Abstract
The Neotropics are highly diverse in avian species. Neotropical countries contribute a large part of the estimated diversity of haemosporidian parasites reported for the planet’s tropical zones. However, sampling is limited and biased, illustrated by only 30% of the genetic records (barcodes) from non-passerines, most of them not linked to a nominal species. This paper aimed to perform the molecular and morphological characterization of the haemosporidians that infect non-passerine birds from Colombia deposited in the biological collection named “Grupo de Estudio Relación Parásito Hospedero (GERPH)”. We analyzed 1239 samples from twelve biomes and two animal care facilities. Phylogenetic relationships using barcodes and mitochondrial genomes were estimated. In addition, the reports of haemosporidian infections in non-passerine birds from the Neotropics recorded after 1978 were summarized. We reported the presence of thirteen morphological haemosporidian species, four potential new species deposited in GERPH, a host range expansion for two Plasmodium species, and a barcode sequence for Haemoproteus caprimulgi. We confirmed the species associated with 56 molecular lineages reported in other neotropical countries at the genus level. Thus, biological collections and curated databases such as MalAvi are essential to support integrative approaches demanded in modern taxonomy.
1. Introduction
Haemosporidia are a highly diverse group of parasites hosted by reptiles, mammals, and birds [1]. These protozoans have been confirmed to infect many avian families on all continents [2]. Traditionally, research on avian haemosporidians relies on species that can be captured using mist nets as a trapping method, thus skewing the information obtained towards passerine birds [3].
The Neotropical region in about ten terrestrial ecoregions [4,5,6] harbors more than 4000 avian species, representing almost half of the world’s species [7]. In fact, the three most biodiverse countries in the world’s Aves are found in this region [8]. Agreeing with this, the avian Haemosporida are highly diverse at low latitudes [9], where the Neotropical countries contribute about 40% of the unique lineages reported for tropical countries (Malavi database [10]). However, recent findings of new lineages [11,12,13] suggest that higher values of parasite diversity can be expected to be found compared to the known host diversity in Central and South American countries [14].
Haemosporidian research in the Neotropics began at the dawn of the 20th century with the characterization of the Haemoproteus columbae life cycle ([15] cit. [16]). White et al. [17] published the first comprehensive compilation of studies on the avian haematozoa of the Neotropics, including infection reports from 31 bird orders from Mexico to Argentina. In that article, the haemosporidian genera Plasmodium, Leucocytozoon, and Haemoproteus were reported in 22 of the 30 non-passerine orders of birds. Then, Gabaldon et al. [18] reported the presence of a fourth parasite genus named Fallisia in a Columbiform species for the first time. Later, Valkiūnas [2] reported fourteen Plasmodium, twelve Haemoproteus, and three Leucocytozoon species in non-passerine hosts for this geographic area, most of them described before 2000 [16]. The findings reported between 1908 and 2005 were made using blood smears as a diagnostic tool (Figure 1). Currently, haemosporidian biodiversity characterization uses combined information from blood smears, mitochondrial lineages, host-parasite interactions, and ecological traits [16]. From the first studies in avian haemosporidians in the Neotropics until now, at least twenty-four species have been described in non-passerine hosts [2,16,19,20,21,22,23].
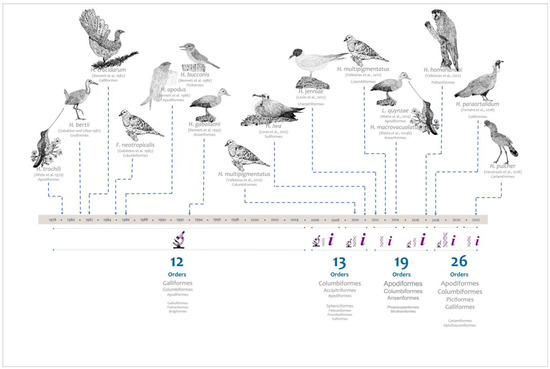
Figure 1.
Schematic representation of the findings of haemosporidians in Neotropical non-passerine birds since the last comprehensive revision in 1978. Over the timeline, the parasite species are described with the host and references. Under the timeline, brown thin lines represent the time periods for the number of studies carried out using only microscopy (represented by the figure of the microscope), methods based on PCR (represented by the DNA molecule), or combined evidence (represented by the letter i). The size of the icons represents the number of studies carried out with each methodology. The thin blue lines represent the time spans for which the number of orders studied is counted. At the bottom, the size of the name represents the number of records associated with each order, the smallest being those with the fewest number of studies in the specified time range.
The first record of a PCR-based diagnosis of haemosporidian parasites in neotropical non-passerine birds was done by Durrant et al. [24] in 2006 (Figure 1). Given the higher detection rate of molecular methods over microscopy (e.g., [25]), the number of explored avian orders has increased (Figure 1). The first Leucocytozoon species reported in Apodiformes (Hummingbirds) came from Colombia, and was published in 2014 [20]. In that report, the authors emphasize a change in the distribution of this parasitic genus, commonly found only in the highlands. Then, several new Leucocytozoon lineages were reported in South America’s highlands for non-passerines [13,21,26,27], etc. To date, according to MalAvi [10], nearly 700 lineages that remain identified at the genus level have been reported for neotropical non-passerine hosts.
In Colombia, the biological collection named Grupo de Estudio Relación Parásito Hospedero (GERPH) hosts samples from twenty non-passerine bird orders collected in at least ten different ecosystems since 1999. This study aimed to perform a molecular and morphological characterization of the Haemosporidians infecting non-passerine birds deposited in the GERPH biological collection. We also present an update on the haemosporidian infection reports in non-passerine birds from Mexico to Argentina for the last 40 years. That would provide a more accurate perception of the state of Neotropical research, allowing future studies to focus efforts on the species and ecosystems less sampled to date.
2. Materials and Methods
2.1. Samples
This study analyses samples of 1239 individuals belonging to 178 species, 34 families, and 20 orders of non-passerine birds deposited in the biological collection GERPH. These samples have been obtained mainly from wild birds collected in 23 localities distributed in 12 of the 32 continental biomes of Colombia (Table S1, Figure 2). There are also samples from animals treated and housed in animal care facilities after being found injured or seized from illegal traffickers. It is essential to highlight that these birds were sampled at the time of admission to the rescue center before receiving treatment or having contact with other specimens in the facilities. A detailed description of the sampling areas is provided in Table S1.
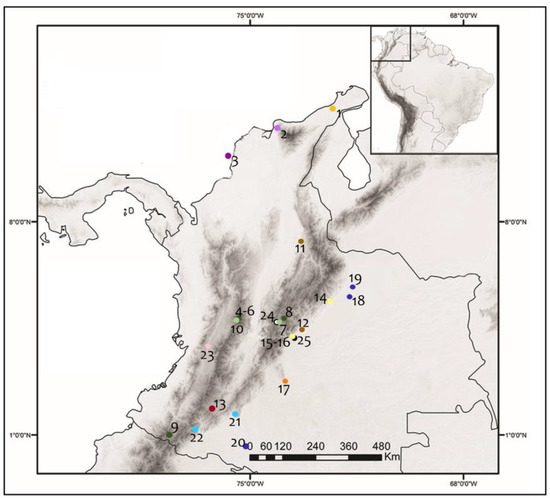
Figure 2.
Geographical location of the sampling localities. Dot colors represent the biomes in each locality. Then, Manaure (1) with a dark yellow spot is placed in the Tropical desert zonobiome of the Guajira and Santa Marta; Cienaga (2) represented by a pale purple circle is located in the Caribbean halobiome; and Barú (3) represented with a dark purple belongs to the Caribbean tropical dry zonobiome. The dark green dots indicate the places included in the High orobiome of the Andes: Los Nevados National Natural Park (NNP) (4), Chingaza National Natural Park (NNP) (5), and Aldana (6). The localities sampled in the middle orobiomes of the Andes represented by light green dots were Universidad Nacional de Colombia- Bogotá Campus (UNAL) (7), Ucumarí National Regional Park (NRP) (8), El Cedral (9), and Quimbaya Flora and Fauna Sanctuary (FFS) (10); while in the low orobiomes of the Andes, brown dots indicate the location of San Gil (11) and Medina (12). The dark red dot represents San Agustín (13) in the upper Magdalena alternate hygric or subxerophytic zonobiome. Yopal (14), Villavicencio (15), and San Miguel (16) where the sampled places in the Amazon-Orinoco peinobiome represented by light yellow dots, while La Macarena NNP (17) represented in orange corresponds to the Macarena Orobiome. Sampling places in the helobiomes of the Orinoco and the Amazon (18–20), and tropical humid Zonobiome of the Orinoco and Amazon (21–22) are represented by dark and pale blue respectively. For helobiomes of the Cauca Valley, the pink dot indicates the Sonso Lagoon (23). As for the animal care facilities, they are represented by a white dot (URRAS–24) and a black dot (Ocarros–25).
2.2. Microscopic Examination
Microscopic examination was performed at low 400× and high magnification 1000×. Positive smears were photographed using the CellSens software (Olympus Corporation). We used the taxonomic keys provided in Valkiūnas [2], Valkiūnas and Iezhova [28] and the original descriptions for parasite taxonomic identification. In addition, graphic material from parasitic species deposited in the collection of Dr. G. Valkiūnas at the Institute of Ecology (CDVA) and specimens from the International Reference Centre for Avian Haematozoa (IRCAH) were revised to compare the parasite species morphologies observed in these blood smears to corroborate species determinations.
Overall and individual parasite genus frequencies were calculated as the positive individuals over the total sampled, expressed as a percentage.
