Abstract
The distinction between shallow coral reefs and mesophotic coral ecosystems (MCEs) has not been fully clarified yet. The original definition of MCEs, by depths of 30–150 m, fixes their bathymetrical limits and fails to accommodate environmental and biological variation. Recent studies have indicated that water transparency and light availability may explain why MCEs do not occur at fixed depths but vary among localities. This study aimed to evaluate the presence and distribution of MCEs, along the central coast of Oaxaca, through optical depths and the associated benthic community. Using MODIS-Aqua satellite data (Kd490), we estimated the mesophotic optical depths monthly (z10%, z1%, z0.1%), over the last four years. In addition, to characterize benthic community structure, we conducted underwater photo quadrat surveys at two locations on the southern Mexican Pacific coast from 10 to 55 m depth. Significant differences between depths and locations were found in benthic communities. Furthermore, the lower distribution of photosynthetic taxa was different between the two locations but indicative to the z10% and z1% in both cases. Those differences were associated with the upwelling season, which reduces, drastically and differentially, the light availability for benthic communities between the two locations and limits the development of MCEs on the central coast of Oaxaca.
1. Introduction
Coral reefs are considered a priority for conservation as biodiversity hotspots [1], although they are threatened by multiple human activities and natural impacts with local, regional, and global effects [2]. In the Eastern Tropical Pacific (ETP), these threats include prolonged sea warming, which results in El Niño–Southern Oscillation (ENSO) episodes, mass coral bleaching, and the consequent coral mortality and reduction in coral cover [3,4]. These events may result in regional and local extinctions of certain coral species [5,6]. Coral communities, however, can persist with low impacts in areas, where environmental variation can be buffered from extreme conditions [6]. This has led to the consideration that possible refuges for shallow coral communities could be found at moderate depths, where temperature oscillations are lower, and the impact of global warming could be less severe [3]. This hypothesis has stimulated the study of mesophotic coral ecosystems (MCEs), initially seen as simple extensions of coral reef communities between 30 and 150 m depth [7]. The distinction between shallow and mesophotic coral communities is not clear yet, as these two ecosystems do not always overlap [8]. In fact, since the earliest uses of the word mesophotic, there has been no solid, objective definition [9,10]. The most criticized elements of MCEs definition have been the bathymetric limits, since they have been determined arbitrarily and fail to explicitly accommodate environmental variation [11]. Nevertheless, the word mesophotic has spread widely among the scientific community to refer to any underwater habitat found in both tropical and temperate zones between 30 and 150 m. These include octocoral fields, seamounts, slope walls, seagrass meadows, deep kelp forests, and rhodolith beds [12,13,14]. All these habitats have been named mesophotic ecosystems (MEs) and, like MCEs, they can harbor important biodiversity [14,15].
Water transparency, temperature, and substrate availability are the main factors identified as favorable or limiting for the development of MCEs [16]. As photosynthesis is the main source of energy and carbon acquisition in photosynthetic corals, and thus, a fundamental component of their biology [17,18], light plays a central role in the structuring of coral reefs and MCEs [17]. Light has been also recognized as a key driving force in the evolution and diversification of photosynthetic coral skeletons, with important implications for the control of the internal light environment of the symbionts and, hence, the physiology of the holobiont [19]. Accordingly, light availability can be considered as a source of energy and also as the main driver of the changes observed in benthic communities [11,20]. Changes in water transparency may explain why MCEs do not occur at fixed depths but vary among localities, as has been recently documented [11,20,21,22].
Optical depths are referred to those physical depths where a certain percentage of surface irradiance (%Es) reaches. In previous work [22], we used three optical depths (z10%, z1%, and z0.1%) as proxies of bathymetric boundaries for the MCEs in the Eastern Tropical Pacific (ETP). Comparing these boundaries with published depth records of photosynthetic corals, we observed that the upper boundary (z10%) in the continental zone of the Mexican Pacific is located between 10 and 20 m depth, and that the MCEs can extend along the upper mesophotic zone, down to 40 m depth (z1%), while the lower mesophotic zone extends down to 60 m (0.1%).
In the ETP, coral reef communities have a clear zonation pattern. Reef flats built up from different species of the Pocillopora genus dominate in shallow areas (0–6 m); whereas species of the genus Pavona dominate in deeper areas (6–14 m) together with small and scattered colonies of Porites panamensis [23,24]. The oceanographic conditions in the ETP and Mexican Pacific are not favorable for the development of shallow coral reefs [3,6,24] and MCEs [25]. However, the central coast of Oaxaca, localized at the southern Mexican Pacific coast, is considered biogeographically a key point of access for coral fauna from Central America [24]. This region is influenced by intermittent winds from November to April, called “Nortes” or “Tehuanos” (with speeds above 10 m/s), which generate significant changes in the water column, resulting in seasonal upwellings that transport subsurface water with low pH, low Ω aragonite, and large variations in sea-surface temperatures (SST) [26]. Upwelling events also affect water transparency, generating seasonal turbidity through the proliferation of algal blooms associated with local nutrients enrichments [27].
A pioneering study on mesophotic environments of the central coast of Oaxaca has documented higher richness of gorgonian species at depths of 40–70 m with respect to shallow areas [28]. The study also documented changes in species composition along the shallower depth gradient (0–25 m). Nonetheless, the presence of MCEs remains to be established as well as an understanding of the environmental factors that could explain the depth-related changes observed in the benthic communities.
We hypothesized that the maximum depth distribution of some photosynthetic and benthic species/genera would indicate the mesophotic optical depths (z10%, z1%, z0.1%) and thus the utility to recognize the location for the mesophotic zone based on light. In summary, this study aimed to evaluate the presence and distribution of MCEs along the central coast of Oaxaca through mesophotic optical depths and the associated benthic community. In addition, other variables, such as location, depth strata, or the type of underwater relief were analyzed.
2. Materials and Methods
This study was carried out on the central coast of Oaxaca, located on the southern Mexican Pacific coast. The study area covers 40 km of coastline and includes two main locations Puerto Ángel and Bahías de Huatulco, with contrasting economic activities. Puerto Ángel is mainly dedicated to intensive artisanal fishing and has moderate tourist activity that seasonally stimulates the local economy [29]. Bahías de Huatulco, in contrast, depends primarily on tourism and includes a protected natural reserve area that limits fishing activity, the Parque Nacional Huatulco (PNH).
To analyze the variability of the apparent optical properties of the study area, we used time-averaged maps and monthly time series (4 km resolution) of the diffuse attenuation coefficient (Kd) at 490 nm (Kd490) from the MODIS-AQUA satellite and Goddard Earth Sciences Data and Information Services Center (https://giovanni.gsfc.nasa.gov 15 March 2022). These values were converted to the downwelling attenuation coefficient (Kd) for the photosynthetic active radiation, PAR (KdPAR, m−1), as documented previously [22], using the following equation [30]:
KdPAR = 0.0864 + 0.884 Kd490 − 0.00137 (Kd490)−1
This equation is only suitable for cases with Kd (PAR) less than ~0.5 m−1. Since light attenuation during upwelling months is very high, we used the Kd (PAR) conversion model for turbid coastal waters [31] for the months with estimated values for KdPAR above 0.5 m−1.
