Abstract
In contrast to birds, the relationship between migration and immunity has been scarcely studied in bats. We examined how the expression of the humoral portion of the constitutive immunity varied in a bat with partial, sex-biased migration: the lesser long-nosed bat (Leptonycteris yerbabuenae (Phyllostomidae)). The lesser long-nosed bat is a nectarivorous species distributed in the arid and semi-arid regions of North and Central America. We evaluated the bacteria-killing abilities (BKAs) of the plasma of male and female lesser long-nosed bats on the Pacific coast in different periods of the year. Because adult males are resident, they were used to explore the effect of reproductive activity on BKA, and we predicted higher values in mating males (i.e., individuals presenting scrotal testicles and a fresh dorsal patch). In contrast to males, most females migrate to cactus deserts in northern Mexico during pregnancy and lactation, and then return to the dry forests of west-central Mexico to mate. We predicted that the combined effect of breeding and migration would have an adverse effect on BKA; therefore, migratory pregnant and lactating females were expected to exhibit a lower BKA than mating females in west-central Mexico. We compared the BKAs of females captured in October and December in central Mexico, and we predicted that migratory females that had recently arrived in October should exhibit a lower BKA than females captured two months later. In addition, we compared the BKAs between lactating females and young in northern Mexico and predicted lower values in recently born individuals. We found that the BKAs of males were higher in reproductive individuals than in non-reproductive individuals. We found a significant difference in the BKAs between females at the two extremes of their migratory range: the values of pregnant females in Sonora and females in December were higher than those of females captured in October. Finally, we found no difference in BKAs between lactating females and young individuals. Our findings indicate that the basal levels of the innate humoral component are heightened in mating males, that this response is reduced in females that recently returned to their mating grounds, and that the constitutive immunity of young individuals matures early, probably in anticipation of the potential to encounter pathogens during their migration to west-central Mexico.
1. Introduction
Migration is the seasonal movement of animals from one habitat to another in search of food, better environmental conditions, or reproductive needs [1]. Migratory habits might allow animals to escape from infected habitats or individuals, remove infected individuals, and recover from infection [2,3,4]. Even if migration enables animals to reduce their pathogen exposure, several studies have shown that migratory species harbor a greater diversity of pathogens than non-migratory species [5,6]. There is therefore a close relationship between migration and the way in which animals deal with infectious agents. Migrating animals face a conflict between the allocation of the resources needed to move to a new site and the allocation of the resources needed to maintain and activate their immune system [7]. Vertebrates are equipped with innate and adaptive immunity to fight pathogens, but both components differ in the way they execute the recognition of danger signals. Innate immunity is the first line of defense against new pathogens, and adaptive immunity is more effective at defending against pathogens to which the animal has already been exposed. Migratory individuals are expected to maintain a strong innate response because they are potentially exposed to a high diversity of pathogens [8].
Although migration is an extensive phenomenon found among mammals [9], the effects of this life strategy on immunity have been scarcely studied. Bats are ideal organisms for studying the relationship between the immune system and migration since several bat species have independently adopted the migratory strategy [10,11]. Bats are associated with a wide variety of infectious agents [12,13], and they have immune adaptations that allow their coexistence with infectious agents that are lethal to other mammals [14,15]. Given that migratory bats are probably exposed to a higher diversity of infectious agents than non-migratory bats, the immune systems of both groups might be under different selective pressures [5,16]. The context under which the immunity of migratory bats is tuned to confront pathogens is further complicated because in some species, migratory individuals must face the additional costs of reproductive activities [10]. In contrast to birds [17,18,19,20], only a couple of studies have examined the relationship between migration and the immune system in bats [21,22]. Nathusius’ pipistrelle (Pipistrellus nathusii) presents higher baseline lymphocyte levels, lower neutrophil levels, and similar haptoglobin levels during its migration period compared to the levels during its pre-migration period, but its baseline plasma haptoglobin levels increase significantly only in migratory bats after an immune challenge [21]. Migratory male silver-haired bats (Lasionycteris noctivagans) have lower IgG levels, higher neutrophil levels, and higher neutrophil/lymphocyte ratios than resident males, yet the bacteria-killing ability (BKA) is similar between the migratory and resident males [22]. Similarly scarce are studies examining the link between reproductive activity and the immune response in bats in general [22,23,24,25]. The inflammatory responses of Myotis myotis [23] and M. vivesi [24] and the BKA of M. vivesi [24] are lower in pregnant females, and females have higher IgG concentrations during pregnancy and higher hemolysis during lactation in Myotis daubentonii [25]. Non-reproductive males have higher concentrations of white blood cells in M. daubentonii [25], higher inflammatory responses in M. vivesi [24], and lower BKAs in Desmodus rotundus [26] than reproductive males.
We explored the relationships between the constitutive innate humoral immunity and the migratory condition and reproductive activity of male and female lesser long-nosed bats (Leptonycteris yerbabuenae (Phyllostomidae)). The lesser long-nosed bat is a nectarivorous species distributed in the arid and semi-arid regions of North and Central America [27]. The population genetic structure of L. yerbabuenae is strongly influenced by the migratory habits of females and the philopatric tendency of males [28,29,30,31]. Individuals of the lesser long-nosed bat along the Pacific coast present a sex-biased migration that is synchronized with reproductive activity. Pregnant females of this species migrate around 1500 km along the Pacific coast to northern Mexico and the south-western USA in spring–summer, where they have their young, while males and some females remain throughout the year in west-central Mexico [32]. We evaluated the BKAs of plasma of male and female lesser long-nosed bats on the Pacific coast in different periods throughout the year. The BKA of plasma measures the capacity of the humoral component of the blood (specifically the complement system) to inhibit the growth of a certain microorganism [33,34,35,36], and it is thus a critical element, as the first line of defense against potential pathogens. Because adult males are resident, they were used to explore the effect of reproductive activity on BKA. In general, testosterone functions as an immunosuppressor [37], but BKA and its main associated immunological component, the complement system, are upregulated by testosterone [38,39]; therefore, we hypothesized that BKA would be lower in non-reproductive males than in mating males. In contrast to males, most females migrate to cactus deserts during pregnancy and lactation, and then return to dry forests to mate. Conflicting predictions arise for the effect on the immune system of these females: physiological changes during pregnancy can increase the complement activity [40,41], but the high nutritional demands of pregnancy could be detrimental to immune function [42,43]. Additionally, these females must maintain a strong immune system to confront the diversity of the potential pathogens found during their journey [44]. We compared the BKAs of females on the two legs of their migratory journey. We hypothesized that the combined effect of breeding and migration would have an adverse effect, resulting in a lower BKA when they migrated north to give birth than in females that returned to mate to west-central Mexico. We also expected that non-pregnant or lactating females that recently arrived in west-central Mexico would exhibit lower BKA values than females captured a few months after their arrival. We took advantage of the presence of young born in northern Mexico to test the hypothesis that their immune system was still immature [45] and that their BKA would therefore be lower than in lactating females. Finally, we measured the BKA of females that did not migrate to the north to breed, but we did not formally compare them with the migratory females due to the small sample size.