2.3. DNA Extraction, Amplification of Cytochrome b (Cytb) Barcodes and mtDNA Genomes
DNA was extracted using the DNeasy blood and tissue kit (Qiagen, Hilden, Germany) when collected samples were stored in EDTA, or the standard phenol chloroform protocol [29] for samples collected in ethanol or SET buffer. Molecular diagnosis was made only for samples whose smears were positive (n = 108). It is important to mention that for some of these positive samples, there was no blood available for molecular tests (n = 20). Once DNA was obtained, amplification of cytochrome b (cytb) fragments was performed using the primers and protocols suggested by Hellgren et al. [30] and Pacheco et al. [31]. To check for non-patent coinfections with other haemosporidians, independent amplifications were conducted using the primers of Pacheco et al. [31]. Amplicons were cleaned with ammonium acetate [32] and sequenced in both senses on an ABI 3730xl System (Applied Biosystems, Foster City, CA, USA). A total of 17 partial cytb gene sequences were reported in this study and submitted to GenBank under accession numbers OL689174, OL689176, OL689177, and OP087637-OP087649.
Second, for those positive samples with good DNA quality and parasitemia partial parasite mtDNA genomes (≈6 kb) were amplified by a PCR protocol with Takara LA Taq™ Polymerase (TaKaRa Mirus Bio, Madison, WI, USA) following Pacheco et al. (2018b) using the oligos forward AE170-5′-GAGGATTCTCTCCACACTTCAATTCGTACTTC-3′ and reverse AE171-5′-CAGGAAAATWATAGACCGAACCTTGGACTC-3′ [33]. PCRs were performed in 50 µL using 2 µL of total genomic DNA. PCR conditions were a partial denaturation at 94 °C for 1 min and 30 cycles with 30 s at 94 °C and 7 min at 67 °C, followed by a final extension of 10 min at 72 °C. PCR products (50 µL for each sample) were visualized in agarose gels (1%). Excised bands (bands of ≈6 kb) were purified using a QIAquick® Gel extraction kit (Qiagen, GmbH, Hilden, Germany). Finally, these products were cloned using pGEM®-T Easy Vector systems (Promega, Madison, WI, USA) following the manufacturer’s directions. A minimum of three to four clones per sample (both strands) were sequenced at GENEWIZ from Azenta Life Sciences (Middlesex County, NJ, USA). A total of five new mtDNA genomes for morphospecies were reported in this study and submitted to GenBank under accession numbers OP701681-OP701685.
2.4. Phylogenetic Analysis
For cytb gene phylogenetic analysis, the partial sequences obtained from non-passerine birds were manually edited in MEGA v7.0. [34]. Three alignments were constructed, one for each of the three haemosporidian genera. Those alignments included lineages obtained from the positive specimens deposited in the biological collection GERPH, along with parasite sequences isolated from passerine and non-passerine birds deposited in GenBank and MalAvi [10]. Thus, for Haemoproteus, an alignment of 465 bp composed of 15 sequences from GERPH specimens, 83 sequences from public databases, and 3 Leucocytozoon species as outgroup was performed. In this alignment, 76 lineages were associated with morphospecies.
For Plasmodium, 59 partial cytb gene sequences were used, 8 from GERPH specimens, and 24 linked with morphospecies. Three Leucocytozoon lineages were added as an outgroup. The total length for this alignment was 448 bp.
Leucocytozoon phylogenetic reconstruction included 48 lineages of this parasite’s genus, along with 2 sequences of Plasmodium and 2 of Haemoproteus. Seven lineages were isolated from specimens deposited in the GERPH collection, while the remaining forty-one were obtained from public databases. Also, 23 lineages were associated with identified morphospecies. The total length for this analysis was 456 bp. All sequences were aligned with MAFFT (Ref. [35], available at https://www.ebi.ac.uk/Tools/msa/mafft/, accessed on 13 September 2022).
For the phylogenetic analysis, the general time-reversible model was used with gamma-distributed substitution rates; a proportion of invariant sites (GTR + G + I) was selected in Jmodeltest [36] as the best fit model of nucleotide substitution, according to the Akaike information criterion. The phylogenetic relationships were estimated using maximum-likelihood (ML) implemented in W-IQ-TREE (Ref. [37], available at http://iqtree.cibiv.univie.ac.at/, accessed on 13 September 2022), performing a bootstrap of 1000 replicates. In addition, a Bayesian inference analysis was conducted with MrBayes v 3.2 [38] using two MCMC simulations 5 × 106 generations, with samplings every 100 generations. The convergence was checked by the average standard deviation of split frequencies inferior to 0.01. A majority consensus rule was applied to obtain a phylogenetic hypothesis of over 75,000 trees.
In addition, a fourth alignment was performed using 70 partial avian parasite mtDNA genomes (5234 bp excluding gaps). The alignment included sequences from the Genbank (N = 65) of the three genera (Leucocytozoon, Haemoproteus, and Plasmodium) and the new mtDNA genome sequences (N = 5) obtained in this study for some well-identified morphospecies using morphology. This alignment was done using MUSCLE, as implemented in SeaView v4.3.5 [39], with manual editing. Then, using both the Bayesian (MrBayes) and maximum likelihood (W-IQ-TREE) methods, phylogenetic relationships were inferred using six partitions [33]. These partitions corresponded to the three non-protein coding regions between the ORFs (fragmented SSU rRNA and LSU rRNA) and the three protein-coding genes, keeping their order in the mtDNA genome. Also, a general time-reversible model was used with gamma-distributed substitution rates and a proportion of invariant sites (GTR + G + I) for each partition of the alignments. This model had the lowest Bayesian information criterion (BIC) scores estimated by jModelTest [36]. The Bayesian support for the nodes was inferred by sampling every 1000 generations from two independent chains lasting 104 Markov chain Monte Carlo steps. Convergence between chains was assumed once the average standard deviation of the posterior probability was <0.01, and the value of the potential scale reduction factor was between 1.00 and 1.02 [38]. Then, as a ‘burn-in’, 25% of the sample was discarded once convergence was reached.
For each of the four alignments constructed in this investigation, genetic distances were calculated using the Kimura 2 parameters (K2P) model of nucleotide substitution implemented in MEGA v.7.0 [34].
3. Results
A total of 108 specimens from 11 avian orders were found infected with haemosporidian parasites. Such infected individuals were distributed in nine of the twelve biomes studied and from the two rescue centers. For Haemoproteus, there were 82 positive birds (6.62%), while for Plasmodium and Leucocytozoon there were 15 and 11 (1.21% and 0.89%), respectively.
Haemoproteus parasites were distributed in all the biomes except for those located in the Caribbean region (tropical desert zonobiome–I; Caribbean halobiome–II; and Caribbean tropical dry zonobiome–III). Meanwhile, Plasmodium was found only for middle orobiomes of the Andes (V), the helobiomes of the Orinoco and the Amazon (X), and the tropical humid zonobiome of the Orinoco and Amazon (XI). Leucocytozoon was restricted to places whose altitudes exceeded 2100 m asl; then, the genus was found only in the Andean mountains in the high altitude (IV) middle altitude (V) orobiomes and in the Upper Magdalena alternate hygric or subxerophytic zonobiome (VII). For the rescue centers, only the infections caused by Plasmodium and Haemoproteus are registered (Table 1).

Table 1.
Non-passerine bird samples deposited in the “Grupo de estudio relación parásito hospedero” biological collection (GERPH). The number of tested specimens is indicated as nT. Localities and biomes are as in Figure 2 and Table S1. P: Plasmodium, H: Haemoproteus, L: Leucocytozoon. Lineages obtained are provided. a: cytb fragments (478 pb), b: Mitochondrial complete genomes, *: New lineages reported in this study. The remaining negative birds are registered at the end of the table, along with the biome (super index), and number of sampled specimens in parenthesis.
Regarding the avian orders, the largest number of available samples of non-passerine birds in the GERPH biological collection corresponded to the Apodiformes and the Columbiformes, while less than five individuals were from Falconiformes, Tinamiformes, Podicipediformes, and Suliformes (Table 1, Tables S1 and S2).
Thirteen haemosporidian morphological species (Figure 3 and Figure 4) were identified in the positive specimens (n = 108 specimens). In addition, four specimens should be studied in more detail for taxonomic confirmation, since they do not fit into previously described morphospecies (Table 1). To date, twenty-two parasite cytb gene lineages and eight partial mtDNA genomes (including the five new mtDNA genomes reported in this research) were obtained from non-Passeriformes blood samples deposited in the biological collection GERPH. Such sequences were associated with ten of the thirteen morphospecies identified in this study. We also report for the first time two new lineages of Plasmodium and three of Haemoproteus in Strigiformes, Pelecaniformes, and Caprimulgiformes (Table 1, Figure 5, Figure 6, Figure 7 and Figure 8). Detailed information on the infections detected in each order explored is presented below.
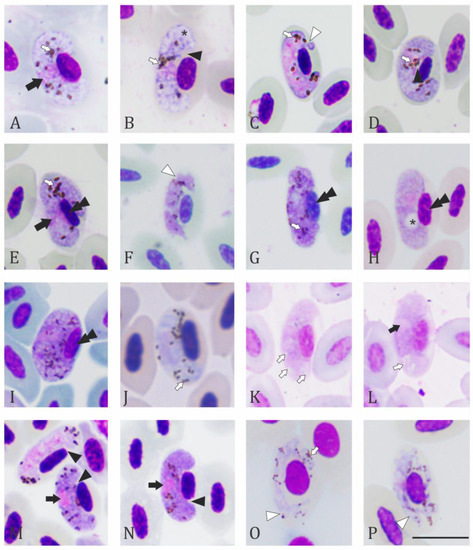
Figure 3.