KdPAR = 0.8045 (Kd490)0.917
Time series data were downloaded from November 2017 to December 2020 at Puerto Ángel (96°28′45.12″ W, 15°38′44″ N; 96°26′15″ W, 15°41′15″ N) and at Huatulco (96°13′45.1″ W, 15°41′15″ N; 96°11′15″ W, 15°43′45.1″ N). The monthly KdPAR-derived values were used to estimate the optical depths z10%, z1%, and z0.1%, and the percentage of surface irradiance at the maximum depth of photosynthetic corals using the following equation [32]:
where Ez and Es are the values of downward irradiance at z m depth, and just below the surface, respectively.
Ez = Es·e−Kd·z
Sampling comprised four sites for Puerto Ángel, and four sites for Huatulco located inside the marine protected area (PNH). Transects, ~100 m long, were run parallel to the underwater relief at each seascape. The transects were recorded mainly at two different depth strata (10 < 20 m, 20 < 30 m) and conducted by SCUBA diving using a photo-quadrat camera system with a connected dive computer to confirm depth. Only one transect was carried out at 40 < 50 m, at the site of Puerto Ángel. The photo-frame system was used as a reference for the transect length (leaving two times the frame length between each photo-plot) and substrate slope grade categories (see Figure S1). In total, 1120 photo-plots (70 × 50 cm) from all transects were analyzed for coral abundance, presence–absence taxa, and maximum deep records of photosynthetic corals. These records were adjusted to the average mean sea-level data. Out of ~60 photo plots at each site and depth, 40 photo plots were randomly selected for community structure analysis, recording abundance of corals.
Genera/species of corals were identified based on the authors’ experience and by a visit to the Natural Museum of Universidad del Mar, campus Puerto Ángel (Oaxaca). For macroalgae, we use the AlgaeBase [33] and assistance from the literature. In addition, local experts in marine algae were consulted. In case of uncertainty at the species level, identification was restricted to genus level.
Statistical Analyses
The benthic communities, identified as macroalgae and corals from 26 independent transects were treated as a sample. The variation in community species composition (so-called taxonomic β-diversity) was quantified by measuring the dissimilarities between each pair of transects with Jaccard’s index [34]. A cluster analysis [35] was conducted based on Jaccard (presence/absence) and Bray Curtis (coral abundance) dissimilarity matrix to visualize assemblages’ spatial structure and their association or lack of association between samples with the predicted variables (location, depth strata, slope grade). We also performed non-metric multidimensional scaling (nMDS) to visualize the overall taxonomic β-diversity [34]. Permutational multivariate analysis of variance (PERMANOVA) on the Jaccard dissimilarity matrix was used to test the hypothesis that benthic communities’ composition differed between depth strata, as well as between locations and slope grade [36]. The analysis consisted of a three-way model with depth as a fixed factor (2 levels), location (Puerto Ángel vs. Huatulco) as a fixed factor crossed with depths, and slope grade (3 levels) as a random factor nested in location and crossed with depth. Three categories were used to describe the slope grade, soft (0 < 20°), moderate (20–50°), and mixed, which includes a combination of different categories in the transect, including the steep slopes (>50°). A unique transect of the deepest stratum (40–50 m) was excluded from the analyses due to a lack of representativeness and variability. In addition, two-way ANOVA analyses was used to test the significance of the variation in species richness between the benthic communities from Puerto Ángel and Huatulco and the two-depth strata.
To quantify coral abundance in the different assemblages from the 25 independent transects, we used log (x + 1) transformed data to Bray-Curtis dissimilarity matrices. Three analyses of similarity (ANOSIM; 999 permutations) were used to test for differences in a multivariate coral community matrix using pairwise dissimilarities between (1) depth stratum, (2) locations and (3) slope grade. For a graphical representation of data, non-metric multidimensional scaling (nMDS) ordination was carried out [34]. We presented the results according to the groups suggested by cluster analyses based on the Bray–Curtis dissimilarity matrix. We called these groups “seascape”, which includes four categories, shallow terraces, shallow slopes, deep terraces, and deep slopes. The SIMPER method was used to identify taxa contributing most to differences between seascapes [35].
Additionally, we carried out a multivariate test for homogeneity in dispersions among groups of predicted variables [37] to corroborate the information obtained from PERMANOVA and ANOSIM (see Figure S2). All analyses were carried out in the programming language R [38]; indices and ordination plots were performed using the “vegan” package [39]; and statistical tests were assessed using the “Tidyverse” and “rstatix” packages.
3. Results
Water transparency was clearer in Puerto Ángel in comparison with Huatulco bays (Figure 1A). The red-framed areas in Figure 1 correspond to KdPAR time series from MODIS-A, shown in Figure S3A. Light attenuation was significantly higher during the upwelling season relative to no-upwelling months at both locations (Figure 1B,C and Figure S3B; two-way ANOVA, F = 3.83, p < 0.05). In addition, during the upwelling season, the mesophotic optical depths (shallow-upper and lower) were all located deeper in Puerto Ángel (14.1–42.3 m) compared to Huatulco (10.1–30.3 m), whereas during the non-upwelling season, these depths were shallower and more homogeneous between both localities (Table S1).
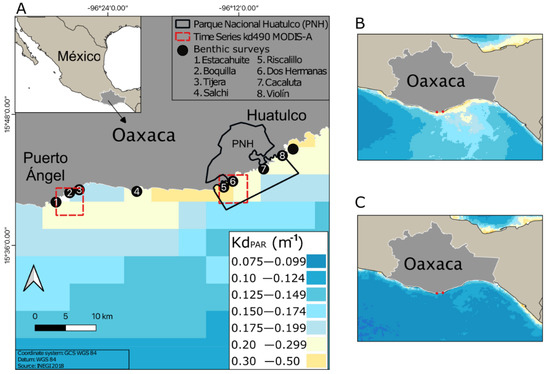
Figure 1.
Time average map for the spatial and temporal variability of satellite-derived KdPAR values of downwelling irradiance in the study area (monthly 4 km resolution; MODIS- Aqua L3m_Kd490 v2018): (A) from November 2019 to October 2021; (B) during upwelling season from November 2020 to April 2021; and (C) during no upwelling months from May 2021 to October 2021.
