Cinetochilides minimus sp. nov., a Tiny Benthic Ciliate (Protozoa, Ciliophora) from Brackish Water in Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Identification
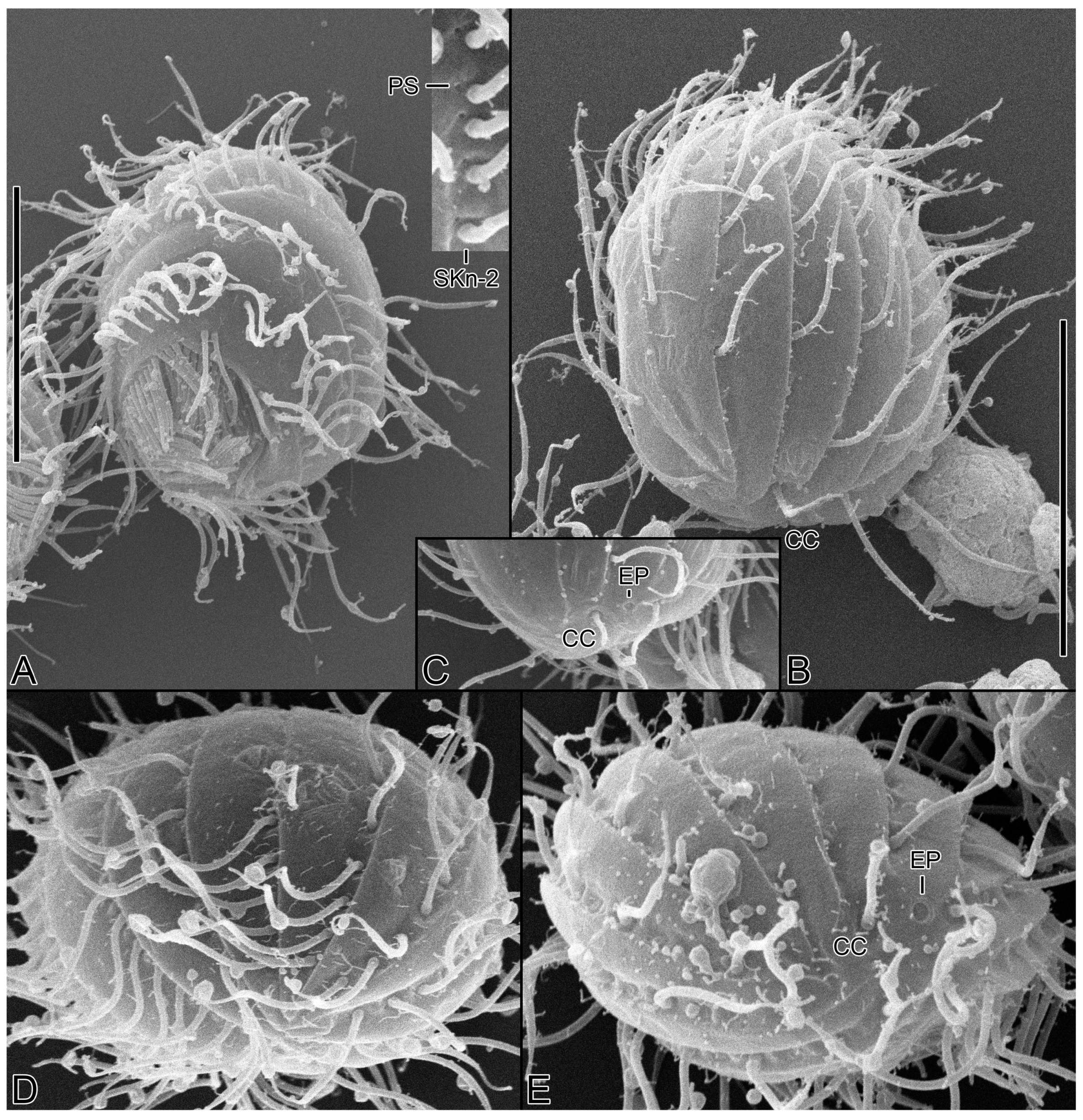
2.2. Scanning Electron Microscopy
2.3. DNA Extraction, PCR Amplification, and Sequencing
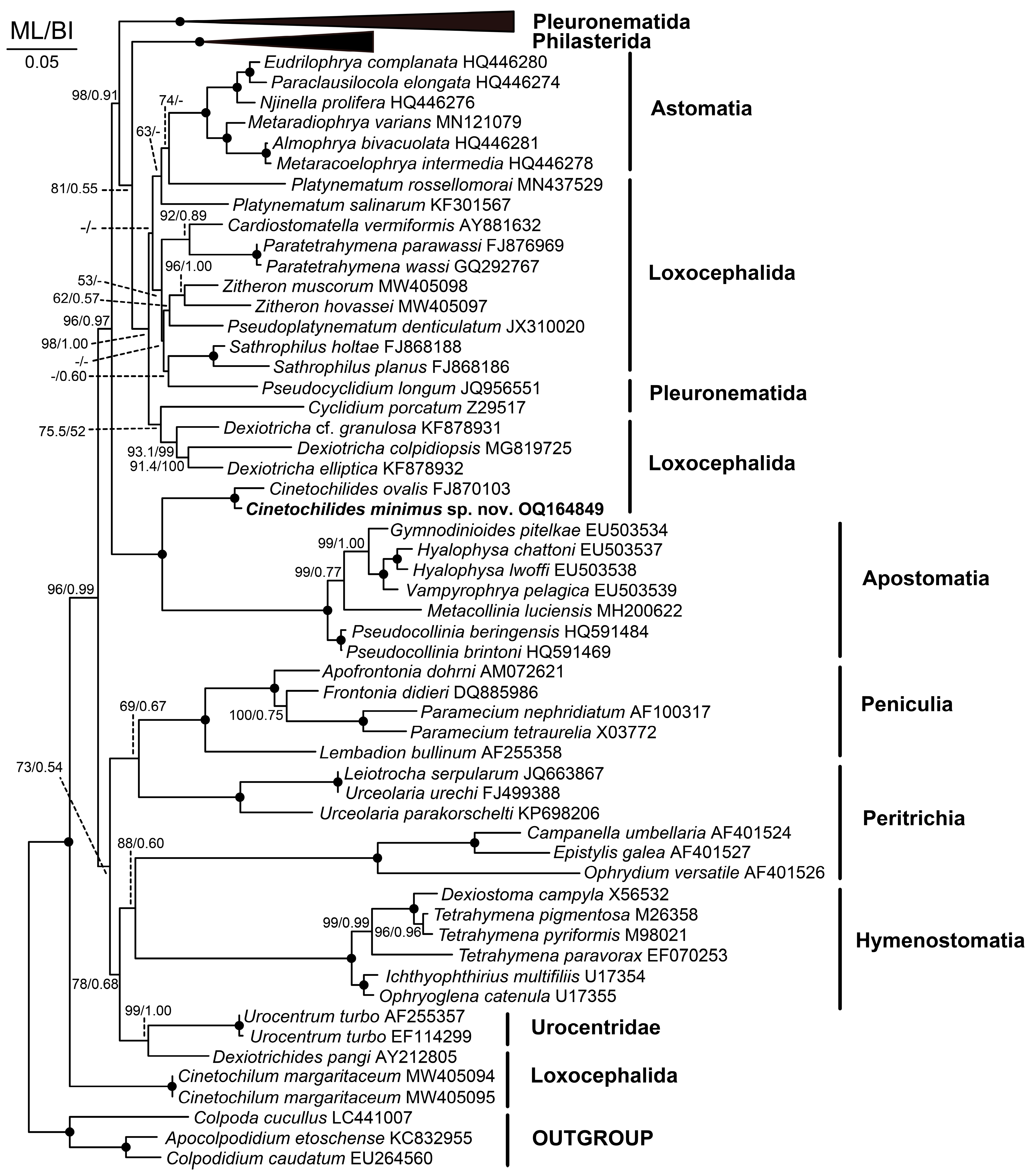
2.4. Phylogenetic Analyses
3. Results
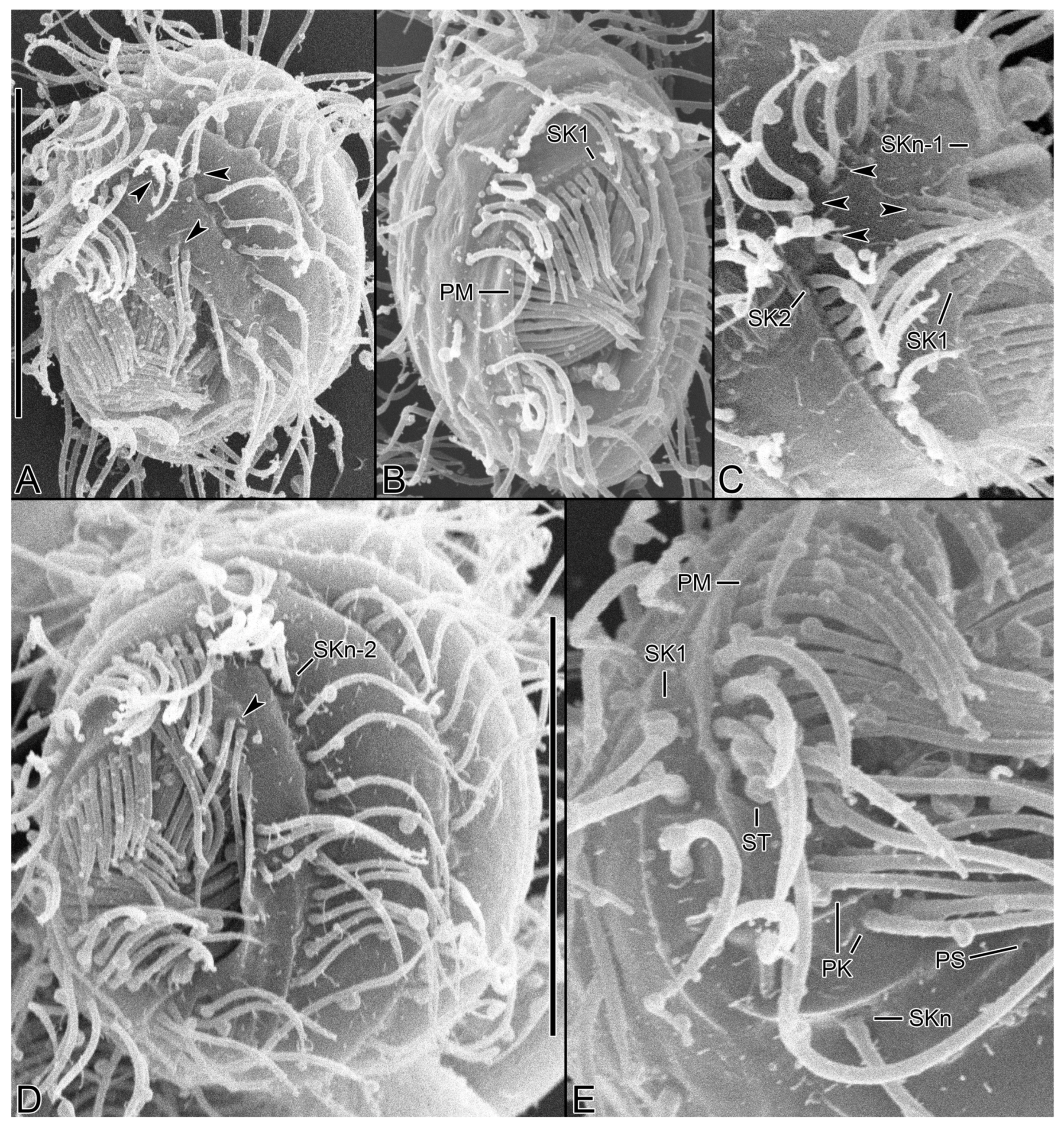
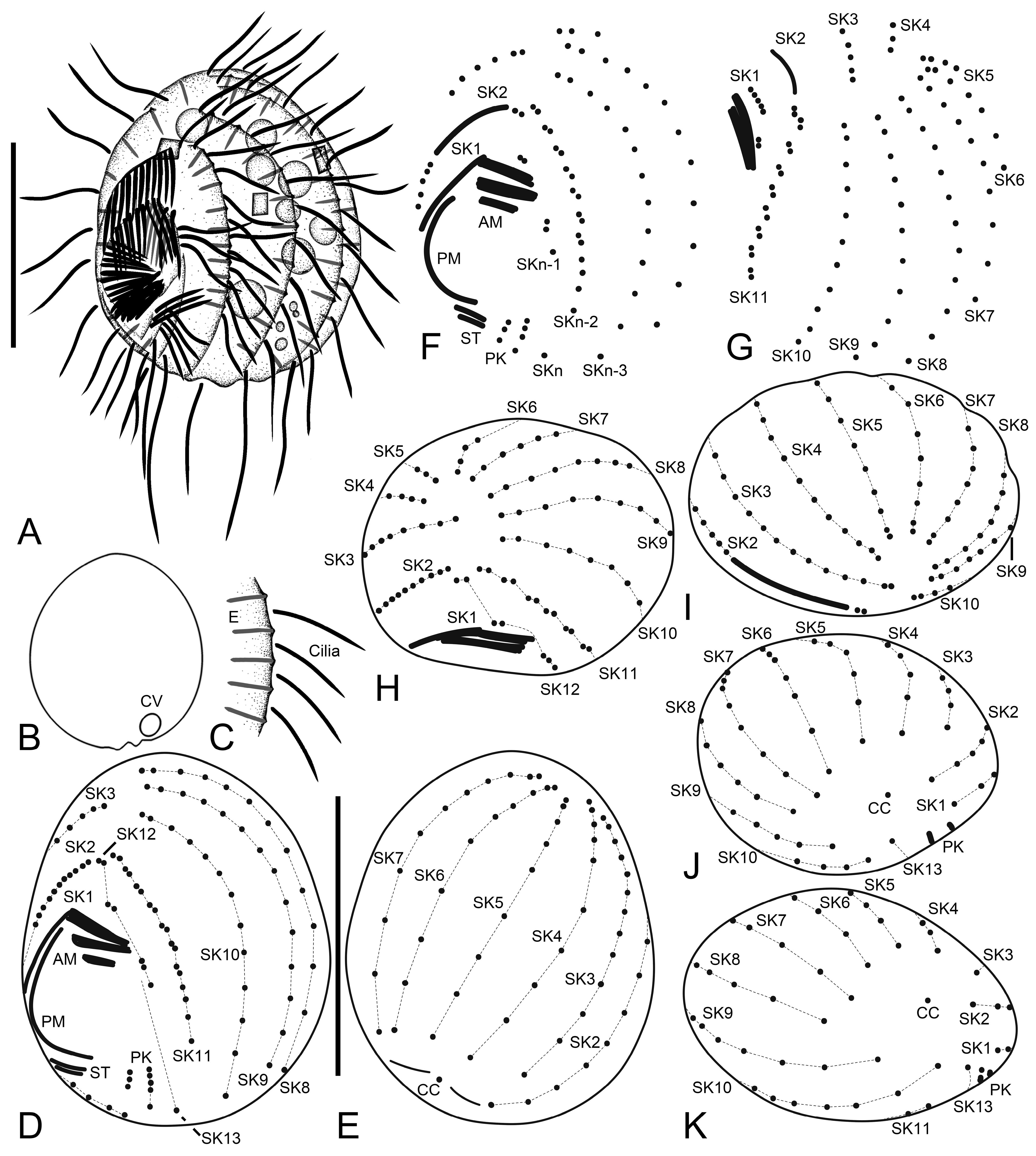
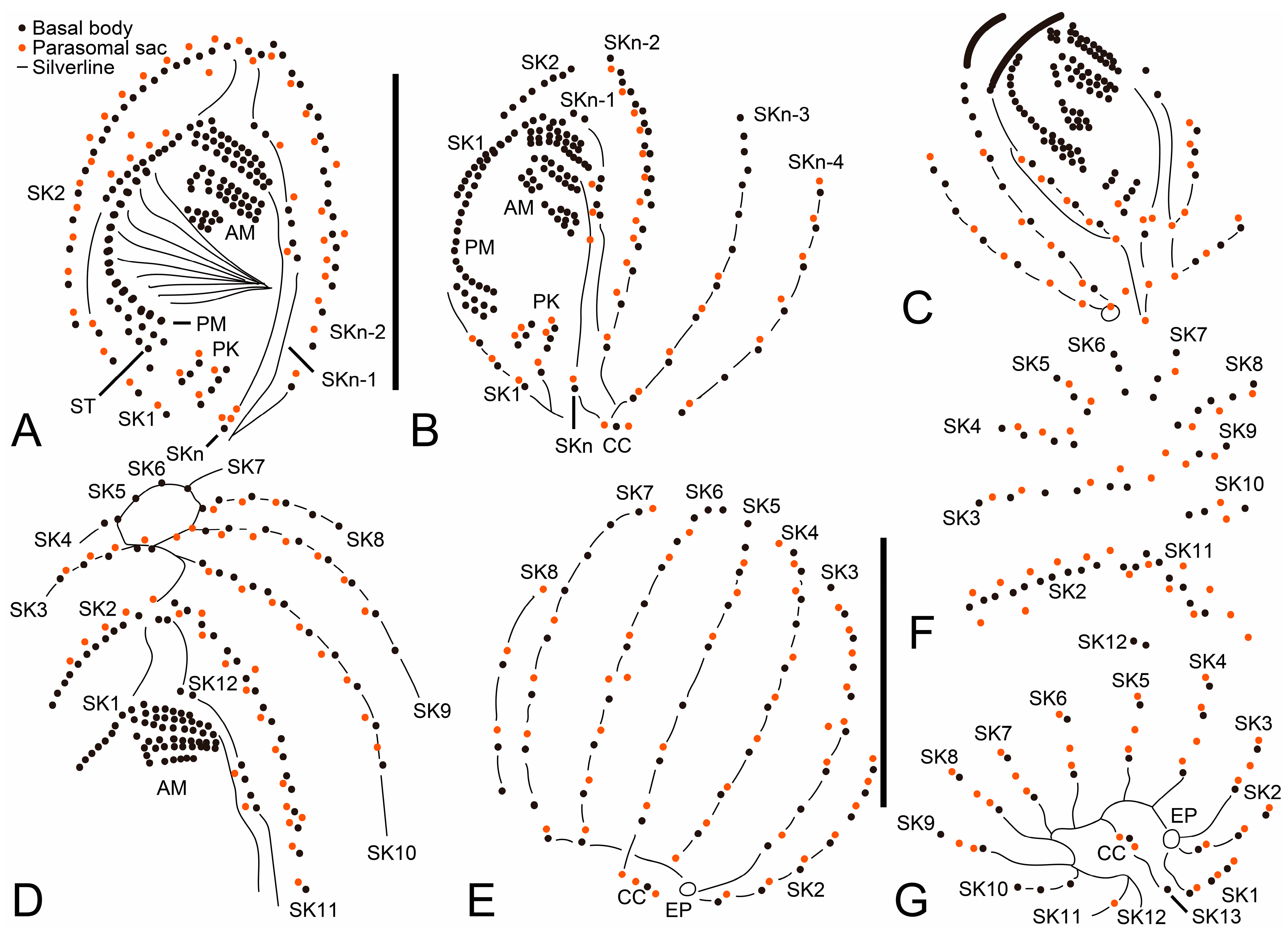
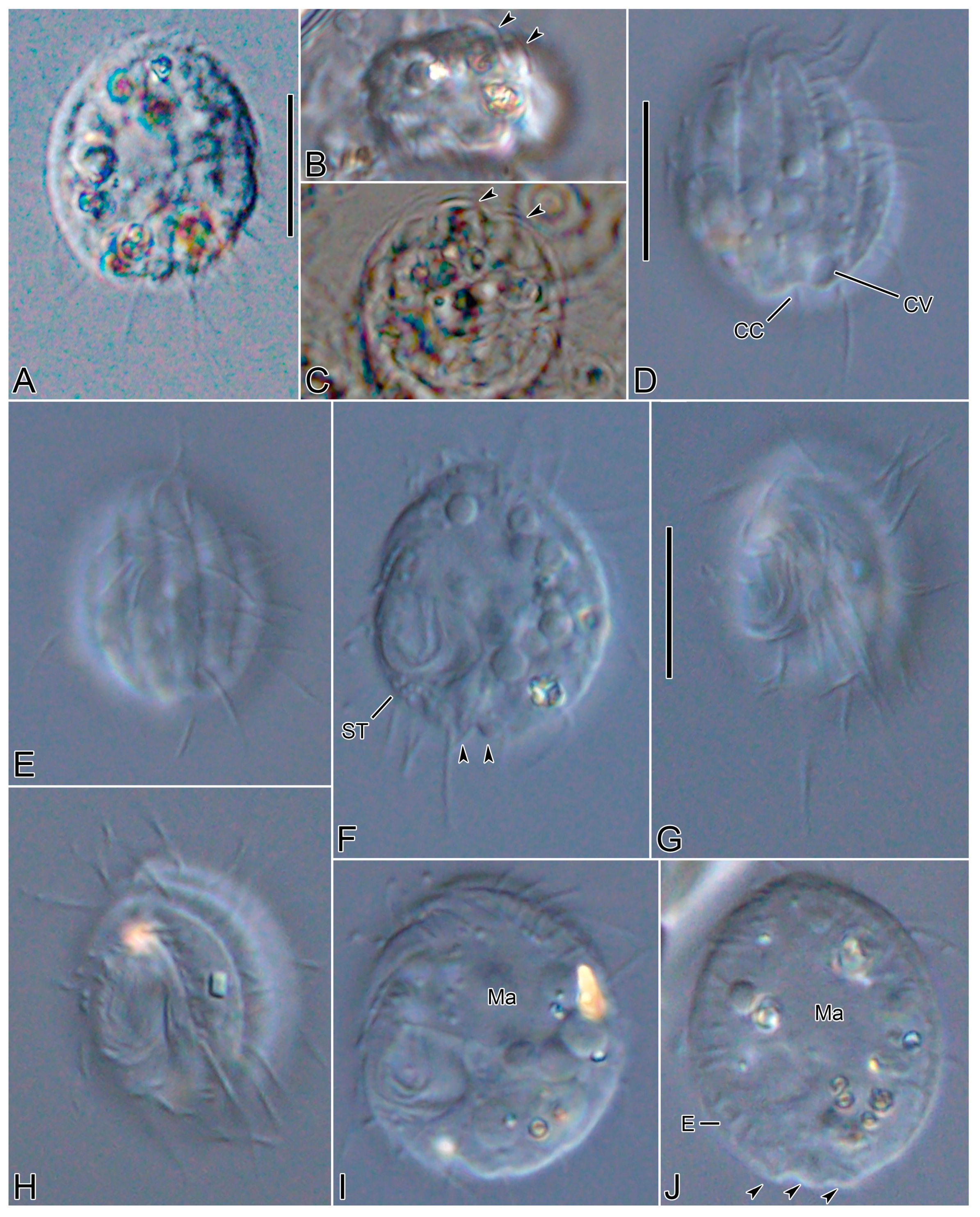
3.1. Systematics
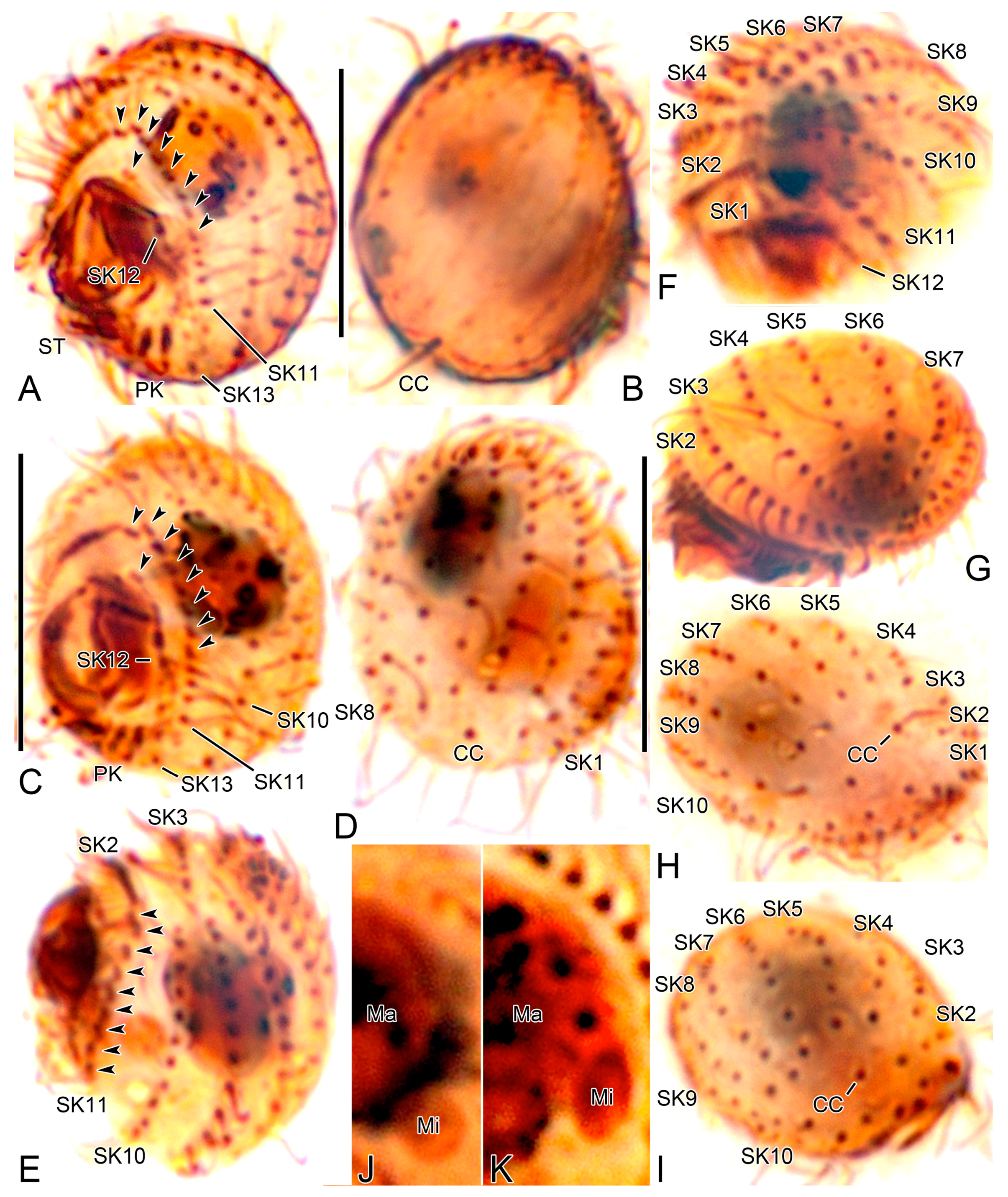
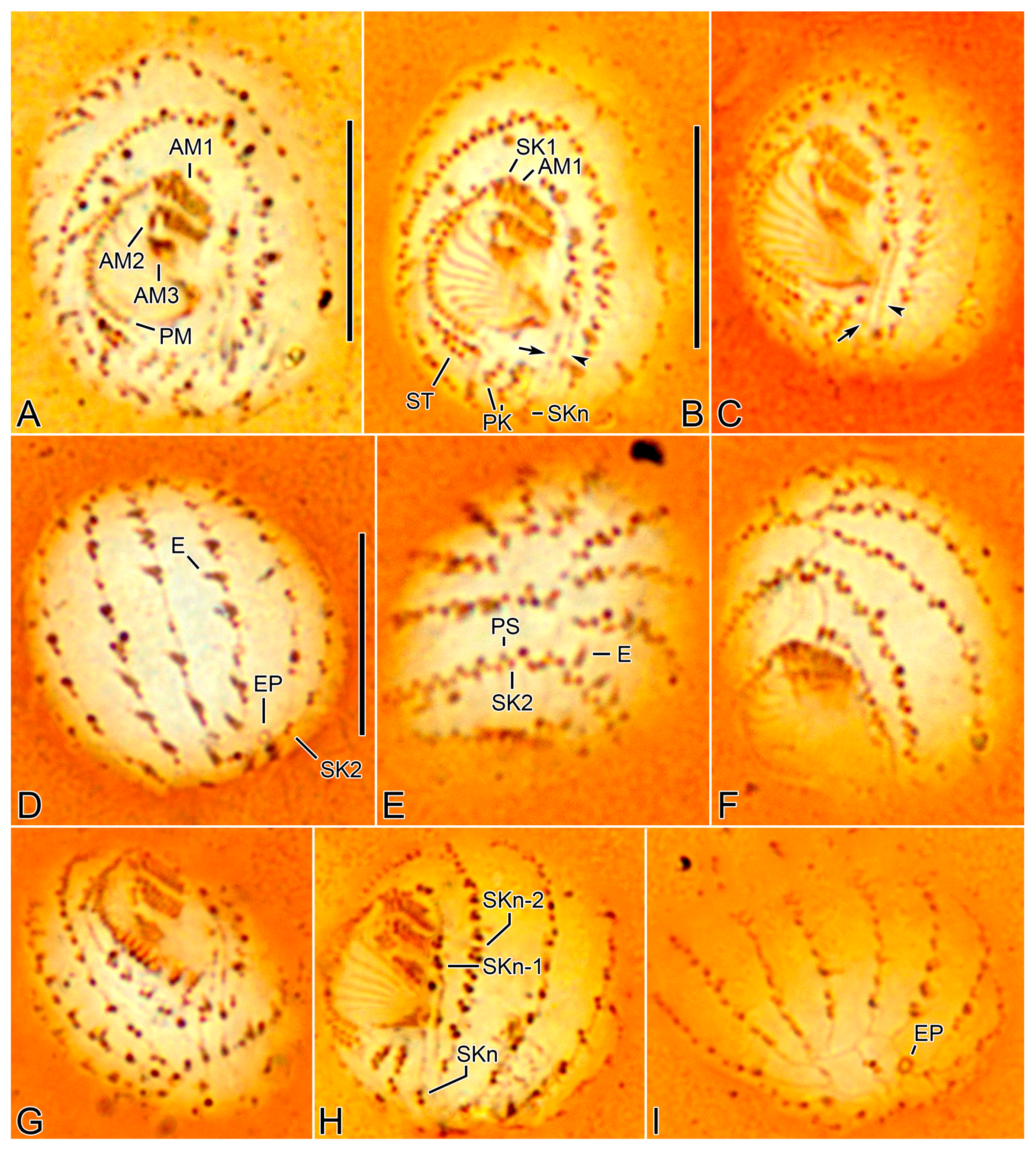
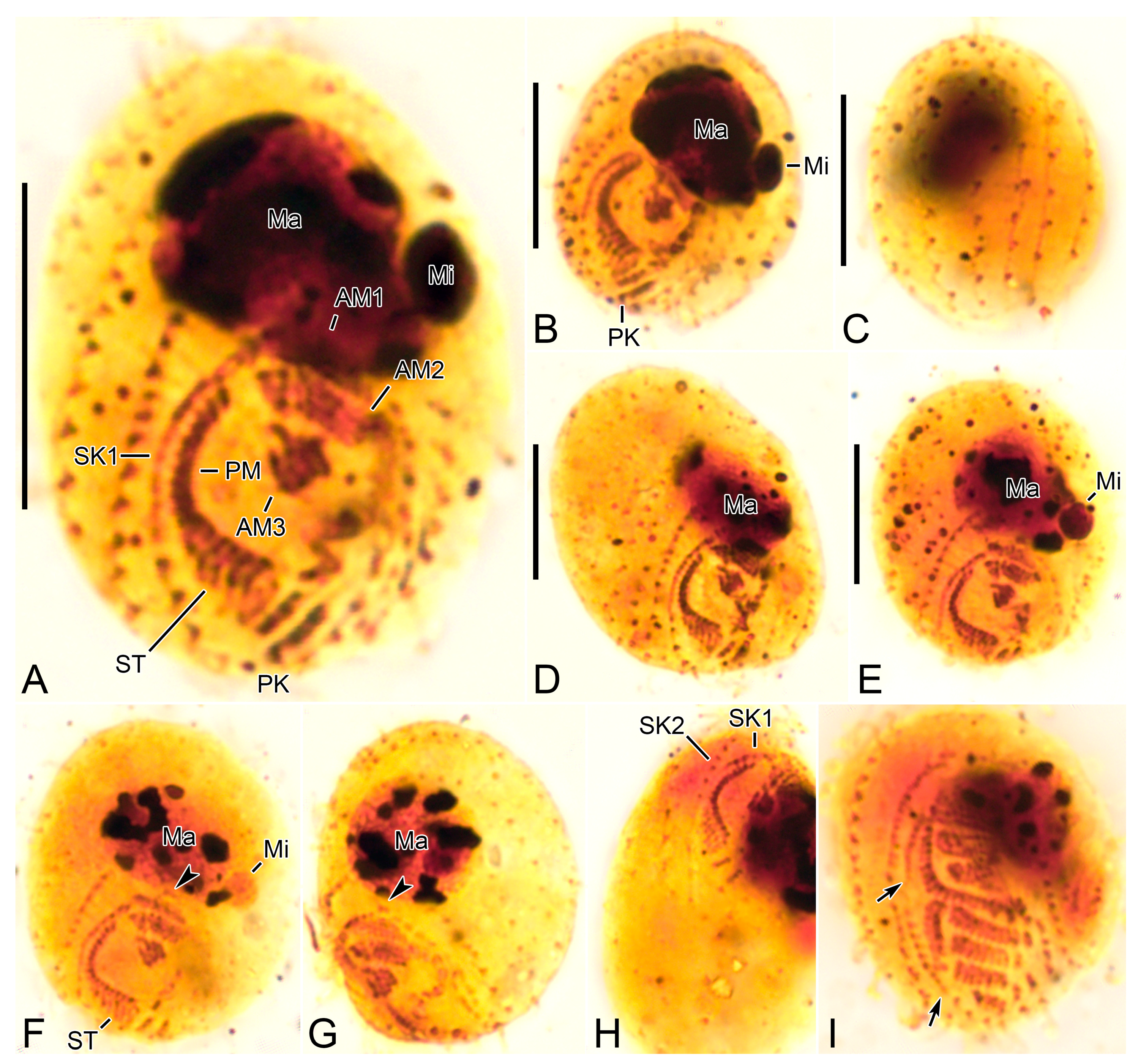
3.2. Species Diagnosis
3.3. Type Locality
3.4. Type Material
3.5. Etymology
3.6. Description
3.7. 18S rRNA Gene Phylogeny
4. Discussion
4.1. Comparison with Related Species
4.2. Phylogeny Based on 18S rRNA Gene Sequences
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pomp, R.; Wilbert, N. Taxonomic and ecological studies of ciliates from Australian saline soils: Colpodids and hymenostomate ciliates. Mar. Freshw. Res. 1988, 39, 479–495. [Google Scholar] [CrossRef]
