Abstract
Studies of the effects of elevation and associated habitat changes on biodiversity have a rich history in conservation biology and have seen a resurgence of interest in recent decades. Mountainous regions are particularly important because they harbour endemic species and are relatively spared from anthropogenic effects. The rather drastic temperature decrease as elevation increases in tropical regions has generated more research on the effects of elevation, especially since global warming could negatively impact the biodiversity of tropical forest mountainous species. Fireflies, especially the solitary species, represent a challenge to work with because though they are biologically diverse, they typically occur at low densities and have rarely been studied across elevations. Many are habitat specialists and have limited dispersal abilities. Firefly diversity changes on five mountains located on the main mountain range of Peninsular Malaysia, which is highly representative of five major elevational forest types, were assessed. Fireflies were restricted to a certain elevational range of mountains, and the turnover of species was significant among forest types across elevations. The forest type and canopy closure were the main characteristics affecting species diversity, although other habitat characteristics may be significant. The ability to reveal any possible associations was limited, as strong statistical associations were not possible due to their low abundance and difficulty in detecting their presence. The firefly species occurrence across elevations is restricted, and habitat loss could pose a risk to lower-elevation species, while global warming could be a threat to high-elevation species.
1. Introduction
Studies of the effects of elevation and associated habitat changes on biodiversity have a rich history in conservation biology and have seen a resurgence of interest in recent decades [1,2]. Mountainous regions are particularly important because they harbour endemic species as a consequence of their elevation profile [3,4,5] and because they are relatively spared from expanding agriculture and development. This isolation from development could provide refuge to numerous species and associated habitats. This is significant in light of upslope range shifts, which have been recently identified to occur across tropical elevations due to global warming [6,7].
Unlike the less variable temperature change with latitudinal increases in tropical regions, the relatively drastic temperature decrease with an increase in altitude for the region has triggered a rise in research on the effects of elevation on species distributions. This has become more apparent after the increase in the awareness of global warming impacts on species distributions and biodiversity [8,9,10]. As the global temperature gradually rises, more evidence is showing the upslope shift of plants and animals to escape the increasing temperature at lower elevations [6,7,11]. The extinction of thermal specialist species occurring at high elevations is also anticipated with the change in global climate. The detrimental effect would be larger on species in tropical montane areas than on their temperate counterparts [3,12,13].
Malaysian Fireflies across Elevation as a Case Study
Fireflies (Coleoptera: Lampyridae) can occur in mass congregations, where they are easily observed and collected, or as solitary species, where they are observed in low densities. It is the solitary species that present a challenge to work with because though they are biologically diverse, their occurrence at low densities makes their collection and observation more difficult, and they have rarely been studied along environmental gradients, including elevation. Fireflies, however, are potentially valuable because their light displays offer a compelling opportunity to develop eco-tourism in forested landscapes, including forests at higher elevations [14,15]. Fireflies are also increasingly becoming popular indicator species promoting environmental awareness among the general public through citizen science projects [16,17,18].
Most species in this group are highly specialised. They are obligate predators of snails, earthworms and slugs during the larval stage [19,20], with larval habitats of either the aquatic, semi-aquatic or terrestrial type [21,22,23]. The habitats of adults and larvae are not necessarily confined to the same microhabitats. Flying adult species are fully terrestrial, requiring herbaceous plants as their resting and mating places. Adults with flightless females are confined to forest floors and are also fully terrestrial [24,25,26]. Ecological theory suggests that such specialisation will increase solitary fireflies’ sensitivity to environmental disturbances [27,28]. Furthermore, solitary fireflies also appear to have low dispersal capacities [25,29], which may limit their ability to colonise other areas or adapt to the changes caused by climate change. Information on fireflies along elevation gradients [5,30] and at high elevations [31,32,33] is very limited; therefore, this study will be able to provide further information on firefly species diversity across elevational gradients.
Here, we focused on the solitary firefly diversity within tropical elevational forests in Malaysia. Using a combination of analyses of species partitioning (beta diversity), the relationships between species richness and diversity (via the rarefaction and extrapolation of Hill numbers) and several habitat characteristics, the firefly species richness and diversity changes along elevation gradients were assessed. The variation associated with forest types, which can be tied to the natural history of the fireflies, was also identified.
2. Materials and Methods
2.1. Study Area
The study was conducted in the main mountain range of Peninsular Malaysia, Banjaran Titiwangsa. This range extends across the middle of the region from the border between Thailand and Malaysia in the north towards the southern tip of the peninsula, separating the east and west coasts. Only areas on the western slopes of the mountain range were sampled in the periods of May and July (when the western region has less rainfall) to minimise the influence of weather on the behaviour and sampling of the fireflies. Weather conditions differ between the eastern and western slopes of the mountain range [34].
Five mountains located between 12 and 47 km apart latitudinally (latitude 3°48′ N–latitude 2°48′ N) were sampled to represent the mountain range (Figure 1a). The existing trails from the lowest points of the mountains consisted mainly of nature trails of 1 m width or less going up mountain summits. The mountains sampled for the study were Gunung Nuang (189–1459 m above sea level, a.s.l.), Bukit Kutu (200–1000 m a.s.l.), Gunung Besar Hantu (225–1409 m a.s.l.), Gunung Berembun (264–1057 m a.s.l.) and Gunung Liang (224–1933 m a.s.l.). A total of 110, 80 m transects were sampled, extending into 5 climatic forest formations of tropical forest, as described by Whitmore [35], i.e., lowland dipterocarp (at 0–330 m a.s.l.), hill dipterocarp (at 330–830 m a.s.l.), upper dipterocarp (830–1350 m a.s.l.), oak-laurel (1200–1500m a.s.l.) and montane ericaceous (1500–2100m a.s.l) (Figure 1b).
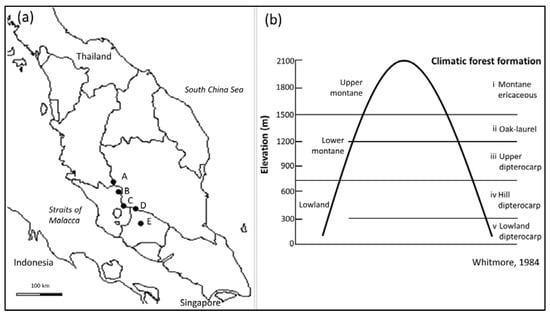
Figure 1.