Associated with the differences found in the optical depths between locations, we observed that in the most eastern sites of the study area (Huatulco), the coral records were shallower than in the western sites (Puerto Ángel). Maximum depths for Pocillopora, Pavona, and Porites genera in the Puerto Ángel area were determined at 17.3 m, 25.55 m, and 27.8 m, respectively, whereas in Huatulco these depths were recorded shallower, with a difference of ~4 m in each genus. The averaged light levels described as the percentage of surface irradiance (%Es) at the maximum depth of distribution of coral genera in each of the sites investigated are illustrated in Figure 2A. The Puerto Ángel sites presented the lowest light levels (zX%) for all coral genera (Pocillopora = 13.7%, Pavona = 6.2% and Porites = 5% Es). These values declined to 8.5%, 3% and 2%, respectively, during the upwelling season, to similar values of the Huatulco sites, especially for Pavona and Porites (Figure 2B). This reduction was larger in the last upwelling season (November 2020–April 2021) when the mean of (Es%) in Pavona and Porites colonies was reduced to 0.9% and 0.6%, respectively, in both locations. During the no upwelling months, the light levels in Pocillopora colonies rose more than two-fold in Puerto Ángel and almost three-fold in Huatulco. Concerning Pavona and Porites, these rises were above three-fold in Puerto Ángel and four-fold in Huatulco (Figure 2C).
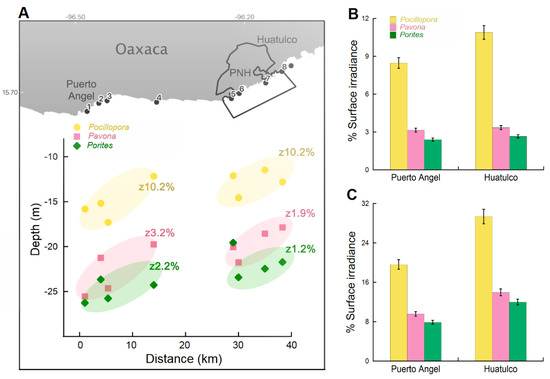
Figure 2.
(A) Maximum depths of coral genera recorded for each site of Puerto Ángel and Huatulco and their average value for the percentage of surface light at each depth (zEs%). The plot also illustrates the distance (km) between sites, where 0 km is the reference for Puerto Ángel bay, located in the west. On the opposite side, in a straight line, is located, at 40 km, La Blanca Islet, outside of the Huatulco protected natural area (PNH). Numbers given with the locations are the same as in Figure 1; (B) variation of the percentage of surface irradiance, %Es (monthly averaged) at the maximum depths recorded for coral distribution during the upwelling season; and (C) the no-upwelling months.
Data recorded for the four year-period November 2017 to October 2021, showed that the monthly optical depths for z10%, z1%, and z0.1% presented a great variability over time. The optical depth at z10% in the last two years was found at ~14–17 m, which matches with the maximum depth of distribution recorded for Pocillopora colonies in both locations. Below, the upper confidence interval of z1%, matches with the deepest distribution of Porites panamensis at both locations (27.8 m at Puerto Ángel and 24.5 m at Huatulco) (Figure 3C,D). At the end of the mesophotic zone, the lower confidence interval limit for z0.1% was found at ~65–70 m (Figure 3A,B). In this study, we observed the presence of crustose coralline algae, CCA, along the entire depth gradient from 10 m to their lower depth at 55 m, which corresponded to the z0.1%.
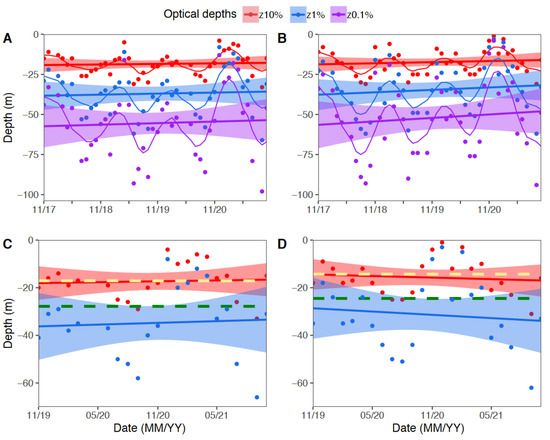
Figure 3.
Monthly variation of mesophotic depths: (A,C) Puerto Ángel; (B,D) Huatulco. Dots represent the monthly optical depths at z10%, z1%, and z0.1% in red, blue, and purple, respectively. Horizontal lines denote the 4-year average values, with 95% confidence interval area; and (C,D) describe the variability of the optical depths for the last two years, from 2020–2021 (same color used in the figures above for z10% and z1%), yellow and green dashed lines correspond to the maximum depths of distribution recorded for Pocillopora and Porites, respectively.
Benthic Characterization
Globally, we identified 65 species of benthic organisms, including 11 species of macroalgae, 24 species of corals, on which this work focused, seven species of sponges, 12 echinoderms, and the remaining species included bivalves, gastropods, crustaceans, plus a single species of ascidian, hydrozoan, and elasmobranch (Table S2).
With regard to the underwater relief, gentle and null slopes (both shallow and deep) were characterized by ledges and terraces, in agreement with Nichol et al. [40]. Moderate, steep, and mixed slopes were characterized by walls associated with edges of cliffs, islands, and ridges. Additionally, we identified “bajos” which present a mix of these slope grade categories. The “bajos” are shallow elevations composed of unconsolidated material that may constitute a hazard to surface navigation [41].
The cluster analyses with the Jaccard and Bray Curtis dissimilarity matrix showed a clear clustering of transects by shallower terraces (10 > 20 m) and slopes. In dendrograms based on the Jaccard dissimilarity matrix that included corals and macroalgae, the deeper slopes were grouped by locations (Figure S4). In the dendrogram based on the Bray–Curtis matrix, the shallow slopes and deeper slopes were divided into different groups (Figure S5). Deep terraces were clustered with shallow slopes mainly. PERMANOVA analysis showed significant differences between depth, locations, and slope grade for these communities (Table 1), whereas nMDS analysis showed a clear separation between deep and shallow terraces, and between terraces and slopes.

Table 1.
PERMANOVA based on the Jaccard measures of similarity performed on species/samplematrix.
Specific richness for macroalgae was statistically significant among depth and location (two-way ANOVA, F203.71, p < 0.01), where Puerto Ángel shows greater richness relative to Huatulco (Figure 4A). For photosynthetic corals, the specific richness was statistically significant only with depth (two-way ANOVA, 8.86, p < 0.01); however, in the deep stratum, lower species richness can be observed at Huatulco compared with Puerto Ángel (Figure 4B). and no significant differences were found with depth or location for non-photosynthetic corals, although in the deeper level (2030 m) of Huatulco we observed a trend for higher number of species than in Puerto Ángel (Figure 4C).