- Gong, J.; Song, W. Morphology and infraciliature of a new marine ciliate, Cinetochilum ovale n. sp. (Ciliophora: Oligohymenophorea). Zootaxa 2008, 1939, 51–57. [Google Scholar] [CrossRef]
- Foissner, W. Terrestrial and semiterrestrial ciliates (Protozoa, Ciliophora) from Venezuela and Galápagos. Denisia 2016, 35, 1–912. [Google Scholar]
- Poláková, K.; Čepička, I.; Bourland, W.A. Phylogenetic position of three well-known ciliates from the controversial order Loxocephalida Jankowski, 1980 (Scuticociliatia, Oligohymenophorea) and Urozona buetschlii (Schewiakoff, 1889) with improved morphological descriptions. Protist 2021, 172, 125833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Miao, M.; Strüder-Kypke, M.C.; Al-Rasheid, K.A.S.; Al-Farraj, S.A.; Song, W. Molecular evolution of Cinetochilum and Sathrophilus (Protozoa, Ciliophora, Oligohymenophorea), two genera of ciliates with morphological affinities to scuticociliates. Zool. Scr. 2011, 40, 317–325. [Google Scholar] [CrossRef]
- Foissner, W. An update of ‘basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa’. Int. J. Syst. Evol. Microbiol. 2014, 64, 271–292. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, J.-H. Cytological staining of protozoa: A case study on the impregnation of hypotrichs (Ciliophora: Spirotrichea) using laboratory-synthesized protargol. Anim. Cells Syst. 2017, 21, 412–418. [Google Scholar] [CrossRef]
