Eco-Coenotic and Diversity Patterns in Artemisia alba Open Scrubs from Romania within the Context of Similar Communities from Neighbouring Regions
Abstract
1. Introduction
2. Materials and Methods
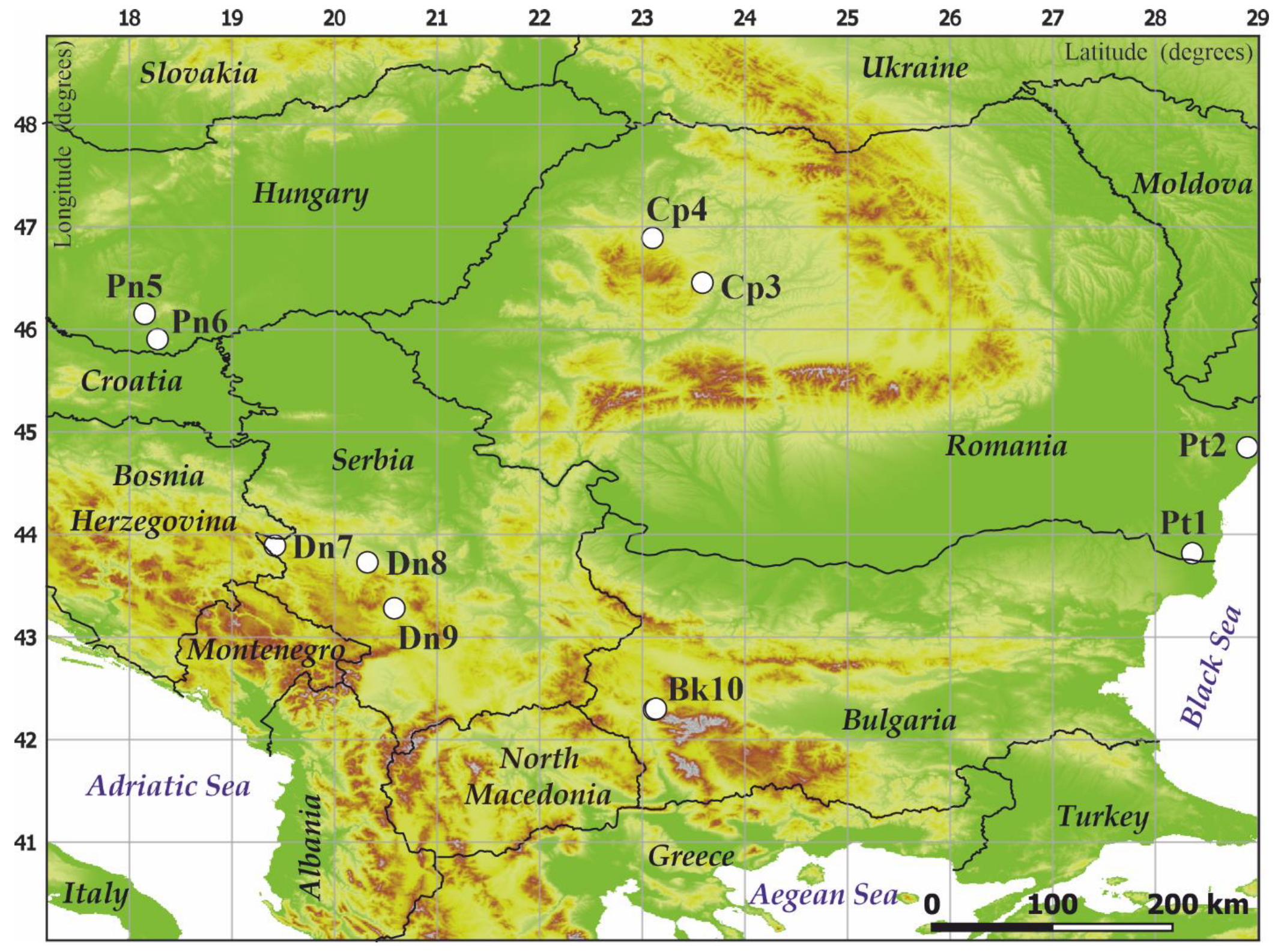
2.1. Study Areas
2.2. Data Collection and Transformation
2.3. Numerical Data Analysis
3. Results
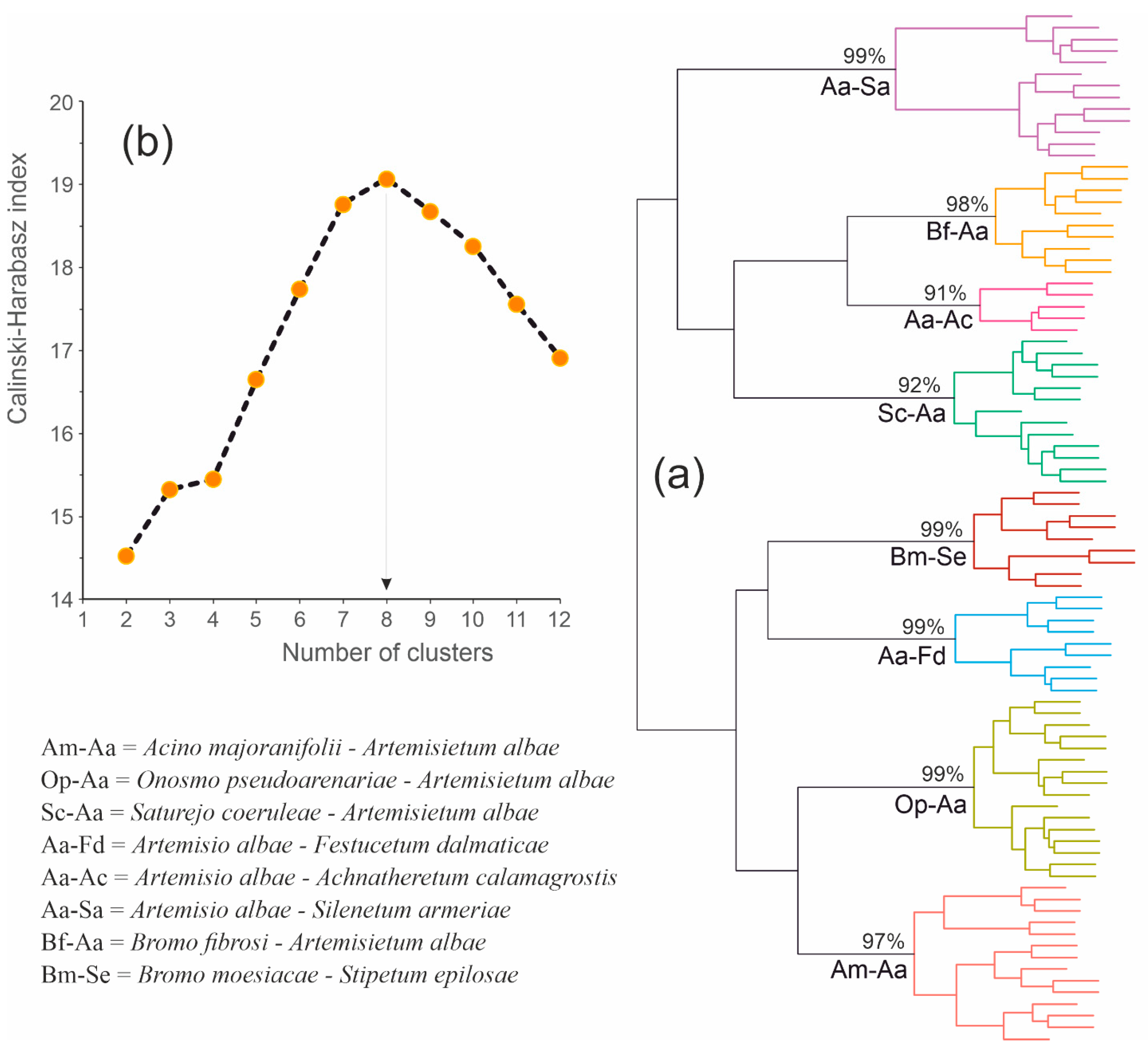
3.1. Classification of All Relevés under Study
3.2. Syntaxonomic Assignation of Artemisia alba Scrubs from the Carpathian and Pontic Regions

3.3. Floristic and Habitat Features of the Carpathian and Pontic Communities (Co)Dominated by Artemisia alba
3.3.1. Acino majoranifolii—Artemisietum albae ass. nova hoc loco
3.3.2. Onosmo pseudoarenariae—Artemisietum albae ass. nova hoc loco
3.3.3. Saturejo coeruleae—Artemisietum albae ass. nova hoc loco
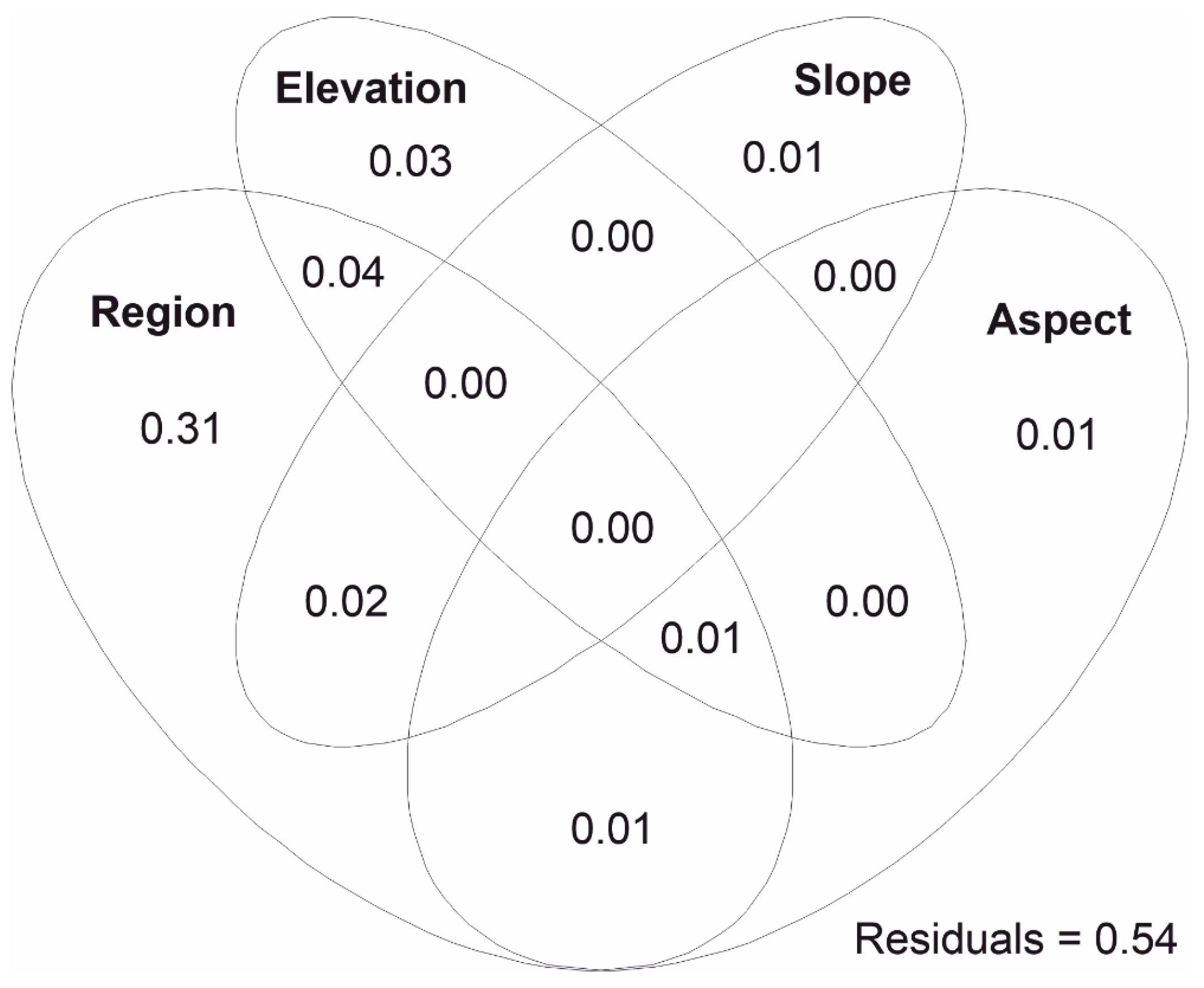
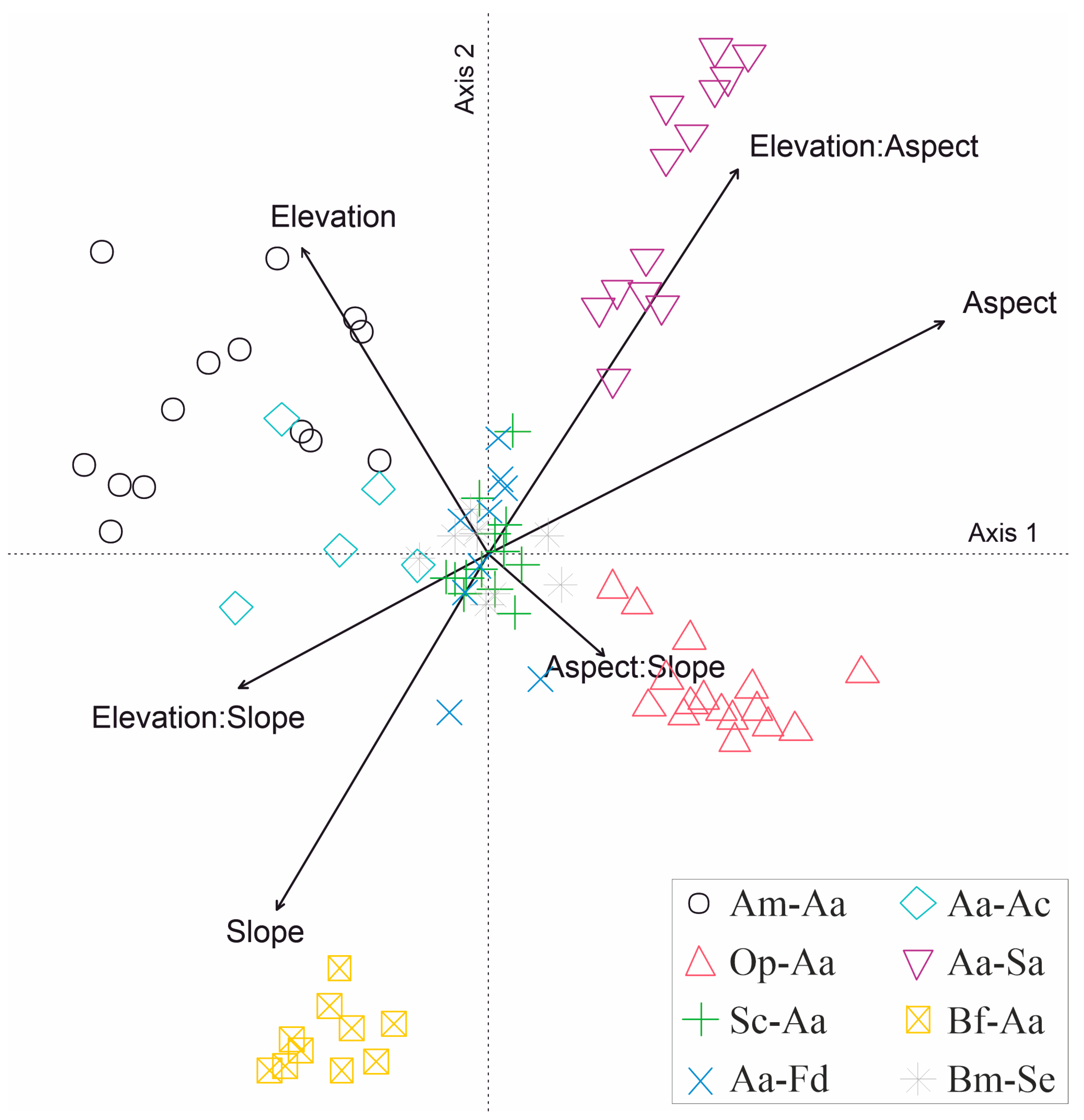
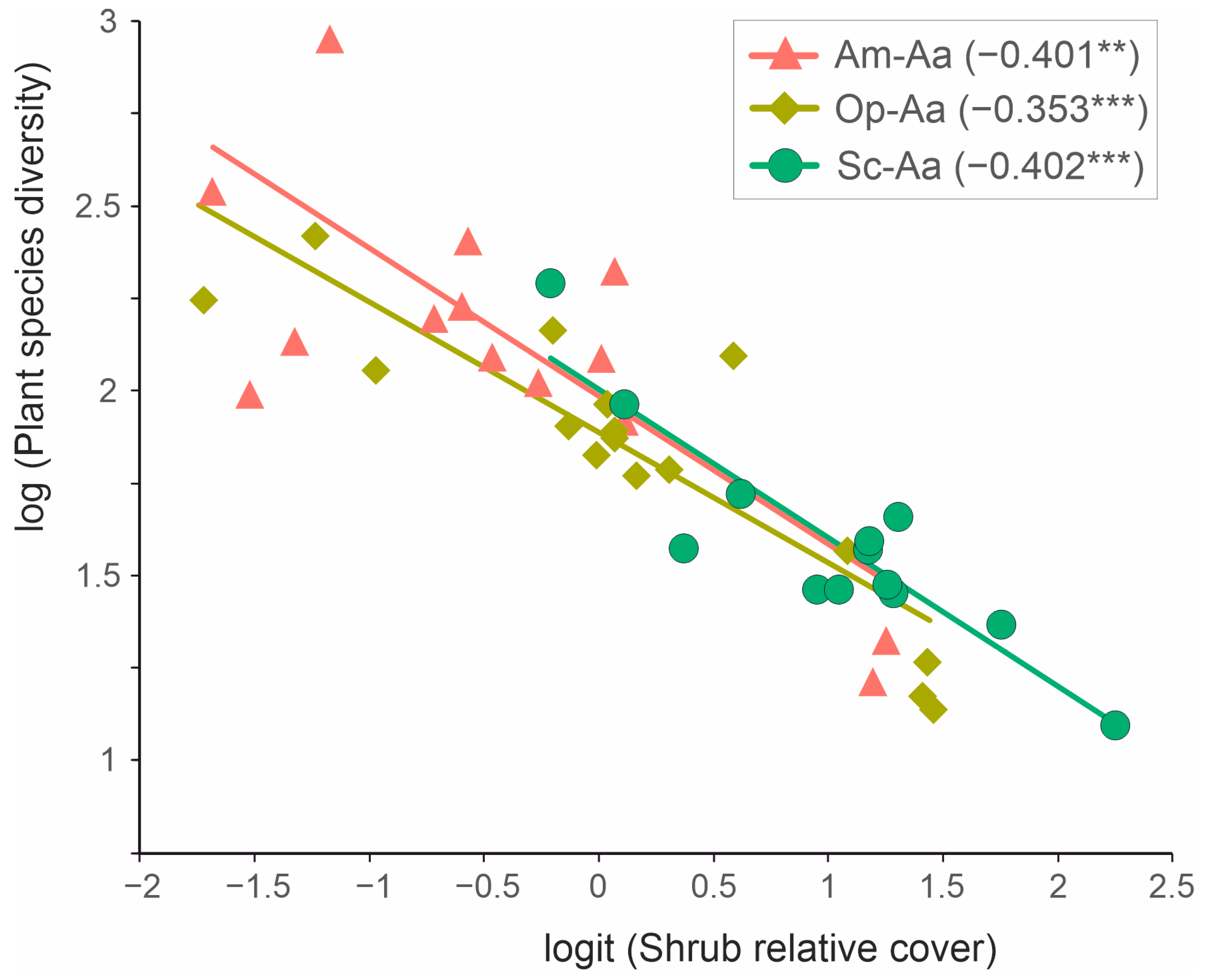
3.4. Predictors Contribution to Floristic Dissimilarities between Communities
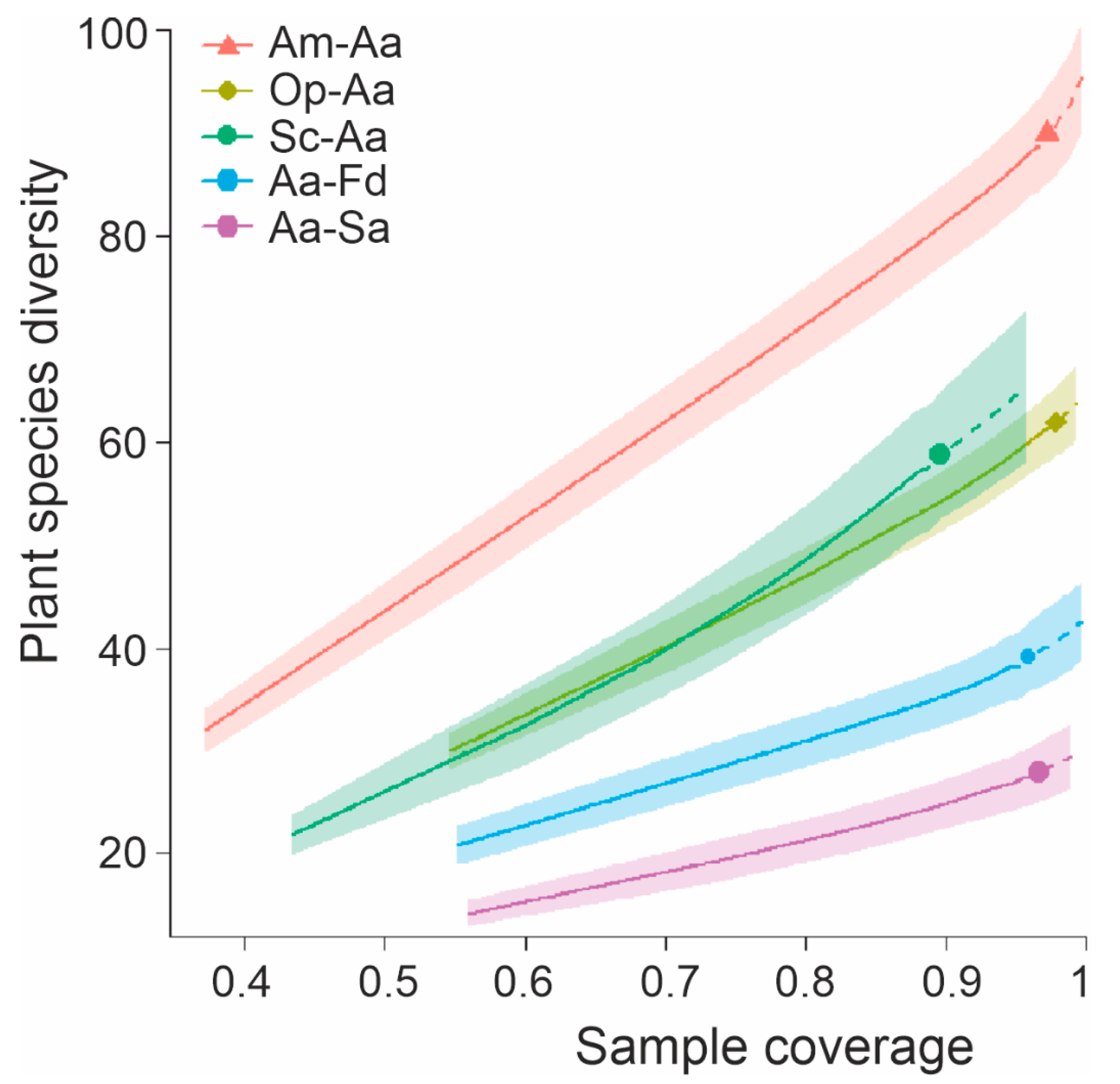
3.5. Patterns in Species Diversity
4. Discussion
4.1. Floristic and Syntaxonomic (Dis)Similarities
4.2. Regional and Local Site Effects on Species Composition/Diversity
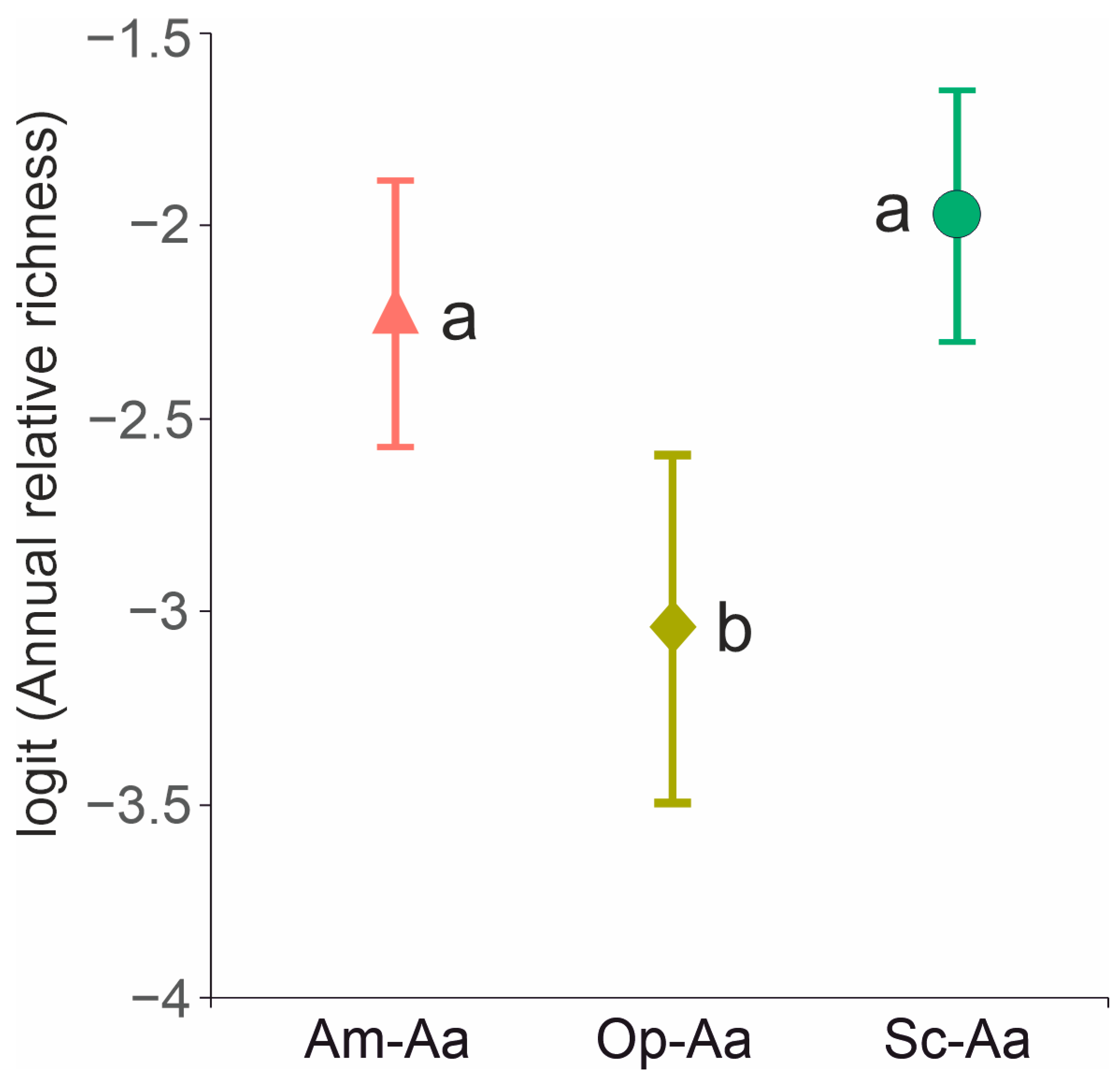
4.3. Annual and Shrub Contribution to Species Diversity
4.4. Limitations and Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, Plantaginaceae to Compositae; Cambridge University Press: Cambridge, UK, 1975; Volume 4, p. 534. [Google Scholar]
- Erschbamer, B.; Grabherr, G.; Reisigl, H. Spatial pattern in dry grassland communities of the Central Alps and its ecophysiological significance. Vegetatio 1983, 54, 143–151. [Google Scholar] [CrossRef]
- Schaminée, J.H.J.; Chytry, M.; Hennekens, S.M.; Janssen, J.A.M.; Jimenez-Alfaro, B.; Knollova, I.; Marceno, C.; Mucina, L.; Rodwell, J.S.; Tichy, L. Review of Grassland Habitats and Development of Distribution Maps of Heathland, Scrub and Tundra Habitats of EUNIS Habitats Classification; Report EEA/NSV/15/005; Alterra: Wageningen, The Netherlands, 2016; p. 379. [Google Scholar]
- Witschel, M. Zur Synsystematik der Trinia glauca reichen Trockenrasen im südlichen Oberrheinraum. Carolinea 1993, 51, 27–40. [Google Scholar]
- Matevski, V.; Čarni, A.; Cušterevska, R.; Kostadinovski, M.; Mucina, L. Syntaxonomy and biogeography of the grasslands on calcareous substrates in the central and southern Balkans. Appl. Veg. Sci. 2018, 21, 488–513. [Google Scholar] [CrossRef]
- Royer, J.M.; Ferrez, Y. Contribution au prodrome des végétation de France: Les Festuco-Brometea Br.-Bl. & Tx. ex Klika et Hadać 1944. Doc. Phytosociol. 2020, 13, 1–304. [Google Scholar]
- Terzi, M. Numerical analysis of the order Scorzoneretalia villosae. Phytocoenologia 2015, 45, 11–32. [Google Scholar] [CrossRef]
- Kuzmanović, N.; Kabaš, E.; Jovanović, S.; Vukojičić, S.; Aćić, S.; Surina, B.; Lakušić, D. Syntaxonomy and nomenclatural adjustments of steppe-like vegetation on shallow ultramafic soils in the Balkans included in the order Halacsyetalia sendtneri. Tuexenia 2016, 36, 293–320. [Google Scholar]
- Csűrös, Ș.; Gergely, I. Stațiuni noi ale speciei Artemisia lobelii All. în România. Stud. Cercet. Biol. 1959, 10, 123–127. [Google Scholar]
- Karácsonyi, C. Vegetația teritoriului. In Patrimoniul natural al Sălajului—Flora, micobiota și vegetația; Negrean, G., Karácsonyi, C., Szatmari, P.M., Eds.; Editura Someșul: Satu Mare, Romania, 2017; Volume 1, pp. 1102–1271. [Google Scholar]
- Tilman, D. Plant Strategies and the Dynamics and Structure of Plant Communities; Princeton University Press: Princeton, NJ, USA, 1988; p. 360. [Google Scholar]
- Callaway, R.M. Positive Interactions and Interdependence in Plant Communities; Springer: Dordrecht, The Netherlands, 2007; p. 415. [Google Scholar]
- Moeslund, J.E.; Arge, L.; Bøcher, P.K.; Dalgaard, T.; Odgaard, M.V.; Nygaard, B.; Svenning, J.-C. Topographically controlled soil moisture is the primary driver of local vegetation patterns across a lowland region. Ecosphere 2013, 4, 91. [Google Scholar] [CrossRef]
- Cook, W.M.; Lane, K.T.; Foster, B.L.; Holt, R.D. Island theory, matrix effects and species richness patterns in habitat fragments. Ecol. Lett. 2002, 5, 619–623. [Google Scholar] [CrossRef]
- Zobel, M. The relative role of species pools in determining plant species richness: An alternative explanation of species coexistence? Trends Ecol. Evol. 1997, 12, 266–269. [Google Scholar] [CrossRef]
- Cody, M.L. Diversity, rarity and conservation in Mediterranean-climate regions. In Conservation Biology. The Science of Scarcity and Diversity; Soule, M.E., Ed.; Sinauer: Sunderland, MA, USA, 1986; pp. 122–152. [Google Scholar]
- Cowling, R.M.; Holmes, P.M.; Rebelo, A.M. Plant diversity and endemism. In The Ecology of Fynbos. Nutrients, Fire and Diversity; Cowling, R.M., Ed.; Oxford University Press: Cape Town, South Africa, 1992; pp. 62–112. [Google Scholar]
- Hacker, S.D.; Gaines, S.D. Some implications of direct positive interactions for community species diversity. Ecology 1997, 78, 1990–2003. [Google Scholar] [CrossRef]
- Arroyo, A.I.; Pueyo, Y.; Saiz, H.; Alados, C.L. Plant–plant interactions as a mechanism structuring plant diversity in a Mediterranean semi-arid ecosystem. Ecol. Evol. 2015, 5, 5305–5317. [Google Scholar] [CrossRef] [PubMed]
- Vega-Alvarez, J.; Garcia-Rodriguez, J.A.; Cayuela, L. Facilitation beyond species richness. J. Ecol. 2019, 107, 722–734. [Google Scholar] [CrossRef]
- Xiao, S.; Michalet, R.; Wang, G.; Chen, S.-Y. The interplay between positive and negative interactions shapes the “community biomass—Species richness” relationship. Oikos 2009, 118, 1343–1348. [Google Scholar] [CrossRef]
- Michalet, R.; Bagousse-Pinguet, L.; Maalouf, J.P.; Lortie, C.J. Two alternatives to the stress-gradient hypothesis at the edge of life: The collapse of facilitation and the switch from facilitation to competition. J. Veg. Sci. 2014, 25, 609–613. [Google Scholar] [CrossRef]
