S.; et al. Discovery of a new population of the federally endangered Alabama Cave Shrimp, Palaemonias alabamae Smalley, 1961, in northern Alabama. Subterr. Biol. 2019, 32, 43–59. [Google Scholar] [CrossRef]
- Hedin, M.; Milne, M.A. New species in old mountains: Integrative taxonomy reveals ten new species and extensive short-range endemism in Nesticus spiders (Araneae, Nesticidae) from the southern Appalachian Mountains. ZooKeys 2023, 1145, 1–30. [Google Scholar] [CrossRef]
- Sket, B. Can we agree on an ecological classification of subterranean animals? J. Nat. Hist. 2008, 42, 1549–1563. [Google Scholar] [CrossRef]
- Trajano, E. Ecological classification of subterranean organisms. In Encyclopedia of Caves, 2nd ed.; White, W.B., Culver, D.C., Eds.; Academic/Elsevier Press: Amsterdam, The Netherlands, 2012; pp. 275–277. [Google Scholar]
- Trajano, E.; de Carvalho, M.R. Towards a biologically meaningful classification of subterranean organisms: A critical analysis of the Schiner-Racovitza system from a historical perspective, difficulties of its application and implications for conservation. Subterr. Biol. 2017, 22, 1–26. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T. Ecological and evolutionary classifications of subterranean organisms. In Encyclopedia of Caves, 3rd ed.; Culver, D.C., White, W.B., Pipan, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 376–379. [Google Scholar]
- Alabama Department of Conservation and Natural Resources. Alabama’s Wildlife Action Plan 2015–2025; Division of Wildlife and Freshwater Fisheries, Alabama Department of Conservation and Natural Resources: Montgomery, AL, USA, 2015. [Google Scholar]
- Barr, T.C., Jr.; Holsinger, J.R. Speciation in cave faunas. Ann. Rev. Ecol. Syst. 1985, 16, 313–337. [Google Scholar] [CrossRef]
- Peck, S.B. A summary of diversity and distribution of the obligate cave-inhabiting faunas of the United States and Canada. J. Caves Karst Stud. 1998, 60, 18–26. [Google Scholar]
- Snowman, C.V.; Zigler, K.S.; Hedin, M. Caves as islands: Mitochondrial phylogeography of the cave-obligate spider species Nesticus barri (Araneae: Nesticidae). J. Arachnol. 2010, 38, 49–56. [Google Scholar] [CrossRef]
- Niemiller, M.L.; Zigler, K.S.; Fenolio, D.B. Cave Life of TAG: A Guide to Commonly Encountered Species in Tennessee, Alabama and Georgia; Biology Section of the National Speleological Society: Huntsville, AL, USA, 2013. [Google Scholar]
- Keyserling, E.G. Neue Spinnen aus Amerika, III. Verh. Zool. Bot. Ges. Wien 1881, 31, 269–314. [Google Scholar] [CrossRef]
- Platnick, N.I. A revision of the Appalachian spider genus Liocranoides (Araneae: Tengellidae). Am. Mus. Novit. 1999, 3285, 1–13. [Google Scholar]
- Holsinger, J.R.; Peck, S.B. The invertebrate cave fauna of Georgia. Nat. Speleolog. Soc. Bull. 1971, 33, 23–44. [Google Scholar]
- Reeves, W.K.; Jensen, J.B.; Ozier, J.C. New faunal and fungal records from caves in Georgia, USA. J. Cave Karst Stud. 2000, 62, 169–179. [Google Scholar]
- Zigler, K.S.; Niemiller, M.L.; Stephen, C.D.R.; Ayala, B.N.; Milne, M.A.; Gladstone, N.S.; Engel, A.S.; Jensen, J.B.; Camp, C.D.; Ozier, J.C.; et al. Biodiversity from caves and other subterranean habitats of Georgia, USA. J. Cave Karst Stud. 2020, 82, 125–167. [Google Scholar] [CrossRef]
- Shear, W.A. Studies in the milliped order Chordeumida (Diplopoda): A revision of the family Cleidogonidae and a reclassification of the order Chordeumida in the New World. Bull. Mus. Comparat. Zool. 1972, 144, 151–352. [Google Scholar]