Haemoproteidae of non-passerine birds of Colombia: (A) macrogametocyte; (B) microgametocyte of Haemoproteus (H.) buteonis-like in Rupornis magnirostris; (C,D) immature microgametocytes; (E) nearly mature macrogametocyte of H. nisi of Buteo platypterus; (F) young gametocyte and (G) mature gametocyte of H. gabaldoni in Cairina moschata; (H) macrogametofyte of H. macrovacuolatus in Dendrocygna autumnalis; (I) fully grown gametocyte of H. nettionis in Anas discors; (J) microgametocyte of H. witti in E. vestita; (K) micro and (L) macrogametocyte of H. caprimulgi from Lyncornis macrotis in the specimen IRCAH G403126_9396; (M) nearly mature macro and microgametocytes and (N) mature macrogametocyte of H. caprimulgi from Nyctidromus albicollis; and (O,P) gametocytes of H. contortus in Numenius phaeopus. Black arrowheads: cleft between parasite and nucleus membranes; black arrows: parasite nucleus; white arrow: hemozoin granules; white arrowhead: parasite membrane; asterisk: vacuole; black double arrowheads: host cell nucleus. Giemsa-stained blood films. Scale bar = 10 μm.
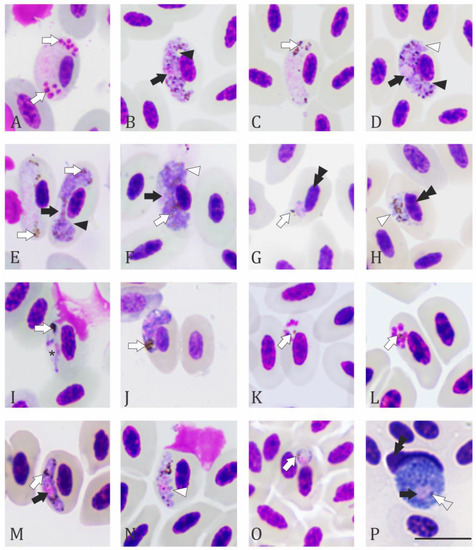
Figure 4.
Haemosporidia of non-passerine birds of Colombia: (A) mature microgametocyte of Haemoproteus (H.) columbae in Columba livia; (B,C) young macro and microgametocytes; (D) nearly mature macrogametocyte of H. paramultipigmentatus from Columbina talpacoti; (E,F) young gametocytes of H syrnii-like in Asio clamator; (G) Erythrocytic meront; and (H) gametocyte of Plasmodium (P.) nucleophilum from Glaucis hirsutus; (I) young gametocyte of Plasmodium sp. from Nyctidromus albicollis; (J) erythrocytic meront of P. tejerai in Galbula ruficauda; (K) fan-shaped and (L) roundish erythrocytic meronts; (M,N) gametocytes of P. elongatum in Porphyrio martinica; (O) parasite stage of Plasmodium sp. in Butorides striata; (P) macrogametocyte of Leucocytozoon (L.) quynzae from Heliangelus exortis. Black arrowheads: cleft between parasite and nucleus menbranes; black arrows: parasite nucleus; white arrow: hemozoin granules; white arrowhead: parasite membrane; asterisk: vacuole; black double arrowheads: host cell nucleus; white double arrowheads: volutin granules. Giemsa-stained blood films. Scale bar = 10 μm.
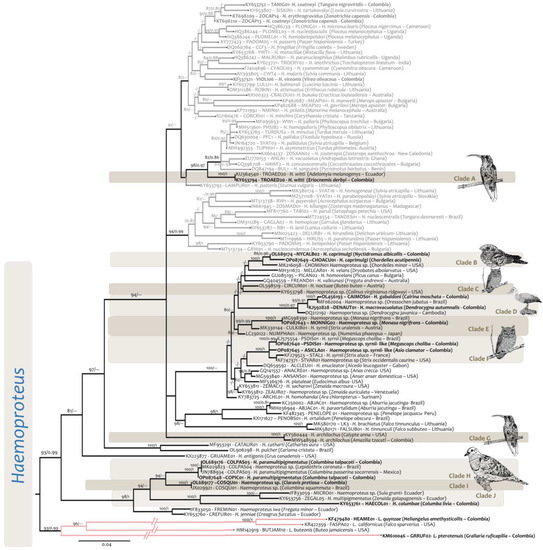
Figure 5.
Phylogenetic reconstructions of Haemoproteus sp. obtained with the Cytochrome b (cytb) barcode fragments of 479 bp. Bold tip labels correspond to sequences obtained from specimens of GERPH biological collection. Parasites isolated from passerines are indicated by grey tip labels, while lineages for non-passerine birds are in black. The nodal support values are indicated above the branches as BI/ML. Nodal supports below 0.8/80 not shown.
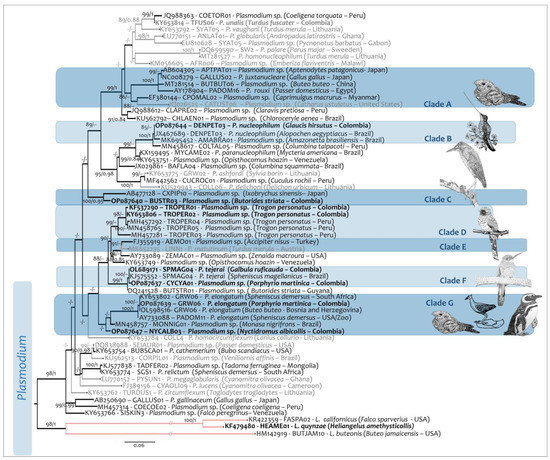
Figure 6.
Phylogenetic reconstructions of Plasmodium sp. obtained with the Cytochrome b (cytb) barcode fragments of 479 bp. Bold tip labels correspond to sequences obtained from specimens from the GERPH biological collection. Parasites isolated from passerines are indicated by grey tip labels, while lineages from non-passerine birds are in black. The nodal support values are indicated above the branches as BI/ML. Nodal supports below 0.8/80 not shown.
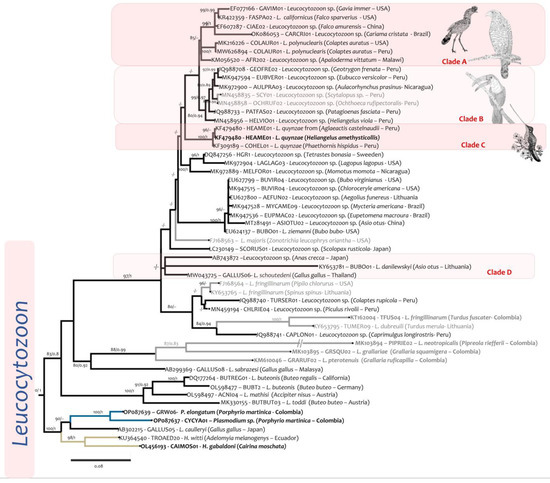
Figure 7.
Phylogenetic reconstructions of Leucocytozoon sp. obtained with the Cytochrome b (cytb) barcode fragments of 479 bp. Bold tip labels correspond to sequences obtained from specimens of the GERPH biological collection. Parasites isolated form passerines are indicated by grey tip labels, while lineages form non-passerine birds are in black. The nodal support values are indicated above the branches as BI/ML. Nodal supports below 0.8/80 are not shown.
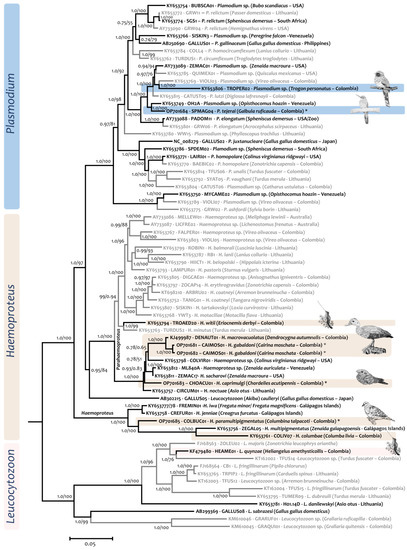
Figure 8.
Phylogenetic reconstructions of Haemosporida using mtDNA genomes. The Bayesian and maximum likelihood phylogenetic analyses were performed using 70 partial avian parasite mtDNA genomes (5234 bp excluding gaps). The values above branches are posterior probabilities/bootstraps. Parasites reported in non-passerines are indicated in black and bold, and parasites in passerines are indicated in gray. The new mtDNA genomes (N = 5) reported in this study are marked with an asterisk. Sequences obtained from samples deposited in the biological collection named “Grupo de estudio relación parásito hospedero” (GERPH) are indicated by colored rectangles.
3.1. Accipitriformes
Six individuals from three species were analyzed (Table 1), and only two infections caused by Haemoproteus were found. One of them was in a Roadside Hawk (Rupornis magnirostris, n = 1) from the eastern plains, and the other in a specimen of a Broad-winged Hawk (Buteo platypterus, n = 1) from URRAS (Table 1). The infection registered in the Rupornis magnirostris was caused by an Haemoproteus buteonis-like parasite (Figure 3A,B) that needs to be further studied in more detail (when more positive samples are available) in order to determine if it corresponds to a new species, since this parasite differed from H. buteonis in one of its main diagnostic characters. Young parasites adhere to the host cell nucleus, growing longitudinally along it. Outlines are usually even; then, they may form angular terminals at the tips of the gametocytes (Figure 3A). Also, wavy or ameboid margins were observed (Figure 3A). Host-cell nucleus displacement was observed in nearly mature or mature gametocytes. Although mature gametocytes are closely appressed to the envelope and host cell nucleus, they do not encircle it completely.