- Köchy, M.; Wilson, S.D. Competitive effects of shrubs and grasses in prairie. Oikos 2000, 91, 385–395. [Google Scholar] [CrossRef]
- Holzapfel, C.; Tielbörger, K.; Parag, H.A.; Kigel, J.; Sternberg, M. Annual plant–shrub interactions along an aridity gradient. Basic Appl. Ecol. 2006, 7, 268–279. [Google Scholar] [CrossRef]
- Osem, Y.; Perevolotsky, A.; Kigel, J. Interactive effects of grazing and shrubs on the annual plant community in semi-arid Mediterranean shrublands. J. Veg. Sci. 2007, 18, 869–878. [Google Scholar] [CrossRef]
- Weedon, J.T.; Facelli, J.M. Desert shrubs have negative or neutral effects on annuals at two levels of water availability in arid lands of South Australia. J. Ecol. 2008, 96, 1230–1237. [Google Scholar] [CrossRef]
- Maestre, F.T.; Bautista, S.; Cortina, J. Positive, negative, and net effects in grass–shrub interactions in Mediterranean semiarid grasslands. Ecology 2003, 84, 3186–3197. [Google Scholar] [CrossRef]
- Pugnaire, F.I.; Armas, C.; Valladares, F. Soil as a mediator in plant-plant interactions in a semi-arid community. J. Veg. Sci. 2004, 15, 85–92. [Google Scholar] [CrossRef]
- Tewksbury, J.J.; Lloyd, J.D. Positive interactions under nurse-plants: Spatial scale, stress gradients and benefactor size. Oecologia 2001, 127, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Keeley, J.E.; Fotheringham, C.J. Species–area relationships in Mediterranean-climate plant communities. J. Biogeogr. 2003, 30, 1629–1657. [Google Scholar] [CrossRef]
- Petrescu, M.; Cuzic, V.; Panait, V.; Cuzic, M. Cercetări privind patrimoniul natural al comunei Sarichioi. Delta Dunării. Stud. Cercet. Ştiinţele Nat. Muzeol. 2012, 6, 153–162. [Google Scholar]
- Heuffel, J. Enumeratio Plantarum in Banatu Temisiensis Sponte Crescentium et Frecquentius Culturum; Typis C. Ueberreuter: Vienna, Austria, 1858. [Google Scholar]
- Schur, F. Enumeration Plantarum Transsilvaniae; Apud G. Braumüller: Vienna, Austria, 1866. [Google Scholar]
- Schrött, L.; Purdela, L. Considerații asupra florei și vegetaţiei rezervației naturale Valea Ciclovei, Jud. Caraș-Severin. Ocrotirea Nat. Mediu. Înconj. 1993, 37, 25–31. [Google Scholar]
- Nechita, N. Flora și Vegetația Cormofitelor din Masivul Hășmaș, Cheile Bicazului și Lacul Roșu; Editura Constantin Matasă: Piatra Neamț, Romania, 2003; p. 383. [Google Scholar]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at High Resolution for the Earth’s Land Surface Areas. EnviDat. 2021. Available online: https://www.envidat.ch/#/metadata/chelsa-climatologies (accessed on 15 March 2023).
- Braun-Blanquet, J. Fitosociología. Bases para el Estudio de las Comunidades Vegetales; Blume: Madrid, Spain, 1979; 820p. [Google Scholar]
- World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Update 2015; World Soil Resources Reports 106; FAO: Rome, Italy, 2015; p. 192. Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 12 January 2023).
- Borhidi, A.; Dénes, A. A Mecsek és a Villányi hegység sziklagyep társulásai. Stud. Phytol. Jubil. Pécs 1997, 4, 45–65. [Google Scholar]
- Todorova, S.; Tzonev, R. Bromo moesiacae—Stipetum epilosae a new association from the relict mountain steppe vegetation in south-western Bulgaria. Hacquetia 2010, 9, 185–206. [Google Scholar] [CrossRef]
- Marković, A. Stepske fitocenoze u Šumadiji; Univerzitet u Kragujevcu, Prirodna-Matematički Fakultet: Kragujevac, Serbia, 2007; p. 115. [Google Scholar]
- Jovanović, S.; Kabas, E.; Kuzmanović, N.; Jakovlyević, K.; Vukojičić, S.; Lukušić, D. Phytosociological characteristics of seven poorly known associations of serpentine rocky grassland vegetation of the order Halacsyetalia sendtneri in Serbia. Bot. Serb. 2017, 41, 221–247. [Google Scholar]
- Sârbu, I.; Ștefan, N.; Oprea, A. Plante Vasculare din România. Determinator Ilustrat de Teren; Victor B. Victor: București, Romania, 2013; p. 1320. [Google Scholar]
- Beers, T.W.; Dress, P.E.; Wensel, L.C. Aspect transformation in site productivity research. J. For. 1966, 64, 691–692. [Google Scholar]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Roswell, M.; Dushoff, J.; Winfree, R. A conceptual guide to measuring species diversity. Oikos 2021, 130, 321–338. [Google Scholar] [CrossRef]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M. ’cluster’ (v2.1.4), Finding Groups in Data: Cluster Analysis Extended. Available online: https://cran.r-project.org/package=cluster (accessed on 12 January 2023).
- Hennig, C. ’fpc’ (v2.2-10), Flexible Procedures for Clustering. Available online: https://cran.r-project.org/package=fpc (accessed on 12 January 2023).
- Liaw, A.; Wiener, M. ‘randomForest’ (v4.7-1.1), Breiman and Cutler’s Random Forests for Classification and Regression. Available online: https://cran.r-project.org/web/packages/randomForest/randomForest.pdf (accessed on 12 January 2023).
- De Cáceres, M.; Jansen, F.; Dell, N. ’indicspecies’ (v1.7.12), Relationship Between Species and Groups of Sites. Available online: https://cran.r-project.org/package=indicspecies (accessed on 12 January 2023).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. ’vegan’ (v2.6-4), Community Ecology Package. Available online: https://cran.r-project.org/package=vegan (accessed on 12 January 2023).
- Hsieh, T.C.; Ma, K.H.; Chao, A. ‘iNEXT’ (v3.0.9), Interpolation and Extrapolation for Species Diversity. Available online: https://cran.r-project.org/web/packages/iNEXT/iNEXT.pdf (accessed on 12 January 2023).
- SAS Institute Inc. SAS/STAT® User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2022; p. 11051. Available online: https://documentation.sas.com/api/collections/pgmsascdc/v_035/docsets/statug/content/statug.pdf?locale=en#nameddest=titlepage (accessed on 12 January 2023).
