Abstract
We document unrecognised diversity within the Tetramorium spininode Bolton group of the Australian monsoonal tropics, which has a single described species. At the time of its description, T. spininode was known from just two collections, but there have since been hundreds of collections from throughout monsoonal Australia. We document morphological and genetic (CO1) variation within the group’s fauna of the Northern Territory (NT), in the centre of its range, where collection intensity has been highest. We recognise 20 species among 124 CO1-sequenced specimens, and 32 species in total from the NT. A key to these species is provided. The most intensively sampled regions within the NT are the mesic (>1000 mm mean annual rainfall) Top End in the far north (with 14 species) and the semi-arid (500–900 mm) Sturt Plateau region to its south (13 species). Only one species is known from both regions. Given such high regional turnover and highly patchy sampling, we estimate that at least 40 species of the T. spininode group occur in the NT. Similar diversity appears to occur in Western Australia, especially in the Kimberley region, but less in Queensland. Our findings suggest that the total number of species in the T. spininode group is likely to be around 100. Our study provides further evidence that monsoonal Australia is an unrecognised global centre of ant diversity.
1. Introduction
Monsoonal Australia, the northern third of the continent that experiences a seasonal tropical climate, is a global centre of ant diversity but is largely unrecognised as such because the great majority of species are undescribed. An early estimate of the total size of the fauna was 1500 species [1], but it more likely numbers in the several thousand given more comprehensive sampling over recent decades, and, informed by extensive DNA barcoding, the realisation that many taxonomically recognised ‘species’ are in fact hyperdiverse species groups [2,3]. For example, Melophorus rufoniger Heterick, Castalanelli and Shattuck was described as a single species occurring throughout mainland Australia but represents dozens of species just in the monsoonal tropics [4]. The similarly widespread Monomorium ‘fieldi’ Forel possibly contains 200 species in the region [5].
Tetramorium is another ant genus that has unrecognised Australian hyperdiversity. Inland Australia is believed to harbour up to 500 species or more of the genus [6], but fewer than 10 of these have been described. One such species from the monsoonal tropics is T. spininode Bolton, which is characterised by uniquely reflexed petiolar and postpetiolar nodes and a highly distinctive first gastral tergite that has pronounced basal flanges and conspicuous sculpture (Figure 1). As such, it was celebrated as ‘the most distinctive and spectacular tetramoriine’ ever described [7].
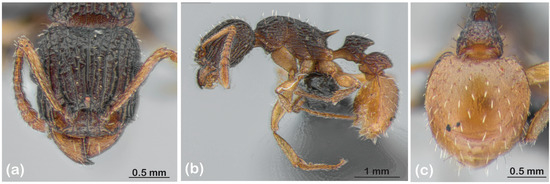
Figure 1.
Tetramorium spininode. (a) Head in frontal view. (b) Lateral view. (c) Gaster in dorsal view. All images are of sequenced specimen OZBOL 6501-22, collected from the type locality.
At the time of its description in 1977, T. spininode was known from just two collections: its type series from the Kimberley region of far northern Western Australia (WA), and a single specimen (differing in colour) from Newcastle Waters, 1000 km southeast in the Northern Territory (NT) [7]. There have since been hundreds of collections of the taxon, almost entirely from the monsoonal zone, and it clearly represents dozens of species [6]. Tetramorium spininode was originally placed in the T. striolatum Viehmeyer group [7] but given its diversity has since been considered to represent its own species group [1]. The key morphological characters that vary among species are:
- Metapleural lobes (following the nomenclature of their original description [7], although they are actually part of the propodeum), which range from inconspicuous and rounded to prominently spinose (Figure 2);
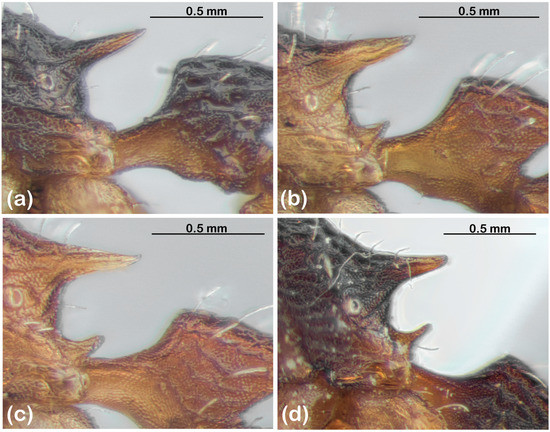 Figure 2. Variation in metapleural lobes among species of the T. spininode group. (a) T. spininode; OZBOL 6501-22. (b) sp. 21; OZBOL 8250-22. (c) sp. 3; OZBOL 3953-21. (d) sp. 14; TET 088-17.