Buteo platypterus showed infection by Haemoproteus nisi [40]. The parasite’s cytoplasm was granular; outlines were ameboid developing small projections (Figure 3C). Growing gametocytes do not or only slightly touch the erythrocyte membranes (Figure 3D), and parasites are closely appressed to both envelope and nucleus, displacing it (Figure 3E). Fully grown gametocytes encircling the host cell nucleus were not observed. Unfortunately, we could not obtain the cytb lineages from these Haemoproteus species infecting these raptors (n = 2).
3.2. Anseriformes
Currently, there are 82 individuals of nine species of Anatidae deposited in the GERPH biological collection (Table 1 and Table S2). According to González et al. [41], two individuals are infected with Haemoproteus gabaldoni (Ref. [19]—Figure 3F,G), fourteen with Haemoproteus macrovacuolatus (Ref. [20]—Figure 3H), and two with Haemoproteus nettionis (Ref. [42]—Figure 3I). Unfortunately, for the specimen infected with H. nettionis, there was no blood available for molecular diagnosis. Haemoproteus gabaldoni (lineage OL456193-CAIMOS01) and H. macrovacuolatus (KJ592828-DENAUT01) were placed in a well-supported clade (clade D, Figure 5), closely related to clades B and C, that contained haemoproteids from nightjars, woodpeckers, frigate birds, and owls. The genetic distance between the taxa in clade D and clades B and C ranged between 0.031 and 0.056 (Table S4).
Regarding the mtDNA genome analysis, three partial parasite mtDNA genomes were obtained for H. macrovacuolatus (n = 1, KJ592828-DENAUT01) and H. gabaldoni (n = 2, OP701681 and OP701682-CAIMOS01). Both parasites appeared as a sister taxon and shared a common ancestor with a Haemoproteus sp. (Figure 8) detected in Colinus virginianus ridgwayi from Arizona (KY653798-COLVIR01; Ref. [43]). The genetic distance between H. macrovacuolatus and H. gabaldoni was 0.008 (Table S5). Although both partial cytb sequences (480 bp) for H. gabaldoni were identical and identified as the CAIMOS01 lineage, the mtDNA genome genetic distance between both was 0.002, indicating some population differences.
3.3. Apodiformes
There are 546 samples from 59 Trochilidae species deposited in the biological collection GERPH, and 14 of them are infected with Haemosporidians. Previously, a specimen of the White-vented Plumeleteer (Chalybura buffonii) was reported infected with Haemoproteus sp. [44]. Unfortunately, for this sample there was no blood for molecular diagnosis (Table 1 and Table S2). Haemoproteus witti [23] morphology was characterized in the Black-thighed Puffleg (Eriocnemis derbyi) that was associated with the TROAED02 lineage in González et al. [45]. In the same report, this species was found infecting two more species of the same genus—the Coppery-bellied Puffleg (E. cupreoventris) and the Glowing Puffleg (E. vestita) (Table S2).
In this study, three more species were positive for H. witti (Figure 3J): the Buff-tailed Coronet (Boissonneaua flavescens), the Sword-billed Hummingbird (Ensifera ensifera), and the Mountain Velvetbreast (Lafresnaya lafresnayi) (Table 1 and Table S2). Furthermore, a Haemoproteus that could not be identified by morphology or molecular methods because of the low parasitemia, and an absence of mature parasites was reported for Rufous-breasted Hermit (Glaucis hirsutus) (Table 1 and Table S2).
Haemoproteus witti (KY653794-TROAED02) has been identified in other hummingbirds as well as in passerine birds [13,46] (Table S6). In the phylogenetic reconstructions, this species (Clade A, Figure 3 and Figure 8) is located basal to other lineages from parasites isolated from passerine species.
Currently, three valid species of haemoproteids infect hummingbirds: H. witti, H. archilochus and H. trochili [23]. Recent records of H. archilochus (Clade G, Figure 5) are for Colombia and the United States [47,48] associated with the lineages KY560444, and MW548594-SETGRA01 (Table S2). Cytb genetic distances between H. witti and H. archilochus were estimated as 0.073 (Table S4).
In addition, the Rufous-breasted Hermit was infected with P. nucleophilum (Ref. [49]—Figure 4G,H). Although scarce, trophozoites were seen in a subpolar position. Such structures had tiny pigment granules and two small vacuoles with even contours. Further stages were observed to adhere to the host cell nucleus growing longitudinally from subpolar positions. Pigment granules increased to four, and outlines became wavy. Scanty nearly mature meronts with up to eight merozoites and pigment granules were clumped in a big spot at the periphery of the meront. The outline was ameboid (Figure 4G). Gametocytes were slender and macrogametocytes with irregular cytoplasm were located lateral to the parasite nucleus to which they discontinuously adhere, as well as to the host cell membrane, possibly due to the scalloped edges of the parasite. The pigment granules were rounded to ovoid and numbered about 10. They were grouped towrds the center of the gametocyte (Figure 4H). In contrast, in microgametocytes the cytoplasm seemed more homogeneous, with wavy contours and pigment granules clumped in two or three spots of different sizes. All the development was observed in mature erythrocytes. The lineage obtained from this hummingbird (OP087644-DENPET03) was identical to that reported by Chagas [50] in the Egyptian Goose (Alopochen aegyptiaca—JX467689-DENPET03) and was placed in clade B (Figure 6) along with the Plasmodium parasites MK695452-AMABRA01 from Anseriformes (Genetic distance 0.006) and MN458617-COLTAL05) from Columbiformes (Genetic distance 0.01) (Table S4).
Leucocytozoon quynzae (Ref. [21], Figure 5P) was the only leucocytozoid detected before in the non-passerine birds deposited in the GERPH biological collection. However, in this study, in addition to the host species reported by Matta et al. [21], two more species were found infected: the Speckled Hummingbird (Adelomyia melanogenys) and the Tourmaline Sunangel (Heliangelus exortis). All infections were detected in localities that exceeded 2100 m in altitude (Table 1 and Table S2).
The new infections reported here were identical to the lineage KF479480-HEAME01 isolated from the type specimen of the species Amethyst-throated Sunangel (Heliangelus amethysticollis) [21]. This lineage was also reported in a White-tufted Sunbeam (Aglaeactis castelnaudii) from Peru, while the lineage KF309189-COHEL01 from L. quynzae was found in the White-bearded Hermit (Phaethornis hispidus) from Peru [13] (Clade C, Figure 8).
Leucocytozoon quynzae was placed basal to the parasites of non-passerine birds such as ducks, raptors, toucans, and woodpeckers in clades A and B (Figure 7). The median genetic distances between those clades were 0.061 (A vs. C) and 0.062 (B vs. C). Although Leucocytozoon polynuclearis was placed in clade A (Gen dist KF471028-HEAME01 vs. MW626894-COLAUR01: 0.06, Table S4), the closest species to L. quynzae was L. schoutedeni in clade D (gen dist 0.05) (Table S4). In the mtDNA phylogenetic tree (Figure 8), L. quynzae (from Heliangelus amethysticollis, KF479480-HEAME01) appeared as the sister taxon of Leucocytozoon sp. (from Turdus fuscater, KT162002-TFUS14); both shared a common ancestor with L. majoris (from Zonotrichia leucophrys oriantha, FJ168563-ZOLEU02). Genetic distances between these species are shown in Table S5.
3.4. Caprimulgiformes
Fourteen individuals of six species were evaluated for haemosporidian infections. The presence of the Haemoproteus sp. and Plasmodium sp.—registered here in the blood of the Lesser Nighthawk (Chordeiles acutipennis), the Pauraque (Nyctidromus albicollis), and the Blackish or Roraiman Nightjar (Nyctipolus nigrescens)—constituted the first report of infection caused by haemosporidians for each species. The Haemoproteids observed in these three species (Figure 3M,N) fit the description of Haemoproteus caprimulgi [51]. Indeed, the comparison of these specimens with the paratype of H. camprimulgi (IRCAH specimen G403126_9396, Figure 3K,L) from the Great-Eared Nightjar (Lyncornis macrotis) indicated that the macrogametocytes were slightly longer in the IRCAH specimen than in the specimens in the Colombian samples (media specimen IRCAH: 16.4 ± 2.01, media specimen N. nigrescens: 15.17 ± 1.42). For the reviewed specimens from the wild (Figure 3M,N), growing parasites often partially touch the host cell nucleus, but some spaces are left between those two membranes (Figure 3M macrogametocyte), particularly in gametocytes with slightly ameboid contours. Parasites in the IRCAH reference material (Figure 3K,L) showed even outlines in the different stages of the development of the parasite, while the specimens in C. acutipennis and N. albicollis sometimes showed ameboid outlines. Fully grown gametocytes were found in mature erythrocytes. Pigment granules are either scattered (see the macrogametocyte in Figure 3M) in the cytoplasm or grouped at the tips (Figure 3M microgametocytes), or different places of the parasite (Figure 3N). For the IRCAH material, small roundish white spots grouped at the tips of the parasites (Figure 3L) could correspond to what once were pigment granules.