- Borhidi, A. An annotated checklist of the Hungaricum plant communities I. The non-forest vegetation. In Critical Revision of the Hungarian Plant Communities; Borhidi, A., Ed.; Janus Pannonius University: Pecs, Hungary, 1996; pp. 43–94. [Google Scholar]
- Chytry, M. Vegetation of the Czech Republic. 1. Grassland and Heathland Vegetation; Academia Praha: Praha, Czech Republic, 2007; p. 526. [Google Scholar]
- Skodova, I.; Janisova, M.; Dubrakova, D.; Ujhazy, K. Festuco-Brometea. In Rastlinne spolocenstva Slovenska 5. Travinnobylinna vegetacia; Hegedusova Vantarova, K., Skodova, I., Eds.; Veda: Bratislava, Slovakia, 2014; pp. 43–62. [Google Scholar]
- Borhidi, A. Magyarország Növenytársulásai; Akadémiai Kiado: Budapest, Hungary, 2003; p. 610. [Google Scholar]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; Gavilán García, R.; et al. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19 (Suppl. S1), 3–264. [Google Scholar] [CrossRef]
- Dihoru, G.; Doniță, N. Flora și vegetația Podișului Babadag; Editura Academiei R.P.R.: București, Romania, 1970; p. 438. [Google Scholar]
- Botti, D. A Phytoclimatic Map of Europe. Cybergeo Eur. J. Geogr. 2018, 867. [Google Scholar] [CrossRef]
- Chiarucci, A.; Robinson, B.H.; Bonini, I.; Petit, D.; Brooks, R.R.; De Dominicis, V. Vegetation of Tuscan ultramafic soils in relation to edaphic and physical factors. Folia Geobot. 1998, 33, 113–131. [Google Scholar] [CrossRef]
- Coldea, G.; Gafta, D.; Negrean, G.; Stoica, A.I.; Hurdu, B.I. Southern Carpathian ultramafic grasslands within the central-southeast European context: Syntaxonomic classification and overall eco-coenotic patterns. Bot. Stud. 2022, 63, 29. [Google Scholar] [CrossRef]
- McCain, C.M.; Grytnes, J.A. Elevational gradients in species richness. In Encyclopedia of Biodiversity; John Wiley & Sons: Chichester, UK, 2010; pp. 1–10. [Google Scholar]
- Pueyo, Y.; Moret-Fernández, D.; Saiz, H.; Bueno, C.G.; Alados, C.L. Relationships between plant spatial patterns, water infiltration capacity, and plant community composition in semi-arid Mediterranean ecosystems along stress gradients. Ecosystems 2013, 16, 452–466. [Google Scholar] [CrossRef]
- Gergely, I. Contribuții la studiul fitocenologic al pădurilor din partea nordică a Munților Trascăului. Contrib. Bot. 1962, 3, 263–298. [Google Scholar]
- Gavrilov, M.B.; Radaković, M.G.; Sipos, G.; Mezősi, G.; Gavrilov, G.; Lukić, T.; Basarin, B.; Benyhe, B.; Fiala, K.; Kozák, P.; et al. Aridity in the Central and Southern Pannonian Basin. Atmosphere 2020, 11, 1269. [Google Scholar] [CrossRef]
- Ratajczak, Z.; Nippert, J.B.; Collins, S.L. Woody encroachment decreases diversity across North American grasslands and savannas. Ecology 2011, 93, 697–703. [Google Scholar] [CrossRef]
- Soliveres, S.; Maestre, F.T.; Eldridge, D.J.; Delgado-Baquerizo, M.; Quero, J.M.; Bowker, M.A.; Gallardo, A. Plant diversity and ecosystem multifunctionality peak at intermediate levels of woody cover in global drylands. Glob. Ecol. Biogeogr. 2014, 23, 1408–1416. [Google Scholar] [CrossRef]
- Görzen, E.; Borisova, K.; Fenesi, A.; Ruprecht, E.; Donath, T.W. Effects of woody species encroachment and fire on vegetation and the soil seed bank in dry grasslands of Transylvania. Appl. Veg. Sci. 2019, 22, 409–422. [Google Scholar] [CrossRef]
- Enyedi, Z.M.; Ruprecht, E.; Deák, M. Long-term effects of the abandonment of grazing on steppe-like grasslands. Appl. Veg. Sci. 2008, 11, 55–62. [Google Scholar] [CrossRef]
- Maestre, F.T.; Callaway, R.M.; Valladares, F.; Lortie, C.J. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 2009, 97, 199–205. [Google Scholar] [CrossRef]



| Site | Latitude (Degrees) | Longitude (Degrees) | Elevation (m) | Aspect | Plant Assoc. | pH | Mg (mg/kg) | Ca (mg/kg) | Co (mg/kg) | Cd (mg/kg) | Cu (mg/kg) | Ni (mg/kg) | Zn (mg/kg) | Cr (mg/kg) | Pb (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colțești (Cp3) | 46.25283 | 23.32494 | 700 | W | Am-Aa | 7.36 | 6.08 | 180.00 | 21.40 | 7.47 | 41.31 | 44.63 | 91.64 | 55.81 | 45.65 |
| Remetea (Cp3) | 46.44625 | 23.58159 | 900 | N | Am-Aa | 7.60 | 18.20 | 210.00 | 21.33 | 5.71 | 40.57 | 18.26 | 101.59 | 51.27 | 30.38 |
| Jebuc (Cp4) | 46.53600 | 23.06160 | 394 | NW | Op-Aa | 7.76 | 24.30 | 250.00 | 12.27 | 11.64 | 32.72 | 19.64 | 45.29 | 39.83 | 4.95 |
| Sfăraș (Cp4) | 46.53490 | 23.06112 | 390 | SW | Op-Aa | 7.86 | 6.08 | 220.00 | 17.08 | 13.43 | 34.09 | 29.78 | 50.00 | 43.62 | 12.66 |
| Cotu Văii (Pt1) | 43.81048 | 28.33560 | 65 | NE | Sc-Aa | 8.07 | 24.30 | 120.00 | 17.22 | 1.66 | 14.11 | 31.06 | 45.32 | 26.71 | 0.57 |
| Enisala (Pt2) | 44.87890 | 28.84540 | 75 | S | Sc-Aa | 8.12 | 6.10 | 110.00 | 21.77 | 2.75 | 16.53 | 55.41 | 67.07 | 43.35 | 9.98 |
| Relevé No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 * | 11 | 12 | 13 | 14 | Frequency (%) |
| Altitude (m) | 680 | 670 | 690 | 680 | 890 | 1000 | 776 | 733 | 680 | 690 | 670 | 660 | 650 | 660 | |
| Aspect | S | SE | E | SE | SV | SE | V | V | S | V | S | V | SE | NE | |
| Slope (degrees) | 45 | 60 | 55 | 70 | 50 | 10 | 25 | 35 | 25 | 20 | 15 | 30 | 15 | 25 | |
| Herb cover (%) | 85 | 60 | 50 | 45 | 55 | 65 | 75 | 60 | 75 | 80 | 70 | 65 | 75 | 55 | |
| Sample area (sq. m) | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 20 | 25 | 15 | 15 | 15 | 15 | 15 | |
| Diagnostic species at association level | |||||||||||||||
| Artemisia alba | 2.3 | 3.4 | 3.4 | 3.3 | 1.2 | 1.2 | 3.4 | 2.3 | 3.4 | 3.4 | 3.4 | 3.4 | 3.4 | 3.3 | 100 |
| Acinos alpinus subsp. majoranifolius | + | + | +.1 | + | +.1 | + | + | + | . | + | 1.2 | . | . | . | 71 |
| Cytisus albus | . | . | . | + | 1.2 | 1.2 | + | . | + | + | . | . | + | + | 57 |
| Silene nutans subsp. dubia | + | . | . | . | . | . | . | . | + | + | . | + | + | + | 43 |
| Bromo—Festucion pallentis | |||||||||||||||
| Phleum montanum | . | . | . | . | + | . | + | + | . | + | + | + | . | + | 50 |
| Anthyllis vulneraria subsp. alpestris | . | . | . | . | . | . | 1.2 | 1.2 | + | . | . | . | + | + | 36 |