2. Materials and Methods
2.1. Study Sites and Sample Collection
We followed the guidelines of the American Society of Mammalogists [46] to capture and handle bats. Individuals of L. yerbabuenae were captured in west-central Mexico in a cave located on Don Panchito Island, ~1 km off the coast of Chamela, Jalisco (19°32′08.4″ N, 105°05′18.8″ W), in March 2019 and 2020 and in May, October, and December 2019 (Figure 1). The population of L. yerbabuenae on Don Panchito Island has seasonal changes in its size and composition [32,47]. During October–January, the population is at its largest number and is equally composed of males and females. In February, most adult females migrate to northern Mexico and the south-western USA to give birth. The remaining population in west-central Mexico is nearly exclusively composed of males throughout August, although some females remain in the cave. Lactating females have been reported in the cave at Don Panchito Island in January–July [32,47], but is it uncertain if they are part of the resident population of this cave or if they originate from other caves in the region. In September, the size of the population increases abruptly with the return of the females from the north to mate with the resident males [32,47]. Individuals in northern Mexico were collected in Mariana cave near Carbó, Sonora (29°35′25.9″ N, 110°48′8.9″ W), in May and July–August 2019 (Figure 1). The population in this cave is exclusively composed of pregnant and lactating females with their young. We cannot be certain that females migrate specifically between Don Panchito Island and Mariana cave, but we assumed that the study sites are representative of the general migratory range of L. yerbabuenae in this part of its distribution. We captured bats with mist nets placed near the entrances of both caves. We recorded the sex, age category (adult or subadults), reproductive condition, and body mass (Ohaus, Parsippany-Troy Hills, NJ, USA, ±0.1 g) of captured individuals and placed each bat in an individual cloth bag. We estimated the age category of a bat by examining the epiphyseal–diaphyseal fusion of the 4th metacarpal–phalangeal joint: bats with open joints were classified as subadults, and bats with fused joints were classified as adults [32,48]. Young individuals that were recently born in Sonora were distinguished from older subadults because they presented short grayish hair, a broad epiphalangeal suture, and a smaller body size. Males of L. yerbabuenae and its congener L. curasoae develop a dorsal patch during the mating season [49], and the odor released by this structure is involved in mate choice by females [50]. Adult males on Don Panchito Island only produce semen and present dorsal patches in September–January [51]. Therefore, we classified adult males as reproductive when they presented a fresh dorsal patch and scrotal testicles and as non-reproductive when they presented inguinal or scrotal testicles with no dorsal patch or with a dry dorsal patch (Figure 2). We classified females as pregnant by palpating their abdomens and as lactating if they presented conspicuous, pink, hairless nipples that released milk after being pressed. Females with dark and keratinized nipples were considered post-lactating. We punctured the antebrachial vein of each bat with a needle (30G × 1/2″) to collect ~100 μL of blood with a micropipette with a sterile tip that was previously treated with heparin (Inhepar®, 5000 Ul/mL). The blood was placed in sterile heparin-impregnated Eppendorf tubes and centrifuged at 6000 rpm for 4 min, after which the plasma fraction was separated, placed in cryotubes submerged in liquid nitrogen, and transported to a laboratory, where the samples were maintained at −70 °C. Analyses of the BKA of bat plasma are resilient to the stress caused by manipulation before the samples are collected [52,53], and our samples were collected, on average, in less than half an hour after capture.
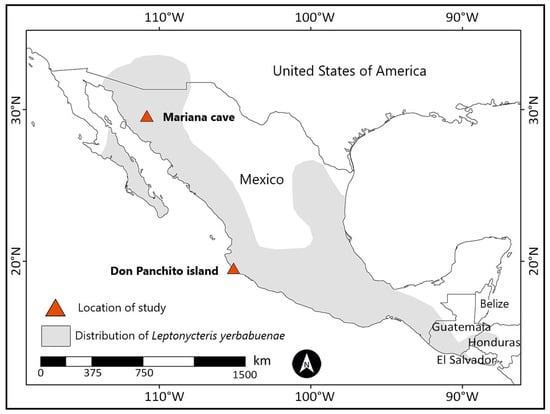
Figure 1.
Geographical distribution of the lesser long-nosed bat (Leptonycteris yerbabuenae) [26], with sample collection sites in Mexico during 2019–2020.
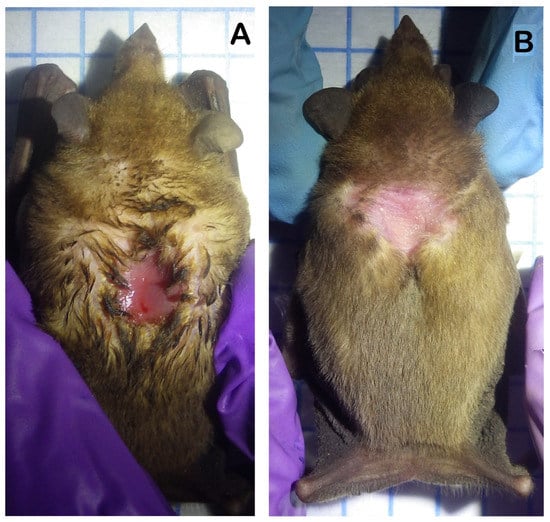
Figure 2.
Male lesser long-nosed bats (Leptonycteris yerbabuenae) captured in west-central Mexico presenting different stages of development of their dorsal sebaceous patches. (A) shows a male with a fresh dorsal patch characterized by the irritation of the tissue and the presence of body fluids in the hair surrounding the patch. (B) shows a male with a dry dorsal patch in which there are no signs of body fluids in the surrounding hair and there is no tissue irritation.