Figure 2. Variation in metapleural lobes among species of the T. spininode group. (a) T. spininode; OZBOL 6501-22. (b) sp. 21; OZBOL 8250-22. (c) sp. 3; OZBOL 3953-21. (d) sp. 14; TET 088-17. - Gastral sculpture, which ranges from finely striate (typically longitudinally, but sometimes radiating from the base, and with varying degrees of punctate background sculpture) to spectacularly costate (Figure 3);
 Figure 3. Variation in gastric sculpture among species of the T. spininode group from the NT. Species are arranged with increasing sculptural coarseness. (a) sp. 15; Arnhem Land, NT (not sequenced). (b) sp. 21; OZBOL 8250-22. (c) sp. 20; TET 086-17. (d) sp. 14; TET 088-17. (e) sp. 11; Manbulloo Stn, NT (not sequenced); (f) sp. 5; OZBOL 8227-21.
Figure 3. Variation in gastric sculpture among species of the T. spininode group from the NT. Species are arranged with increasing sculptural coarseness. (a) sp. 15; Arnhem Land, NT (not sequenced). (b) sp. 21; OZBOL 8250-22. (c) sp. 20; TET 086-17. (d) sp. 14; TET 088-17. (e) sp. 11; Manbulloo Stn, NT (not sequenced); (f) sp. 5; OZBOL 8227-21. - Structure of the first gastral tergite, including the extent of basal flanging (Figure 3) and the presence of an antero-medial ridge (Figure 4);
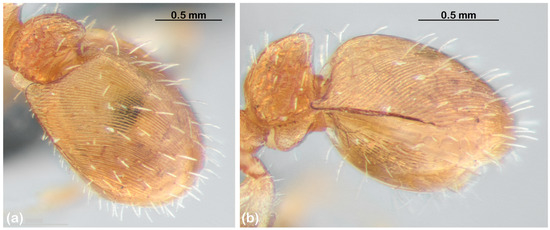 Figure 4. Ridged gaster. (a) Oblique view, showing dorsal ridge. (b) Lateral view, showing perpendicular anterior face. Both images are of sp. 12; King River, NT (not sequenced).
Figure 4. Ridged gaster. (a) Oblique view, showing dorsal ridge. (b) Lateral view, showing perpendicular anterior face. Both images are of sp. 12; King River, NT (not sequenced). - Rugosity of the head and mesosoma (Figure 5);
 Figure 5. Variation in morphology, sculpture and colour among species of the T. spininode group. (a) sp. 12; King River, NT (not sequenced); (b) sp. 8; nr Manyallaluk, NT (not sequenced); (c) sp. 6; Manbulloo Stn, NT (not sequenced); (d) sp. 9; nr Beswick, NT (not sequenced); (e) sp. 11; OZBOL 3950-21; (f) sp. 26; TET 073-17.
Figure 5. Variation in morphology, sculpture and colour among species of the T. spininode group. (a) sp. 12; King River, NT (not sequenced); (b) sp. 8; nr Manyallaluk, NT (not sequenced); (c) sp. 6; Manbulloo Stn, NT (not sequenced); (d) sp. 9; nr Beswick, NT (not sequenced); (e) sp. 11; OZBOL 3950-21; (f) sp. 26; TET 073-17. - Body colour, which ranges from uniformly yellowish or orange-brown to bicoloured, with dark brown head, mesosoma and waist, contrasting with yellowish legs and gaster (Figure 5).