| Bromus pannonicus | . | . | . | . | . | . | + | +.1 | . | + | + | . | . | + | 36 |
| Festuca pallens | . | + | . | + | . | + | . | + | . | 1.2 | . | . | . | . | 36 |
| Seseli osseum | . | . | . | . | . | . | + | + | + | + | . | . | + | . | 36 |
| Carduus defloratus subsp. glaucus | . | . | . | . | . | . | + | + | . | . | . | . | . | + | 21 |
| Thalictrum minus | . | . | . | . | . | . | . | + | . | + | . | . | . | + | 21 |
| Jurinea transsilvanica | . | . | . | + | . | . | . | . | . | . | + | . | . | . | 14 |
| Sempervivum marmoreum | + | . | . | + | . | . | . | . | . | . | . | . | . | . | 14 |
| Sesleria heufleriana | . | . | . | . | . | + | . | . | . | . | . | . | . | + | 14 |
| Stipo—Festucetalia pallentis | |||||||||||||||
| Alyssum murale | + | + | +.1 | + | . | . | + | + | . | 1.2 | . | . | . | + | 57 |
| Scabiosa columbaria | . | . | . | . | . | . | + | + | . | + | . | + | + | + | 43 |
| Anthericum ramosum | . | . | . | . | . | . | + | 1.1 | + | + | . | . | . | + | 36 |
| Melica ciliata | . | . | . | . | . | . | . | + | 1.2 | 1.1 | 2.3 | 2.3 | . | . | 36 |
| Sedum hispanicum | . | . | + | . | . | . | . | . | 1.2 | + | 2.2 | + | . | . | 36 |
| Stipa pulcherrima | . | . | . | + | . | . | . | . | 1.1 | . | 2.2 | 1.2 | 1.1 | . | 36 |
| Thymus comosus | . | . | . | . | . | . | + | + | . | + | + | . | . | + | 36 |
| Erysimum odoratum | . | + | . | . | + | + | + | . | . | . | . | . | . | . | 29 |
| Teucrium montanum | . | . | . | . | . | . | 1.2 | 1.1 | . | 1.2 | . | . | 2.3 | . | 29 |
| Helianthemum canum | . | . | . | . | . | . | . | 1.3 | + | + | . | . | . | . | 21 |
| Inula ensifolia | . | . | . | . | + | . | . | . | . | . | . | . | . | . | 7 |
| Festuco—Brometea (including Festucetalia valesiacae) | |||||||||||||||
| Festuca rupicola | 3.3 | 1.2 | 1.2 | + | 1.2 | . | . | . | 2.3 | 2.2 | + | + | 2.2 | + | 79 |
| Stachys recta | + | +.1 | . | + | . | . | + | +.1 | + | + | + | + | + | + | 79 |
| Helianthemum nummularium subsp. obscurum | . | + | . | + | + | + | . | 1.2 | + | + | + | + | . | + | 71 |
| Koeleria macrantha | 1.2 | + | + | + | . | . | + | . | + | . | 1.2 | 2.3 | + | 1.1 | 71 |
| Teucrium chamaedrys | . | 2.3 | . | . | . | + | 1.1 | 2.3 | + | + | + | + | 2.3 | 1.2 | 71 |
| Euphorbia cyparissias | + | + | . | . | . | + | . | . | + | + | + | + | + | + | 64 |
| Leontodon crispus | . | + | + | + | . | + | . | + | . | + | + | . | + | + | 64 |
| Campanula sibirica subsp. divergentiformis | + | + | +.1 | + | + | . | +.1 | + | . | . | . | . | . | . | 50 |
| Carex humilis | . | . | . | . | 1.2 | . | . | 1.1 | 2.2 | 2.2 | 1.2 | + | + | . | 50 |
| Dianthus carthusianorum | + | . | . | + | . | . | . | . | . | + | + | + | 1 | + | 50 |
| Avenula pratensis | . | . | + | . | . | . | . | + | + | + | . | + | . | 1.1 | 43 |
| Centaurea stoebe subsp. australis | + | . | . | + | . | . | . | + | . | . | . | + | + | + | 43 |
| Eryngium campestre | + | + | + | . | . | . | . | . | . | . | + | + | + | . | 43 |
| Potentilla cinerea | . | + | . | + | . | . | . | . | 2.2 | 2.2 | + | . | 2.2 | . | 43 |
| Alyssum alyssoides | + | + | + | . | . | . | . | . | . | . | . | . | + | + | 36 |
| Asperula cynanchica | . | . | . | . | + | . | . | + | . | + | . | + | + | . | 36 |
| Potentilla heptaphylla | + | . | . | . | + | . | . | . | . | . | + | + | + | . | 36 |
| Allium flavum | . | . | . | + | . | . | . | . | . | + | + | . | + | . | 29 |
| Bothriochloa ischaemum | 1.1 | 1.2 | . | . | . | . | . | . | + | . | . | . | . | + | 29 |
| Bromus erectus | . | . | . | . | . | . | . | + | . | + | + | . | . | + | 29 |
| Salvia pratensis | + | . | . | . | . | . | . | + | . | . | . | + | + | . | 29 |
| Aster amellus | . | . | . | . | + | . | . | . | . | . | + | . | . | + | 21 |
| Centaurea triumfettii | . | . | . | . | + | + | . | + | . | . | . | . | . | . | 21 |
| Fragaria viridis | . | . | . | . | . | . | + | . | . | . | . | + | . | + | 21 |
| Medicago falcata | . | . | + | . | . | . | . | . | . | . | 1.2 | . | + | . | 21 |
| Phleum phleoides | . | + | + | + | . | . | . | . | . | . | . | . | . | . | 21 |
| Sanguisorba minor | . | . | . | . | . | . | + | + | . | . | . | . | + | . | 21 |
| Thymus glabrescens | 2.2 | . | . | . | . | . | . | . | + | . | . | + | . | . | 21 |
| Veronica prostrata | . | + | . | . | . | . | . | . | + | . | . | + | . | . | 21 |
| Veronica spicata | . | . | . | . | . | . | . | . | + | + | . | + | . | . | 21 |
| Astragalus onobrychis | . | . | . | . | . | . | . | 1.2 | . | . | . | . | + | . | 14 |
| Minuartia verna | . | . | . | . | . | . | . | . | +.1 | + | . | . | . | . | 14 |
| Onobrychis viciifolia | . | . | . | . | . | . | + | 1.2 | . | . | . | . | . | . | 14 |
| Pulsatilla montana subsp. jankae | . | . | . | . | + | . | . | . | . | . | . | . | + | . | 14 |
| Bromus riparius | . | . | . | . | . | . | . | + | . | . | . | . | . | . | 7 |
| Cephalaria radiata | . | . | . | . | + | . | . | . | . | . | . | . | . | . | 7 |
| Pilosella officinarum | . | + | . | . | . | . | . | . | . | . | . | . | . | . | 7 |
| Plantago argentea | . | . | . | . | + | . | . | . | . | . | . | . | . | . | 7 |
| Prunella grandiflora | . | . | . | . | . | . | . | + | . | . | . | . | . | . | 7 |
| Thesium linophyllon | . | . | . | . | . | . | . | + | . | . | . | . | . | . | 7 |
| Seslerion rigidae | |||||||||||||||
| Helianthemum rupifragum | + | + | + | . | . | . | . | . | + | + | . | . | . | . | 36 |
| Sesleria rigida | . | . | . | + | . | + | + | 1.1 | . | . | . | . | . | 2.3 | 36 |
| Primula veris subsp. columnae | . | . | . | . | . | + | 1.2 | 1.2 | . | . | . | . | . | + | 29 |
| Seseli gracile | . | . | . | . | . | . | 1.1 | 2.2 | . | + | . | . | . | + | 29 |
| Helictotrichon decorum | . | . | . | . | 2.2 | 2.2 | 1.2 | . | . | . | . | . | . | . | 21 |
| Seseli rigidum | . | . | . | + | . | . | . | . | + | + | . | . | . | . | 21 |
| Viola jooi | . | . | . | . | + | + | + | . | . | . | . | . | . | . | 21 |