(a) Map of Peninsular Malaysia showing locations of the five mountains sampled on Banjaran Titiwangsa mountain range: (A) Gunung Liang, Perak; (B) Bukit Kutu, Selangor; (C) Gunung Nuang, Selangor; (D) Gunung Besar Hantu, Negeri Sembilan; and (E) Gunung Berembun, Negeri Sembilan. (b) The five climatic forest formations spanning elevations of Malaysian mountains, adapted from Whitmore [35].
2.2. Firefly Sampling and Identification
A total of 110 transects were sampled, representing the 5 mountains and 5 forest types. Sampling was conducted at every 50 m altitude increase from the lowest possible elevation band at 200 m a.s.l. and to the highest possible at 1900 m a.s.l. The determination of elevation was based on altimeter readings (Suunto, altimeter watch, error reading of less than 1 m).
Within each elevation band, the sampling of fireflies was conducted along an 80 m long strip and within a 3 m wide belt on both sides of the transect. Due to the varying topography of the mountains, it was not possible to standardise the sampling time within each elevation band. However, the sampling of fireflies was performed for 20 to 30 min for each transect. The sampling of fireflies at night consisted of collecting firefly adults and larvae beginning at nautical twilight, which ends between 20:04 and 20:16 h in the period between May and July 2015 [36].
The detection of fireflies was performed based on the lights they produced whilst in flight, on the ground or on plants. Two persons walked along the transect, with headlamps switched off most of the time to improve their ability to detect the firefly lights. Sweep nets and soft insect forceps were used to collect the adults and larvae, respectively. Fireflies fall into two main groups—light-producing adults that are active at night (mainly of the Luciolinae and Lampyrinae subfamilies) and those in which adults have reduced light organs and therefore are active during the day (the Ototretinae subfamily). Both groups have light-emitting larvae that are active at night, and thus, our sampling method enabled both groups to be collected during a single sampling period. All specimens were immediately preserved in 70% ethanol.
Adult fireflies in the Luciolinae were identified to the species level using firefly taxonomic identification keys [21,37,38]. Adult fireflies in the Lampyrinae and larvae of Ototretinae were assigned to genera using the taxonomic identifications by Jeng et al. [24,39,40] and Kawashima et al. [41] and later grouped into morphospecies, as species-level guides are not available for either subfamily.
2.3. Habitat Characteristics’ Recording
Along each transect, habitat assessments at 0, 20, 40 and 60 m points were conducted to record four key habitat features hypothesised to influence the firefly distribution and abundance, viz., canopy closure, leaf litter depth, the height of understorey plants and the number of trees with DBH > 40 cm.
Canopy closure was recorded using a spherical, convex densiometer following Lemmon [42]. Uncompressed leaf litter thickness was measured (to the nearest cm) at the corners of a 1 m2 quadrat located in the 3 m belt parallel to the transect (16 readings per transect). The height of the understorey plants was measured using a 150 cm long ruler with 15 alternating 10 cm white and red bands placed at the habitat assessment point. An observer stood three metres away from the ruler and counted the visible sections of it. The value was then deducted from the total 15 to give the approximate height of the understorey plants to the nearest 10 cm. Readings were taken at 2 points, with the ruler remaining at 1 point while the observer moved parallel to the transect line (8 readings per transect). The height of understorey plants was taken in the 3 m belt of the transect. The number of trees with DBH > 40 cm was counted for the total length of the transect. Only trees that were <3 m parallel to the transect on either side were counted.
2.4. Data Analysis
2.4.1. Species Beta-Diversity Analysis
Firefly species abundance as a function of both the forest type and mountain was modelled by fitting the following model using the Poisson family, which produced better diagnostics than a negative binomial distribution: abundance ~ forest type + mountain. The interactions were not fitted because there were too many missing combinations of the forest type and mountain at high elevations. An ANOVA table was obtained to test the hypothesis that the forest type and mountain influenced species relative abundances (e.g., turnover/beta diversity). Univariate tests identifying which species were responsible for the change were also obtained. These results were complemented by figures showing species abundances among forest types.
Beta diversity was partitioned into turnover and nestedness and combined with a permanova analysis to produce a graphical representation of these data. The components of beta diversity in our data were first estimated using the betapart package for R [43]. Turnover vastly exceeded nestedness (see results); therefore, the resulting matrix was used as the dependent variable, and a permanova analysis using the adonis2() function from the package vegan, version 2.5-2 for R [44], was performed. As indicated above, the model turnover_matrix ~ forest type + mountain was fitted.
2.4.2. Species-Diversity–Habitat Relationships
The firefly species diversity and habitat relationships were analysed in a two-step process. First, iNEXT version 2.0.15 for R [45] was used to estimate species richness. The estimated function from iNEXT was used to calculate the values for a standardised coverage of 60% for each forest-type–mountain combination. In iNEXT, the maximum recommended extrapolation for species richness is suggested to be double the reference sample size. Across 16 samples for this analysis, 60% was the maximum coverage that could be specified without influencing the predication bias for species richness. This resulted in 19 estimates of species richness (3–5 estimates from among transects within 5 forest types from 5 mountains).
Second, these estimates were combined with the habitat characteristics data, i.e., the mean value of the canopy cover percentage, leaf litter depth, understorey height and large tree abundance estimated for each forest type (Figure 1b). These data were input into a linear model of richness as a function of the habitat characteristics (no interactions). The model was fitted to allow quadratic relationships among the four continuous habitat characteristics. p-values are reported based on Type II sums of squares from the Anova() function in the car package, version 3.0-0 in R [46], and effect sizes are reported as Partial eta squared (Partial η2) values from the etasq() function in the heplots package, version 1.3-5 for R [47].
3. Results
3.1. Sampling Effort and Species Found
Between 15 and 34 transects were sampled per mountain. For all mountains, the most transects were sampled in hill dipterocarp forests (ten transects per mountain), while the lowest number of transects were sampled in lowland dipterocarp forests (mean: 1.8 transects per mountain). A total of 19 species were recorded in this study (Table 1), of which 6 species have females that cannot fly, as their flying wings are reduced or non-existent (flightless), while 3 of the flight-capable (flighted) species were diurnal fireflies. This study also discovered three firefly species new to science, which were subsequently described [48,49]. Between 3 and 12 species of fireflies were collected along the elevations of each mountain (mean: 6.4 species), with the largest species collection obtained in Gunung Nuang, Selangor (12 species). The highest number of collections were obtained for Pygoluciola dunguna (n = 15), followed by Abscondita pallescens (n = 12) and Stenocladius sp. 1 (n = 12). The rest of the firefly species collections were between one and eight individuals.