Here we assess the extent of unrecognised diversity within the T. spininode group by providing an integrated morphological and genetic (CO1) analysis of diversity within the NT, which has by far the highest number of collections of the taxon. We specifically address two questions. First, how many species are likely to be represented by collections of the T. spininode group from the NT? Second, what is the extent of geographical turnover of species within the NT? We then draw on this information, along with collections from elsewhere in northern Australia, to discuss the likely total number of species in the T. spininode group. A formal taxonomic analysis and species descriptions will be provided in a following paper.
2. Materials and Methods
Our study was based on the approximately 800 pinned specimens of the T. spininode-group in the ant collection held at the CSIRO laboratory in Darwin (subsequently referred to as the Darwin collection), which holds the vast majority of collected specimens of the taxon. More than 80% of the T. spininode-group specimens in the Darwin collection are from the NT. Geographic coverage of samples within the NT is extremely patchy, being heavily concentrated in the central north–south corridor of the monsoonal region (Figure 6). Most samples are from either the very mesic (1000–1500 mm mean annual rainfall) Top End (especially the central Kakadu/Nitmiluk region) or the semi-arid Sturt Plateau region (500–900 mm) to its south (Figure 6). Most other regions were only sparsely sampled or not represented at all.
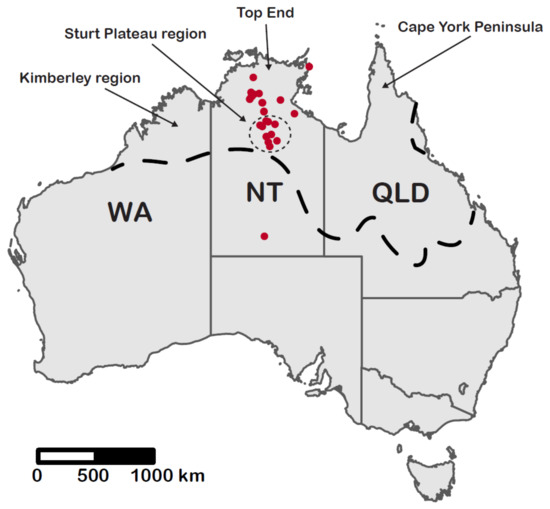
Figure 6.
Map of Australia showing collection localities (red dots) for sequenced specimens of the Tetramorium spininode group. Many localities represent multiple sites. The four major biogeographic regions in northern Australia are indicated. The dashed line represents the approximate southern boundary of the monsoonal zone, where rainfall is very heavily concentrated in a summer wet season. Total annual rainfall ranges from about 2000 mm on the Tiwi Islands in the Top End to 500 mm on the southern boundary with the central arid zone.
We first conducted a preliminary sorting of NT specimens based on morphological variation. This sorting was then refined through analysis of CO1 sequences obtained from 124 of the specimens that covered a wide range of morphological variation among relatively recent (<15 years) collections (Supplementary Table S1). We also sequenced a specimen of T. spininode collected from its type locality, along with a specimen of the T. striolatum group for use as an outgroup in CO1-tree construction. DNA extraction (from foreleg tissue) and CO1 sequencing were conducted through the Barcode of Life Data (BOLD) System (for extraction details, see http://ccdb.ca/resources; accessed on 20 March 2023). Each sequenced specimen was assigned a unique identification code that combines the batch within which it was processed, its number within the batch and the year of sequencing (e.g., OZBOL6501-22 for the T. spininode specimen). All specimens were labelled with their respective BOLD identification numbers and sorted to the species documented here, in the Darwin collection.
DNA sequences were checked and edited in MEGA [8]. Sequences were aligned using the UPGMB clustering method in MUSCLE [9] and then translated into (invertebrate) proteins to check for stop codons and nuclear paralogues. The aligned sequences were trimmed accordingly, resulting in 1056 base pairs. MUSCLE was also used to construct a maximum-likelihood tree.