| Centaurea atropurpurea | . | . | . | . | + | . | + | . | . | . | . | . | . | . | 14 |
| Centaurea reichenbachii | . | . | . | . | + | . | + | . | . | . | . | . | . | . | 14 |
| Dianthus spiculifolius | . | . | . | . | + | + | . | . | . | . | . | . | . | . | 14 |
| Ranunculus oreophilus | . | . | . | . | . | + | . | + | . | . | . | . | . | . | 14 |
| Geranion sanguinei | |||||||||||||||
| Hypericum perforatum | + | + | + | . | + | . | + | . | . | + | . | + | . | . | 50 |
| Vincetoxicum hirundinaria | . | . | . | . | + | + | + | + | . | + | + | . | . | + | 50 |
| Geranium sanguineum | . | . | . | . | + | + | + | + | . | . | . | + | . | . | 36 |
| Verbascum lychnitis | + | + | + | + | . | . | . | . | . | . | . | 1.1 | . | . | 36 |
| Coronilla varia | . | + | + | . | . | . | + | . | + | . | . | . | . | . | 29 |
| Trifolium alpestre | . | . | . | . | 1.2 | . | + | . | . | + | . | + | . | . | 29 |
| Cruciata glabra | . | . | . | . | . | . | + | 1.1 | . | . | . | . | . | + | 21 |
| Cnidium silaifolium | . | . | . | . | + | . | . | . | + | . | . | . | . | . | 14 |
| Origanum vulgare | . | . | . | . | . | . | + | + | . | . | . | . | . | . | 14 |
| Dictamnus albus | . | . | . | . | . | . | . | . | . | . | + | . | . | . | 7 |
| Companion | |||||||||||||||
| Galium album | . | + | . | + | + | + | 1.1 | + | . | + | . | + | . | + | 64 |
| Bromus arvensis | 1.1 | + | . | . | . | . | . | . | . | . | . | + | + | . | 29 |
| Echium vulgare | . | + | . | . | . | . | + | + | . | . | . | . | + | . | 29 |
| Lactuca quercina | + | + | . | + | . | . | . | . | . | . | . | . | . | + | 29 |
| Poa angustifolia | . | + | + | + | . | . | . | . | . | . | + | . | . | . | 29 |
| Caucalis platycarpos | . | + | . | . | . | . | . | . | . | . | 1.1 | + | . | . | 21 |
| Cytisus nigricans | . | . | . | . | . | . | + | + | . | . | . | . | . | + | 21 |
| Orobanche alba | + | . | . | . | . | . | . | . | . | + | . | . | . | + | 21 |
| Plantago lanceolata | + | . | . | . | . | . | . | . | . | . | . | + | + | . | 21 |
| Vicia pannonica | . | + | . | . | . | . | + | . | . | . | + | . | . | . | 21 |
| Asplenium ruta-muraria | . | . | . | . | + | . | . | . | . | + | . | . | . | . | 14 |
| Cytisus austriacus | . | . | . | . | . | . | . | + | . | + | . | . | . | . | 14 |
| Lepidium campestre | . | + | + | . | . | . | . | . | . | . | . | . | . | . | 14 |
| Medicago lupulina | + | . | . | . | . | . | + | . | . | . | . | . | . | . | 14 |
| Rhamnus saxatilis subsp. tinctorius | . | . | . | . | . | . | . | + | . | + | . | . | . | . | 14 |
| Trifolium pratense | . | + | . | . | . | . | . | . | . | . | . | . | + | . | 14 |
| Artemisia campestris | . | . | . | . | . | . | . | . | + | . | . | . | . | . | 7 |
| Dianthus giganteus | . | . | . | . | . | . | . | . | . | . | + | . | . | . | 7 |
| Peucedanum cervaria | . | . | . | . | . | + | . | . | . | . | . | . | . | . | 7 |
| Picris hieracioides | . | . | + | . | . | . | . | . | . | . | . | . | . | . | 7 |
| Pilosella hoppeana | . | . | . | . | . | + | . | . | . | . | . | . | . | . | 7 |
| Reseda luteola | . | . | . | . | . | + | . | . | . | . | . | . | . | . | 7 |
| Trifolium arvense | . | . | . | . | . | . | . | + | . | . | . | . | . | . | 7 |
| Relevé No. | 15 | 16 | 17 | 18 * | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | Frequency (%) |
| Elevation (m) | 380 | 385 | 390 | 395 | 400 | 380 | 385 | 390 | 395 | 390 | 395 | 410 | 420 | 390 | 385 | 390 | |
| Aspect | SW | W | SW | SW | S | S | S | S | SW | S | SW | S | SW | SW | SW | SW | |
| Slope (degrees) | 35 | 35 | 65 | 65 | 70 | 25 | 30 | 10 | 30 | 20 | 25 | 25 | 20 | 10 | 25 | 20 | |
| Herb cover (%) | 70 | 70 | 65 | 65 | 75 | 60 | 55 | 55 | 60 | 45 | 65 | 65 | 60 | 50 | 60 | 60 | |
| Sample area (sq. m) | 25 | 25 | 20 | 20 | 25 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 15 | |
| Diagnostic species at association level | |||||||||||||||||
| Artemisia alba | 4.4 | 4.4 | 4.4 | 3.4 | 4.4 | 3.5 | 3.4 | 3.5 | 3.5 | 2.5 | 3.5 | 3.5 | 3.5 | 2.3 | 3.4 | 2.3 | 100 |
| Onosma pseudoarenaria | + | + | + | 1.2 | + | + | 1.2 | +.3 | + | 1.2 | +.2 | +.3 | + | + | . | + | 94 |
| Cephalaria radiata | . | + | + | + | . | . | + | + | . | + | + | + | 1.2 | + | . | + | 69 |
| Asyneuma canescens | . | . | . | + | + | + | + | + | + | + | . | . | . | . | . | . | 44 |
| Daphne cneorum | . | . | . | + | + | + | . | . | . | . | + | . | . | + | 1.2 | + | 44 |
| Echinops ritro subsp. ruthenicus | + | . | . | + | + | . | . | . | + | . | . | + | . | 1.1 | . | + | 44 |
| Bromo—Festucion pallentis | |||||||||||||||||
| Seseli osseum | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | . | 94 |
| Linum tenuifolium | + | + | + | + | + | + | +.2 | +.2 | + | + | + | . | . | 1.2 | . | + | 81 |
| Jurinea transsilvanica | + | + | + | . | + | + | . | . | . | + | + | . | + | . | . | + | 56 |
| Scorzonera austriaca | + | + | + | + | . | . | . | + | . | . | + | . | . | . | . | . | 38 |
| Phleum montanum | . | . | . | . | + | . | . | . | + | . | . | . | + | . | + | + | 31 |
| Sesleria heufleriana | . | + | . | + | . | . | . | . | . | + | . | + | . | . | + | . | 31 |
| Festuca pallens | . | . | . | . | . | . | . | . | . | + | . | + | + | . | . | . | 19 |
| Euphorbia seguieriana | . | . | . | . | . | . | + | . | . | + | . | . | . | . | . | . | 13 |
| Seseli gracile | . | . | . | + | . | . | . | . | + | . | . | . | . | . | . | . | 13 |
| Thalictrum minus | . | . | . | . | . | . | + | . | . | . | . | . | . | . | . | + | 13 |
| Stipo—Festucetalia pallentis | |||||||||||||||||
| Anthericum ramosum | + | + | + | + | + | + | + | + | + | + | + | + | . | + | + | + | 94 |
| Inula ensifolia | + | + | + | + | + | . | 1.3 | 2.5 | + | 2.3 | + | + | 1.3 | 2.4 | 1.3 | 2.3 | 94 |
| Teucrium montanum | + | + | + | + | . | + | 1.2 | 1.2 | + | 1.2 | + | + | + | + | . | + | 88 |
| Helianthemum canum | + | + | + | + | + | + | + | . | . | + | 1.2 | + | . | + | . | + | 75 |