- Jung, J.-H.; Min, G.-S. New record of two species in stichotrichous ciliates (Ciliophora: Stichotrichia) from Korea. Korean J. Syst. Zool. 2009, 25, 227–236. [Google Scholar] [CrossRef]
- Sonnenberg, R.; Nolte, A.W.; Tautz, D. An evaluation of LSU rDNA D1-D2 sequences for their use in species identification. Front. Zool. 2007, 4, 6. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Park, M.-H.; Moon, J.H.; Kim, K.N.; Jung, J.-H. Morphology, morphogenesis, and molecular phylogeny of Pleurotricha oligocirrata nov. spec. (Ciliophora: Spirotrichea: Stylonychinae). Eur. J. Protistol. 2017, 59, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-H.; Moon, J.H.; Park, K.-M.; Kim, S.; Dolan, J.R.; Yang, E.J. Novel insights into the genetic diversity of Parafavella based on mitochondrial CO1 sequences. Zool. Scr. 2018, 47, 743–755. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Res. 1999, 41, 95–98. [Google Scholar]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Hillis, D.M.; Bull, J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993, 42, 182–192. [Google Scholar] [CrossRef]
- Alfaro, M.E.; Zoller, S.; Lutzoni, F. Bayes or bootstrap? A simulation study comparing the performance of Bayesian Markov chain Monte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Mol. Biol. Evol. 2003, 20, 255–266. [Google Scholar] [CrossRef]
- Lynn, D.H.; Strüder-Kypke, M.C. The sanguicolous apostome Metacollinia luciensis Jankowski 1980 (Colliniidae, Apostomatia, Ciliophora) is not closely related to other sanguicolous apostomes. J. Eukaryot. Microbiol. 2019, 66, 140–146. [Google Scholar] [CrossRef] [PubMed]
| Characteristics a | Method | Mean | M | SD | SE | CV | Min | Max | n |
|---|---|---|---|---|---|---|---|---|---|
| Body, length | P | 12.6 | 12.7 | 0.8 | 0.2 | 6.3 | 11.4 | 13.9 | 18 |
| SN | 16.1 | 16.5 | 1.5 | 0.3 | 9.2 | 12.9 | 18.9 | 19 | |
| Body, width | P | 10.6 | 10.8 | 0.6 | 0.2 | 6.1 | 9.4 | 11.6 | 18 |
| SN | 13.4 | 13.7 | 1.4 | 0.3 | 10.4 | 9.9 | 15.3 | 19 | |
| Body length–width, ratio | P | 1.2 | 1.2 | 0.1 | 0.0 | 5.3 | 1.1 | 1.3 | 18 |
| SN | 1.2 | 1.2 | 0.1 | 0.0 | 9.8 | 1.1 | 1.5 | 19 | |
| Anterior body end to proximal end of paroral membrane, distance | P | 9.9 | 10.0 | 0.6 | 0.2 | 6.5 | 8.5 | 10.9 | 14 |
| SN | 12.1 | 12.5 | 1.5 | 0.4 | 12.3 | 8.8 | 14.3 | 13 | |
| Anterior body end to proximal end of paroral membrane, % of body length | P | 79.1 | 79.2 | 4.5 | 1.2 | 5.7 | 69.7 | 87.0 | 14 |