2.2. Measurement of Bacteria-Killing Ability
We measured the BKA of plasma with Escherichia coli (ATCC #8739; Microbiologics, St. Cloud, MN, USA) using the spectrophotometric method described by Liebl and Martin [54] and French and Neuman-Lee [55] with some modifications. This Gram-negative bacteria stimulates the constitutive immune response [33], and it has previously been used in several bat studies [24,25,26,56,57,58]. The constitutive immunity is a combination of the humoral (e.g., natural antibodies, complement, and acute phase proteins) and cellular components (e.g., macrophages, heterophils, and thrombocytes) of the immune system that establish the first line of defense against invading pathogens. All the procedures were conducted in a laminar flow hood using ethanol-sterilized and/or autoclaved equipment and disposable materials. Lyophilized pellets of E. coli were reconstituted in a phosphate-buffered solution (PBS) and diluted to a working concentration of 105 bacteria/mL. Plasma samples were thawed only once, and they were used immediately. We used 96-well conical-bottom cell culture microplates (Sarstedt, Nümbrecht, Germany, 83.3926.500) to plate 9 replicates of positive controls (18 µL of 0.01 M sterile PBS; Sigma Aldrich, St. Louis, MO, USA), 6 replicates of negative controls (18 µL of PBS), 9 replicates of an antibiotic control (2 µL of ampicillin at a concentration of 0.05 µg/mL in 16 µL of PBS), and 3 replicates of each sample (2 µL of plasma diluted in 16 µL of PBS). We then added 4 µL of a bacterial suspension with a concentration of 105 bacteria/mL to all wells except the negative controls. The plate was covered while still in the hood and incubated at 200 rpm and 37 °C for 30 min. After this initial incubation, 128 µL of nutrient broth (BD 234,000) was added to each well, and the absorbance of the plate was recorded at a wavelength of 600 nm (Epoch Microplate Reader, Biotek, Winooski, VT, USA). Following this measurement, the plate was incubated for a second time at 200 rpm for 12 h at 37 °C. Then, we measured the absorbance at 600 nm (Epoch Microplate Reader, Biotek). The first set of absorbance measurements was the initial absorbance (IA), and the second set was the final absorbance (FA) of the samples and controls. The negative control was used to ensure that there was no contamination, and the antibiotic control was used to verify that the bactericidal action could be evaluated with the assay, but they were not used in the final calculation of BKA. The calculation of plasma BKA was determined as the percentage of bacteria eliminated by the plasma in relation to the positive control, using the mean of the replicates for each parameter, with the following formula:
2.3. Data Analyses
All analyses were conducted in R [59]. We compared variables with deviance analyses using the ANOVA function in the car package [60]. We used the fitdistrplus package [61] to identify the probability function that best fit the data. Accordingly, we used a general linear model with a lognormal distribution to compare the body masses and used a generalized linear model with a zero-inflated beta distribution to compare the BKAs (Statistical models, Supplementary Materials S1). We used the glmmTMB package for the BKA comparisons [62]. Post hoc comparisons were made, when appropriate, with Tukey’s tests using the multcomp package [63]. For adult males, we compared the body mass and BKA values of individuals with inguinal or scrotal testicles captured in March and May, individuals with scrotal testicles but with no dorsal parches captured in October, and individuals with scrotal testicles and fresh dorsal patches captured in December. We pooled the males from March 2019, May 2019, and March 2020 into a single group (March–May) because there were no significant differences in body mass and BKA among them (results not shown). Adult males that appeared to be in a post-mating stage (i.e., individuals with dry dorsal patches and scrotal testicles captured in December) were not included in the comparison due to the small sample size. For adult females, comparisons were made considering both the reproductive and migratory status; accordingly, we compared pregnant and lactating individuals collected in Sonora in May and July–August and individuals captured in Jalisco with no external signs of pregnancy or lactation captured in October and December. Adult females captured in October and December had no external signs of pregnancy or lactation and were classified as non-reproductive. However, it is possible that these females were in the pre-mating or mating stages based on reports about this population [51]. We did not include post-lactating and non-reproductive females captured in Sonora or lactating, post-lactating, and non-reproductive females captured in Jalisco in March–May in the comparison due to their small sample sizes. We detected outliers using Cook’s distance and removed them from the analyses (3 adult females and 2 adult males for the BKA comparisons and 1 adult male for the body mass comparison). We did not include body mass as a covariate in the BKA comparison of males because the assumption of the independence of the covariate and the treatment effect [64] was not met (see Section 3). Because the mass of the pregnant females was affected by the mass of their fetus, we did not include them in the body mass comparison, and we did not include body mass as a covariate in the BKA comparison of females. A previous bat work reported significant differences in BKAs between sexes [25]; however, we did not compare the BKA values of non-reproductive adult males and females collected in the same period at the same site (March and May in Jalisco) because the sample size for females was low. We compared the body masses and BKAs of young individuals and lactating females collected in May–August in Sonora. We did not compare the age categories in Jalisco because the sample size of the subadult individuals was small for any given period (March 2019: n = 3; March 2020: n = 4; May 2019: n = 2; December 2019: n = 4). All models were validated with residual analyses using the DHARMa package [65].
3. Results
3.1. Reproductive Status and Body Mass
All adult males captured in March 2019 (n = 10) and 2020 (n = 15) had inguinal testicles, and adult males captured in May had inguinal (n = 17) or scrotal testicles (n = 5), males in October had scrotal testicles but no dorsal patches (n = 13), and males captured in December presented scrotal testicles with fresh (n = 7) or dry dorsal patches (n = 3). There were significant differences in body mass among adult males (F2, 60 = 6.43, p = 0.003, n = 63; Figure 3A): reproductive males were heavier than non-reproductive males in March–May (p = 0.001) and non-reproductive males in October (p = 0.021), and no difference was found between the last two groups (p = 0.785). Most adult females in Jalisco were captured in October and December with no external signs of pregnancy or lactation, and a small number of lactating (n = 2), post-lactating (n = 1), and non-reproductive females (n = 4) were captured in March and May. Most adult females captured in Sonora were pregnant in May (n = 10; lactating: n = 6), and most were lactating in July–August (n = 13; pregnant: n = 1; post-lactating: n = 4; non-reproductive: n = 4). There were significant differences in body mass among adult females (F2, 37 = 29.2, p < 0.001, n = 40; Figure 3B): lactating females in May and July–August were heavier than females in Jalisco in October (p < 0.001) and December (p < 0.001), with no significant difference between the last two groups (p = 0.44). Young individuals (n = 18) were captured in July–August in Sonora. Body mass was significantly different between young and adult females (F2, 34 = 34.4, p < 0.001, n = 37; Figure 3C): lactating females had a higher body mass than young males (p < 0.001) and young females (p < 0.001), but body mass did not differ between the sexes in young bats (p = 0.27). The body mass values of groups not included in the comparisons due to small sample sizes are shown in Table 1.
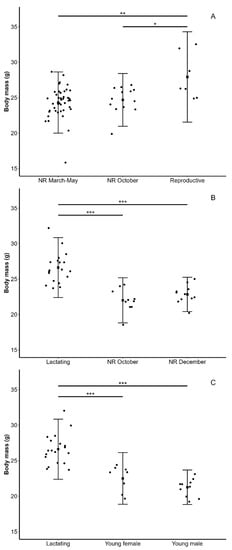
Figure 3.
Body masses of lesser long-nosed bats (Leptonycteris yerbabuenae) for (A) non-reproductive (NR) adult males in March 2019 and 2020 and May 2019 (inguinal or scrotal testicles and no dorsal patches), October 2019 (scrotal testicles and no dorsal patches), and reproductive males in December 2019 (males with scrotal testicles and fresh dorsal patches) captured in west-central Mexico; for (B) lactating females captured in northern Mexico in May and July–August 2019 and adult females with no apparent signs of pregnancy or lactation (NR) captured in west-central Mexico in October and December 2019; and for (C) lactating females and young males and females captured in northern Mexico in July–August 2019. Pregnant females captured in northern Mexico were not included in the comparison because their body mass included the mass of the fetus. Values are means ± standard deviations. Significant differences in pairwise comparisons are shown (p ≤ 0.05 *, p ≤ 0.01 **, p ≤ 0.001 ***) based on a general linear model with a lognormal distribution.

Table 1.