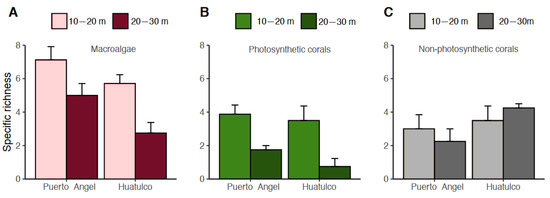
Figure 4.
Specific richness in the two-depth strata and locations: (A) macroalgae; (B) photosynthetic corals; and (C) non-photosynthetic corals.
Coral community structure changes were observed between the depth strata and with the variation in slope grade, which was reflected in different coral compositions through the “seascapes” (Figure 5). The spatial ordination of coral community structure (nMDS) revealed a clear separation between seascapes (Figure 5A), which present significant differences (ANOSIM, R2 = 0.57, p < 0.01), together with the associated variables “slope grade” (ANOSIM, R2 = 0.44, p < 0.01), and depth (ANOSIM, R2 = 0.21, p < 0.05). No differences were found among locations (ANOSIM, R2 = 0.043, p = 0.163).
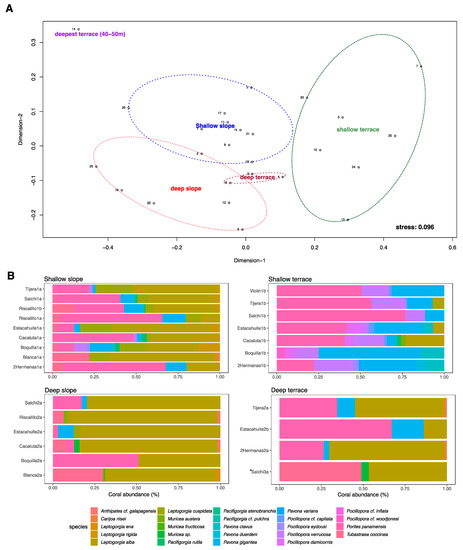
Figure 5.
Non-metric multidimensional scaling ordination using photosynthetic and non-photosynthetic coral composition from the two-depth stratum (10 < 20 m and 20 < 30 m): (A) each ellipse represents one type of seascape based on Bray–Curtis dissimilarity matrices (2D, stress = 0.096); and (B) total cumulative percentage of the relative abundance of the 24 coral taxa in the different seascapes. * Only one transect of 40–50 m located in Puerto Ángel.
SIMPER test showed that Porites panamensis and Pavona gigantea were the photosynthetic corals that contributed most to the differences found between shallow and deep slopes (Figure 5B). Figure 5 also illustrates the differences observed in the photosynthetic coral composition between the benthic communities located on the shallow terrace and shallow slope. Pavona and Pocillopora species were significantly more abundant at the shallow terrace, whereas the differences for non-photosynthetic corals were better explained by Leptogorgia cuspidata and Tubastrea coccinea. However, the species that contributed most to the differences found between the shallow and deep terrace were Pocillopora verrucosa (more abundant in shallow terrece), Porites panamensis, and Leptogorgia alba (the most abundant species in deep terrace), whereas Leptogorgia alba, Leptogorgia cuspidata, and Pocillopora verrucosa were the species that better explained the differences observed between the shallow terrace and the shallow slope (Figure 5B).
Furthermore, differences along the depths in photosynthetic corals densities were also observed between the terraces and slopes (Figure 6). A greater density of colonies was found in Huatulco between 10 and 20 m compared to Puerto Ángel. However, between 20 and 30 m these differences were reverted. Interestingly, the sizes of these coral genera were contrasting. Porites panamensis presented great abundance and density of little colonies, with more than 500 colonies smaller than 25 cm. Among the bigger colonies, Pavona and Pocillopora presented the highest frequency of medium sizes (25 > 50 cm) with about 90 and 70 colonies, respectively. Differentially, Pavona presented bigger colonies (50 and 40) in the largest categories compared to Pocillopora. In the deep layer (20 < 30 m), the differences were very notorious, beginning with the absence of pocilloporids, and Porites panamensis presented a significant reduction by more than 1/3 in its number of colonies; also, Pavona presented a reduction of their colonies of its most frequent size category by 1/6, reducing to only 16 colonies, and registered a total of 47 colonies including all categories (Figure S6).
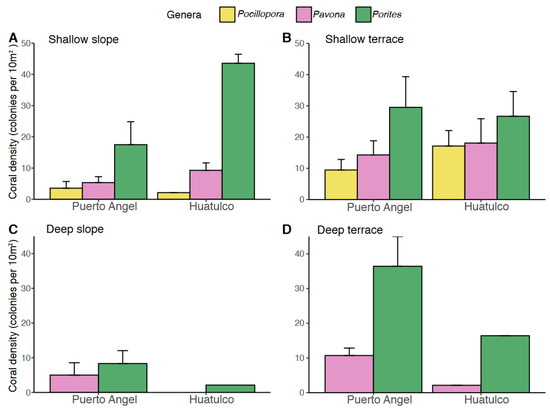
Figure 6.
Variations in coral density at the different seascapes characterized: (A) shallow slope; (B) shallow terrace; (C) deep slope; and (D) deep terrace; in the three genera: Pocillopora (yellow), Pavona (pink) and Porites (green).
Changes in photosynthetic coral morphology were observed associated with the reduction in light availability with depth, at genera and species/morphotype levels. Branching species were absent below the z10% confidence interval limits, where only semi-massive, foliaceus and encrusting morphologies were present. In shallow depths, the branching genus, Pocillopora, dominates, growing in extensive reef-plate shapes shallower than 10 m (>z25%) (Figure 7A). Below these physical and optical depths, Pocillopora morphotypes change to flat and thick branching morphologies, growing as isolated colonies (Figure 7B,C). On the other side, the depth generalist Pavona genus was found as semi-massive species/morphotypes between 5–21 m (Figure 7D). At these depths, foliaceous morphotypes were also present, although they only dominate at the lowest depth distribution limit of this genus (Figure 7E,F). The principal genus and species that contributes significantly to modify the submarine relief in the mesophotic environment is Pavona gigantea. This species can construct large tridimensional structures (>1 m) until 22 m depth. Below this threshold, only colonies smaller than 70 cm were present. The other depth generalist genus was Porites, which presents encrusting colony morphologies of small size from 10 to 28 m (Figure 7G–I).

Figure 7.
Variation in coral morphology with depth for: (A–C) Pocillopora; (D–F) Pavona; (G–I) Porites panamensis; and (J–L) Leptogorgia alba.