| SN | 75.1 | 73.8 | 6.5 | 1.8 | 8.6 | 64.5 | 86.2 | 13 | |
| Anterior end of somatic kinety 1 to proximal end of paroral membrane, distance | P | 4.9 | 4.9 | 0.3 | 0.1 | 6.6 | 4.2 | 5.5 | 18 |
| SN | 6.6 | 6.7 | 0.4 | 0.1 | 6.8 | 5.7 | 7.3 | 19 | |
| Anterior end of somatic kinety 1 to proximal end of paroral membrane, % of body length | P | 39.4 | 39.1 | 3.1 | 0.7 | 7.8 | 32.6 | 43.6 | 18 |
| SN | 41.4 | 40.8 | 4.7 | 1.1 | 11.4 | 34.4 | 50.3 | 19 | |
| Anterior body end to macronucleus, distance | P | 2.4 | 2.6 | 0.6 | 0.2 | 26.5 | 1.4 | 3.2 | 14 |
| SN | 3.5 | 3.5 | 0.5 | 0.1 | 14.2 | 2.4 | 4.3 | 13 | |
| Anterior body end to adoral membranelle 1, distance | SN | 5.5 | 5.5 | 1.4 | 0.4 | 25.3 | 2.4 | 7.6 | 12 |
| Macronuclear nodules, number | P + SN | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 35 |
| Macronuclear nodule, length | P | 5.0 | 5.2 | 0.5 | 0.1 | 10.1 | 3.9 | 5.8 | 18 |
| SN | 4.7 | 4.6 | 0.5 | 0.1 | 10.5 | 3.9 | 5.8 | 17 | |
| Macronuclear nodule, width | P + SN | 4.0 | 4.0 | 0.6 | 0.1 | 14.0 | 3.0 | 5.0 | 18 |
| SN | 3.2 | 3.1 | 0.5 | 0.1 | 16.4 | 2.4 | 4.0 | 17 | |
| Micronuclei, number | P + SN | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 6 |