Bacteria-killing abilities (BKAs; median, interquartile range) and body masses (mean ± SD) of lesser long-nosed bats (Leptonycteris yerbabuenae) not included in the statistical models due to small sample sizes. Individual values are shown when n < 3. Non-reproductive adult males in December 2019 presented scrotal testicles and dry dorsal patches.
3.2. Bacteria-Killing Ability
There were significant differences in BKA among adult males (χ22 = 8.69, p = 0.013, n = 62; Figure 4A): reproductive males had higher values than non-reproductive males in March–May (p = 0.049) and non-reproductive males in October (p = 0.009), with no difference between the last two groups (p = 0.337). There were significant differences in BKA among adult females (χ23 = 9.17, p = 0.027, n = 48; Figure 4B): females that had recently arrived in Jalisco in October had lower values than pregnant females in Sonora (p = 0.021) and females captured a few months after their arrival at Jalisco (p = 0.046); no other pairwise comparison among females was significantly different (p ≥ 0.114). Finally, the BKA values of young male and female individuals and lactating females were not significantly different (χ22 = 1.80, p = 0.41, n = 37; Figure 4C). The BKA values of groups not included in the comparisons due to small sample sizes are shown in Table 1.
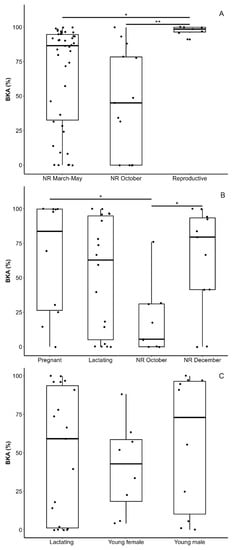
Figure 4.
Bacteria-killing ability (BKA) in plasma of lesser long-nosed bats (Leptonycteris yerbabuenae) for (A) non-reproductive (NR) adult males in March 2019 and 2020 and May 2019 (inguinal or scrotal testicles and no dorsal patches), in October 2019 (scrotal testicles and no dorsal patches), and reproductive males in December 2019 (males with scrotal testicles and a fresh dorsal patches) captured in west-central Mexico; for (B) pregnant and lactating females captured in northern Mexico in May and July–August 2019 and adult females with no external signs of pregnancy or lactation (ANR) captured in west-central Mexico in October and December 2019; and for (C) lactating females and young males and females captured in northern Mexico in July–August 2019. Values are medians (squares), interquartile ranges (boxes), and 5–95% percentiles (whiskers). Significant differences in pairwise comparisons are shown (p ≤ 0.05 *, p ≤ 0.01 **) based on a generalized linear model with a zero-inflated beta distribution.
4. Discussion
4.1. Male BKA
Reproductive male lesser long-nosed bats had higher BKA values than non-reproductive adult males, which might be explained by several factors. First, males with fresh dorsal patches and scrotal testicles were probably mating because spermatogenesis occurs along with these two externals indicators of reproductive activity [51]. Thus, it is possible that the increase in testosterone concentration that mating males experience potentiates BKA [38,39,66,67]. This increase in BKA could be a consequence of an enhancement in complement activity since testosterone increases the production of molecules associated with this immune component [38,39,68]. In line with this idea, reproductive male vampire bats exhibit higher BKA values than non-reproductive males [26], although BKA does not vary with reproductive activity in male Daubenton’s bats [25]. This discrepancy suggests that the effect of the reproductive season on the immune system in bats is not widespread. To corroborate the possible effect that testosterone may have on the immune system in bats, it is essential to evaluate the activity of the immune system and the levels of this hormone in both reproductive and non-reproductive cohorts.
Second, males with fresh dorsal patches had higher body masses than non-reproductive and scrotal males, indicating that their stronger immune response might also be related to a better body condition. Mating in our focal population occurs during the peak of flower resource availability [32,47], which is reflected in the higher body masses of males. Given that some components of the immune system are costly in terms of energetic resources [42,43], the superior body condition of mating males might enable them to show a stronger BKA.
Finally, the increase in population density and of interindividual contacts during the mating season might expand the risk of transmission of pathogenic microorganisms [69,70]. This aspect becomes particularly relevant for mating male lesser long-nosed bats, considering that the microbiota present in the dorsal patch include pathogenic bacteria [71]. This scenario might strengthen the complement system [72,73], leading to differences in BKA values between reproductive and non-reproductive individuals of lesser long-nosed bats. The lower ectoparasite load presented by males developing the dorsal patch relative to males with no patch [74,75,76] adds support to this idea, as the complement system can target ectoparasites [77]. Recent work with other elements of the immune system shows that male southern long-nosed bats (L. curasoae) and lesser long-nosed bats with dorsal patches have a higher inflammatory response than males with no patch [78]. We also captured individuals with scrotal testicles and dry dorsal patches, probably representing post-mating males. Although not formally compared due to their small sample size, males with dry patches had lower BKA values than males with fresh patches, furthering supporting our view that BKA increases during mating. Further work including larger sample sizes of males with fresh dorsal patches and dry dorsal patches is warranted to test the relationship of this structure and the strength of the immune response in long-nosed bats.
4.2. Female BKA
In contrast to males, the BKA of most adult female lesser long-nosed bats was susceptible to the effects of migratory movements in addition to reproductive activity. We found a significant difference in the BKA values between females in the two extremes of their migratory range: the values of pregnant females at their northern breeding grounds were higher than those of females that were captured shortly after returning to west-central Mexico. These results contradict our initial hypothesis that the energetic cost of migration and gestation would lead to lower BKA values in females that migrated to the north. Unlike our findings, previous work showed that BKA does not vary among the reproductive categories in female Daubenton’s bats [25], which contrasts with the finding of lower BKA values in pregnant fish-eating Myotis than in females in other reproductive categories [24]. No previous work has tested changes in BKA values in migratory female bats, but BKA does not differ with migration status in male silver-haired bats [22]. It thus appears that the costs of migration and reproductive activity do not have a detrimental effect of the bactericidal activity of pregnant females, perhaps because the physiological rearrangement of gestation [40,41,79,80] and the high availability of resources during this season [81,82,83,84] may increase their capacity to mount a strong BKA [85]. Similarly, lactation did not appear to have a negative effect on BKA in lesser long-nosed bats, which was congruent with observations in Daubenton’s bats [25] and other terrestrial mammal species [86].
Migration can adversely affect the immune system, although we were not able to fully test this idea due to the low capture rate of females that that did not migrate and remained residents in west-central Mexico. For instance, the BKA of migratory common blackbirds (Turdus merula) is lower than in resident conspecifics [19,20]. It is evident though that the returning journey to west-central Mexico takes a toll on BKA, which is also reflected in the lower body mass of females shortly after their arrival. Female lesser long-nosed bats migrate along a nectar corridor that is predominantly determined by the presence of cactus species [84], and they might depauperate their body reserves when they return to west-central Mexico if they encounter lower densities of blooming cacti [81,82,87]. BKA is positively related to the energy reserves of migratory vertebrates [88], and it might be a factor determining the lower BKA of returning females. However, differences in body mass do not fully explain the lower BKA of females in October because females captured two months later had significantly higher BKA, yet their body masses did not differ. Other physiological factors associated with migration, such as the antioxidant capacity [20], might have played a role in the extent to which BKA varied among females in our study. The increase in BKA in December might have been the result of a higher rate of inter-individual contacts during mating, favoring their exposure to infectious agents and heightening BKA [69,70]. The presence of resident and migratory females in our focal population of lesser long-nosed bat offers an excellent model for further testing these ideas.