3.2. Firefly Elevational Ranges
Fireflies were found to be distributed within certain ranges of elevation (Table 1). Several species exhibited a relatively large elevational range. Stenocladius sp. 1 and Abscondita pallescens were present in the three lower-elevation forest types, while Curtos costipennis was found between elevations of 1100 and 1750 m a.s.l., which covered the three highest-elevation forest types. There were also species that were restricted to the upper sections of the mountains, namely, Pyrocoelia sp. 1, Pyrocoelia sp. 3, Pyrocoelia fumigata and Drilaster sp. There seemed to be a cut-off point at 900 m a.s.l. for the majority of the lower-elevation-occurring species, with 9 out of a total of 11 species not found above 900 m a.s.l. (Table 1).


Table 1.
Average individual numbers per firefly species according to elevation bands sampled. The average was calculated from data at each elevation among the five mountains.
Table 1.
Average individual numbers per firefly species according to elevation bands sampled. The average was calculated from data at each elevation among the five mountains.
| Elevation Bands, m a.s.l. | Firefly Species Found (Average no.) | Forest Types |
|---|---|---|
| 1900 | - | Mountain ericaceous |
| 1850 | - | |
| 1800 | Drilaster sp. (1.00), Pyrocoelia sp. 1 (1.00) | |
| 1750 | Curtos costipennis (1.00), Drilaster sp. (1.00), Pyrocoelia fumigata (3.00) | |
| 1700 | Pyrocoelia sp. 1 (3.00), Pyrocoelia sp. 3 (3.00) | |
| 1650 | - | |
| 1600 | Curtos obscuricolor (1.00), Pyrocoelia sp. 3 (3.00) | |
| 1550 | - | |
| 1500 | - | Oak-laurel |
| 1450 | - | |
| 1400 | Abscondita berembun * (1.00), Luciola jengai *,† (1.00) | |
| 1350 | - | |
| 1300 | - | |
| 1250 | Curtos obscuricolor (0.33), Curtos costipennis (0.33) | |
| 1200 | Curtos costipennis (0.33), Stenocladius sp. 1 † (0.33) | Upper hill dipterocarp |
| 1150 | Curtos costipennis (0.33) | |
| 1100 | Curtos costipennis (0.33) | |
| 1050 | - | |
| 1000 | Abscondita berembun * (1.20), Stenocladius sp. (0.20) | |
| 950 | Stenocladius sp. 1 † (0.20) | |
| 900 | Abscondita pallescens (0.20) | |
| 850 | Abscondita pallescens (0.40), Diaphanes sp. 1 (0.20), Stenocladius sp. 1 † (0.20) | |
| 800 | Abscondita pallescens (0.20) | Hill dipterocarp |
| 750 | Abscondita pallescens (0.60) | |
| 700 | Abscondita pallescens (0.20) | |
| 650 | Diaphanes sp. 1 (0.20), Pyrocoelia sp. 2 (0.20), | |
| 600 | Diaphanes sp. 2 (0.20), Pygoluciola dunguna * (0.20) | |
| 550 | Abscondita pallescens (0.20), Colophotia brevis (0.20), Curtos sp. 1 (0.20), Stenocladius sp. 2 (0.40) | |
| 500 | Abscondita pallescens (0.20), Colophotia brevis (0.20), Pygoluciola dunguna * (0.20) | |
| 450 | Colophotia brevis (0.40), Stenocladius sp. 2 (0.40) | |
| 400 | Pyrocoelia sp. 2 (0.20) | |
| 350 | Abscondita pallescens (0.20), Luciola jengai *† (0.40), Pygoluciola dunguna * (1.80), Stenocladius sp. 2 (0.20) | |
| 300 | Abscondita pallescens (0.20), Diaphanes sp. 1 (0.20), Diaphanes sp. 2 (0.40), Luciola jengai *† (0.20), Pygoluciola dunguna * (0.80) | Lowland dipterocarp |
| 250 | Colophotia brevis (1.00), Luciola pallidipes (1.33), Stenocladius sp. 1 (1.00), Stenocladius sp. 2 (0.20) | |
| 200 | Colophotia brevis (1.00), Pyrocoelia sp. 2 (1.00), Stenocladius sp. 1 † (3.00) |
* Specimens collected during this study subsequently described as new species [48,49]. † Species occurring at ≤900 m a.s.l. and at ≥950 m a.s.l.
3.3. Species Turnover (β-Diversity)—Multivariate Generalised Linear Model Method
Evidence for species turnover associated with the forest type but not the mountain identity was detected (Table 2), indicating that species diversity and relative abundance were changing in a consistent manner up the elevation gradient across the five mountains. Univariate tests indicated that Pygoluciola dunguna (p = 0.001), Abscondita berembun (p = 0.012) and Abscondita pallescens (p = 0.025) were driving these changes.

Table 2.
ANOVA table from the multivariate analysis of abundance (mvabund) testing the influence of mountain and forest types across elevation. Significance level at p < 0.05.
3.4. Species Turnover (β-Diversity)—Distance Matrix Methods
Analysis using β-diversity partitioning indicated that the turnover component dominated the signal of β-diversity (βsim, turnover = 0.89; βsne, nestedness = 0.048; total = 0.94). A permanova analysis of the turnover component suggested both the mountain identity and forest type were significantly associated with species turnover, though the forest type captured approximately 25% more variation (SS 1.76 vs. SS 2.28, Table 3).

Table 3.
PERMANOVA table from the analysis of the beta-diversity turnover component testing the influence of mountain and forest types across elevation. Significance level at p < 0.05.
3.5. Species-Diversity–Habitat Relationships
Figure 2 shows iNEXT-based estimates of species richness among the five forest types across elevational increments, indicating substantial variation in the predicted species richness but with large uncertainties.