- Shear, W.A. The milliped family Trichopetalidae, Part 2: The genera Trichopetalum, Zygonopus and Scoterpes (Diplopoda: Chordeumatida, Cleidogonoidea). Zootaxa 2010, 2385, 1–62. [Google Scholar] [CrossRef]
- Hoffman, R.L. Revision of the milliped genera Boraria and Gyalostethus (Polydesmida: Xystodesmidae). Proc. United States Natl. Mus. 1965, 117, 305–348. [Google Scholar] [CrossRef][Green Version]
- Christiansen, K.; Bellinger, P. Cave Arrhopalites: New to science. J. Cave Karst Stud. 1996, 58, 168–180. [Google Scholar]
- Christiansen, K.; Bellinger, P. The Collembola of North America North of the Rio Grande. A Taxonomic Analysis, 2nd ed.; Grinnell College: Grinnell, IA, USA, 1998. [Google Scholar]
- Zeppelini, D.; Christiansen, K. Arrhopalites (Collembola: Arrhopalitidae) in U.S. caves with the description of seven new species. J. Cave Karst Stud. 2003, 65, 36–42. [Google Scholar]
- Zeppelini, D.; Taylor, S.J.; Slay, M.E. Cave Pygmarrhopalites Vargovitsh, 2009 (Collembola, Symphypleona, Arrhopalitidae) in United States. Zootaxa 2009, 2204, 1–18. [Google Scholar] [CrossRef]
- Park, O. Cavernicolous pselaphid beetles of Alabama and Tennessee. Geol. Surv. Ala. Mus. Pap. 1951, 31, 1–107. [Google Scholar]
- Park, O. New or little-known species of pselaphid beetles, chiefly from southeastern United States. J. Tenn. Acad. Sci. 1958, 33, 39–74. [Google Scholar]
- Park, O. Cavernicolous pselaphid beetles of the United States. Am. Midl. Nat. 1960, 64, 66–104. [Google Scholar] [CrossRef]
- Marshall, S.A.; Peck, S.B. Distribution of cave-dwelling Sphaeroceridae (Diptera) of eastern North America. Proc. Entomol. Soc. Ont. 1984, 115, 37–41. [Google Scholar]
- Marshall, S.A.; Peck, S.B. The origin and relationships of Spelobia tenebrarum Aldrich, a troglobitic, eastern North American sphaerocerid fly. Can. Entomol. 1985, 117, 1013–1015. [Google Scholar] [CrossRef]
- Lewis, J.J. Bioinventory of Caves of the Cumberland Escarpment Area of Tennessee; Final Report to Tennessee Wildlife Resources Agency & The Nature Conservancy of Tennessee; Lewis & Associates, LLC: Clarksville, IN, USA, 2005. [Google Scholar]
- Holsinger, J.R. Systematics of the subterranean amphipod genus Stygobromus (Crangonyctidae): Part II. Species of the eastern United States. Smithson. Contrib. Zool. 1978, 266, 1–144. [Google Scholar]
- Niemiller, M.L.; Near, T.J.; Fitzpatrick, B.M. Delimiting species using multilocus data: Diagnosing cryptic diversity in the southern cavefish, Typhlichthys subterraneus (Teleostei: Amblyopsidae). Evolution 2012, 66, 846–866. [Google Scholar] [CrossRef]
- Cooper, J.E. Ecological and Behavioral Studies in Shelta Cave, Alabama, with Emphasis on Decapod Crustaceans. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 1975. [Google Scholar]
- Niemiller, M.L.; Zigler, K.S. Patterns of cave biodiversity and endemism in the Appalachians and Interior Plateau of Tennessee, USA. PLoS ONE 2013, 8, e64177. [Google Scholar] [CrossRef] [PubMed]
- Culver, D.C.; Deharveng, L.; Bedos, A.; Lewis, J.J.; Madden, M.; Reddell, J.R.; Sket, B.; Trontelj, P.; White, D. The mid-latitude biodiversity ridge in terrestrial cave fauna. Ecography 2006, 29, 120–128. [Google Scholar] [CrossRef]
- Culver, D.C.; Master, L.L.; Christman, M.C.; Hobbs, H.H. Obligate cave fauna of the 48 contiguous United States. Conserv. Biol. 2000, 14, 386–401. [Google Scholar] [CrossRef]