There is no specific level of CO1 divergence that can be used to define a species, but it is typically 1–3% within a species [10]. We delimited species based on the integration of morphological variation, CO1 clustering and distance, and geographic distribution [11]. We followed a species concept based on reproductive isolation and evolutionary independence as evidenced by morphological differentiation between sister (i.e., most closely related) clades (considering all available samples from the same collections as those of sequenced specimens) and sympatric distribution.
We imaged representative morphological characters and specimens using a Leica DMC5400 camera mounted on a Leica M205C dissecting microscope. We took image montages using the Leica Application suite v. 4.13 and stacked them in Zerene stacker.
3. Results
3.1. Species Richness
We recognise 32 species (spp. 1–32) of the T. spininode group among specimens from the NT, 20 of which were represented among the 124 sequenced specimens (Figure 7). Species that were not sequenced are represented only by older (>15 years) collections. A key to species (including T. spininode) is as follows:
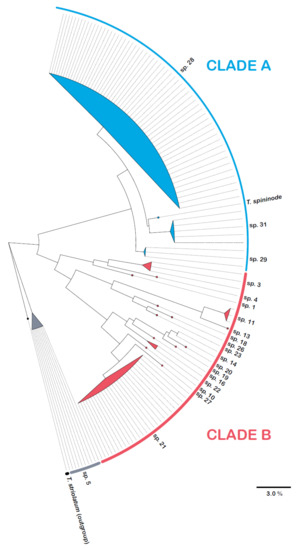
Figure 7.
Summary CO1 tree constructed by maximum likelihood showing the 20 sequenced species of the T. spininode group from the NT, along with T. spininode from WA. All species other than sp. 5 belong to one of two major clades (A and B). The full tree is shown in Figure S1.
- First gastral tergite costate ……………………………………………………….…………………..……………..……….2First gastral tergite striate ..……………………………………………………….………………….…………..………….8
- Metapleural lobes strongly developed and dentiform or spinose .…….……………………………….……………….3Metapleural lobes weakly developed and rounded .……………………………………………………………………..6
- Metapleural spines short and broadly triangular …………………….……………………….…………….………....….4Metapleural spines long and narrow ……………………………………………………..………………………..……….5
- Body reddish brown contrasting with yellowish legs; sides of head behind eyes regularly rugose (Forest Hill and Manbulloo stns)…………………………………………………..….……..….……..….……..….……..……...….…….sp. 1Body uniformly yellowish; sides of head behind eyes irregularly sculptured (Manbulloo Stn) ....……...……....sp. 2
- Sides of head behind eyes regularly rugose; gastral costae more strongly radial anteriorly (Hayfield Shenandoah Stn) ……….………….……………………..………………..……………..……………..………………………….…….sp. 3Sides of head behind eyes irregularly sculptured; gastral costae less strongly radial anteriorly (Lorella Springs) …………………………………………………………..………………………………………………………..sp. 4
- Gastral costae transverse anteriorly (throughout Sturt Plateau) …………………………………………...………..sp. 5Gastral costae never transverse ………………………………………..…………………………………………………….7
- Small species with short petiolar and postpetiolar nodes that are not so strongly reflexed (Katherine region; Figure 5c) …...…….…………………………………….…………………………….…..…………….……………………..sp. 6Larger species with more strongly reflexed petiolar and postpetiolar nodes (Groote Eylandt, Gulf region) ..………….……………………………….…………………….…………………..………….…………………….sp. 7
- Metapleural lobes strongly developed and dentiform or spinose …….………………………………………..……….9Metapleural lobes not strongly developed, rounded or very weakly dentate …………………………………………26
- Mesosoma with a deep metanotal groove (Manyallaluk; Figure 5b) ……………………………………………….sp. 8Mesosoma with at most a weak metanotal notch ………………………..……………..……………..………………….10
- First gastral tergite with a rounded medial ridge such that in profile it is angled anteriorly, with a perpendicular anterior face ………..…………………………………………………………………………………………..…………….11First gastral tergite without a medial ridge such that its profile lacks a distinct anterior face ……..……..………..15
- Mesosoma conspicuously rugose throughout most of its lateral faces …..…………..…………..…………….………2Mesosoma without conspicuous rugae on most of its lateral faces ………….………….………….………….………..13
- Petiole and postpetiole conspicuously darker than mesosoma and gaster (Manyallaluk, Beswick; Figure 5d) ……………………………………………………………..………………………………………………...……….……..sp. 9Petiole and postpetiole concolorous with mesosoma and gaster (Nitmiluk NP) .…….……….……….…………sp. 10