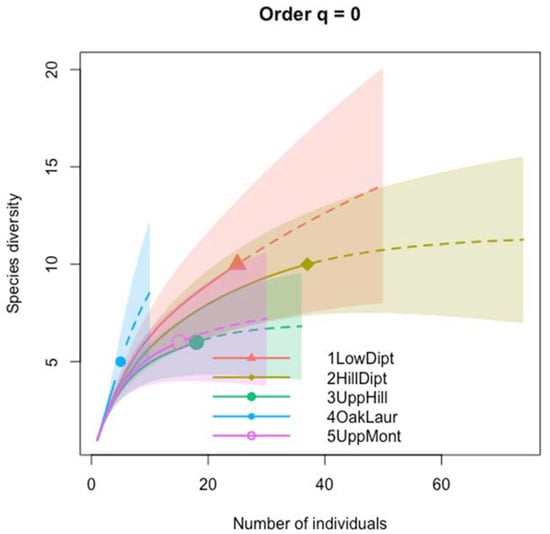
Figure 2.
Species richness of fireflies estimated by iNEXT via extrapolation and rarefaction of Hill number (q = 0) for the five forest types across elevational gradients. Each shape is the observed species richness of fireflies in that forest type. Solid lines represent the sample-size-based rarefactions made by iNEXT. Dashed lines are the extrapolated curves produced by iNEXT up to double the value of reference sample. The 95% confidence interval (shaded regions) was obtained by a bootstrap method.
The species richness of fireflies was influenced by the following: a large effect of forest type, a moderate effect of canopy closure and a small effect of leaf litter (Table 4).

Table 4.
Effect sizes and ANOVA table derived from the linear model estimating the effect of forest type and habitat characteristics on species richness of fireflies. Effect sizes >0.3 are deemed large.
4. Discussion
4.1. Firefly Elevational Ranges
This study has highlighted the importance of exploring the biodiversity of the inland tropical forest solitary fireflies specifically across elevational gradients. At present, only two known studies on firefly species diversity across tropical elevations have been conducted [5,30], both of which were in Amazonia. On the general inspection of the pattern of the firefly species distribution in the five mountains sampled in Peninsular Malaysia (Table 1), several preliminary findings on the solitary firefly habitat range across elevations were identified. Certain species of fireflies were restricted to higher elevations, and some were at lower elevations, while the rest were able to adapt to a larger range size across elevations.
For fireflies that occur within the lower ranges of elevation, habitat loss could primarily be the cause of concern for their conservation, as more areas, typically the lowland and hill dipterocarp forests, are either being degraded from logging or rapidly being converted into vast agricultural landscapes [50,51,52]. An assessment by the World Resource Institute identified that Peninsular Malaysia experienced forest cover loss at an average of 1609.1 km2/year between 2001 and 2010, and the rate was not expected to decrease in the near future, although forest reserves remained relatively intact [53]. Aside from habitat loss from deforestation, the fragmentation of natural habitats will also pose a risk to low-elevation firefly species, as in order for them to be able to shift from an unconducive area, a forest corridor or continuous forest is required. The importance of habitat continuity linking low-elevation to higher-elevation areas for thermal specialists has been highlighted by previous studies on amphibians, reptiles and insects [54,55,56]. The ability of fireflies to adapt to higher elevations, however, remains unknown and requires further research.
Several solitary fireflies collected in this study were also found to be confined to the highest elevation ranges. Of the six species observed in the montane ericaceous forest, three were Pyrocoelia, which has flightless females. The dispersal limitations of flightless females may contribute to their restricted elevational range, as well as the suitability of abiotic and biotic conditions of the montane ericaceous forest for their survival. Mountain passes, a term describing the physiological barrier to thermally restricted taxa, may also explain their isolation at the highest or lowest points of mountains [12,57,58].
The influence of temperature could explain the restriction of certain firefly species to certain elevational zones. Temperature plays an important role in the rate of enzyme-catalyst reactions and has been shown to modulate light-flashing intervals, flash intensity and the onset of flashing of firefly lights [59,60]. The production of firefly flashes has been found to be strongly influenced by ambient temperature, therefore regulating flashing signal patterns [61,62,63]. As the elevation increases, the temperature decreases, and the different temperatures experienced by fireflies in their specific habitat could possibly restrict their elevation and habitat ranges.
Based on this study, there were two main groups of fireflies present across the elevational gradients of the five mountains, where almost half of the species collected did not occur above 900 m a.s.l. (Table 1). Macedo et al. [5] compared the elevational ranges of seven families of beetles in Brazil, and fireflies were found to be the most specialised among groups of beetles, i.e., highly specialised in lower or upper elevational zones. Aside from the temperature, these restricted distributions across elevations could be explained by the fireflies requiring obligate prey at the larval stage and specific microhabitat requirements at different stages of their lifecycles [23,38,64,65].
Although Whitmore [35] was able to identify five elevational forest zones linked to changes in the floristic composition and structure, the three lowest elevation forest types share many common tree species that influence the canopy structure, the forest environment and its microhabitats. Ashton [66] further explained that the ecotone of the floristic zones of the lowland and lower montane forests (Figure 1) is gradual, happening approximately between 800–1300 m a.s.l. However, the ecotone is more obvious between the lower montane and upper montane areas, which commonly comprise warm temperate species, i.e., Fagaceae (oak) and Lauraceae (laurel). The more distinct difference in the floristic composition between the lower montane and upper montane areas, but a subtler change from lowland to lower montane areas, could explain why the majority of lower-elevation fireflies were not found beyond 900 m a.s.l.
4.2. β-Diversity of Fireflies between Mountains and between Forest Types
Our results suggest that there was a lower turnover of species among the five mountains, but substantial turnover among forest types along the elevation gradient (Table 3), which gives further support to our general analysis of the firefly species collected across the elevations sampled (Table 1). The results indicated that the β-diversity of firefly species among the forest types was strongly attributed to species replacement rather than species loss based on the much higher turnover value, βsim = 0.89. The firefly beta-diversity pattern across elevational forest types is the result of species substitution in a forest type by different species in the other forest types (species replacement).
There were relatively few species responsible for the turnover. The mvabund models identified Abscondita pallescens, Abscondita berembun and Pygoluciola dunguna as having significant influences on the patterns. Among the species of fireflies collected, Abscondita pallescens (n = 12) was the most widely distributed across elevations (200–1200 m a.s.l.), occurring in four of the mountains and three of the forest types. Their wider distribution may be explained by the species having flighted females and a relatively bigger size in comparison to other Abscondita species [48]. Abscondita berembun was described as a new species subsequent to the sampling performed in this study [48] and was only collected at the highest sections of two mountains (elevation between 1750 and 1800 m a.s.l.). Abscondita is a genus from Southeast Asia, described in 2013 [38], with biology and behaviour described for only two species, i.e., Abs. chinensis and Abs. terminalis. Both have been recorded in mountains in China, although they were also found in other types of habitats, e.g., grasslands.