- Niemiller, M.L.; Bichuette, E.; Taylor, S.J. Conservation of cave fauna in Europe and the Americas. In Ecological Studies: Cave Ecology; Moldovan, O.T., Kovac, L., Halse, S., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 451–478. [Google Scholar]
- Mammola, S.; Cardoso, P.; Culver, D.C.; Deharveng, L.; Ferreira, R.L.; Fiŝer, C.; Galassi, D.M.P.; Griebler, C.; Halse, S.; Humphreys, W.F.; et al. Scientists’ warning on the conservation of subterranean ecosystems. BioScience 2019, 69, 641–650. [Google Scholar] [CrossRef]
- Barbour, M.S. Paint Rock River Watershed Nonpoint Source Pollution; Unpublished Report to Alabama Department of Environmental Management, Montgomery, Alabama; Alabama Natural Heritage Program: Montgomery, AL, USA, 2003; 184p. [Google Scholar]
- Sokolov, I.M. Four new species of the genus Anillinus Casey (Coleoptera, Carabidae, Anillini) from Alabama, U.S.A., with a revised key to the Alabama species. Zootaxa 2020, 4808, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Barr, T.C., Jr. A Classification and Checklist of the Genus Pseudanophthalmus Jeannel (Coleoptera: Carabidae: Trechinae); Special Publication 11; Virginia Museum of Natural History: Martinsville, VA, USA, 2004. [Google Scholar]
- Ober, K.A.; Niemiller, M.L.; Philips, T.K. Cave trechine (Coleoptera: Carabidae) diversity and biogeography in North America. In Cave Life—Drivers of Diversity and Diversification; Wynne, J.J., Ed.; John Hopkins Press: Baltimore, MD, USA, 2022; pp. 192–223. [Google Scholar]
- Gladstone, N.S.; Carter, E.T.; McKinney, M.L.; Niemiller, M.L. Status and distribution of the cave-obligate land snails in the Appalachians and Interior Low Plateau of the eastern United States. Am. Malacol. Bull. 2018, 36, 62–78. [Google Scholar] [CrossRef]
- Gladstone, N.S.; Niemiller, M.L.; Pieper, E.B.; Dooley, K.E.; McKinney, M.L. Morphometrics and phylogeography of the cave-obligate land snail Helicodiscus barri (Gastropoda, Stylommatophora, Helicodiscidae). Subterr. Biol. 2019, 30, 1–32. [Google Scholar] [CrossRef]
- Niemiller, M.L.; Taylor, S.J.; Slay, M.E.; Hobbs, H.H., III. Biodiversity in the United States and Canada. In Encyclopedia of Caves, 3rd ed.; Culver, D.C., White, W.B., Pipan, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–176. [Google Scholar]
- Miller, B.T.; Niemiller, M.L. Distribution and relative abundance of Tennessee cave salamanders (Gyrinophilus palleucus and G. gulolineatus) with an emphasis on Tennessee populations. Herpetol. Conserv. Biol. 2008, 3, 1–20. [Google Scholar]
- Miller, B.T.; Niemiller, M.L. Gyrinophilus palleucus. Cat. Am. Amphib. Reptiles 2012, 884, 1–7. [Google Scholar]
- Niemiller, M.L.; Niemiller, K.D.K. Species Status Assessment for the Tennessee Cave Salamander (Gyrinophilus palleucus) McCrady, 1954. Version 1.0; Tennessee Wildlife Resources Agency: Nashville, TN, USA, 2020; 59p.
- Ethridge, J.Z.; Gibson, J.R.; Nice, C.C. Cryptic diversity within and amongst spring-associated Stygobromus amphipods (Amphipoda: Crangonyctidae). Zool. J. Linn. Soc. 2013, 167, 227–242. [Google Scholar] [CrossRef]
- Devitt, T.J.; Wright, A.M.; Cannatella, D.C.; Hillis, D.M. Species delimitation in endangered groundwater salamanders: Implications for aquifer management and biodiversity conservation. Proc. Natl. Acad. Sci. USA 2019, 116, 2624–2633. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).