| Thymus comosus | . | + | + | + | + | . | + | + | . | 1.2 | + | + | . | + | 1.2 | . | 69 |
| Astragalus monspessulanum | . | + | . | + | + | . | + | . | + | + | . | + | . | . | . | + | 50 |
| Stipa pulcherrima | + | + | . | . | . | . | . | . | . | . | . | . | . | . | + | . | 19 |
| Leontodon crispus | . | + | . | . | . | . | . | . | . | . | . | . | + | . | . | . | 13 |
| Festuco—Brometea (including Festucetalia valesiacae) | |||||||||||||||||
| Carex humilis | 1.2 | + | + | + | 1.2 | 1.2 | 1.2 | 1.3 | 1.2 | 1.2 | 2.3 | 2.3 | 2.3 | 2.5 | 2.3 | 3.4 | 100 |
| Asperula cynanchica | . | + | . | + | + | + | + | + | + | + | + | + | + | + | . | 1.2 | 81 |
| Stipa capillata | + | 1.2 | . | . | + | 1.3 | 2.3 | + | + | 2.3 | 1.1 | + | 1.3 | + | . | 2.4 | 81 |
| Teucrium chamaedrys | + | . | 1.2 | + | + | + | + | + | + | + | . | + | 1.2 | . | + | 1.3 | 81 |
| Allium flavum | . | + | + | + | + | 1.2 | + | . | + | 1.2 | + | + | 1.1 | . | + | . | 75 |
| Campanula sibirica | + | + | + | + | . | . | + | +.2 | + | . | + | + | + | . | . | + | 69 |
| Adonis vernalis | . | + | . | . | 1.1 | 1.3 | + | . | + | . | . | + | + | . | . | + | 50 |
| Bothriochloa ischaemum | 1.1 | . | . | . | + | . | . | + | + | + | 2.3 | + | + | . | . | . | 50 |
| Dorycnium pentaphyllum subsp. herbaceum | + | + | + | + | + | . | . | . | . | + | + | . | . | . | + | . | 50 |
| Eryngium campestre | . | + | . | . | + | . | + | . | + | . | . | + | + | . | + | + | 50 |
| Helianthemum nummularium subsp. obscurum | + | . | . | + | + | + | + | . | . | . | . | . | + | . | . | + | 44 |
| Sanguisorba minor subsp. minor | . | + | + | + | . | . | + | . | + | . | . | . | + | . | + | . | 44 |
| Scabiosa ochroleuca | . | . | + | . | + | . | + | + | + | . | . | + | . | . | + | . | 44 |
| Thesium linophyllon | . | . | + | + | . | . | + | . | . | + | . | + | . | + | . | + | 44 |
| Potentilla cinerea | . | . | . | . | . | 2.3 | + | . | 2.4 | . | . | 2.3 | 1.3 | . | . | 1.3 | 38 |
| Aster amellus | . | . | . | + | . | . | . | . | + | . | . | + | . | . | + | + | 31 |
| Euphorbia cyparissias | . | . | + | + | . | . | . | . | + | . | . | . | . | . | + | + | 31 |
| Festuca rupicola | . | . | . | + | . | + | . | . | . | . | + | + | 1.1 | . | . | . | 31 |
| Plantago argentea | + | . | + | . | + | . | . | . | . | . | . | . | . | + | + | . | 31 |
| Salvia pratensis | . | + | + | + | + | . | . | . | . | . | . | . | . | . | + | . | 31 |
| Euphorbia glareosa | . | . | + | + | + | . | . | . | . | + | . | . | . | . | . | . | 25 |
| Kengia serotina | + | . | + | . | + | . | . | . | + | . | . | . | . | . | . | . | 25 |
| Onobrychis viciifolia | . | + | . | . | + | . | . | . | . | . | . | . | . | + | + | . | 25 |
| Thymus glabrescens | . | . | . | + | + | . | . | . | . | . | . | + | . | . | . | + | 25 |
| Bromus erectus | . | . | . | . | . | . | . | . | . | + | . | + | + | . | . | . | 19 |
| Centaurea scabiosa subsp. scabiosa | . | . | . | . | . | . | . | . | + | . | . | . | + | + | . | . | 19 |
| Odontites luteus | . | . | . | . | . | . | . | + | . | . | . | . | + | . | + | . | 19 |
| Bromus riparius | . | . | . | . | . | . | . | . | . | . | . | . | . | + | . | + | 13 |
| Festuca valesiaca | . | . | . | + | . | . | . | + | . | . | . | . | . | . | . | . | 13 |
| Koeleria macrantha | . | . | . | . | . | + | . | . | . | . | . | . | + | . | . | . | 13 |
| Acinos arvensis | . | . | . | . | . | . | . | . | . | . | . | . | + | . | . | . | 6 |
| Avenula pratensis | . | . | . | . | . | . | . | . | . | . | . | . | . | + | . | . | 6 |
| Bromus squarrosus | . | . | . | . | . | . | . | + | . | . | . | . | . | . | . | . | 6 |
| Cytisus albus | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | . | 6 |
| Polygala major | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | . | 6 |
| Pulsatilla montana | . | . | . | + | . | . | . | . | . | . | . | . | . | . | . | . | 6 |
| Stachys recta | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | + | 6 |
| Thlaspi perfoliatum | . | . | . | . | . | . | . | . | . | . | . | . | + | . | . | . | 6 |
| Veronica spicata | . | . | . | . | . | + | . | . | . | . | . | . | . | . | . | . | 6 |
| Geranion sanguinei | |||||||||||||||||
| Dictamnus albus | . | + | . | + | + | + | . | . | . | . | . | + | . | . | . | . | 31 |
| Agrimonia eupatoria | . | . | . | . | + | . | . | + | + | . | . | . | . | . | . | . | 19 |
| Hypericum perforatum | . | . | . | . | . | . | . | + | + | . | . | . | . | . | . | . | 13 |
| Cirsio—Brachypodion pinnati | |||||||||||||||||
| Salvia verticillata | . | . | . | . | . | + | + | + | + | + | + | + | + | . | . | 1.2 | 56 |
| Brachypodium pinnatum | . | . | + | + | + | . | + | 1.2 | 1.2 | + | . | + | . | . | . | . | 50 |
| Linum flavum | . | + | + | . | + | . | . | +.2 | . | + | . | . | . | . | . | 1.2 | 38 |
| Gypsophila collina | + | . | . | 1.1 | . | . | . | . | . | . | + | . | . | . | + | + | 31 |
| Prunella grandiflora | . | + | . | . | + | . | . | . | . | . | . | . | . | + | . | . | 19 |
| Filipendula vulgaris | . | . | . | + | + | . | . | . | . | . | . | . | . | . | . | . | 13 |
| Companion | |||||||||||||||||
| Echium vulgare | + | . | + | + | + | . | . | + | . | . | + | . | + | . | . | . | 44 |
| Cytisus nigricans | + | + | . | + | . | . | + | + | . | . | . | . | . | . | . | . | 31 |
| Peucedanum oreoselinum | . | . | . | + | + | . | . | + | . | + | . | . | . | . | . | . | 25 |
| Carduus hamulosus | . | . | . | . | . | . | . | . | . | . | + | + | . | . | . | . | 13 |
| Linum hirsutum | . | . | + | . | . | . | . | . | . | . | . | . | . | . | + | . | 13 |
| Polygala vulgaris | . | . | . | + | + | . | . | . | . | . | . | . | . | . | . | . | 13 |
| Lactuca quercina | + | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 6 |
| Quercus pubescens | . | . | . | . | . | . | . | . | . | . | . | . | . | + | . | . | 6 |
| Rhamnus saxatilis subsp. tinctorius | . | + | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 6 |