Pygoluciola dunguna, also recently described [49], was the most frequently collected in this study (n = 15) and occurred in the lowland and hill dipterocarp forests at elevations of 300–600 m a.s.l. Until recently, Pygoluciola was thought to be a rare genus [67]. Nineteen species, all confined to the Southeast Asian region, have been described thus far [48,68]. Details on the habitat, behaviour and distribution of this genus can be referenced to two species. Pygoluciola dunguna is confined between the two lowest-elevation forests, i.e., lowland and hill dipterocarp forests [49], while P. qinyu has been recorded at 532m a.s.l. at E Mei Mountain [32]. The larvae of both of these species are semiaquatic [32,69].
Our results were similar to those of other studies of invertebrates, which often showed different groups within taxa having different elevational ranges and occurring in habitats with different elevation heights. For example, studies of ant species diversity in Mediterranean mountains found a strong correlation with elevation and identified three distinct groups: generalists spanning all elevations; cold-tolerant restricted to high elevations; and low-elevation specialists [70]. Dung beetles of Costa Rica, in contrast, revealed high levels of specialisation with species-specific, narrow elevational ranges [11]. This comparison suggests that while fireflies are challenging to study, they largely behave as other invertebrates, with evidence of generalists, broad specialists at high or low altitudes and instances of high specialisation.
4.3. Firefly Species Diversity and Habitat Relationships
In contrast to many other studies of invertebrates across elevations, e.g., Blatrix et al. [70], Bhardwaj et al. [71], Carneiro et al. [72] and Chamberlain et al. [73], strong statistical associations with habitat characteristics, which were hypothesised to be important to fireflies, were not detected. However, the substantial effects of the size of the forest type and canopy closure suggested that these factors are important, although the power to detect significant relationships was lacking. The low abundance and our capacity to detect firefly species probably limited the ability to reveal associations that likely existed.
Based on firefly biology, we expected canopy closure to influence diversity because it regulates the ambient and lower canopy temperatures of the forest and filters light penetration to the forest floor [74]. Ground-dwelling insects, including dung beetles, were found to decrease in species richness when canopy closure decreases [75,76,77]. With respect to fireflies, canopy closure could influence the forest’s microclimatic conditions at the understorey level where the firefly at different life stages survives, and increasing canopy closure could provide a conducive environment for fireflies and especially their food source, which largely depends on shaded and moist conditions [21,38,78].
Author Contributions
Conceptualization, K.L.E.; methodology, A.P.B. and B.N.; validation, L.A.B.; formal analysis, A.P.B.; investigation, B.N.; resources, L.A.B.; data curation, B.N.; writing—original draft preparation, B.N.; writing—review and editing, A.P.B. and L.A.B.; visualization, A.P.B. and B.N.; supervision, A.P.B. and K.L.E.; project administration, B.N.; funding acquisition, B.N. and A.P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lomolino, M.V. Elevation Gradients of Species-Density: Historical and Prospective Views. Glob. Ecol. Biogeogr. 2001, 10, 3–13. [Google Scholar] [CrossRef]
- Guo, Q.; Kelt, D.A.; Sun, Z.; Liu, H.; Hu, L.; Ren, H.; Wen, J. Global Variation in Elevational Diversity Patterns. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, C.; Rapini, A.; Santos Damascena, L.; De Marco Junior, P. The Worrying Future of the Endemic Flora of a Tropical Mountain Range under Climate Change. Flora 2016, 218, 1–10. [Google Scholar] [CrossRef]
- Chaves, A.V.; Freitas, G.H.S.; Vasconcelos, M.F.; Santos, F. Biogeographic Patterns, Origin and Speciation of the Endemic Birds from Eastern Brazilian Mountaintops: A Review. Syst. Biodivers. 2015, 13, 1–16. [Google Scholar] [CrossRef]
- Macedo, M.V.; Monteiro, R.F.; Flinte, V.; Almeida-Neto, M.; Khattar, G.; da Silveira, L.F.L.; Araújo, C.d.O.; Araújo, R.d.O.; Colares, C.; Gomes, C.V.S.; et al. Insect Elevational Specialization in a Tropical Biodiversity Hotspot. Insect Conserv. Divers. 2017, 11, 240–254. [Google Scholar] [CrossRef]
- Freeman, B.G.; Class Freeman, A.M. Rapid Upslope Shifts in New Guinean Birds Illustrate Strong Distributional Responses of Tropical Montane Species to Global Warming. Proc. Natl. Acad. Sci. USA 2014, 111, 4490–4494. [Google Scholar] [CrossRef]
- Molina-Martínez, A.; León-Cortés, J.L.; Regan, H.M.; Lewis, O.T.; Navarrete, D.; Caballero, U.; Luis-Martínez, A. Changes in Butterfly Distributions and Species Assemblages on a Neotropical Mountain Range in Response to Global Warming and Anthropogenic Land Use. Divers. Distrib. 2016, 22, 1085–1098. [Google Scholar] [CrossRef]
- Cusack, D.F.; Karpman, J.; Ashdown, D.; Cao, Q.; Ciochina, M.; Halterman, S.; Lydon, S.; Neupane, A. Global Change Effects on Humid Tropical Forests: Evidence for Biogeochemical and Biodiversity Shifts at an Ecosystem Scale. Rev. Geophys. 2016, 54, 523–610. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, Y.; Zhou, L.; Li, B.; Luo, Y. An Imperative Need for Global Change Research in Tropical Forests. Tree Physiol. 2013, 33, 903–912. [Google Scholar] [CrossRef]
- Menendez, R.; Gonzalez-Megias, A.; Jay-Robert, P.; Marquez-Ferrando, R. Climate Change and Elevational Range Shifts: Evidence from Dung Beetles in Two European Mountain Ranges. Glob. Ecol. Biogeogr. 2014, 23, 646–657. [Google Scholar] [CrossRef]