Here, we reported for the first time the cytb and mtDNA genome sequences associated with H. caprimulgi. The cytb lineages obtained in this study for this morphospecies were placed in a well-supported clade along with the sequence CHOMIN01-MK216058 obtained from the Common or Antillean Nighthawk (Chordeiles minor) from the USA (clade B, Figure 5). Genetic distances between the last-mentioned sequence and the sequences obtained in this study ranged from 0.013 to 0.02 (Table S4). Interestingly, in the mtDNA genomic analysis (Figure 8), H. caprimulgi appeared to share a common ancestor with parasites found in other non-passerine birds (Anseriformes, Galliformes, Columbiformes, and Strigiformes). Indeed, all Haemoproteus (except H. witti) mtDNA genomes available from species found in non-Passeriformes are part of a monophyletic group with high posterior probability and bootstrap at the node (1.0/100). The genetic distance between these parasites is shown in Table S5.
Nyctidromus albicollis, additionally, was found infected with Plasmodium sp. Although the morphospecies of this infection could not be identified because of the low number of parasite stages in the peripheral blood (Figure 4I). Molecular tests indicated that the lineage OP087647-NYCTALB03 shared a common ancestor with the parasites of the clade G (Figure 6) that included the lineage OL598516 of P. elongatum, which differed by 0.037, and a lineage of Plasmodium sp. (AY733088) isolated from an African Penguin (Spheniscus demersus) separated by a genetic distance of 0.034. There is only one additional record of a Plasmodiidae infecting a Caprimulgiform species in Myanmar, the lineage COPMAL02 (EF380144) isolated from Caprimulgus macrurus (clade A, Figure 6) that differed from OP087647-NYCTALB03 by 0.067 (Table S4).
3.5. Charadriiformes
As for Charadriiformes, 228 individuals from six families and twenty-four species were screened for haemosporidian parasites. Only one was found infected. Haemoproteus contortus [52] was the only parasite detected in a Whimbrel (Numenius phaeopus), the same host species in which this parasite was described [52]. The cytoplasm was homogeneous and faintly stained, so the macrogametocytes were recognized by the more compact nucleus and a slightly more bluish tone of cytoplasm (Figure 3P). Growing and fully mature gametocytes showed highly ameboid outlines (Figure 3O,P) that makes the parasite touch the host cell nucleus at some points but leave the characteristic cleft between them (Figure 3P). We did not find ring-like, fully closed parasites as published in the original description, but all mature forms did completely encircle the host cell nucleus (Figure 3O). Unfortunately for this specimen (infected Whimbrel), there was no material available for molecular analysis.
Currently, there is only one lineage from a haemoproteid species isolated from N. phaeopus in Japan (NUMPHA01 GenBank Acc. num LC230122) that lies basal in clade E (Figure 5).
3.6. Columbiformes
One hundred and thirty Columbiformes representing twelve species were analyzed (Table 1), from which thirty-nine individuals from eleven species were infected with H. columbae [53], H. paramultipigmentatus [54] or Haemoproteus sp.
The Rock Pigeon (Columba livia), an introduced species in Colombia, showed a prevalence of 41% (16/39, Table 1) of H. columbae. Most of the specimens infected with H. columbae (Ref. [53] Figure 4A) had high parasitemia, with 8% as the maximum parasitemia recorded for a host in our biological collection [55]. All the sampled specimens had the lineage HAECOL01 (KY653761) that share a common ancestor with H. multipigmentatus [54] (clade J, Figure 5), differing with a genetic distance of 0.098 (Table S4).
Haemoproteus paramultipigmentatus (Ref. [54] Figure 4B–D), was detected in the Ruddy Ground Dove (Columbina talpacoti). Young or nearly mature stages predominate (Figure 4B) in the individuals infected with this species of parasite. Very early stages were seen in subpolar positions. The young parasites were located lateral to the host cell nucleus touching it in some portions (Figure 4B). Outlines were predominantly ameboid, and the cytoplasm was irregular with some small vacuoles visible. In nearly mature gametocytes, outlines were wavy. Few pigment granules were observed, particularly for microgametocytes that seem to aggregate in clumps throughout the cytoplasm or at the poles of the parasites (Figure 4C). The more mature parasites observed fill the erythrocyte to the poles, displacing the host cell nucleus considerably. However, gametocytes still showed clefts between the membranes of gametocytes and the host cell nucleus (Figure 4D), and the contours were still slightly wavy. However, from the early stages, macrogametocytes may show multiple pigment granules scattered throughout the cytoplasm that was a distinctive character of the species [54].
Four sequences were obtained from the Ruddy Ground Dove specimens sampled in Colombia (n = 13). Two of them were identical to the lineage obtained from the type specimen of H. paramultipigmentatus (JN788934-COLPAS03) in Mexico, while the other (OP087648-COPIC01) differed by a genetic distance of 0.002 (clade H, Figure 5; Table S4).
In addition, a Haemoproteus sp. was detected in a Claravis pretiosa (Blue Ground Dove) with low parasitemia. This lineage (OL689177-COSQU01) lay basal to H. paramultipigmentatus, along with JX029921-COSQU01, a Haemoproteus parasite that was found in the Scaled Dove (Columbina squamata) in Brazil, which was identical (clade I, Figure 5; Table S4). In addition, the mtDNA lineage obtained in this study (OP701685) was identified as COLBUC01 and appeared as a sister taxon of H. columbae from Columba livia (KY653761-COLIV07, Colombia). The genetic distance for all the subgenus Haemoproteus species are shown in Table S5.
A White-throated Quail-Dove (Zentrygon frenata) was found infected with Haemoproteus sp.; however, given the few stages observed and the small additional sample available, molecular analysis and further determination were not possible.
3.7. Galbuliformes
Only one specimen of the individuals of Galbulidae had a Haemosporidian infection (Table 1). A specimen of Rufous-tailed Jacamar (Galbula ruficauda) had an infection caused by Plasmodium tejerai [56]. Few morphological stages were found in the smear; however, the erythrocytic meront observed (Figure 4J) in a polar position of the erythrocyte was elongate with even outlines. Although appressed with the host nucleus and outer membrane, no displacement of it or perceptible deformation was evident. The large vacuole was not evident in this species, however. Such structures fade as meronts mature. In addition, pigment granules were clumped, as in P. tejerai, once the large vacuoles disappeared [2]. In addition, 10 merozoites were observed, fitting the description of P. tejerai, since this species of parasite usually showed 10 to 15 nuclei. Yet, the parasite cytoplasm was not basophilic as has been reported for the species. In any case, the lineage obtained (OL689171-SPMAG04) was identical to KJ575552 and MF953290 (SPMAG04) (clade E, Figure 6), and both were related to P. tejerai, detected in Spheniscus magellanicus [57,58] and Galbula ruficauda [59] from Brazil (Table S2). These lineages were placed in a well-supported clade (clade F, Figure 6) along with Plasmodium species found in Gruiformes in Colombia and Pelecaniformes n Guyana. Genetic distances between these taxa were 0.0021 in both cases (Table S4). In the mtDNA phylogenetic analysis, P. tejerai appeared as a sister taxon of Plasmodium sp. found in Opisthocomus hoazin from Venezuela (KY653749-OH2A; Ref. [60]); it was part of the monophyletic group that contains P. lutzi, P. elongatum, and Plasmodium sp. of a Trogon personatus from Colombia (KY653806-TROPER02). The genetic distances among these parasites can be found in Table S5.
In addition to the three species of Bucconidae deposited in the GERPH biological collection (Table 1), only one infection caused by a Haemoproteus parasite was found in a Black-fronted Nunbird (Monasa nigrifrons). The morphology of this parasite must be studied in more detail since it does not fit completely with the previously described species (Haemoproteus bucconis) for this family [2]. Yet, the lineage OP087643 was identical to the sequence MONNIG02-MG598390 found in Brazil in the same host species. Such lineages were placed as the sister group of H. syrnii (clade E, Figure 5). The genetic distance between the lineage CULKIB01 of H. syrnii and the lineage found in M. nigrifrons was 0.038 (Table S4).
3.8. Gruiformes
Thirty-six individuals from three families of Gruiformes were screened for haemosporidian parasites (Table 1). Two Plasmodium species were detected in the American Purple Gallinule (Porphyrio martinica). The first of the Plasmodium species was compatible with P. elongatum (Ref. [61] Figure 4K–N). Erythrocytic meronts were found in only one specimen and developed predominantly in mature erythrocytes containing six or nine merozoites arranged in a fan (Figure 4K) or a circumference (Figure 4L) with no displacement of the host cell nucleus. Gametocytes located in a lateral position had even (Figure 4M) or highly ameboid (Figure 4N) outlines; in contrast to those reported by [62], they were slightly separated from the envelope of the erythrocyte (Figure 4M). None or only a few medium-sized pigment granules were observed clumped in the parasite cytoplasm (Figure 4N). The amplified lineage OP087639-GRW06 was identical to OL598516, identified as P. elongatum and reported by Harl et al. [63]. It was closely related to the Plasmodium sp. lineage AY733088 from Sphenciformes, while differed from lineages of Passeriformes and Caprimulgiformes (clade G, Figure 6) by a genetic distance ranging between 0.0021 and 0.036 (Table S4).
The second species of Plasmodium sp. needs to be studied in more detail, since few diagnostic characters could be seen due to the low parasitaemia. The lineage (CYACYA02-OP087637) was placed in clade F (Figure 6), closely related to the lineage SPMAG04 (OP087638) P. tejerai found in Galbuliformes (genetic distance of 0.0021—Table S4).