| Relevé No. | 31 | 32 | 33 | 34 | 35 * | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | Frequency (%) |
| Elevation (m) | 65 | 65 | 60 | 60 | 65 | 70 | 70 | 65 | 81 | 65 | 65 | 65 | 70 | |
| Aspect | NW | NE | NE | N | N | NE | NE | E | W | W | W | W | S | |
| Slope (degrees) | 8 | 5 | 5 | 8 | 5 | 5 | 3 | 10 | 3 | 3 | 3 | 5 | 10 | |
| Herb cover (%) | 90 | 90 | 90 | 80 | 75 | 70 | 80 | 65 | 60 | 55 | 90 | 80 | 80 | |
| Sample area (sq. m) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Diagnostic species at association level | ||||||||||||||
| Artemisia alba | 2.3 | 2.5 | 2.5 | 4.5 | 3.5 | 3.5 | 4.5 | 3.5 | 3.5 | 1.3 | 2.5 | 3.3 | 2.3 | 100 |
| Satureja coerulea | 4.5 | 4.5 | 4.5 | 1.1 | 3.5 | 2.5 | 2.3 | 2.4 | 1.3 | 4.5 | 4.5 | 3.4 | 2.5 | 100 |
| Koeleria splendens | + | 2.4 | . | + | 1.2 | + | . | 2.3 | 2.3 | + | 1.3 | 1.3 | . | 77 |
| Euphorbia dobrogensis | + | . | . | + | . | . | + | + | + | . | + | + | + | 62 |
| Jurinea dobrogensis | . | + | + | +.2 | . | + | . | . | + | 1.3 | + | + | . | 62 |
| Agropyron ponticum | . | . | . | + | + | 1.2 | 1.3 | + | + | . | + | . | . | 54 |
| Hyacinthella leucophaea | + | + | . | . | . | + | . | . | + | . | . | + | . | 38 |
| Pimpinello—Thymion zygioidi | ||||||||||||||
| Thymus zygioides | + | 1.2 | 1.2 | 1.2 | 1.2 | 2.5 | + | 1.2 | 1.2 | + | . | . | +.2 | 85 |
| Potentilla bornmuelleri | + | + | . | . | + | 1.2 | 1.3 | + | + | + | + | 1.3 | . | 77 |
| Dianthus pseudarmeria | + | . | . | . | + | . | + | + | + | + | 1.2 | . | . | 54 |
| Astragalus spruneri | . | . | . | . | . | . | . | . | . | . | + | + | . | 15 |
| Cytisus jankae | . | . | . | . | . | . | . | . | . | 2.3 | . | + | . | 15 |
| Agropyron brandzae subsp. ciliatum | . | . | . | . | . | . | . | . | . | . | . | . | 2.4 | 8 |
| Dianthus nardiformis | . | . | . | . | . | . | . | . | . | . | . | . | + | 8 |
| Euphorbia myrsinites | . | . | . | . | . | . | . | . | . | . | . | . | 1.3 | 8 |
| Koeleria lobata | . | . | . | . | . | . | . | . | . | . | . | . | + | 8 |
| Bromo—Festucion pallentis | ||||||||||||||
| Linum tenuifolium | + | + | + | + | . | + | . | + | . | . | . | + | + | 62 |
| Euphorbia seguieriana | . | . | . | . | . | . | . | . | . | . | . | + | + | 15 |
| Thalictrum minus | . | . | . | . | . | . | . | . | . | . | . | + | . | 8 |
| Saturejon montanae | ||||||||||||||
| Leontodon crispus | + | + | + | + | . | . | . | . | . | . | . | + | . | 38 |
| Achillea clypeolata | . | . | + | . | . | . | . | + | . | . | . | + | + | 31 |
| Convolvulus cantabrica | . | . | . | . | + | . | . | . | . | . | . | . | . | 8 |
| Onobrychis alba | . | . | + | . | . | . | . | . | . | . | . | . | . | 8 |
| Festuco—Brometea (including Festucetalia valesiacae) | ||||||||||||||
| Festuca valesiaca | 1.3 | 1.3 | . | 1.3 | + | 1.2 | . | + | . | + | 1.3 | 1.4 | 1.2 | 77 |
| Sanguisorba minor | + | . | + | + | + | 2.3 | 1.2 | . | 1.2 | + | + | . | . | 69 |
| Filipendula vulgaris | 1.4 | 1.3 | 1.3 | 1.4 | . | . | . | . | + | + | 1.2 | 1.2 | . | 62 |
| Polygala major | 1.3 | + | 1.3 | + | +.3 | + | . | +.2 | . | + | . | . | . | 62 |
| Adonis vernalis | 1.2 | . | 1.2 | . | . | . | . | . | + | + | 1.3 | + | . | 46 |
| Bromus riparius | + | + | . | . | + | . | . | + | + | . | + | . | . | 46 |
| Onosma visianii | + | . | + | . | + | . | + | . | . | . | + | + | . | 46 |
| Bothriochloa ischaemum | + | + | + | . | . | . | . | . | . | . | + | . | + | 38 |
| Campanula sibirica | 1.3 | + | 1.3 | + | . | . | . | . | + | . | . | . | . | 38 |
| Eryngium campestre | . | + | . | + | . | . | . | . | + | . | + | . | + | 38 |
| Salvia nutans | . | . | + | . | . | . | + | . | + | + | +.3 | . | . | 38 |
| Euphorbia agraria | . | . | + | + | + | + | . | . | . | . | . | . | . | 31 |
| Euphorbia glareosa | + | . | + | . | . | + | + | . | . | . | . | . | . | 31 |
| Haplophyllum suaveolens | . | . | + | . | . | . | + | . | + | . | . | + | . | 31 |
| Paeonia tenuifolia | . | . | . | + | + | + | . | . | . | . | . | + | . | 31 |
| Stipa lessingiana | . | . | . | + | + | + | 1.2 | . | . | . | . | . | . | 31 |
| Asperula tenella | . | . | . | + | . | . | . | . | . | . | . | + | + | 23 |
| Carex halleriana | . | + | + | + | . | . | . | . | . | . | . | . | . | 23 |
| Gypsophila pallasii | . | . | . | . | . | . | + | . | . | + | . | + | . | 23 |
| Sideritis montana | . | . | . | + | . | + | . | . | . | . | + | . | . | 23 |
| Tanacetum corymbosum | . | . | . | 1.2 | . | + | . | . | + | . | . | . | . | 23 |
| Tanacetum millefolium | . | . | . | . | + | . | . | + | . | . | . | . | 1.2 | 23 |
| Teucrium chamaedrys | . | + | . | +.3 | . | . | 1.1 | . | . | . | . | . | . | 23 |
| Anthemis tinctoria | . | . | + | . | . | + | . | . | . | . | . | . | . | 15 |
| Crupina vulgaris | . | . | . | . | . | . | + | + | . | . | . | . | . | 15 |
| Echinops ritro subsp. ruthenicus | . | . | . | . | . | + | . | . | . | . | . | . | + | 15 |
| Erysimum diffusum | + | . | . | + | . | . | . | . | . | . | . | . | . | 15 |
| Ferulago confusa | . | . | . | . | + | + | . | . | . | . | . | . | . | 15 |
| Jasminum fruticans | . | . | + | . | . | . | . | + | . | . | . | . | . | 15 |
| Orlaya grandiflora | . | . | . | + | . | . | . | . | . | . | . | . | + | 15 |
| Pilosella officinarum | . | . | . | . | . | . | + | + | . | . | . | . | . | 15 |
| Veronica spicata subsp. barrelieri | + | . | + | . | . | . | . | . | . | . | . | . | . | 15 |
| Bromus squarrosus | . | . | . | . | . | . | . | . | . | . | . | . | + | 8 |
| Cephalaria uralensis | . | . | . | . | . | . | . | . | . | . | . | . | 1.2 | 8 |
| Coronilla varia | . | . | + | . | . | . | . | . | . | . | . | . | . | 8 |
| Galium flavescens | . | . | . | . | . | . | . | + | . | . | . | . | . | 8 |
| Medicago minima | + | . | . | . | . | . | . | . | . | . | . | . | . | 8 |
| Stipa capillata | . | . | . | . | . | . | . | . | . | . | + | . | . | 8 |