- Ventral processes of postpetiole yellowish, distinctly lighter than node ……………..…...……...……...…...……….14Ventral processes of postpetiole reddish to dark brown, concolorous with node (Forest Hill and Manbulloo stns; Figure 5e) ……………………….………………………………..….……………..….……………..….…..….………..sp. 11
- First gastral tergite more strongly flanged, and lateral striations strongly radiating towards the medial line (King River; Figure 4) ………………………………………………………………………………………………………….sp. 12First gastral tergite less strongly flanged, and lateral striations less strongly radiating towards the medial line (Lakefield, Manbulloo and Mataranka stns ……………….…………….…………….…………….……………….sp. 13
- Mesosoma yellowish brown, concolorous with gaster ………………….…………….…..…………………………….16Mesosoma reddish or blackish brown, distinctly darker than gaster ………………….…………….……….………...20
- Gastral striations coarse ………………………………………………….……..………….…………….…...………..…...17Gastral striations fine, often faint …………………………………………………………….…………….…..………….18
- Metapleural spines long and narrow (Kakadu NP) .……………………….…………………………….………….sp. 14Metapleural spines short and triangular (Vermalha Stn) ………………..…………………………………..……...sp. 15
- First gastral tergite primarily punctate anteriorly, with only feeble striations (Kakadu NP) ………………..….sp. 16First gastral tergite finely but conspicuously striate throughout …………..………………………..………..…………19
- First gastral tergite with punctate background sculpture, giving it a somewhat matt appearance; rugae on frontal area finer and more closely approximated (Kakadu NP) ……………………………………………...…………....sp. 17First gastral tergite with feeble background sculpture, giving it a shiny appearance; rugae on frontal area very coarse and widely separated (Kakadu NP, Nitmiluk NP, Arnhem Land) ………………………………………...sp. 18
- Head, mesosoma and waist orange-brown; metapleural lobes spinose; first gastral tergite without striations on its posterior half medially (Gove) …………………………………………………………………………...…..………...sp. 19Head, mesosoma and waist darker reddish or blackish brown ……………..…………..……………..……………....21
- First gastral tergite conspicuously striate throughout …………………………………..……………..………………..22First gastral tergite mostly smooth and shiny medially on posterior half ……..…………..……………..……………24
- Metapleural spines especially long and narrow; first gastral tergite with feeble background sculpture, giving it a shiny appearance (Kakadu NP) ……………….………………..……………..……………..………………………...sp. 20Metapleural spines not so long and narrow; first gastral tergite with punctate background sculpture, giving it a more matt appearance ……………………………..……………..……………..……………..……..…………………….23
- Basal flanges of first gastral tergite weakly developed; in dorsal view, lateral margins of first gastral tergite only weakly curved (Sturt Plateau region, Kakadu NP) .……………..……………..……………..……………………..sp. 21Basal flanges of first gastral tergite strongly developed; in dorsal view, lateral margins of first gastral tergite strongly curved (eastern Top End) .….……………………………………..……………..……………..…………...sp. 22
- Metapleural spines short and broadly triangular (Kakadu NP) …………..…………..……………..…..………...sp. 23Metapleural spines long and narrow ………………………..…………………..……………..………………………….25
- First gastral tergite with very feeble background punctation, giving it a shiny appearance (Cobourg Peninsula, Blue Mud Bay) …………………………………..……………………..……………..……………..……………..…….sp. 24First gastral tergite with stronger background punctation, giving it a more matt appearance (Limmen NP) …………………………………………………………..……………..……………..……………………………..sp. 25
- Mesosoma dark brown, contrasting with light honey-brown legs and gaster ………………..……………..………..27Mesosoma yellowish or reddish brown, more or less concolorous with legs and gaster ……………………………………….……………………………………………..……..…………..……….………….28
- Anterior half of pronotal dorsum with regular, coarse rugae (Kimberley, WA) ……..….………….……….spininodeAnterior half of pronotal dorsum irregularly sculptured (Kakadu NP) …………………..……………..………..sp. 26
- Colour darker reddish brown, rugae on frontal area coarser ……………………………..……...………..…………..29Colour paler yellowish or orange-brown; rugae on frontal area not so coarse ..…………..……………..…………..31