4. Discussion
The vertical distribution of photosynthetic corals in the Southern Mexican Pacific was found in this study to reach deeper depths relative to the values documented previously. The deepest records of Pavona and Porites in the Parque Nacional Huatulco, were found at 20 and 24 m, respectively, and at 25.5 and 27.8 m in Puerto Ángel (Oaxaca), whereas they were previously determined at 15 m and 18 m, respectively [23]. These maximum depths were correlated with local changes in light attenuation within the water column, in agreement with previous works for clear waters [42], or turbid waters [22,43]. Associated with this pattern in this study we documented a greater algal richness at Puerto Ángel, but no difference in coral cover at 10–20 m depth. The loss of coral diversity with depth occurred at a shallower threshold at Huatulco than at Puerto Ángel (Figure 4 and Figure 6). From Figure 1, we can see that the spatial variability in water transparency (more transparent waters in Puerto Angel) suggests a correlation with the loss of diversity of photosynthetic organisms. This interpretation is supported by recent studies indicating the importance of water transparency and the photosynthetic capacity to use the available energy in promoting coral diversity and structuring coral communities [44]. However, other factors, such as species interactions, or the role of the intermediate disturbance hypothesis [45,46], have been traditionally more accepted in coral reef research for explaining the maintenance of species diversity.
Although photosynthetic corals can complement their dietary requirements through heterotrophy, especially with increasing depth [47], a logical reasoning could be that these photosynthetic corals with mixotrophic abilities reach greater depths than the phototrophic specialists (macroalgae). However, we found crustose coralline algae closer to z0.1%, and photosynthetic corals were limited to z1%. Thus, the dependence on the quality and quantity of light is important for maintaining their photosynthesis at a lower distribution, which could be related to the minimum amount of time above an absolute threshold of light intensity [42]. In our study, Porites panamensis could survive six months with an estimated average of 0.6% Es during the upwelling season. In addition, our results indicate that Pocillopora and Porites differentially adjust their depth limits towards a minimum percentage of surface irradiance, which in the central coast of Oaxaca are constrained to z10% and z1%, respectively.
In contrast, non-photosynthetic corals did not show the same pattern. The Huatulco area presents the highest species richness, with a notable difference in the deep stratum (20 < 30 m) compared to Puerto Angel. The reason for these differences deserves more attention in future studies. At present, there is no evidence that upwellings induce coral richness in Huatulco since previous studios on gorgonians [28] found greater species richness with depth at Puerto Ángel. However, gorgonians are non-photosynthetic, and may respond positively to high upwelling due to increased food delivery. The differences found in our study could be related to the historically intensive artisanal fishing at Puerto Ángel and the extraction of black coral for jewelry [29,48] before its prohibition in 2001 [49], together with the declaration of a natural protected area at Huatulco in 1998 [50].
The relevance of linking the variability of light levels with ecological data for understanding mesophotic boundaries has been pointed out previously [11,20,51]. Furthermore, some of these studies have presented robust methodologies for detecting potential boundaries between shallow and mesophotic scleractinian assemblages [20,52]. However, these methodologies are generally expensive and difficult to replicate.
Considering that light availability is the primary factor determining the operation and biological composition of aquatic ecosystems [32], we emphasized in our analysis the use of light metrics for investigating their possible association with the location and structure of the mesophotic zone. Given the controversy over the bathymetric boundaries of MCEs and the different terms that have emerged [16,53], it is necessary first to define a common mesophotic zone. For this, focus should be directed towards the semantics of the word “mesophotic”, which refers to intermediate light conditions, and on the variable nature of the distribution of photosynthetic benthic communities along the entire depth gradient, where light levels encompass five orders of magnitude, from z100% on the surface to z0.01%. By excluding the extremes of this variability (shallow areas where coral reefs could develop from z100% to z10%; and the deeper “photic” zone, which ranges from z0.1% to z0.01%, where crusty coralline algae have been reported [32,54], the mesophotic zone for the establishment of MCEs or MEs prevails as the intermediate light zone for benthic marine photosynthesis and primary production (Figure 8). This image only shows the mesophotic boundaries based on optical depths, that should be the same worldwide. However, in turbid waters, the mesophotic zone is shallower and narrower (12–70 m in the central coast of Oaxaca) than the clearest waters (see our previous work [22] for a comparison of the mesophotic optical boundaries worldwide with different values of KdPAR).
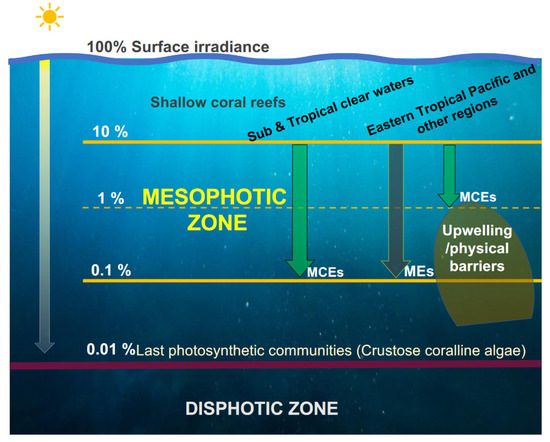
Figure 8.
Total ranges of photosynthetic communities in the percentage of surface irradiance (Es%). In the middle of this variability (mesophotic zone), the MCEs or MEs are developed. In the Mexican Pacific and most of the ETP, the MCEs are limited in the upper mesophotic zone, while the clearest waters reach the bottom of the lower mesophotic zone.
Our results confirmed this understanding, underlining the use of intermediate light levels (z10%, z1%, and z0.1%) for benthic photosynthesis as proxies of mesophotic boundaries, determined by specific taxa, which can be used as bioindicators. Our analysis also revealed that the importance of light for the determination of mesophotic coral communities not only requires attention to spatial variation in the light field but also to the integrative consequences of temporal variation of light availability. For example, higher variability in water transparency over time results in wider mesophotic boundaries, as we described in the waters of the central coast of Oaxaca reported in this study (Figure 3). In contrast, the lower seasonal variation reported in the Red Sea, which results in narrower mesophotic boundaries (see Tamir et al. [20], Figure 1).
Long-term records in water transparency using satellite Kd data have been confirmed to be powerful tools (almost two decades for Aqua-MODIS), encompassing great variability over time [21,55]. These data have been found to present fewer associated errors than the Secchi disk data [56]. Furthermore, they allow estimating spatial and temporal variability in the light levels at different locations and seasons and correlate this variation with local changes in the mesophotic zone boundaries. Remote sensing approaches, therefore, facilitate descriptions of changes in water transparency at larger scales and over larger periods of time, compensating for the disadvantage of local, in-situ, ecological descriptions, enhancing the capacity of defining the MCEs indicated by Laverick et al. [52].
Understanding the role of light in the establishment of mesophotic boundaries requires investigating the minimum light requirements for the development of MCEs i.e., the maximum level of light attenuation (Kd) that allows the development of MCEs at their upper boundaries, without compromising the potential role of MCEs as coral refuges.