3.9. Pelecaniformes
From the ten sampled species of Pelecaniformes, only the Striated Heron (Butorides striata) showed infection by a Plasmodium sp. species. However, it could not be identified due the low parasitemia (Figure 4O), where only a few immature parasite stages were found. Yet, the lineage OP087640-BUTSTR03 was placed in a well-supported clade, along with AB477128-CXPIP10 that was isolated from a Yellow Bittern (Ixobrychus sinensis)—a Pelecaniform species in Japan (clade C, Figure 6). Both lineages showed a genetic distance of 0.03 (Table S4). Another, the lineage of Plasmodium was isolated from B. striata in Guyana (DQ241528-BUTSTR01, clade F, Figure 6); however, it is distantly related to BUTSTR (OP087640), differing from it with a genetic distance of 0.052 (Table S4).
3.10. Strigiformes
Two of the three species of Strigiformes sampled were positive for haemosporidians (Table 1). A species closely similar to Haemoproteus syrnii, henceforth H syrnii-like, was detected in the Striped Owl (Asio clamator) and the Tropical Screech-Owl (Megascops choliba). Most of the parasites in Asio clamator (Figure 4E,F) were immature. Early stages were observed in lateral or polar position to the host cell nucleus. Young gametocytes were identified by the presence of a space between the host cell nucleus and the parasite membrane, and by the spaces at the host cell poles (Figure 4E). Even (Figure 4E) or slightly ameboid (Figure 4F) outlines were observed in equal proportions. Parasites caused a slight to moderate displacement of the host cell nucleus without encircling it completely (Figure 4F) as has been reported by [64]. The specimen in M. choliba was slightly different from that of Asio clamator, so it needs to be studied in more detail to confirm the parasite species’ identity.
Two closely related cytb lineages were obtained from the infected owls—OP087645-ASICLA010 from A. clamator and OP087641-PSDIS01 from Megascops choliba. (genetic distance 0.0084—Table S4)—the last one identical to the lineage KJ575554 identified as H. syrnii, isolated from Megascops choliba in Brazil [58].
The lineage of H. syrnii was located in two distantly related clades (clade E and clade F, Figure 5). The sequences OP087645-ASICLA01 and OP087641-PSDIS01 were placed in the well-supported clade F (Figure 6) along with the lineages KF279523-STAL2 (H. syrnii) and KF747371-STVAR01 (Haemoproteus sp.). Both infections were isolated from owls from Europe and North America. The genetic distances between the lineages in the Colombian hosts and the group comprising KF279523-STAL2 and KF747371-STVAR01 were 0.0127 and 0.0084 (Table S4). Meanwhile, the genetic distance with the lineage MK330144-CULKIB01 in clade E was 0.0322.
3.11. Trogoniformes
Only one out of the six Trogoniformes samples analyzed was found infected. The sample showed the two Plasmodium species (Table 1) already reported by González et al. [45]. A blood smear of a Masked Trogon (Trogon personatus) showed parasite stages of Plasmodium (Huffia) sp. along with a Plasmodium (Novyella) sp. which was found in coinfection. The sequences TROPER01 (KF537290) and TROPER02 (KY653806) obtained for this specimen have no other known host. Such sequences were placed in clade D (Figure 6; see also Figure 8 for comparison), along with the other lineages isolated from Peruvian T. personatus (MH457281-TROPER03, MH457292-TROPER04, and MN458765-TROPER05) that differed by genetic distances ranging from 0.0021 to 0.023. The most closely related species identified in the phylogenetic tree was P. matutinum in clade E (MK652235-LINN1), separated by genetic distances of 0.034 and 0.036 (Table S4).
4. Discussion
At a global scale, the diversity patterns of avian haemosporidian species seem similar to those of their hosts [9,65]. Thus, the Neotropics, as the world’s most diverse area for birds, is expected to harbor diverse haemosporidian parasite communities. However, at the regional level, biodiversity patterns are also driven by ecological and biogeographical contexts resulting from the local and regional evolutionary histories. Thus, as expected, the diversity of these hematozoa is unevenly distributed throughout the continent and along elevational gradients in mountainous areas [13,14,45,59]. In general, such patterns have been studied using data from passerine birds. Thus, due to the scarcity of information, estimates of diversity, distribution, and life cycles, as well as the characterization of prevalence and their relationship with life histories, environmental conditions, and specific traits, are unknown for the vast majority of parasites found in non-passerine birds (see [66]).
There are five major results from this study. (1) It added valuable information on the hosts, morphology, barcode sequences, and phylogenetic relationships of parasites infecting non-passerine birds of 11 bird orders and 41 species, of which 34 are endemic in the Neotropical region. In addition, we reported the barcode sequence for H. caprimulgi while reporting the host range expansion for P. elongatum, and P. nucleophilum, reported for the first time infecting Porphyrio martinica (Gruiformes) and Glaucis hirsutus. (2) From the 22 lineages isolated from samples deposited in the biological collection at GERPH, five are newly reported in this study. In this repository, we have identified thirteen morphological species, and four more need to be investigated in the future since they seem to constitute new species. (3) Furthermore, 14 of the 22 lineages amplified in this study were identical to 78 cytb sequences previously reported in GenBank (Table S6), which have been isolated from 274 host species around the globe. Due to the tremendous volume of information published today, compilations such as the one presented here greatly aid to avoid the duplication of information released in public databases. (4) Also, we are reporting for the first time five new parasite mtDNA genomes for the morphospecies P. tejerai, H. gabaldoni (n = 2), H. caprimulgi, and H. paramultipigmentatus and their phylogenetic relationships. (5) In this study, we present a summary of all the reports on non-passerine birds examined for haemosporidians over the last 40 years for the Neotropics (Tables S2 and S3).
Following the general tendencies observed in the last 40 years of research from Neotropical countries, Haemoproteus parasites were the most prevalent in the study. Indeed, these parasites were found in most of the biomes sampled, with Columbiformes being the most infected birds, followed by the Anseriformes and the Apodiformes (Trochilidade). This data should be interpreted with precaution since the Apodiformes and Columbiformes are the non-passerine birds most sampled using mist nets.
According to our results, the Columbiformes sampled in the Amazon-Orinoco peinobiome (VIII) were in the bird order where Haemoproteus is the most frequent parasite genus, similar to what has been reported by [59]. In that article, the prevalence in doves was high in Amazonia and in the tropical and subtropical grassland great biome located close to the area of the Amazon-Orinoco peinobiome [5,59,67]. Most of the species of Columbiformes exhibit a gregarious behavior that may facilitate the vector to easily locate and feed on different individuals of the group [55,68].
In the case of Trochilidae, our results indicate the highest frequency of infections by Haemoproteus in the high altitude orobiomes of the Andes (IV). Fecchio et al. [59] reported the Peruvian Andes as one of the biomes with the highest prevalence of these parasites in hummingbirds; McNew et al. [13] reported the highest prevalence at altitudes that fit with the high and middle altitude orobiomes of the Andes (IV and V respectively).
Meanwhile, Plasmodium was found in biomes under 2500 m asl. In all these places, plenty of breeding places for Culicidae vectors are available, from bromeliads in the middle altitude orobiomes of the Andes to large tracts of flooded land in the helobiomes of the Orinoco and the Amazon. Temperatures in these areas exceed 12 °C, which is suitable for Plasmodium development [69].
As for Leucocytozoon, our results contrast the recent findings where this parasite has been reported to infect resident birds under 2100 m asl in Brazil [70,71]. Gametocytes from in situ captured birds have not corroborated these infections, and they may correspond to non-patent chronic infections that were probably overlooked on blood smears, or they may correspond to abortive infections. However, the fact that those reports are in territorial species suggests the presence of a competent vector that is maintaining the infection from either migratory birds or resident chronically infected hosts in such places, defying the proposed theory of the existence of an altitudinal gradient of distribution of these parasites [21,27,72].
Current research of haemosporidian diversity, particularly in non-passerine birds, faces three methodological challenges to be resolved. First, mist nets of medium-size mesh reaching less than three meters of height above the ground (i.e., ref. [73,74]) are the usual method for host sampling [3]. Such methodology biases the capture towards small non-passerine birds such as Columbiformes and Trochilidae. There is a paucity of studies using alternative methods like bow-nets [75] rocket nets, and swim-in bait traps [76] that are used by hand at nests during the breeding season [3,77], as opposed to samples obtained from animals collected by guns [78] and collisions with different objects [79]. They may bias the information obtained towards the orders under investigation that can be collected using these methods (i.e., raptors and ducks). The joint work with animal protection entities such as rescue centers represents an alternative for obtaining samples of rare specimens that are not regularly captured in mist nets, or for which there is some restriction on sampling. Valuable information has been obtained from these specimens in different recent studies (i.e., ref. [21,61,62]). However, parasite transmission can occur at these facilities, and the captive birds can become “accidental hosts” of parasites with which they are not infected in their natural environment [80,81,82,83]. So, it is essential to settle protocols of sampling that lead us to detect the infections truly acquired in the wild.
Second, the low parasitemias common in birds with chronic infections represent a challenge for estimating the prevalence and diversity of haemosporidians in wild birds using morphology. Although the association of parasite lineages with a morphological parasite species was the main aim of this investigation, particularly for this study, such values were underestimated since the molecular determination was made only on samples whose smear was positive. As PCR methods are more sensitive than microscopy [25,84], they can detect infections that are non-patent and might be part of the transmission cycles [25]. This is common in other vertebrates, such as humans, where asymptomatic patients with submicroscopic infections can infect malaria vectors [85]. However, detection based only on PCR may compromise the reliability of the host–parasite relationships since such methodologies can detect the parasite DNA at any development stage, even if the life cycle was interrupted before the parasite reaches the gametocyte stage [86]. Despite the fact that molecular methods to detect specific Haemosporidian developmental stages such as gametocytes are more reliable than microscopy [87], those are not available in avian Haemosporida due to limited genomic information.