- García-López, A.; Micó, E.; Galante, E. From Lowlands to Highlands: Searching for Elevational Patterns of Species Richness and Distribution of Scarab Beetles in Costa Rica. Divers. Distrib. 2012, 18, 543–553. [Google Scholar] [CrossRef]
- Sheldon, K.S.; Huey, R.B.; Kaspari, M.; Sanders, N.J. Fifty Years of Mountain Passes: A Perspective on Dan Janzen’s Classic Article. Am. Nat. 2018, 191, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Raxworthy, C.J.; Pearson, R.G.; Rabibisoa, N.; Rakotondrazafy, A.M.; Ramanamanjato, J.B.; Raselimanana, A.P.; Wu, S.; Nussbaum, R.A.; Stone, D.A. Extinction Vulnerability of Tropical Montane Endemism from Warming and Upslope Displacement: A Preliminary Appraisal for the Highest Massif in Madagascar. Glob. Chang. Biol. 2008, 14, 1703–1720. [Google Scholar] [CrossRef]
- Nada, B.; Mohd Salleh, S.; Khirul Faizal, O. A Survey of Fireflies (Coleoptera: Lampyridae) in Fraser’s Hill, Pahang. In Forest R&D: Meeting National and Global Needs, Proceedings of the Conference on Forestry and Forest Products Research 2013, Kuala Lumpur, Malaysia, 12–13 November 2013; Rahim, S., Lim, H.F., Huda Farhana, M.M., Mahmudin, S., Eds.; Forest Research Institute Malaysia: Kuala Lumpur, Malaysia, 2014; pp. 14–19. [Google Scholar]
- Wong, C.H. In the Blink of a Firefly. Malays. Nat. 2014, 26–32. [Google Scholar]
- Chow, A.; Chong, J.H.; Cook, M.; White, D. Vanishing Fireflies: A Citizen-Science Project Promoting Scientific Inquiry and Environmental Stewardship. Sci. Educ. Civ. Engagem. 2014, 6, 23–31. [Google Scholar]
- Bonney, R.; Dickinson, J.L. Overview of Citizen Science. In Citizen Science: Public Participation in Environmental Research; Dickinson, J.L., Bonney, R., Eds.; Cornell University Press: Ithaca, NY, USA, 2012; pp. 19–26. [Google Scholar]
- Xing, Y. Local Environment Attachment and the Possibility of Using Citizen Science Approaches to Measure Firefly Populations in Time and Place. Unpublished Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2012; p. 170. [Google Scholar]
- Viviani, V.R.; Rosa, S.P.; Martins, M.A. Aspisoma Lineatum (Gyllenhal) (Coleoptera: Lampyridae) Firefly: Description of the Immatures, Biological, and Ecological Aspects. Neotrop. Entomol. 2012, 41, 89–94. [Google Scholar] [CrossRef]
- Fu, X.H.; Meyer-Rochow, V.B. Larvae of the Firefly Pyrocoelia Pectoralis (Coleoptera: Lampyridae) as Possible Biological Agents to Control the Land Snail Bradybaena Ravida. Biol. Control 2013, 65, 176–183. [Google Scholar] [CrossRef]
- Fu, X.H.; Ballantyne, L.A.; Lambkin, C.L. The External Larval Morphology of Aquatic and Terrestrial Luciolinae Fireflies (Coleoptera: Lampyridae). Zootaxa 2012, 3405, 1–34. [Google Scholar] [CrossRef]
- Ballantyne, L.A.; Lambkin, C.L.; Luan, X.; Boontop, Y.; Nak-Eiam, S.; Pimpasalee, S.; Silalom, S.; Thancharoen, A. Further Studies on South Eastern Asian Luciolinae: 1. Sclerotia Ballantyne, a New Genus of Fireflies with Back Swimming Larvae 2. Triangulara Pimpasalee, a New Genus from Thailand (Coleoptera: Lampyridae). Zootaxa 2016, 4170, 201–249. [Google Scholar] [CrossRef]
- Fu, X.H.; Meyer-Rochow, V.B. An Investigation into the Morphological and Behavioral Adaptations of the Aquatic Larvae of Aquatica Leii (Coleoptera: Lampyridae) to Prey upon Freshwater Snails That Serve as Intermediate Hosts for the Liver Fluke. Biol. Control 2012, 62, 127–134. [Google Scholar] [CrossRef]
- Jeng, M.-L.; Lai, J.; Yang, P.-S.; Sato, M. Revision of the Genus Diaphanes Motschulsky (Coleoptera, Lampyridae, Lampyrinae) of Taiwan. Jpn. J. Syst. Entomol. 2001, 7, 203–235. [Google Scholar]
- Fu, X.H.; South, A.; Lewis, S.M. Sexual Dimorphism, Mating Systems, and Nuptial Gifts in Two Asian Fireflies (Coleoptera: Lampyridae). J. Insect Physiol. 2012, 58, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.; Schlindwein, M.N.; Viviani, V.R. Survey of Bioluminescent Coleoptera in the Atlantic Rain Forest of Serra Da Paranapiacaba in São Paulo State (Brazil). Biota Neotrop. 2016, 16, e0045. [Google Scholar] [CrossRef]
- Firebaugh, A.; Haynes, K.J. Experimental Tests of Light-Pollution Impacts on Nocturnal Insect Courtship and Dispersal. Oecologia 2016, 182, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Kazama, S.; Matsumoto, S.; Ranjan, S.P.; Hamamoto, H.; Sawamoto, M. Characterization of Firefly Habitat Using a Geographical Information System with Hydrological Simulation. Ecol. Modell. 2007, 209, 392–400. [Google Scholar] [CrossRef]
- Kakehashi, K.; Kuranishi, R.B.; Kamata, N. Estimation of Dispersal Ability Responding to Environmental Conditions: Larval Dispersal of the Flightless Firefly, Luciola Parvula (Coleoptera: Lampyridae). Ecol. Res. 2014, 29, 779–787. [Google Scholar] [CrossRef]
- Smith, B.W. Firefly Diversity in Colombia: Patterns Across a Dynamic Landscape. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2009; p. 123. [Google Scholar]
- Wattanachaiyingcharoen, W.; Nak-Eiam, S.; Phanmuangma, W.; Boonkiaew, S.; Nimlob, N. Species Diversity of Firefly (Coleoptera: Lampyridae) in the Highlands of Northern Thailand. NU Int. J. Sci. 2016, 13, 24–32. [Google Scholar]
- Fu, X.H.; Ballantyne, L.A. Taxonomy and Behaviour of Lucioline Fireflies (Coleoptera: Lampyridae: Luciolinae) with Redefinition and New Species of Pygoluciola Wittmer from Mainland China and Review of Luciola LaPorte. Zootaxa 2008, 1733, 1–44. [Google Scholar] [CrossRef]
- Jeng, M.-L.; Engel, M.S.; Yang, P.-S. Discovery of the Female of Pyrocoelia Prolongata in Taiwan (Coleoptera, Lampyridae). Zookeys 2011, 116, 49–57. [Google Scholar] [CrossRef]
- Jamaludin, S.; Sayang, M.D.; Wan Zawiah, W.Z.; Abdul Aziz, J. Trends in Peninsular Malaysia Rainfall Data during the Southwest Monsoon and Northeast Monsoon Seasons: 1975–2004. Sains Malays. 2010, 39, 533–542. [Google Scholar]
- Whitmore, T.C. Tropical Rain Forest of the Far East; Oxford University Press: Oxford, UK, 1984; p. 352. [Google Scholar]
- Timeanddate.Com. Available online: www.timeanddate.com (accessed on 12 September 2015).