4.3. BKA of Young and Lactating Females
Immunity is an evolving system in which young individuals experience immune maturation [45]. A few bat studies have examined the effect of age on immunity, with contrasting findings. The white blood cell count decreased, the IgG concentration increased, and the bactericidal activity did not change with age in greater sac-winged bats (Saccopteryx bilineata) when comparing individuals that were at least 1 year old [56]. The leucocyte profile varied with age in the Egyptian rousette bat (Rousettus aegyptiacus), with higher abundances of neutrophils, CD3+ T cells, and CD206+ mononuclear myeloid cells in adults and higher abundances of CD79a+ B cells and CD11b+ T cells in juveniles [89]. Previous work with birds has found that BKA [90] and other elements of the constitutive immune system [91] increase with age in nestlings, eventually reaching the levels found in adults. In contrast, young male and female lesser long-nosed bats had similar BKA values to lactating females. The young individuals in our study were probably no older than 2–3 months and, although they were flying independent of their mothers, we were not certain that they were already weaned. It is thus likely that the young BKA was passively determined by the milk obtained from their mothers [92]. In sum, our findings indicate that the constitutive immune system of young lesser long-nosed bats matures early in life, probably in anticipation of their potential exposure to pathogens during their migration to central Mexico.
4.4. Concluding Remarks
We found compelling evidence showing that BKA varies throughout the year in male and female lesser long-nosed bats in response to different factors. Interestingly, interindividual variation was large in all examined groups, including individuals with ~100% bactericidal activity and individuals with no bactericidal activity or almost no bactericidal activity. It is beyond the scope of this study to uncover what drives interindividual variation in plasma BKA, but factors intrinsic to the individuals may contribute to the observed results. For instance, variation in BKA in vampire bats (D. rotundus) is determined by the abundance of some core microbiota members [93], and recent work has shown that DNA methylation profiles match the chronological ages of bats (including the lesser long-nosed bat [94]). Populations of migratory bats are composed of individuals of different ages that probably have contrasting microbiota compositions, and further work can easily incorporate these elements to understand interindividual variations in immune responses during the migratory cycle that cannot be attributed to other factors.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d15040530/s1, Supplementary Materials S1: Statistical models.
Author Contributions
Conceptualization, D.A.R.-R. and L.G.H.M.; Methodology: D.A.R.-R., C.R. and L.G.H.M.; Formal Analysis, D.A.R.-R. and L.G.H.M.; Investigation, D.A.R.-R., C.R., J.J.F.-M. and L.G.H.M.; Resources, D.A.R.-R. and L.G.H.M.; Data Curation: D.A.R.-R. and L.G.H.M.; Writing—Original Draft Preparation, D.A.R.-R. and L.G.H.M.; Writing—Review and Editing, C.R. and J.J.F.-M.; Supervision, L.G.H.M.; Project Administration, L.G.H.M.; Funding Acquisition, L.G.H.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, Dirección General de Asuntos del Personal Académico-UNAM (#IN204219) to L.G.H.M. The Consejo Nacional de Ciencia y Tecnología supported D.A.R.-R. with a graduate student grant.
Institutional Review Board Statement
The study was conducted in strict accordance with the recommendations and permit from Dirección General de Vida Silvestre (#8053/19).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is readily available under request to correspondent author.
Acknowledgments
This article is part of the requirements for D.A.R.-R. to obtain a PhD degree in the Posgrado en Ciencias Biológicas-UNAM. We are thankful for the logistic support of the personnel of the Chamela Biological Station and Omar Calva during the field work in Jalisco and Sonora, respectively. We thank Marco Tulio Solano De la Cruz (Unidad de Genética Molecular of the Instituto de Ecología-UNAM), and Osiris Gaona Pineda and Luisa Falcón (Laboratorio de Ecología Bacteriana of the Instituto de Ecología-UNAM) for their technical support during the bactericide laboratory analyses, and Wilmar Alexander Torrez López and Jesús Mendoza Badillo for their help with the statistical analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Neuweiler, G. Ecology. In Biology of Bats; Oxford University Press: New York, NY, USA, 2000; ISBN 0195099508. [Google Scholar]
- Altizer, S.; Bartel, R.; Han, B.A. Animal migration and infectious disease risk. Science 2011, 331, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.K.; Binning, S.A. Migratory recovery from infection as a selective pressure for the evolution of migration. Am. Nat. 2016, 187, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.K.; Binning, S.A. Recovery from infection is more likely to favour the evolution of migration than social escape from infection. J. Anim. Ecol. 2020, 89, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R.; de Angeli Dutra, D. Animal migrations and parasitism: Reciprocal effects within a unified framework. Biol. Rev. 2021, 96, 1331–1348. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.J.; Altizer, S.; Peacock, S.J.; Shaw, A.K. Animal migration and infection dynamics: Recent advances and future frontiers. In Animal Behavior and Parasitism; Ezenwa, V., Altizer, S.M., Hall, R., Eds.; Oxford University Press: Oxford, UK, 2022; pp. 111–132. ISBN 9780192895561. [Google Scholar]
- Dingle, H.; Drake, A. What is migration? Bioscience 2007, 57, 113–121. [Google Scholar] [CrossRef]