Third, according to recent molecular characterizations, the haemosporidian parasites infecting non-passerine birds are highly diverse [22,64,68,88,89].The Neotropics may harbor a great diversity of haemosporidian lineages that might be overlooked due to the mis-priming of the oligonucleotides routinely used for the detection of these parasites [63,90,91]. Indeed, in this study we failed to amplify the Haemoproteus parasites of raptors, probably due to a mismatch between the primers and the cytb sequence of the parasites. To overcome this issue different primer sets have been developed aiming to characterize the genetic diversity of particular areas and hosts [31,63,91,92].
Biological collections constitute large information banks on the specimens deposited in these repositories [93]. In this sense, the specimens stored in collections such as the “GERPH collection” have allowed for the obtaining of informative molecular data linked to morphospecies. Furthermore, multiple parasite mtDNA genomes from morphospecies found in non-passerine and passerine birds deposited in biological collections have yielded phylogenies with a better resolution (Figure 8). Although mtDNA phylogenetic hypothesis lacks sequences of species that are available only for cytb fragments, which may affect branch length in some of the taxa, the main topologies are similar to those obtained with 478 bp. lineages (for example, the clade of Haemoproteus (Haemoproteus) parasites and their position regarding the Haemoporteus (Parahaemoproteus) species or lineages included in both analyses). Thus, future directions of research with this repository will encompass the molecular screening of all samples and will increase the efforts to get more mitochondrial genomes. These perspectives will provide new insights into diversity and more robust phylogenetic hypotheses of haemosporidian parasites [33,94].
For the Neotropics, 1779 species of non-passerine birds have been reported [95]. To date, about 30% of these species have been explored for haemosporidian infections. In the case of Colombia, 195 of a total of 878 non-passerine bird species have been tested for avian malaria and related parasites. Many host taxa have been tested with only a handful of individuals that do not represent the species’ geographic range nor allow for the estimation of the prevalence of haemosporida parasites. This number of taxa is equivalent to only 22% of the biodiversity of non-passerine hosts in the country. Although significant advances have been made in the last twenty years, there are still nine bird orders under-sampled. Then, for a more precise understanding of parasite diversity and dynamics in Colombia and the Neotropics, deeper sampling of the great host biodiversity reported for this area will be needed.
We confirmed infections using morphology that were previously reported using only PCR in other neotropical countries. So, the use of collections allows the implementation of integrative approaches prevailing in modern taxonomy. Although limited in number of sampled individuals per host species, our findings exemplify the importance of the information deposited in repositories and collections as data sources to support the study of the geographic distribution of host-parasite diversity associations. Indeed, surveys directed to inform biodiversity assessments of vertebrates can better provide insights into regional processes by considering parasites and other symbionts in order to document host-symbiont species assemblages. Biological collections where specimens are curated with their associated metadata are indispensable for incorporating neglected symbiont taxa such as haemosporidian parasites into biodiversity science.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d15010057/s1: Table S1: Biomes and specific localities of origin of the non-passerine bird samples deposited in the GERPH biological collection included in this study. Table S2: Non-passerine species found infected with Haemosporidian parasites in the Neotropics after the comprehensive review of White et al. [17]. Table S3: Non-passerine species explored and found negative for Haemosporidian parasites in the Neotropics after the comprehensive review of White et al. (1978). Table S4: Kimura-two-parameters pairwise genetic distances between lineages in Figure 5, Figure 6 and Figure 7. Table S5: Estimates of evolutionary divergence between haemosporidian parasites’ mtDNA genome sequences (5234 bp excluding gaps). Table S6: Lineages identified in this study as identical with others previously reported. Table S7: Database of papers reviewed for data compilation of Haemosporidian parasite infections in neotropical birds after 1978. References [96,97,98,99,100,101,102,103,104,105,106,107,108] are cited in Supplementary Materials.
Author Contributions
Conceptualization, I.A.L.-A., A.D.G., M.A.P., A.A.E. and N.E.M.; methodology, I.A.L.-A., A.D.G., M.A.P. and N.E.M.; formal analysis, I.A.L.-A., A.D.G., M.A.P. and N.E.M.; investigation, I.A.L.-A., M.A.P., A.C., B.A.G.-S., A.D.G. and N.E.M.; resources, N.E.M., A.D.G., I.A.L.-A., B.A.G.-S., C.M., O.R.-F. and A.C.; data curation, I.A.L.-A., A.D.G., N.E.M. and M.A.P.; writing—original draft preparation, I.A.L.-A., M.A.P. and N.E.M.; writing—review and editing, A.D.G., M.A.P., A.A.E., B.A.G.-S., C.M., O.R.-F., A.C. and N.E.M.; visualization, I.A.L.-A. and M.A.P.; funding acquisition, N.E.M., A.D.G., O.R.-F., C.M. and I.A.L.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This compilation was supported by Minciencias contract No 572-2020.
Institutional Review Board Statement
All the assays described in this study were performed using blood samples and smears deposited and stored over the years in the “Grupo de Estudio Relación Parásito Hospedero—GERPH” biological collection. The methods for sample collection used by the GERPH research group were approved by the bioethics committee of the Department of Animal Health Sciences of the Faculty of Veterinary Medicine of the National University of Colombia (Permit number CBE-FMVZ-016), the Bioethics Committee of the Sciences Faculty of the National University of Colombia (Act number: 04 of 2017 and 03 of 2018), and the institutional bioethics committee of the UNITROPICO University (Permit number 431-CI-2019-121).
Informed Consent Statement
Not applicable.
Data Availability Statement
The sequence data produced in this study are available in the GenBank database under accession numbers OL689174, OL689176, OL689177, OP087637-OP087649, and OP701681-OP701685. Also, this lineage information was submitted to the MalAvi database.
Acknowledgments
The authors would like to thank the past and present members of GERPH for their collaboration in the field and laboratory. Through their efforts, the biological collection of the Host Parasite Relationship Study Group (GERPH) has been maintained. Special thanks to Gustavo Fuentes, Natalia Basto, David Pinto and Jhon Macías. Also, we would like to thank the URRAS staff, especially Miguel Nova, Juan Guerrero, Libny Bolaños and Kelly Diaz, WCS and Ocarros Biopark. A special thanks to Gypsy G. Lotta of the Galactic Monkey Collective for illustration of the birds used in all the figures in this article, and Thomas Defler for language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Levine, N. The Protozoan Phylum Apicomplexa; CRC Press: Boca Ratón, FL, USA, 1988. [Google Scholar] [CrossRef]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Ratón, FL, USA, 2005; ISBN 0-203-64379-8. [Google Scholar] [CrossRef]
- Svobodová, M.; Weidinger, K.; Peške, L.; Volf, P.; Votýpka, J.; Voříšek, P. Trypanosomes and Haemosporidia in the Buzzard (Buteo buteo) and Sparrowhawk (Accipiter nisus): Factors Affecting the Prevalence of Parasites. Parasitol. Res. 2015, 114, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Zizka, A.; Carvalho, F.A.; Scharn, R.; Bacon, C.D.; Silvestro, D.; Condamine, F.L. Amazonia Is the Primary Source of Neotropical Biodiversity. Proc. Natl. Acad. Sci. USA 2018, 115, 6034–6039. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C. Terrestrial Ecoregions of the World: A New Map of Life on EarthA New Global Map of Terrestrial Ecoregions Provides an Innovative Tool for Conserving Biodiversity. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E. The Global 200: Priority Ecoregions for Global Conservation. Ann. Mo. Bot. Gard. 2002, 89, 199–224. [Google Scholar] [CrossRef]
- Birds of the World—Comprehensive Life Histories for All Bird Species and Families. Available online: https://birdsoftheworld.org/bow/home (accessed on 27 October 2022).