- Ballantyne, L.A.; Lambkin, C.L. Systematics and Phylogenetics of Indo-Pacific Luciolinae Fireflies (Coleoptera: Lampyridae) and the Description of New Genera. Zootaxa 2013, 3653, 1–162. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, L.A.; Fu, X.H.; Lambkin, C.L.; Jeng, M.-L.; Faust, L.F.; Wijekoon, W.M.C.D.; Li, D.; Zhu, T. Studies on South-East Asian Fireflies: Abscondita, a New Genus with Details of Life History, Flashing Patterns and Behaviour of Abs. Chinensis (L.) and Abs. Terminalis (Olivier) (Coleoptera: Lampyridae: Luciolinae). Zootaxa 2013, 3721, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Jeng, M.-L.; Lai, J.; Yang, P.-S.; Sato, M. On the Validity of the Generic Name Pyrocoelia Gorham (Coleoptera, Lampyridae, Lampyrinae) with Review of Taiwanese Species. Jpn. J. Syst. Entomol. 1999, 5, 347–362. [Google Scholar]
- Jeng, M.-L.; Yang, P.-S.; Engel, M.S. The Firefly Genus Vesta in Taiwan (Coleoptera: Lampyridae). J. Kans. Entomol. Soc. 2007, 80, 265–280. [Google Scholar] [CrossRef]
- Kawashima, I.; Satou, F.; Sato, M. The Lampyrid Genus Drilaster (Coleoptera, Lampyridae, Ototretinae) of the Ryukyu Archipelago, Southwest Japan. Jpn. J. Syst. Entomol. 2005, 11, 225–262. [Google Scholar]
- Lemmon, P.E. A Spherical Densiometer for Estimating Forest Overstory Density. For. Sci. 1956, 2, 314–320. [Google Scholar]
- Baselga, A.; Orme, C.D.L. Betapart: An R Package for the Study of Beta Diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Oksanen, A.J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, R.B.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package Vegan: Community Ecology Package, Version 2.5-2. R Package. 2018. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 15 June 2018).
- Hsieh, T.C.; Ma, K.H.; Chao, A. Package ‘INEXT’ Version 2.0.15. R Package. 2018. Available online: http://chao.stat.nthu.edu.tw/wordpress/software_download/ (accessed on 30 July 2018).
- Fox, J.; Weisberg, S.; Price, B.; Adler, D.; Bates, D.; Baud-Bovy, G.; Bolker, B.; Ellison, S.; Firth, D.; Friendly, M.; et al. Package Car: Companion to Applied Regression, Version 3.0-0. R Package. 2018. Available online: https://CRAN.R-project.org/package=car (accessed on 1 May 2018).
- Fox, J.; Friendly, M.; Monette, G. Package Heplots: Visualising Hypothesis Tests in Multivariate Linear Models, Version 1.3-5. R Package. 2018. Available online: https://CRAN.R-project.org/package=heplots (accessed on 1 May 2018).
- Ballantyne, L.A.; Lambkin, C.L.; Ho, J.-Z.; Jusoh, W.F.A.; Nada, B.; Nak-Eiam, S.; Thancharoen, A.; Wattanachaiyingcharoen, W.; Yiu, V. The Luciolinae of S. E. Asia and the Australopacific Region: A Revisionary Checklist (Coleoptera: Lampyridae) Including Description of Three New Genera and 13 New Species. Zootaxa 2019, 4687, 1–174. [Google Scholar] [CrossRef]
- Nada, B.; Ballantyne, L.A. A New Species of Pygoluciola Wittmer with Unusual Abdominal Configuration, from Lowland Dipterocarp Forest in Peninsular Malaysia (Coleoptera: Lampyridae: Luciolinae). Zootaxa 2018, 4455, 343–362. [Google Scholar] [CrossRef]
- Saiful, I. Effects of Selective Logging on Tree Species Composition, Richness and Diversity in a Hill Dipterocarp Forest in Malaysia. J. Trop. For. Sci. 2014, 26, 188–202. [Google Scholar]
- Abdullah, S.A.; Hezri, A.A. From Forest Landscape to Agricultural Landscape in the Developing Tropical Country of Malaysia: Pattern, Process, and Their Significance on Policy. Environ. Manage. 2008, 42, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Peh, K.S.-H.; de Jong, J.; Sodhi, N.S.; Lim, S.L.-H.; Yap, C.A.-M. Lowland Rainforest Avifauna and Human Disturbance: Persistence of Primary Forest Birds in Selectively Logged Forests and Mixed-Rural Habitats of Southern Peninsular Malaysia. Biol. Conserv. 2005, 123, 489–505. [Google Scholar] [CrossRef]
- Hamid, W.A.; Rahman, S.B.W.A. Comparison Results of Forest Cover Mapping of Peninsular Malaysia Using Geospatial Technology. IOP Conf. Ser. Earth Environ. Sci. 2016, 37, 012027. [Google Scholar] [CrossRef]
- Şekercioğlu, H.; Primack, R.B.; Wormworth, J. The Effects of Climate Change on Tropical Birds. Biol. Conserv. 2012, 148, 1–18. [Google Scholar] [CrossRef]
- Brodie, J.; Post, E.; Laurance, W.F. Climate Change and Tropical Biodiversity: A New Focus. Trends Ecol. Evol. 2012, 27, 145–150. [Google Scholar] [CrossRef]
- Beier, P.; Brost, B. Use of Land Facets to Plan for Climate Change: Conserving the Arenas, Not the Actors. Conserv. Biol. 2010, 24, 701–710. [Google Scholar] [CrossRef]
- Ghalambor, C.K.; Huey, R.B.; Martin, P.R.; Tewksbury, J.J.; Wang, G. Are Mountain Passes Higher in the Tropics? Janzen’s Hypothesis Revisited. Integr. Comp. Biol. 2006, 46, 5–17. [Google Scholar] [CrossRef]