| Micronucleus, length | P | 1.6 | 1.6 | 0.3 | 0.1 | 18.2 | 1.2 | 1.9 | 4 |
| SN | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 2 | |
| Micronucleus, width | P | 1.3 | 1.3 | 0.2 | 0.1 | 17.8 | 1.1 | 1.5 | 4 |
| SN | 0.8 | 0.8 | 0.0 | 0.0 | 0.0 | 0.7 | 0.9 | 2 | |
| Somatic kineties, number (without postoral kineties | P + SN | 12.9 | 13.0 | 0.3 | 0.1 | 2.0 | 12.0 | 13.0 | 27 |
| Somatic kinety 1, length of polymerized portion | SN | 4.5 | 4.5 | 0.4 | 0.1 | 9.3 | 3.8 | 5.0 | 18 |
| Somatic kinety 1, number of polymerized kinetids | SN | 12.4 | 13.0 | 1.0 | 0.2 | 8.1 | 11.0 | 14.0 | 17 |
| Somatic kinety 2, length of polymerized portion | SN | 5.9 | 6.0 | 0.6 | 0.1 | 10.3 | 4.9 | 7.1 | 18 |
| Somatic kinety 2, number of polymerized kinetids | SN | 14.6 | 14.0 | 1.6 | 0.4 | 10.8 | 12.0 | 18.0 | 18 |
| Somatic kinety n-2, number of dikinetids | P + SN | 7.1 | 7.0 | 0.3 | 0.1 | 4.5 | 7.0 | 8.0 | 35 |
| Somatic kinety n-2, number of monokinetids | P + SN | 4.0 | 4.0 | 0.5 | 0.1 | 13.6 | 3.0 | 5.0 | 35 |
| Somatic kinety n-1, number of dikinetids | P + SN | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 36 |
| Somatic kinety n-1, number of basal bodies below third dikinetid | P + SN | 3.5 | 3.5 | 0.5 | 0.1 | 14.5 | 3.0 | 4.0 | 32 |