- Lateral striations on first gastral tergite diverging from the base (Tandidgee Stn)………………………..……...sp. 27Striations on first gastral tergite parallel throughout ……………………...…………………..……………..…………..30
- Gastral striations finer (Sturt Plateau, Victoria River District, Gulf region) ………………..……………...……..sp. 28Gastral striations coarser (Newcastle Waters and Hidden Valley stns) ......……..……...…...…...…...…...…….sp. 29
- Striations on first gastral tergite parallel throughout (Vermhala Stn) ………..…………...…...…...…....…...…..sp. 30Lateral striations on first gastral tergite either diverging or converging from the base ……...…...…...…....….......32
- Lateral striations on first gastral tergite diverging from the base (Tandidgee, Newcastle Waters and Henbury Stations, Alice Springs, Uluru NP) ……………………..…………………………………………..………..………...….sp. 31Lateral striations on first gastral tergite converging from the basal lobes (Limmen NP) ………………………..sp. 32
The CO1 tree is structured into three primary clades: clade A (three NT species and T. spininode), clade B (sixteen species) and sp. 5 (Figure 7). Key morphological characters show strong structure within the CO1 tree. Species 5 has a unique combination of a lack of metapleural spines and costate gastral sculpture. All species in clade A lack a metapleural spine, whereas, with one exception (sp. 27, which is morphologically very similar to sp. 28 in clade A), the metapleural lobe is spinose in all species in clade B. The three sequenced species with costate gastral sculpture other than sp. 5 (sp. 1, sp. 3 and sp. 4; all with spinose metapleural lobes) form a distinct subclade within clade B. The non-sequenced sp. 2 shares these characters and therefore likely belongs in this clade. The two other non-sequenced species with costate gasters (spp. 6 and 7) lack spinose metapleural lobes and so are likely allied to sp. 5. The two sequenced species with a ridged gaster (sp. 11 and sp. 13; both also with spinose metapleural lobes) form a subclade within clade B. The three non-sequenced species with a ridged gaster (sp. 12, sp. 14 and sp. 15) also have spinose metapleural lobes and therefore likely belong in this subclade. There is also geographic structure among the characters. For example, none of the 7 species with costate gastral sculpture are among the 14 species known from the mesic (>1000 mm mean annual rainfall) Top End. On the other hand, a subclade of five species within clade B (sp. 14, sp. 16, sp. 19, sp. 20 and sp. 22), all with spinose metapleural lobes and a finely striate gaster, is known only from the Top End.
3.2. Species Turnover
There is almost complete species turnover between the mesic Top End (14 species) and the semi-arid Sturt Plateau region to the south (13 species), with only 1 (sp. 21) in common. Of the four species from the Gulf region (sp. 4, sp. 7, sp. 28 and sp. 32), only one (sp. 28) has been recorded elsewhere. The two remaining species (sp. 8 and sp. 9) are from the Beswick/Manyallaluk area southeast of Katherine, and neither is known from elsewhere. Only one species (sp. 31) is known to extend beyond the NT monsoonal zone into the central arid zone (Figure 6).
4. Discussion
We have recognised 32 species of the T. spininode group from NT specimens. None of the species are T. spininode, and so all are undescribed. None are cryptic in the sense of being unable to be differentiated morphologically [12], and there was a very strong match between morphological and CO1 variation.
Sampling was heavily concentrated in two regions, the mesic (>1000 mean annual rainfall) Top End and semi-arid (500–900 mm) Sturt Plateau region to the south, and this is where most (26) of the species were collected. A similar number of species occur in each region (14 and 13, respectively), and only one is known from both. The remaining six species are all known from very restricted locations. Such high rates of geographic turnover suggest that many additional species occur in regions that have not been sampled. It is likely that the total number of species occurring in the NT is at least 40 and possibly > 50.