- Kelly, T.R.; MacGillivray, H.L.; Hobson, K.A.; MacDougall-Shackleton, S.A.; MacDougall-Shackleton, E.A. Immune profiles vary seasonally, but are not significantly related to migration distance or natal dispersal, in a migratory songbird. J. Exp. Zool. 2017, 327, 284–292. [Google Scholar] [CrossRef]
- Avgar, T.; Street, G.; Fryxell, J.M. On the adaptive benefits of mammal migration. Can. J. Zool. 2014, 92, 481–490. [Google Scholar] [CrossRef]
- Fleming, T.H.; Eby, P. Ecology of Bat Migration. In Bat Ecology; Kunz, T.H., Fenton, B.M., Eds.; The University of Chicago Press: Chicago, IL, USA, 2003; pp. 156–197. ISBN 0-226-46206-4. [Google Scholar]
- Popa-Lisseanu, A.G.; Voigt, C.C. Bats on the move. J. Mammal. 2009, 90, 1283–1289. [Google Scholar] [CrossRef]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef]
- Mühldorfer, K. Bats and bacterial pathogens: A review. Zoonoses Public Health 2013, 60, 93–103. [Google Scholar] [CrossRef]
- Paweska, J.T.; Storm, N.; Grobbelaar, A.A.; Markotter, W.; Kemp, A.; van Vuren, P.J. Experimental inoculation of Egyptian fruit bats (Rousettus aegyptiacus) with ebola virus. Viruses 2016, 8, 29. [Google Scholar] [CrossRef]
- Kuzmin, I.V.; Schwarz, T.M.; Ilinykh, P.A.; Jordan, I.; Ksiazek, T.G.; Sachidanandam, R.; Basler, C.F.; Bukreyev, A. Innate immune responses of bat and human cells to filoviruses: Commonalities and distinctions. J. Virol. 2017, 91, e02471-16. [Google Scholar] [CrossRef]
- Hegemann, A.; Fudickar, A.M.; Nilsson, J.Å. A physiological perspective on the ecology and evolution of partial migration. J. Ornithol. 2019, 160, 893–905. [Google Scholar] [CrossRef]
- de Angeli Dutra, D.; Fecchio, A.; Martins Braga, É.; Poulin, R. Migratory birds have higher prevalence and richness of avian haemosporidian parasites than residents. Int. J. Parasitol. 2021, 51, 877–882. [Google Scholar] [CrossRef]
- Owen, J.C.; Moore, F.R. Swainson’s thrushes in migratory disposition exhibit reduced immune function. J. Ethol. 2008, 26, 383–388. [Google Scholar] [CrossRef]
- Eikenaar, C.; Hegemann, A. Migratory common blackbirds have lower innate immune function during autumn migration than resident conspecifics. Biol. Lett. 2016, 12, 78–81. [Google Scholar] [CrossRef]
- Eikenaar, C.; Isaksson, C.; Hegemann, A. A hidden cost of migration? innate immune function versus antioxidant defense. Ecol. Evol. 2018, 8, 2721–2728. [Google Scholar] [CrossRef]
- Voigt, C.C.; Fritze, M.; Lindecke, O.; Costantini, D.; Pētersons, G.; Czirják, G. The immune response of bats differs between pre-migration and migration seasons. Sci. Rep. 2020, 10, 17384. [Google Scholar] [CrossRef]
- Rogers, E.J.; McGuire, L.; Longstaffe, F.J.; Clerc, J.; Kunkel, E.; Fraser, E. Relating wing morphology and immune function to patterns of partial and differential bat migration using stable isotopes. J. Anim. Ecol. 2022, 91, 858–869. [Google Scholar] [CrossRef]
- Christe, P.; Arlettaz, R.; Vogel, P. Variation in intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis). Ecol. Lett. 2000, 3, 207–212. [Google Scholar] [CrossRef]
- Otálora-Ardila, A.; Flores-Martínez, J.J.; Rosales, C.; Salame-Méndez, A.; Herrera M., L.G. Physiological and ecological correlates of the cellular and humoral innate immune responses in an insular desert bat: The fish-eating myotis (Myotis vivesi). Diversity 2022, 14, 781. [Google Scholar] [CrossRef]
- Ruoss, S.; Becker, N.I.; Otto, M.S.; Czirják, G.; Encarnação, J.A. Effect of sex and reproductive status on the immunity of the temperate bat Myotis daubentonii. Mamm. Biol. 2019, 94, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.J.; Czirják, G.; Volokhov, D.V.; Bentz, A.B.; Carrera, J.E.; Camus, M.S.; Navara, K.J.; Chizhikov, V.E.; Fenton, M.B.; Simmons, N.B.; et al. Livestock abundance predicts vampire bat demography, immune profiles and bacterial infection risk. Phil. Trans. R. Soc. B 2018, 373, 20170089. [Google Scholar] [CrossRef] [PubMed]
- Medellín, R. Leptonycteris yerbabuenae. The IUCN Red List of Threatened Species. 2016, e.T136659A21988965. Available online: https://www.iucnredlist.org/species/136659/21988965 (accessed on 10 September 2022).
- Morales-Garza, M.R.; del C. Arizmendi, M.; Campos, J.E.; Martínez-Garcia, M.; Valiente-Banuet, A. Evidences on the migratory movements of the nectar-feeding bat Leptonycteris curasoae in Mexico using random amplified polymorphic DNA (RAPD). J. Arid Environ. 2007, 68, 248–259. [Google Scholar] [CrossRef]
- Wilkinson, G.S.; Fleming, T.H. Migration and evolution of lesser longnosed bats Leptonycteris curasoae, inferred from mitochondrial DNA. Mol. Ecol. 1996, 5, 329–339. [Google Scholar] [CrossRef]
- Menchaca, A.; Arteaga, M.C.; Medellin, R.A.; Jones, G. Conservation units and historical matrilineal structure in the tequila bat (Leptonycteris yerbabuenae). Glob. Ecol. Conserv. 2020, 23, e01164. [Google Scholar] [CrossRef]
- Trejo-Salazar, R.E.; Castellanos-Morales, G.; Hernández-Rosales, D.C.; Gámez, N.; Gasca-Pineda, J.; Morales Garza, M.R.; Medellin, R.; Eguiarte, L.E. Discordance in maternal and paternal genetic markers in lesser long-nosed bat Leptonycteris yerbabuenae, a migratory bat: Recent expansion to the north and male phylopatry. PeerJ 2021, 9, e12168. [Google Scholar] [CrossRef]
- Stoner, K.E.; Karla, K.A.; Roxana, R.C.; Quesada, M. Population dynamics, reproduction, and diet of the lesser long-nosed bat (Leptonycteris curasoae) in Jalisco, Mexico: Implications for conservation. Biodivers. Conserv. 2003, 12, 357–373. [Google Scholar] [CrossRef]
- Millet, S.; Bennett, J.; Lee, K.A.; Hau, M.; Klasing, K.C. Quantifying and comparing constitutive immunity across avian species. Dev. Comp. Immunol. 2007, 31, 188–201. [Google Scholar] [CrossRef]
- Tieleman, B.I.; Williams, J.B.; Ricklefs, R.E.; Klasing, K.C. Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. Proc. R. Soc. B 2005, 272, 1715–1720. [Google Scholar] [CrossRef]