| Somatic kinety n, number of basal bodies | P + SN | 1.2 | 1.0 | 0.4 | 0.1 | 32.2 | 1.0 | 2.0 | 37 |
| Postoral kineties, number | P + SN | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 37 |
| Adoral membranelle 1, width | SN | 3.2 | 3.2 | 0.3 | 0.1 | 8.1 | 2.5 | 3.7 | 19 |
| Adoral membranelle 2, width | SN | 2.9 | 2.9 | 0.2 | 0.0 | 7.2 | 2.4 | 3.3 | 19 |
| Adoral membranelle 3, width | SN | 1.6 | 1.5 | 0.2 | 0.1 | 14.7 | 1.2 | 1.9 | 19 |
| Paroral membrane, length of chord | SN | 6.5 | 6.4 | 0.5 | 0.1 | 8.0 | 5.8 | 7.9 | 19 |
| Characteristics | C. australiensis (Type Pop.) | C. monomacronucleatus (Type Pop.) | C. ovalis (Type Pop.) | C. terricola (Type sp., Type Pop.) | C. minimus sp. nov. |
|---|---|---|---|---|---|
| Body, length in vivo | ~28 µm | 15–25 µm | 20–30 µm | 24–30 µm | 13–18 µm |
| Macronucleus, numbers | 2 | 1 | 1 | 2 | 1 |
| Somatic kineties, number | 11 | 11 or 12 | 12 or 13 | 11 | 12 or 13 |
| Fragmentation of somatic kinety 1 | Absent | Absent | Absent | Absent | Present |
| Scutica, position | Behind paroral membrane | Behind paroral membrane | Behind somatic kinety 1 | Behind paroral membrane | Behind paroral membrane |
| Membranelle 1, structure | Continuous | Continuous | Fragmented | Continuous | Continuous |