Diversity in the T. spininode group appears to be at least as high in WA as it is in the NT. The Darwin collection has 19 sorted species from WA, from just 21 collection localities. Notably, none of these have spinose metapleural lobes, and six have a spectacularly costate gaster (Figure 8). The one NT species extending into the arid southern NT (sp. 31) also occurs in WA’s Great Sandy Desert and the southern Kimberley, but otherwise, there appears to be no overlap between the WA and NT species in the Darwin collection. Diversity appears to be lower in Queensland (Qld). Species 38 (occurring throughout the semi-arid NT) appears to occur throughout northern Qld (extending into Cape York Peninsula), but none of the other Qld species are known from the NT. As in WA, none of the Qld species in the Darwin collection have spinose metapleural lobes.

Figure 8.
Variation in coarse gastral sculpture of six species (a–f) of the T. spininode group from the Kimberley region of WA.
Assuming that the total NT fauna consists of more than 40 species and a similar number occurs in WA, given that the group occurs throughout northern Qld and that geographic turnover is extremely high, the total number of species in the T. spininode group would appear to be around 100. We acknowledge that this figure might change somewhat (either up or down) following more detailed taxonomic analysis, but we believe that our finding of extremely high unrecognised diversity is robust. Such a finding further builds the case that monsoonal Australia should be recognised as a global centre of ant species diversity. Unrecognised diversity occurs throughout the monsoonal fauna of Tetramorium, especially in the T. striolatum group, which is far more diverse than the T. spininode group [6]. The initial figure of about 1500 ant species occurring in monsoonal Australia [1] was based on estimates that have now proven to be far too low. For example, the Melophorus rufoniger group was initially estimated to include ten species and the Monomorium nigrius group twenty [1], whereas their true diversity is an order of magnitude higher [4,5]. The original estimate for the number of species in the T. spininode group was 8 and for the T. striolatum group 15 [1], both just small fractions of the actual faunas. All these findings point to Australia’s monsoonal ant fauna numbering in the several thousand species, which would make it by far the most species-rich known anywhere in the world.
5. Conclusions
The extraordinary hyperdiversity of ants in monsoonal Australia is not reflected in a recent analysis of ant global diversity patterns, either within Australia or internationally [13]. This is because the analysis is based on described species only. The analysis shows ant diversity within Australia to be highest along the eastern coast and in the temperate southeast and southwest. These are the areas where the great majority of the Australian population lives and where taxonomic effort has been heavily concentrated. The Australian map is one of sampling intensity and taxonomic effort rather than of species richness. The global analysis shows species richness in monsoonal Australia as being lower than for most of the United States, and that the Neotropics is the clear global hotspot for ant diversity [13]. A very different picture would emerge if undescribed species were included. A high number of undescribed species is characteristic of tropical ant faunas throughout the world, but there is no evidence that the extreme level of unrecognised diversity in monsoonal Australia occurs anywhere else. For example, an extensive CO1 analysis of the fauna of Brazil’s southern Atlantic Forest revealed that its size was likely only 6–10% larger than currently recognised [14]. The Australian monsoonal tropics thus appears to represent a major biogeographic anomaly in terms of global patterns of ant diversity. This is even more remarkable given that the vast majority of species are epigaeic, with relatively few specialist arboreal or cryptobiotic species that feature so strongly in tropical rainforest [3,15].
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d15040476/s1, Figure S1: full CO1 tree, showing all sequenced specimens, Table S1: list of specimens sequenced in this study and their collection locations. Specimens are identified by their BOLD ID codes and arranged according to species.
Author Contributions
A.N.A. conceived the study, led the development of the Darwin ant collection and wrote the first draft of the manuscript. F.B. prepared the figures and contributed to the writing of the paper. B.D.H. helped develop the Darwin ant collection and contributed to the writing of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
François Brassard was supported by a University Research Training Scheme, a Research Training Program Stipend Scholarship, and a Holsworth wildlife research endowment grant.