The most conspicuous changes in the structure of photosynthetic coral communities along the depth gradient were the loss of branching species in shallow areas. This is the case of Pocillopora genera, the shallow specialists. In contrast, the rise in dominance of depth-generalists was expressed, first, as a reduction of Pavona species with depth, and later in rises in foliaceous morphologies of Pavona gigantea and encrusting colonies of Porites panamensis. This pattern was previously reported for the Mexican Pacific [22,57] and similarly for the Red Sea [20]. However, the variation of colony morphology could also be used in the recognition of mesophotic boundaries. For example, the distribution of the branching genera in the study area (i.e., Pocillopora) matches with the upper mesophotic limit (~z10%), similarly to Acropora genera distribution in the Red Sea [20].
On the other hand, field observations and the spatial ordination of mesophotic community revealed that geofeatures with different slope grades influence the spatial patterns of benthic biodiversity, as previously indicated [58]. In addition, our study also indicates that the degree of slope of different geofeatures has significantly impacted corals and macroalgae assemblages. The last was previously pointed out by Lesser et al. [47,59], who indicated that the variation in irradiance induced by the changes in substrate angle could explain the organization of different MCE communities at the same depths, in agreement with our interpretation (Figure 5 and Figure 6).
In addition to the role of light in explaining the structural variation of coral reef communities, we cannot overlook in this study the potential contribution of other environmental factors associated with the upwellings, such as the reductions in temperatures and pH or the potential contribution of coastal hydrodynamics in driving coral larvae far offshore and reducing coral recruitment of the local community. All these factors can also contribute synergically to modifying this community structure in vertical [60] and horizontal distributions.
Finally, it is also important to remark that the presence of MCEs is not so evident due to their deep distribution and distance from the coast, two factors that have received low attention from local researchers in the Mexican Pacific. In addition, the distribution of the coral reefs of the central coast of Oaxaca is generally limited to the bays, where the depths do not exceed 15 m. This characteristic has facilitated the investigation of these shallow coral ecosystems. In contrast, beyond 15 m, the Mexican Pacific still has scarce published data on coral communities and explorations outside the bays on the central coast of Oaxaca. Moreover, in this study, we did not find substrate availability in moderate, gentle or null slopes between 28 and 40 m which suggest that Pavona and Porites panamensis could colonize slightly deeper areas than the reported values in this study.
5. Conclusions
It has been recognized that the optical properties of the water column have strong correlations with MCE community patterns [42,47]. Here, we found that it is possible to set for the Southern Mexican Pacific and ETP the bathymetric boundaries of MCEs and MEs using the intermediate optical depths for benthic photosynthesis, directly associated with the water column optical properties, as they determine differences in photoacclimation and species distribution with depth. Therefore, our analysis concluded that these bathymetric boundaries could be recognized using these optical depths together with taxa-specific indicators. Nevertheless, more worldwide research is needed to confirm the utility of light metrics to determine MCE zonation patterns and reach a more solid definition for MCEs and other mesophotic environments.
Our study supports the primary role of light in explaining the structural variation and depth distribution of Mesophotic Coral Ecosystems. However, it requires attention in future studies in their spatial and temporal variation implications linked with the variability of optical properties of the water column. On the central coast of Oaxaca, the seasonal upwellings induce significant changes in species composition and abundance of mesophotic communities, macroalgal richness, and photosynthetic corals densities by reducing light availability, depending on the magnitude of these events on light attenuation in the water column. This results in a limited development of MCEs constrained at z1% in this area of the Mexican Pacific and could be similar in other upwelling zones worldwide, mainly in some areas of ETP with similar characteristics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15040531/s1, Figure S1: Geofeatures and their slope grade; Figure S2. Boxplots showing b-dispersion test; Figure S3. Variability of KdPAR calculated from MODIS-Aqua satellite data (Kd490); Figure S4. Dendrogram for hierarchical clustering of the 26 sites using group-average linking of Jaccard dissimilarities matrix (presence/absence). Figure S5. Dendrogram for hierarchical clustering of the 26 sites, using group-average linking of Bray-Curtis dissimilarities calculated on log (x + 1) transformed abundance data. Figure S6. Size frequency distributions of photosynthetic corals genera in the two depth strata. Table S1: Mesophotic optical depths during upwelling and non-upwelling months; Table S2. List of benthic species in the study locations.
Author Contributions
Conceptualization, methodology, formal analysis: M.Á.P.-C.; investigation: M.Á.P.-C. and G.E.L.-M.; writing—original draft preparation: M.Á.P.-C.; writing—review and editing: M.Á.P.-C., S.E., G.E.L.-M., G.E. and G.H.-A.; visualization, M.Á.P.-C. and S.E.; supervision: S.E. and G.E.; project administration and funding acquisition: G.H.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a National Geographic Society grant (#NGS-62129C-19).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Acknowledgments
We appreciate the support of Edgar Cruz-Acevedo on the statistical analyses and we thank Israel Moreno for his assistance during the fieldwork. In addition, we are grateful for the assistance of Cindy Fernández-García and Julio A. Acosta-Calderón in the identification of macroalgae. The author M.Á.P-C appreciates the advice of C. Tatiana Galindo-Martínez during the submission process. The Mexican Consejo Nacional de Ciencia y Tecnología (CONACyT) is acknowledged for providing a four-year PhD fellowship to M.Á.P.-C. We thank CIIDIR for the access provided to its facilities and the UMAR for the access to its Museum of Natural History during the research internship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roberts, C.M.; McClean, C.J.; Veron, J.E.; Hawkins, J.P.; Allen, G.R.; McAllister, D.E.; Werner, T.B. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 2002, 295, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Barnes, M.L.; Bellwood, D.R.; Cinner, J.E.; Cumming, G.S.; Jackson, J.B.C.; Kleypas, J.; Van de Leemput, I.A.; Lough, J.M.; Morrison, T.H.; et al. Coral reefs in the Anthropocene. Nature 2017, 546, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Glynn, P.W. Coral reef bleaching: Facts, hypotheses, and implications. Glob. Change Biol. 1996, 2, 495–509. [Google Scholar] [CrossRef]