- Moore, M.S.; Reichard, J.D.; Murtha, T.D.; Zahedi, B.; Fallier, R.M.; Thomas, H. Specific alterations in complement protein activity of little brown myotis (Myotis lucifugus) hibernating in white-nose syndrome affected sites. PLoS ONE 2011, 6, e27430. [Google Scholar] [CrossRef]
- Merchant, M.E.; Roche, C.; Elsey, R.M.; Prudhomme, J. Antibacterial properties of serum from the american alligator (Alligator mississippiensis). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 136, 505–513. [Google Scholar] [CrossRef]
- Foo, Y.Z.; Nakagawa, S.; Rhodes, G.; Simmons, L.W. The effects of sex hormones on immune function: A meta-analysis. Biol. Rev. 2016, 92, 551–571. [Google Scholar] [CrossRef]
- Ruiz, M.; French, S.S.; Demas, G.E.; Martins, E.P. Food supplementation and testosterone interact to influence reproductive behavior and immune function in Sceloporus graciosus. Horm. Behav. 2010, 57, 134–139. [Google Scholar] [CrossRef]
- Weintraub, R.M.; Churchill, W.H.; Crisler, C.; Rapp, H.J.; Borsos, T. Mouse complement: Influence of sex hormones on its activity. Science 1966, 152, 783–785. [Google Scholar] [CrossRef]
- Derzsy, Z.; Prohászka, Z.; Rigó, J.; Füst, G.; Molvarec, A. Activation of the complement system in normal pregnancy and preeclampsia. Mol. Immunol. 2010, 47, 1500–1506. [Google Scholar] [CrossRef]
- Richani, K.; Soto, E.; Romero, R.; Espinoza, J.; Chaiworapongsa, T.; Nien, J.K.; Edwin, S.; Kim, Y.M.; Hong, J.S.; Mazor, M. Normal pregnancy is characterized by systemic activation of the complement system. J. Matern. Neonatal Med. 2005, 17, 239–245. [Google Scholar] [CrossRef]
- Lochmiller, R.L.; Deerenberg, C. Trade-offs in evolutionary immunology: Just what is the cost of immunity? Oikos 2000, 88, 87–98. [Google Scholar] [CrossRef]
- Demas, G.E.; Greives, T.; Chester, E.; French, S. The Energetics of Immunity. In Ecoimmunology; Oxford University Press: New York, NY, USA, 2012; pp. 259–296. ISBN 9780199737345. [Google Scholar]
- Buehler, D.M.; Tieleman, B.I.; Piersma, T. How do migratory species stay healthy over the annual cycle? A conceptual model for immune function and for resistance to disease. Integr. Comp. Biol. 2010, 50, 346–357. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; Mcmichael, A. Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B 2015, 282, 20143085. [Google Scholar] [CrossRef]
- Sikes, R.S.; Gannon, W.L. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011, 92, 235–253. [Google Scholar] [CrossRef]
- Ceballos, G.; Fleming, T.H.; Chavez, C.; Nassar, J. Population dynamics of Leptonycteris curasoae (Chiroptera: Phyllostomidae) in Jalisco, Mexico. J. Mammal. 1997, 78, 1220–1230. [Google Scholar] [CrossRef]
- Anthony, E.L.P. Age Determination in Bats. In Ecological and Behavioral Methods for the Study of Bats; Kunz, T.H., Ed.; Smithsonian Institution Press: Washington, DC, USA; London, UK, 1988; pp. 47–58. [Google Scholar]
- Nassar, J.M.; Salazar, M.V.; Quintero, A.; Stoner, K.E.; Gómez, M.; Cabrera, A.; Jaffé, K. Seasonal sebaceous patch in the nectar-feeding bats Leptonycteris curasoae and L. yerbabuenae (Phyllostomidae: Glossophaginae): Phenological, histological, and preliminary chemical characterization. Zoology 2008, 111, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-romo, A.M.; Burgos, J.F.; Kunz, T.H. Smearing behaviour of male Leptonycteris curasoae (Chiroptera) and female responses to the odour of dorsal patches. Behaviour 2011, 148, 461–483. [Google Scholar]
- Rincón-Vargas, F.; Stoner, K.E.; Vigueras-Villaseñor, R.M.; Nassar, J.M.; Chaves, Ó.M.; Hudson, R. Internal and external indicators of male reproduction in the lesser long-nosed bat Leptonycteris yerbabuenae. J. Mammal. 2013, 94, 488–496. [Google Scholar] [CrossRef]
- Strobel, S.N.; Becker, J.A. No short-term effect of handling and capture stress on immune responses on bats assessed by bacterial killing assay. Mamm. Biol. 2015, 80, 312–315. [Google Scholar] [CrossRef]
- Becker, D.J.; Czirják, G.Á.; Rynda-apple, A.; Plowright, R.K. Handling stress and sample storage are associated with weaker complement-mediated bactericidal ability in birds but not bats. Physiol. Biochem. Zool. 2019, 92, 37–48. [Google Scholar] [CrossRef]
- Liebl, A.L.; Ii, L.B.M. Simple quantification of blood and plasma antimicrobial capacity using spectrophotometry. Funct. Ecol. 2009, 23, 1091–1096. [Google Scholar] [CrossRef]
- French, S.S.; Neuman-lee, L.A. Improved ex vivo method for microbiocidal activity across vertebrate species. Biol. Open 2012, 1, 482–487. [Google Scholar] [CrossRef]
- Schneeberger, K.; Courtiol, A.; Czirja Gabor, A.; Voigt, C.C. Immune profile predicts survival and reflects senescence in a small, long-lived mammal, the greater sac-winged bat (Saccopteryx bilineata). PLoS ONE 2014, 9, e108268. [Google Scholar] [CrossRef]
- Hernández-Arciga, U.; Herrera M., L.G.; Ibañez-Contreras, A.; Miranda-Labra, R.U.; Flores-Martínez, J.J.; Königsberg, M. Baseline and post-stress seasonal changes in immunocompetence and redox state maintenance in the fishing bat Myotis vivesi. PLoS ONE 2018, 13, e0190047. [Google Scholar] [CrossRef]
- Becker, D.J.; Chumchal, M.M.; Bentz, A.B.; Platt, S.G.; Czirják, G.; Rainwater, T.R.; Altizer, S.; Streicker, D.G. Predictors and immunological correlates of sublethal mercury exposure in vampire bats. R. Soc. Open Sci. 2017, 4, 170073. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; ISBN 9781544336466. [Google Scholar]
- Delignette-Muller, M.L.; Dutang, C. Fitdistrplus: An r package for fitting distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. GlmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biometr. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Miller, G.A.; Chapman, J.P. Misunderstanding analysis of covariance. J. Abnorm. Psychol. 2001, 110, 40–48. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R. Package Version 0.4.6. 2022. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 17 January 2023).