- Janzen, D.H. Why Mountain Passes Are Higher in the Tropics. Am. Nat. 1967, 101, 233–249. [Google Scholar] [CrossRef]
- Ueda, I.; Shinoda, F.; Kamaya, H. Temperature-Dependent Effects of High Pressure on the Bioluminescence of Firefly Luciferase. Biophys. J. 1994, 66, 2107–2110. [Google Scholar] [CrossRef]
- Dreisig, H. Environmental Control of the Daily Onset of Luminescent Activity in Glowworms and Fireflies (Coleoptera: Lampyridae). Oecologia 1975, 18, 85–99. [Google Scholar] [CrossRef]
- Iguchi, Y. Temperature-Dependent Geographic Variation in the Flashes of the Firefly Luciola Cruciata (Coleoptera: Lampyridae). J. Nat. Hist. 2010, 44, 861–867. [Google Scholar] [CrossRef]
- Iguchi, Y. The Ecological Impact of an Introduced Population on a Native Population in the Firefly Luciola Cruciata (Coleoptera: Lampyridae). Biodivers. Conserv. 2009, 18, 2119–2126. [Google Scholar] [CrossRef]
- Sharma, U.; Goswami, A.; Phukan, M.; Chandra Rajbongshi, S.; Gohain Barua, A. Temperature Dependence of the Flash Duration of the Firefly Luciola Praeusta. Photochem. Photobiol. Sci. 2014, 13, 1788–1792. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Amano, T.; Katoh, K.; Higuchi, H. The Habitat Requirement of the Genji-Firefly Luciola Cruciata (Coleoptera: Lampyridae), a Representative Endemic Species of Japanese Rural Landscapes. Biodivers. Conserv. 2006, 15, 191–203. [Google Scholar] [CrossRef]
- Yuma, M. Effect of Rainfall on the Long-Term Population Dynamics of the Aquatic Firefly Luciola Cruciata. Entomol. Sci. 2007, 10, 237–244. [Google Scholar] [CrossRef]
- Ashton, P.S. Florisitic Zonation of Tree Communities on Wet Tropical Mountains Revisited. Perspect. Plant Ecol. Evol. Syst. 2003, 6, 87–104. [Google Scholar] [CrossRef]
- Ballantyne, L.A.; Lambkin, C.L. A Phylogenetic Reassessment of the Rare S.E. Asian Firefly Genus Pygoluciola Wittmer (Coleoptera: Lampyridae: Luciolinae). Raffles Bull. Zool. 2006, 54, 21–48. [Google Scholar]
- Ballantyne, L.A.; Lambkin, C.L.; Boontop, Y.; Jusoh, W.F.A. Revisional Studies on the Luciolinae Fireflies of Asia (Coleoptera: Lampyridae): 1. The Genus Pyrophanes Olivier with Two New Species. 2. Four New Species of Pteroptyx Olivier and 3. A New Genus Inflata Boontop, with Luciola Indica. Zootaxa 2015, 3959, 1–84. [Google Scholar] [CrossRef]
- Nada, B.; Ballantyne, L.A.; Jusoh, W.F.A. Description of the Larva of a Firefly Species, Pygoluciola Dunguna Nada (Coleoptera: Lampyridae). Zootaxa 2021, 4920, 528–542. [Google Scholar] [CrossRef]
- Blatrix, R.; Lebas, C.; Galkowski, C.; Wegnez, P.; Pimenta, R.; Morichon, D. Vegetation Cover and Elevation Drive Diversity and Composition of Ant Communities (Hymenoptera: Formicidae) in a Mediterranean Ecosystem. Myrmecol. News 2016, 22, 119–127. [Google Scholar]
- Bhardwaj, M.; Uniyal, V.P.; Sanyal, A.K.; Singh, A.P. Butterfly Communities along an Elevational Gradient in the Tons Valley, Western Himalayas: Implications of Rapid Assessment for Insect Conservation. J. Asia. Pac. Entomol. 2012, 15, 207–217. [Google Scholar] [CrossRef]
- Carneiro, E.; Mielke, O.H.H.; Casagrande, M.M.; Fiedler, K. Skipper Richness (Hesperiidae) Along Elevational Gradients in Brazilian Atlantic Forest. Neotrop. Entomol. 2014, 43, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, D.; Tocco, C.; Longoni, A.; Mammola, S.; Palestrini, C.; Rolando, A. Nesting Strategies Affect Altitudinal Distribution and Habitat Use in Alpine Dung Beetle Communities. Ecol. Entomol. 2015, 40, 372–380. [Google Scholar] [CrossRef]
- Nakamura, A.; Kitching, R.L.; Cao, M.; Creedy, T.J.; Fayle, T.M.; Freiberg, M.; Hewitt, C.N.; Itioka, T.; Koh, L.P.; Ma, K. Forests and Their Canopies: Achievements and Horizons in Canopy Science. Trends Ecol. Evol. 2017, 32, 438–451. [Google Scholar] [CrossRef]
- Azevedo-Ramos, C.; De Carvalho, O.; Do Amaral, B.D. Short-Term Effects of Reduced-Impact Logging on Eastern Amazon Fauna. For. Ecol. Manage. 2006, 232, 26–35. [Google Scholar] [CrossRef]
- França, F.M.; Louzada, J.; Barlow, J. Selective Logging Effects on ‘Brown World’ Faecal-Detritus Pathway in Tropical Forests: A Case Study from Amazonia Using Dung Beetles. For. Ecol. Manage. 2018, 410, 136–143. [Google Scholar] [CrossRef]
- Hosaka, T.; Niino, M.; Kon, M.; Ochi, T.; Yamada, T.; Fletcher, C.; Okuda, T. Impacts of Small-Scale Clearings Due to Selective Logging on Dung Beetle Communities. Biotropica 2014, 46, 720–731. [Google Scholar] [CrossRef]
- Kaufmann, T. Ecological and Biological Studies on the West African Firefly Luciola discicollis (Coleoptera: Lampyridae). Ann. Entomol. Soc. Am. 1965, 58, 414–426. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).