Data Availability Statement
The CO1 data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank our many collaborators who collected the specimens analysed in this study, and especially Magen Pettit, Tony Hertog and Jodie Hayward from CSIRO, as well as staff from the Flora and Fauna Division of the NT Department of Environment and Natural Resources, who have included ant sampling at hundreds of sites in the NT as part of ongoing biodiversity assessment. We also thank Ben Aidoo, Sarah Bonney, Prakash Gaudel and Magen Pettit for preparing samples for CO1 analysis, and Francisco Hita Garcia and Julio Chaul for their comments on the draft manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andersen, A. The Ants of Northern Australia: A Guide to the Monsoonal Fauna; CSIRO Publishing: Collingwood, VIC, Australia, 2000; ISBN 0643066039. [Google Scholar]
- Oberprieler, S.K.; Andersen, A.N.; Moritz, C.C. Ants in Australia’s monsoonal tropics: CO1 barcoding reveals extensive unrecognised diversity. Diversity 2018, 10, 36. [Google Scholar] [CrossRef]
- Andersen, A.N.; Vasconcelos, H.L. Historical biogeography shapes functional ecology: Inter-continental contrasts in responses of savanna ant communities to stress and disturbance. J. Biogeogr. 2022, 49, 590–599. [Google Scholar] [CrossRef]
- Andersen, A.N.; Hoffmann, B.D.; Oberprieler, S.K. Megadiversity in the ant genus Melophorus: The M. rufoniger Heterick, Castalanelli and Shattuck species group in the Top End of Australia’s Northern Territory. Diversity 2020, 12, 386. [Google Scholar] [CrossRef]
- Andersen, A.N.; Brassard, F.; Hoffmann, B.D. Ant megadiversity in monsoonal Australia: Diversity and distribution in the hyperdiverse Monomorium nigrius Forel group. Diversity 2022, 14, 46. [Google Scholar] [CrossRef]
- Andersen, A.N. Ant megadiversity and its origins in arid Australia. Austral Entomol. 2016, 55, 132–147. [Google Scholar] [CrossRef]
- Bolton, B. The ant tribe Tetramoriini (Hymenoptera: Formicidae). The genus Tetramorium Mayr in the Oriental and Indo Australian regions, and in Australia. Bull. Br. Mus. (Nat. Hist.) Entomol. 1977, 36, 67–151. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Fisher, B.L.; Hebert, P.D.N. DNA barcoding for effective biodiversity assessment of a hyperdiverse arthropod group: The ants of Madagascar. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Schlick-Steiner, B.C.; Steiner, F.M.; Moder, K.; Seifert, B.; Sanetra, M.; Dyreson, E.; Stauffer, C.; Christian, E. A multidisciplinary approach reveals cryptic diversity in Western Palearctic Tetramorium ants (Hymenoptera: Formicidae). Mol. Phylogenetics Evol. 2006, 40, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Bickford, D.; Lohman, D.J.; Sodhi, N.S.; Ng, P.K.L.; Meier, R.; Winker, K.; Ingram, K.K.; Das, I. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007, 22, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Kass, J.M.; Guénard, B.; Dudley, K.L.; Jenkins, C.N.; Azuma, F.; Fisher, B.L.; Parr, C.L.; Gibb, H.; Longino, J.Y.; Ward, P.S.; et al. The global distribution of known and undiscovered ant biodiversity. Sci. Adv. 2022, 8, eabp9908. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, P.E.; Lavinia, P.D.; Suarez, A.V.; Lijtmaer, D.A.; Leponce, M.; Paris, C.I.; Tubaro, P.L. Mind the gap! Integrating taxonomic approaches to assess ant diversity at the southern extreme of the Atlantic Forest. Ecol. Evol. 2017, 7, 10451–10466. [Google Scholar] [CrossRef] [PubMed]
- Brühl, C.A.; Gunsalam, G.; Linsenmair, K.E. Stratification of ants (Hymenoptera, Formicidae) in a primary rain forest in Sabah, Borneo. J. Trop. Ecol. 1998, 14, 285–297. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).