- Ezenwa, V.O.; Ekernas, L.S.; Creel, S. Unravelling complex associations between testosterone and parasite infection in the wild. Funct. Ecol. 2012, 26, 123–133. [Google Scholar] [CrossRef]
- Walker, W.H. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 2011, 1, 116–120. [Google Scholar] [CrossRef]
- Caren, L.D.; Rosenberg, L.T. Steroids and serum complement in mice: Influence of hydrocortisone, diethylstilbestrol, and testosterone. Science 1966, 152, 782–783. [Google Scholar] [CrossRef]
- Allen, L.C.; Turmelle, A.S.; Mendonça, M.T.; Navara, K.J.; Kunz, T.H.; McCracken, G.F. Roosting ecology and variation in adaptive and innate immune system function in the Brazilian free-tailed bat (Tadarida brasiliensis). J. Comp. Physiol. B 2009, 179, 315–323. [Google Scholar] [CrossRef]
- Nunn, C.L.; Gittleman, J.L.; Antonovics, J. Promiscuity and the primate immune system. Science 2000, 290, 1168–1170. [Google Scholar] [CrossRef] [PubMed]
- Gaona, O.; Cerqueda-García, D.; Falcón, L.I.; Vázquez-Domínguez, G.; Valdespino-Castillo, P.M.; Neri-Barrios, C.X. Microbiota composition of the dorsal patch of reproductive male Leptonycteris yerbabuenae. PLoS ONE 2019, 14, e0226239. [Google Scholar] [CrossRef] [PubMed]
- Heesterbeek, D.A.C.; Angelier, M.L.; Harrison, R.A.; Rooijakkers, S.H.M. Complement and bacterial infections: From molecular mechanisms to therapeutic applications. J. Innate Immun. 2018, 10, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Bagherimoghaddam, A.; Rafatpanah, H.; Mansouritorghabeh, H. Elevated levels of C3, C4, and CH50 of the complement system in ICU and non-ICU patients with COVID-19. Health Sci. Rep. 2022, 5, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Romo, M.; Kunz, T.H. Dorsal patch and chemical signaling in males of the long-nosed bat, Leptonycteris curasoae (Chiroptera: Phyllostomidae). J. Mammal. 2009, 90, 1139–1147. [Google Scholar] [CrossRef]
- Muñoz-Romo, M.; Burgos, J.F.; Kunz, T.H. The dorsal patch of males of the Curaçaoan long-nosed bat, Leptonycteris curasoae (Phyllostomidae: Glossophaginae) as a visual signal. Acta Chiropt. 2011, 13, 207–215. [Google Scholar] [CrossRef]
- Nassar, J.M.; Galicia, R.; Ibarra, A.; Medellin, R.A. Tracking the origin of the smearing behavior in long-nosed bats (Leptonycteris spp.). Mamm. Biol. 2016, 81, 623–627. [Google Scholar] [CrossRef]
- Abbassy, M.; Shaheen, H.; Dykstra, E.; Beavers, G.; Hoel, D.; Hanafi, H.; Afifi, M.; Ibrahim, M.; Karim, K. Identification of Culex pipiens Linnaeus (Diptera: Culicidae) immunogens recognized by host humoral immunity and their impact on survival and fecundity. Egypt J. Immunol. 2007, 14, 43–54. [Google Scholar]
- Herrera M., L.G.; Hernández-Arciga, U.; González-Carcacía, J.A.; Nassar, J.M. Olfactory cues of mate quality in mammals: Inflammatory response is higher in male long-nosed bats with odorous dorsal patch. Mammal Res. in press.
- He, Y.; Xu, B.; Song, D.; Wang, Y.; Yu, F.; Chen, Q.; Zhao, M. Normal range of complement components during pregnancy: A prospective study. Am. J. Reprod. Immunol. 2020, 83, e13202. [Google Scholar] [CrossRef]
- Hopkinson, N.D.; Powell, R.J. Classical complement activation induced by pregnancy: Implications for management of connective tissue diseases. J. Clin. Pathol. 1992, 45, 66–67. [Google Scholar] [CrossRef]
- Cockrum, E.L. Seasonal distribution of northwestern populations of the long-nosed bats, Leptonycteris sanborni family Phyllostomidae. An. Inst. Biol. Ser. Zool. 1991, 62, 181–202. [Google Scholar]
- Valiente-Banuet, A.; Arizmendi, M.D.C.; Rojas-Martínez, A.; Domínguez-Canseco, L. Ecological relationships between columnar cacti and nectar-feeding bats in Mexico. J. Trop. Ecol. 1996, 12, 103–119. [Google Scholar] [CrossRef]
- Fleming, T.H.; Nuñez, R.A.; Sternberg, L.D.S.L. Seasonal changes in the diets of migrant and non-migrant nectarivorous bats as revealed by carbon stable isotope analysis. Oecologia 1993, 94, 72–75. [Google Scholar] [CrossRef]
- Burke, R.A.; Frey, J.K.; Ganguli, A.; Stoner, K.E. Species distribution modelling supports “nectar corridor” hypothesis for migratory nectarivorous bats and conservation of tropical dry forest. Divers. Distrib. 2019, 25, 1399–1415. [Google Scholar] [CrossRef]
- Gilot-fromont, E.; Jégo, M.; Bonenfant, C.; Gibert, P.; Rannou, B.; Klein, F.; Gaillard, J.-M. Immune phenotype and body condition in roe deer: Individuals with high body condition have different, not stronger immunity. PLoS ONE 2012, 7, e45576. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Moreno-Indias, I.; Sánchez-Macías, D.; Morales-delaNuez, A.; Torres, A.; Argüello, A.; Castro, N. Sheep and goats raised in mixed flocks have diverse immune status around parturition. J. Dairy Sci. 2019, 102, 8478–8485. [Google Scholar] [CrossRef]
- Rojas-Martinez, A.; Valiente-Banuet, A.; Del Coro Arizmendi, M.; Alcántara-Eguren, A.; Arita, H.T. Seasonal distribution of the long-nosed bat (Leptonycteris curasoae) in North America: Does a generalized migration pattern really exist? J. Biogeogr. 1999, 26, 1065–1077. [Google Scholar] [CrossRef]
- Eikenaar, C.; Hegemann, A.; Packmor, F.; Kleudgen, I.; Isaksson, C. Not just fuel: Energy stores are correlated with immune function and oxidative damage in a long-distance migrant. Curr. Zool. 2020, 66, 21–28. [Google Scholar] [CrossRef]
- Friedrichs, V.; Toussaint, C.; Schäfer, A.; Rissmann, M.; Dietrich, O.; Mettenleiter, T.C.; Pei, G.; Balkema-Buschmann, A.; Saliba, A.E.; Dorhoi, A. Landscape and age dynamics of immune cells in the Egyptian rousette bat. Cell Rep. 2022, 40, 111305. [Google Scholar] [CrossRef]
- Aastrup, C.; Hegemann, A. Jackdaw nestlings rapidly increase innate immune function during the nestling phase but no evidence for a trade-off with growth. Dev. Comp. Immunol. 2021, 117, 103967. [Google Scholar] [CrossRef] [PubMed]
- Mauck, R.A.; Matson, K.D.; Philipsborn, J.; Ricklefs, R.E. Increase in the constitutive innate humoral immune system in leach’s storm-petrel (Oceanodroma leucorhoa) chicks is negatively correlated with growth rate. Funct. Ecol. 2005, 19, 1001–1007. [Google Scholar] [CrossRef]
- Cacho, N.T.; Lawrence, R.M. Innate immunity and breast milk. Front. Immunol. 2017, 8, 584. [Google Scholar] [CrossRef]
- Ingala, M.R.; Becker, D.J.; Bak Holm, J.; Kristiansen, K.; Simmons, N.B. Habitat fragmentation is associated with dietary shifts and microbiota variability in common vampire bats. Ecol. Evol. 2019, 9, 6508–6523. [Google Scholar] [CrossRef]
- Wilkinson, G.S.; Adams, D.M.; Haghani, A.; Lu, A.T.; Zoller, J.; Breeze, C.E.; Arnold, B.D.; Ball, H.C.; Carter, G.G.; Cooper, L.N.; et al. DNA methylation predicts age and provides insight into exceptional longevity of bats. Nat. Commun. 2021, 12, 1615. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).