| Teucrium polium subsp. capitatum | . | . | . | . | . | . | . | . | . | . | . | + | . | 8 |
| Companion | ||||||||||||||
| Lappula marginata | . | . | . | . | . | . | . | . | . | . | + | . | + | 15 |
| Peucedanum arenarium | . | . | . | . | . | . | + | + | . | . | . | . | . | 15 |
| Salvia ringens | . | . | . | . | . | + | . | 1.2 | . | . | . | . | . | 15 |
| Aegilops cylindrica | . | . | . | . | . | + | . | . | . | . | . | . | . | 8 |
| Ajuga laxmannii | . | . | . | + | . | . | . | . | . | . | . | . | . | 8 |
| Allium rotundum | . | . | . | . | . | . | . | . | . | . | . | . | +.3 | 8 |
| Aster oleifolius | . | . | . | . | . | . | . | + | . | . | . | . | . | 8 |
| Carthamus lanatus | . | . | . | . | . | . | . | . | . | . | . | . | + | 8 |
| Centaurea orientalis | . | . | . | . | . | . | . | . | . | . | . | + | . | 8 |
| Consolida regalis | . | . | . | . | . | . | . | . | . | . | . | . | + | 8 |
| Dactylis glomerata | . | . | . | + | . | . | . | . | . | . | . | . | . | 8 |
| Hypericum elegans | . | . | . | . | . | . | + | . | . | . | . | . | . | 8 |
| Iris pumila | + | . | . | . | . | . | . | . | . | . | . | . | . | 8 |
| Jurinea tzar-ferdinandi | . | . | . | . | . | . | + | . | . | . | . | . | . | 8 |
| Linaria genistifolia | . | . | . | . | . | . | . | . | . | . | . | . | + | 8 |
| Linum austriacum | + | . | . | . | . | . | . | . | . | . | . | . | . | 8 |
| Nonea atra | . | . | + | . | . | . | . | . | . | . | . | . | . | 8 |
| Ononis pusilla | . | . | . | + | . | . | . | . | . | . | . | . | . | 8 |
| Potentilla pedata | . | . | + | . | . | . | . | . | . | . | . | . | . | 8 |
| Tragopogon dubius | . | . | . | . | . | . | . | . | . | . | . | . | + | 8 |
| Xeranthemum annuum | . | . | . | . | . | . | . | . | . | . | . | . | + | 8 |
| Species Name | Phi Coefficient |
|---|---|
| Acino majoranifolii—Artemisietum albae | |
| Acinos alpinus subsp. majoranifolius | 0.770 |
| Festuca rupicola | 0.664 |
| Cytisus albus | 0.641 |
| Scabiosa columbaria | 0.629 |
| Silene nutans subsp. dubia | 0.629 |
| Onosmo pseudoarenariae—Artemisietum albae | |
| Onosma pseudoarenaria | 0.870 |
| Seseli osseum | 0.796 |
| Cephalaria radiata | 0.725 |
| Inula ensifolia | 0.706 |
| Stipa capillata | 0.699 |
| Saturejo coeruleae—Artemisietum albae | |
| Satureja coerulea | 0.911 |
| Thymus zygioides | 0.782 |
| Potentilla bornmuelleri | 0.776 |
| Euphorbia dobrogensis | 0.764 |
| Jurinea dobrogensis | 0.704 |
| Diagnostic Species at Association Level | Am-Aa | Op-Aa | Sc-Aa | Aa-Fd | Aa-Ac | Aa-Sa | Bf-Aa | Bm-Se |
|---|---|---|---|---|---|---|---|---|
| Acinos alpinus subsp. majoranifolius | 71 | . | . | . | . | . | . | . |
| Cytisus albus | 57 | 6 | . | . | . | . | . | . |
| Silene nutans subsp. dubia | 43 | . | . | . | . | . | . | . |
| Onosma pseudoarenaria | . | 94 | . | . | . | . | . | . |
| Cephalaria radiata | 7 | 69 | . | . | . | . | . | . |
| Asyneuma canescens | . | 44 | . | . | . | . | . | . |
| Daphne cneorum | . | 44 | . | . | . | . | . | . |
| Echinops ritro subsp. ruthenicus | . | 44 | 15 | . | . | . | . | . |
| Satureja coerulea | . | . | 100 | . | . | . | . | . |
| Koeleria splendens | . | . | 77 | . | 60 | . | . | . |
| Euphorbia dobrogensis | . | . | 62 | . | . | . | . | . |
| Jurinea dobrogensis | . | . | 62 | . | . | . | . | . |
| Agropyron ponticum | . | . | 54 | . | . | . | . | . |
| Hyacinthella leucophaea | . | . | 38 | . | . | . | . | 22 |
| Festuca dalmatica | . | . | . | 100 | . | . | . | 78 |
| Thymus praecox subsp. clivorum | . | . | . | 67 | . | . | . | . |
| Achnatherum calamagrostis | . | . | . | . | 100 | 8 | . | . |
| Silene armeria | . | . | . | . | 20 | 100 | . | . |
| Galium corrudifolium | . | . | . | . | . | 69 | . | . |
| Koeleria pyramidata | . | . | . | . | 20 | 15 | 100 | . |
| Stipa epilosa | . | . | . | . | . | . | . | 100 |
| Thymus striatus | . | . | . | . | . | . | . | 89 |
| Hypericum rumeliacum | . | . | . | . | . | . | . | 89 |
| Pimpinella tragium subsp. lithophila | . | . | . | . | . | . | . | 89 |
| Asphodeline taurica | . | . | . | . | . | . | . | 89 |
| Bromus moesiacus | . | . | . | . | . | . | . | 56 |
| Tragopogon balcanicus | . | . | . | . | . | . | . | 56 |
| Predictors | AIC | F-Value | Prob(>F) |
|---|---|---|---|
| Elevation (m) | 259.83 | 5.528 | 0.0001 |
| Slope (degrees) | 258.42 | 3.205 | 0.0001 |
| Elevation × Slope | 256.92 | 3.245 | 0.0002 |
| Aspect | 256.61 | 2.109 | 0.0057 |
| Aspect × Slope | 255.79 | 2.543 | 0.0010 |
| Predictors | Coefficient Estimate | t Value | Prob (>t) | Goodness-of-Fit Statistics |
|---|---|---|---|---|
| Intercept | 3.3256 | 118.64 | <0.0001 | Chi-sq./DF = 1 |
| Elevation (m) | 1.9706 | 3.55 | 0.0011 | |
| Slope (degrees) | 2.3010 | 2.93 | 0.0059 | |
| Bare soil (decimals) | −0.4643 | −2.24 | 0.0312 | |
| Elevation × Slope | −2.6791 | −2.34 | 0.0251 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coldea, G.; Gafta, D.; Negrean, G. Eco-Coenotic and Diversity Patterns in Artemisia alba Open Scrubs from Romania within the Context of Similar Communities from Neighbouring Regions. Diversity 2023, 15, 475. https://doi.org/10.3390/d15040475
Coldea G, Gafta D, Negrean G. Eco-Coenotic and Diversity Patterns in Artemisia alba Open Scrubs from Romania within the Context of Similar Communities from Neighbouring Regions. Diversity. 2023; 15(4):475. https://doi.org/10.3390/d15040475
Chicago/Turabian StyleColdea, Gheorghe, Dan Gafta, and Gavril Negrean. 2023. "Eco-Coenotic and Diversity Patterns in Artemisia alba Open Scrubs from Romania within the Context of Similar Communities from Neighbouring Regions" Diversity 15, no. 4: 475. https://doi.org/10.3390/d15040475
APA StyleColdea, G., Gafta, D., & Negrean, G. (2023). Eco-Coenotic and Diversity Patterns in Artemisia alba Open Scrubs from Romania within the Context of Similar Communities from Neighbouring Regions. Diversity, 15(4), 475. https://doi.org/10.3390/d15040475