| Horizontal silverlines | Present | N/A | N/A | N/A | Absent |
| Habitat (salinity) | Saline soil (20–25‰) | Saline soil (~50‰) | Sandy littoral area (30‰) | Saline soil (~30‰) | Sandy littoral area (10‰) |
| Reference | Pomp and Wilbert [1] | Foissner [3] | Gong and Song [2] | Foissner [3] | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.H.; Omar, A.; Jung, J.-H. Cinetochilides minimus sp. nov., a Tiny Benthic Ciliate (Protozoa, Ciliophora) from Brackish Water in Korea. Diversity 2023, 15, 76. https://doi.org/10.3390/d15010076
Moon JH, Omar A, Jung J-H. Cinetochilides minimus sp. nov., a Tiny Benthic Ciliate (Protozoa, Ciliophora) from Brackish Water in Korea. Diversity. 2023; 15(1):76. https://doi.org/10.3390/d15010076
Chicago/Turabian StyleMoon, Ji Hye, Atef Omar, and Jae-Ho Jung. 2023. "Cinetochilides minimus sp. nov., a Tiny Benthic Ciliate (Protozoa, Ciliophora) from Brackish Water in Korea" Diversity 15, no. 1: 76. https://doi.org/10.3390/d15010076
APA StyleMoon, J. H., Omar, A., & Jung, J.-H. (2023). Cinetochilides minimus sp. nov., a Tiny Benthic Ciliate (Protozoa, Ciliophora) from Brackish Water in Korea. Diversity, 15(1), 76. https://doi.org/10.3390/d15010076