- Reyes-Bonilla, H.; Carriquiry, J.; Leyte-Morales, G.; Cupul-Magaña, A. Effects of the El Niño-Southern Oscillation and the anti-El Niño event (1997–1999) on coral reefs of the western coast of México. Coral Reefs 2002, 21, 368–372. [Google Scholar] [CrossRef]
- Leyte-Morales, G.E.; Bonilla, H.R.; Cintra-Buenrostro, C.E.; Glynn, P.W. Range extension of Leptoseris papyracea (Dana, 1846) to the west coast of Mexico. Bull. Mar. Sci. 2001, 69, 1233–1237. [Google Scholar]
- Smith, T.B.; Glynn, P.W.; Maté, J.L.; Toth, L.T.; Gyory, J.A. Depth refugium from catastrophic coral bleaching prevents regional extinction. Ecology 2014, 95, 1663–1673. [Google Scholar] [CrossRef]
- Hinderstein, L.M.; Marr, J.C.A.; Martinez, F.A.; Dowgiallo, M.J.; Puglise, K.A.; Pyle, R.L.; Appeldoorn, R. Theme section on “Mesophotic coral ecosystems: Characterization, ecology, and management”. Coral Reefs 2010, 29, 247–251. [Google Scholar] [CrossRef]
- Baker, E.K.; Puglise, K.A.; Harris, P.T. Mesophotic Coral Ecosystems: A Lifeboat for Coral Reefs? The United Nations Environment Program and GRID-Arendal, Nairobi and Arendal: Nairobi, Republic of Kenya, 2016; 98p. [Google Scholar]
- Pomar, L. Types of carbonate platforms: A genetic approach. Basin Res. 2001, 13, 313–334. [Google Scholar] [CrossRef]
- Puglise, K.A.; Hinderstein, L.; Marr, J.C.A.; Dowgiallo, M.J.; Martinez, F.A. Mesophotic coral ecosystems research strategy: International Workshop to prioritize research and Management needs for Mesophotic Coral Ecosystems. In Proceedings of the NOAA National Centers for Coastal Ocean Science, Jupiter, FL, USA, 12–15 July 2008; p. 24. [Google Scholar]
- Laverick, J.H.; Tamir, R.; Eyal, G.; Loya, Y. A generalized light-driven model of community transitions along coral reef depth gradients. Glob. Ecol. Biogeogr. 2020, 29, 1554–1564. [Google Scholar] [CrossRef]
- Spalding, H.L. Ecology of Mesophotic Macroalgae and Halimeda Kanaloana Meadows in the Main Hawaiian Islands. Ph.D. Dissertation, University of Hawaii, Honolulu, HI, USA, August 2012. [Google Scholar]
- Meirelles, P.M.; Amado-Filho, G.M.; Pereira-Filho, G.H.; Pinheiro, H.T.; De Moura, R.L.; Joyeux, J.C.; Thompson, F.L. Baseline assessment of mesophotic reefs of the Vitória-Trindade Seamount Chain based on water quality, microbial diversity, benthic cover and fish biomass data. PloS ONE 2015, 10, e0130084. [Google Scholar] [CrossRef]
- Soares, M.D.O.; Tavares, T.C.L.; Carneiro, P.B.D.M. Mesophotic ecosystems: Distribution, impacts and conservation in the South Atlantic. Divers. Distrib. 2018, 25, 255–268. [Google Scholar]
- García-Sais, J.R.; Castro-Gomez, R.L.; Sabater-Clavell, J.; Esteves, R.; Williams, S.; Carlo, M. Mesophotic Benthic Seascape and Associated Marine Communities at Abrir La Sierra, Puerto Rico; Draft Final Report NOAA Grant FNA07NMF4410117; Mesophotic.org: Lajas, PR, USA, 2010. [Google Scholar]
- Costa, B.; Kendall, M.S.; Parrish, F.A.; Rooney, J.; Boland, R.C.; Chow, M.; Lecky, J.; Montgomery, A.; Spalding, H. Identifying suitable locations for mesophotic hard corals offshore of Maui, Hawai’i. PLoS ONE 2015, 10, e0130285. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M.P.; Slattery, M.; Leichter, J.J. Ecology of mesophotic coral reefs. J. Exp. Mar. Biol. Ecol. 2009, 375, 1–8. [Google Scholar] [CrossRef]
- Dubinsky, Z.; Falkowski, P. Light as a source of information and energy in zooxanthellate corals. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 107–118. [Google Scholar]
- Enríquez, S.; Méndez, E.R.; Hoegh-Guldberg, O.; Iglesias-Prieto, R. Key functional role of the optical properties of coral skeletons in coral ecology and evolution. Proc. R. Soc. B Biol. Sci. 2017, 284, 20161667. [Google Scholar] [CrossRef] [PubMed]
- Tamir, R.; Eyal, G.; Kramer, N.; Laverick, J.H.; Loya, Y. Light environment drives the shallow-to-mesophotic coral community transition. Ecosphere 2019, 10, e02839. [Google Scholar] [CrossRef]
- Turak, E.; DeVantier, L. Reef-building corals of the upper mesophotic zone of the central Indo-west Pacific. In Mesophotic Coral Ecosystems; Loya, Y., Puglise, K.A., Bridge, T.C.L., Eds.; Springer: New York, NY, USA, 2019; pp. 621–651. [Google Scholar]
- Pérez-Castro, M.Á.; Schubert, N.; De Oca, G.A.M.; Leyte-Morales, G.E.; Eyal, G.; Hinojosa-Arango, G. Mesophotic Coral Ecosystems in the Eastern Tropical Pacific: The current state of knowledge and the spatial variability of their depth boundaries. Sci. Total Environ. 2022, 806, 150576. [Google Scholar] [CrossRef]
- Glynn, P.W.; Leyte- Morales, G.E. Coral reefs of Huatulco, west México: Reef development in upwelling Gulf of Tehuantepec. Rev. Biol. Trop. 1997, 45, 1033–1048. [Google Scholar]
- Reyes-Bonilla, H.R.; López-Pérez, A. Biogeografía de los corales pétreos (Scleractinia) del Pacífico de México. Cienc. Ma. 1998, 24, 211–224. [Google Scholar] [CrossRef]
- Smith, T.B.; Maté, J.L.; Gyory, J. Thermal Refuges and Refugia for Stony Corals in the Eastern Tropical Pacific. In Coral Reefs of the Eastern Tropical Pacific. Coral Reefs of the World; Glynn, P.W., Manzello, D.P., Enochs, I.C., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 501–515. [Google Scholar]
- Chapa-Balcorta, C.; Hernandez-Ayon, J.M.; Durazo, R.; Beier, E.; Alin, S.R.; López-Pérez, A. Influence of post-Tehuano oceanographic processes in the dynamics of the CO2 system in the Gulf of Tehuantepec, Mexico. J. Geophys. Res. Oceans 2015, 120, 7752–7770. [Google Scholar] [CrossRef]
- Garciía-Reyes, M.; Largier, J.L. Seasonality of coastal upwelling off central and northern California: New insights, including temporal and spatial variability. J. Geophys. Res. Oceans 2012, 117, C03028. [Google Scholar] [CrossRef]
- Abeytia, R.; Guzmán, H.M.; Breedy, O. Species composition and bathymetric distribution of gorgonians (Anthozoa: Octocorallia) on the Southern Mexican Pacific coast. Rev. Biol. Trop. 2013, 61, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, C.J.N. Puerto Ángel (Oaxaca en México) en la economía-mundo: Una primera propuesta metodológica que busca el desarrollo regional. In Desigualdad Socio-Espacial, Innovación Tecnológica y Procesos Urbanos; Universidad Nacional Autónoma de México y Asociación Mexicana de Ciencias para el Desarrollo Regional, A.C., Ed.; UNAM: Ciudad de México, México, 2019; Volume 3. [Google Scholar]
- Morel, A.; Gentili, B.; Claustre, H.; Babin, M.; Bricaud, A.; Ras, J.; Tièche, F. Optical properties of the “clearest” natural waters. Limnol. Oceanogr. 2007, 52, 217–229. [Google Scholar] [CrossRef]
- Wang, M.; Son, S.; Shi, W. Evaluation of MODIS SWIR and NIR–SWIR atmospheric correction algorithm using SeaBASS data. Remote Sens. Environ. 2009, 113, 635–644. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems, 3rd ed.; Cambridge University Press: New York, NY, USA, 2011. [Google Scholar]
- AlgaeBase. Available online: https://www.algaebase.org (accessed on 23 May 2022).
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E., Ltd.: Plymouth, UK, 2001. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 7 October 2022).
- Oksanen, J. Vegan: Community Ecology Package, R Package Version 1.8-5. 2007. Available online: http://www.cran.r-project.org (accessed on 7 October 2022).
- Nichol, S.; Huang, Z.; Howard, F.; Porter-Smith, R.; Lucieer, V.; Barrett, N. Geomorphological Classification of Reefs—Draft Framework for an Australian Standard; Report to the National Environmental Science Program; Marine Biodiversity Hub: Geoscience, Australia, 2016; 27p. [Google Scholar]
- INEGI. Available online: https://www.inegi.org.mx/contenidos/temas/relieve/submarino/doc/glosa_relv_sub.pdf (accessed on 25 March 2022).
- Kahng, S.E.; Garcia-Sais, J.R.; Spalding, H.L.; Brokovich, E.; Wagner, D.; Weil, E.; Toonen, R.J. Community ecology of mesophotic coral reef ecosystems. Coral Reefs 2010, 29, 255–275. [Google Scholar] [CrossRef]
- Morgan, K.M.; Perry, C.T.; Smithers, S.G.; Johnson, J.A.; Daniell, J.J. Evidence of extensive reef development and high coral cover in nearshore environments: Implications for understanding coral adaptation in turbid settings. Sci. Rep. 2016, 6, 29616. [Google Scholar] [CrossRef]
- López-Londoño, T.; Gómez-Campo, K.; Hernández-Pech, X.; Enríquez, S.; Iglesias-Prieto, R. Photosynthetic usable energy explains vertical patterns of biodiversity in zooxanthellate corals. Sci. Rep. 2022, 12, 20821. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Dial, R.; Roughgarden, J. Theory of marine communities: The intermediate disturbance hypothesis. Ecology 1988, 79, 1412–1424. [Google Scholar] [CrossRef]
- Lesser, M.P.; Slattery, M.; Mobley, C.D. Biodiversity and functional ecology of mesophotic coral reefs. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 71. [Google Scholar] [CrossRef]
- The Pacific Coast of Mexico. Available online: https://www.tomzap.com/prison_p.html (accessed on 27 February 2023).
- D.O.F. Norma Oficial Mexicana NOM-059-ECOL-2001, Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones Para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo. Available online: https://dof.gob.mx/nota_detalle.php?codigo=735036&fecha=06/03/2002#gsc.tab=0 (accessed on 21 March 2023).
- D.O.F. Decreto Por el Que se Declara Área Natural Protegida, con el Carácter de Parque Nacional, la Región Conocida Como Huatulco, en el Estado de Oaxaca, con una Superficie Total de 11,980-98-00 Hectáreas. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=4888031&fecha=24/07/1998#gsc.tab=0 (accessed on 21 March 2023).
- Eyal, G.; Tamir, R.; Kramer, N.; Eyal-Shaham, L.; Loya, Y. The red sea: Israel. In Mesophotic Coral Ecosystems; Springer: Cham, Switzerland, 2019; pp. 199–214. [Google Scholar] [CrossRef]
- Laverick, J.H.; Andradi-Brown, D.A.; Rogers, A.D. Using light-dependent scleractinia to define the upper boundary of mesophotic coral ecosystems on the reefs of Utila, Honduras. PLoS ONE 2017, 12, e0183075. [Google Scholar] [CrossRef]
- Turner, J.A.; Andradi-Brown, D.A.; Gori, A.; Bongaerts, P.; Burdett, H.L.; Ferrier-Pagès, C.; Voolstra, C.R.; Weinstein, D.K.; Bridge, T.C.L.; Costantini, F.; et al. Key Questions for Research and Conservation of Mesophotic Coral Ecosystems and Temperate Mesophotic Ecosystems. In Mesophotic Coral Ecosystems. Coral Reefs of the World; Loya, Y., Puglise, K., Bridge, T., Eds.; Springer: Cham, Switzerland, 2019; Volume 12, pp. 989–1003. [Google Scholar] [CrossRef]
- Littler, M.M.; Littler, D.S. The Nature of Crustose Coralline Algae and Their Interactions on Reefs. Res. Discov. 2013, 199, 200–212. [Google Scholar]
- Hedley, J.D.; Roelfsema, C.M.; Chollett, I.; Harborne, A.R.; Heron, S.F.J.; Weeks, S.; Mumby, P.J. Remote sensing of coral reefs for monitoring and management: A review. Remote Sens. 2016, 8, 118. [Google Scholar] [CrossRef]
- Lee, Z.P.; Weidemann, A.; Kindle, J.; Arnone, R.; Carder, K.L.; Davis, C. Euphotic zone depth: Its derivation and implication to ocean-color remote sensing. J. Geophys. Res. Oceans 2007, 112, C03009. [Google Scholar] [CrossRef]
- Iglesias-Prieto, R.; Beltrán, V.H.; LaJeunesse, T.C.; Reyes-Bonilla, H.; Thomé, P.E. Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc. R. Soc. B Biol. Sci. 2004, 111, 1757–1763. [Google Scholar] [CrossRef]
- Rees, M.J.; Jordan, A.; Price, O.F.; Coleman, M.A.; Davis, A.R. Abiotic surrogates for temperate rocky reef biodiversity: Implications for marine protected areas. Divers. Distrib. 2014, 20, 284–296. [Google Scholar] [CrossRef]
- Lesser, M.P.; Curtis, D.M.; John, D.H.; Slattery, M. Incident light on mesophotic corals is constrained by reef topography and colony morphology. Mar. Ecol. Prog. Ser. 2021, 670, 49–60. [Google Scholar] [CrossRef]
- Cortés, J. Isla del Coco, Costa Rica, Eastern Tropical Pacific. In Mesophotic Coral Ecosystems. Coral Reefs of the World; Loya, Y., Puglise, K., Bridge, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 12, pp. 465–475. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).