- Develey, P.F. Bird Conservation in Brazil: Challenges and Practical Solutions for a Key Megadiverse Country. Perspect. Ecol. Conserv. 2021, 19, 171–178. [Google Scholar] [CrossRef]
- Clark, N.J.; Clegg, S.M.; Lima, M.R. A Review of Global Diversity in Avian Haemosporidians (Plasmodium and Haemoproteus: Haemosporida): New Insights from Molecular Data. Int. J. Parasitol. 2014, 44, 329–338. [Google Scholar] [CrossRef]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A Public Database of Malaria Parasites and Related Haemosporidians in Avian Hosts Based on Mitochondrial Cytochrome b Lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef]
- Alvarez-Londoño, J.; Cardona-Romero, M.; Martínez-Sánchez, E.T.; Ossa-López, P.A.; Pérez-Cárdenas, J.E.; Gonzalez, A.D.; Rivera-Páez, F.A.; Castaño-Villa, G.J. Avian Haemosporidian (Haemosporida: Plasmodium and Haemoproteus) in the Department of Arauca, Colombian Orinoquia Region. Parasitol. Res. 2022, 121, 1775–1787. [Google Scholar] [CrossRef]
- Anjos, C.C.; Chagas, C.R.; Fecchio, A.; Schunck, F.; Costa-Nascimento, M.J.; Monteiro, E.F.; Mathias, B.S.; Bell, J.A.; Guimarães, L.O.; Comiche, K.J. Avian Malaria and Related Parasites from Resident and Migratory Birds in the Brazilian Atlantic Forest, with Description of a New Haemoproteus Species. Pathogens 2021, 10, 103. [Google Scholar] [CrossRef]
- McNew, S.M.; Barrow, L.N.; Williamson, J.L.; Galen, S.C.; Skeen, H.R.; DuBay, S.G.; Gaffney, A.M.; Johnson, A.B.; Bautista, E.; Ordoñez, P. Contrasting Drivers of Diversity in Hosts and Parasites across the Tropical Andes. Proc. Natl. Acad. Sci. USA 2021, 118, e2010714118. [Google Scholar] [CrossRef]
- González Quevedo, C.; Pabón Vidal, A.L.; Rivera Gutiérrez, H.F. Prevalence of Haemosporidians in a Neotropical Endemic Bird Area. Avian Conserv. Ecol. 2016, 11, 7. [Google Scholar] [CrossRef]
- Aragão, H.D.B. Sobre o Cyclo Evolutivo Ea Transmissão Do Haemoproteus columbae. Rev. Médica De São Paulo 1908, 11, 409–416. [Google Scholar]
- Santiago-Alarcon, D.; Marzal, A. Research on Avian Haemosporidian Parasites in the Tropics before the Year 2000. In Avian Malaria and Related Parasites in the Tropics; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–44. [Google Scholar]
- White, E.M.; Greiner, E.C.; Bennett, G.F.; Herman, C.M. Distribution of the Hematozoa of Neotropical Birds. Rev. De Biol. Trop. 1978, 26, 43–102. [Google Scholar]
- Gabaldon, A.; Ulloa, G.; Zerpa, N. Fallisia (Plasmodioides) neotropicalis Subgen. Nov. Sp. Nov. from Venezuela. Parasitology 1985, 90, 217–225. [Google Scholar] [CrossRef]
- Bennett, G.F. Haemoproteus gabaldoni n. Sp.(Apicomplexa: Haemoproteidae) from the Muscovy Duck Cairina Moschata (Aves: Anatidae). Syst. Parasitol. 1993, 25, 119–123. [Google Scholar] [CrossRef]
- Matta, N.E.; Pacheco, M.A.; Escalante, A.A.; Valkiūnas, G.; Ayerbe-Quiñones, F.; Acevedo-Cendales, L.D. Description and Molecular Characterization of Haemoproteus macrovacuolatus n. Sp. (Haemosporida, Haemoproteidae), a Morphologically Unique Blood Parasite of Black-Bellied Whistling Duck (Dendrocygna autumnalis) from South America. Parasitol. Res. 2014, 11, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Matta, N.E.; Lotta, I.A.; Valkiūnas, G.; González, A.D.; Pacheco, M.A.; Escalante, A.A.; Moncada, L.I.; Rodríguez-Fandiño, O.A. Description of Leucocytozoon quynzae Sp. Nov.(Haemosporida, Leucocytozoidae) from Hummingbirds, with Remarks on Distribution and Possible Vectors of Leucocytozoids in South America. Parasitol. Res. 2014, 113, 457–468. [Google Scholar] [CrossRef]
- Vanstreels, R.E.T.; Dos Anjos, C.C.; Leandro, H.J.; de Moraes Carvalho, A.; Santos, A.P.; Egert, L.; Hurtado, R.; de Carvalho, E.C.Q.; Braga, É.M.; Kirchgatter, K. A New Haemosporidian Parasite from the Red-Legged Seriema cariama cristata (Cariamiformes, Cariamidae). Int. J. Parasitol. Parasites Wildl. 2022, 18, 12–19. [Google Scholar] [CrossRef]
- White, E.M.; Bennett, G.F.; Williams, N.A. Avian Haemoproteidae. 11. The Haemoproteids of the Hummingbird Family Trochilidae. Can. J. Zool. 1979, 57, 908–913. [Google Scholar] [CrossRef]
- Durrant, K.L.; Beadell, J.S.; Ishtiaq, F.; Graves, G.R.; Olson, S.L.; Gering, E.; Peirce, M.A.; Milensky, C.M.; Schmidt, B.K.; Gebhard, C. Avian Hematozoa in South America: A Comparison of Temperate and Tropical Zones. Ornithol. Monogr. 2006, 60, 98–111. [Google Scholar] [CrossRef]
- Pacheco, M.A.; Ferreira, F.C.; Logan, C.J.; McCune, K.B.; MacPherson, M.P.; Albino Miranda, S.; Santiago-Alarcon, D.; Escalante, A.A. Great-Tailed Grackles (Quiscalus mexicanus) as a Tolerant Host of Avian Malaria Parasites. PloS ONE 2022, 17, e0268161. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, R.J.; Sedano, R.; Chasar, A.C.; Chaves, J.A.; Nguyen, J.T.; Whitaker, A.; Smith, T.B. New Host and Lineage Diversity of Avian Haemosporidia in the Northern Andes. Evol. Appl. 2014, 7, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Lotta, I.A.; Pacheco, M.A.; Escalante, A.A.; González, A.D.; Mantilla, J.S.; Moncada, L.I.; Adler, P.H.; Matta, N.E. Leucocytozoon Diversity and Possible Vectors in the Neotropical Highlands of Colombia. Protist 2016, 167, 185–204. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T.A. Keys to the Avian Malaria Parasites. Malar. J. 2018, 17, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; Volume 2. [Google Scholar]
- Hellgren, O.; Waldenström, J.; Bensch, S. A New PCR Assay for Simultaneous Studies of Leucocytozoon, Plasmodium, and Haemoproteus from Avian Blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.A.; Cepeda, A.S.; Bernotienė, R.; Lotta, I.A.; Matta, N.E.; Valkiūnas, G.; Escalante, A.A. Primers Targeting Mitochondrial Genes of Avian Haemosporidians: PCR Detection and Differential DNA Amplification of Parasites Belonging to Different Genera. Int. J. Parasitol. 2018, 48, 657–670. [Google Scholar] [CrossRef]
- Bensch, S.; Stjernman, M.; Hasselquist, D.; Örjan, Ö.; Hannson, B.; Westerdahl, H.; Pinheiro, R.T. Host Specificity in Avian Blood Parasites: A Study of Plasmodium and Haemoproteus Mitochondrial DNA Amplified from Birds. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2000, 267, 1583–1589. [Google Scholar] [CrossRef]
- Pacheco, M.A.; Matta, N.E.; Valkiūnas, G.; Parker, P.G.; Mello, B.; Stanley Jr, C.E.; Lentino, M.; Garcia-Amado, M.A.; Cranfield, M.; Kosakovsky Pond, S.L. Mode and Rate of Evolution of Haemosporidian Mitochondrial Genomes: Timing the Radiation of Avian Parasites. Mol. Biol. Evol. 2018, 35, 383–403. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A Fast Online Phylogenetic Tool for Maximum Likelihood Analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView Version 4: A Multiplatform Graphical User Interface for Sequence Alignment and Phylogenetic Tree Building. Mol. Biol. Evol. 2009, 27, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Peirce, M.; Marquiss, M. Haematozoa of British Birds. VII. Haematozoa of Raptors in Scotland with a Description of Haemoproteus nisi Sp. Nov. from the Sparrowhawk (Accipiter nisus). J. Nat. Hist. 1983, 17, 813–821. [Google Scholar] [CrossRef]
- González, A.D.; Lotta-Arevalo, I.; Fuentes, G.A.; Macías-Zacipa, J.; Acevedo-Cendales, L.D.; Matta, N.E. Haemoproteus gabaldoni a Valid Species? An Approach from Morphology and Molecular Tools Applied to Parasites of Anseriformes. Acta Trop. 2022, 233, 106540. [Google Scholar] [CrossRef]
- Cleland, S.J.B.; Johnston, T.H. Descriptions of New Haemoprotozoa from Birds in New South Wales, with a Note on the Resemblance between the Spermatozoa of Certain Honeyeaters (Fam. Meliphagidae) and Spirochaete-Trypanosomes; Royal Society of New South Wales: Sydney, Australia, 1909. [Google Scholar]
- Pacheco, M.A.; Escalante, A.A.; Garner, M.M.; Bradley, G.A.; Aguilar, R.F. Haemosporidian Infection in Captive Masked Bobwhite Quail (Colinus virginianus ridgwayi), an Endangered Subspecies of the Northern Bobwhite Quail. Vet. Parasitol. 2011, 182, 113–120. [Google Scholar] [CrossRef][Green Version]
- Rodríguez, O.A.; Matta, N.E. Blood Parasites in Some Birds from Eastern Plains of Colombia. Mem. Do Inst. Oswaldo Cruz 2001, 96, 1173–1176. [Google Scholar] [CrossRef]
- González, A.D.; Lotta, I.A.; García, L.F.; Moncada, L.I.; Matta, N.E. Avian Haemosporidians from Neotropical Highlands: Evidence from Morphological and Molecular Data. Parasitol. Int. 2015, 64, 48–59. [Google Scholar] [CrossRef]
- Galen, S.C.; Witt, C.C. Diverse Avian Malaria and Other Haemosporidian Parasites in Andean House Wrens: Evidence for Regional Co-diversification by Host-switching. J. Avian Biol. 2014, 45, 374–386. [Google Scholar] [CrossRef]
- Bradshaw, A.; Tell, L.A.; Ernest, H.B.; Bahan, S.; Carlson, J.; Sehgal, R. Detection and Prevalence of Haemoproteus archilochus (Haemosporida, Haemoproteidae) in Two Species of California Hummingbirds. Parasitol. Res. 2017, 116, 1879–1885. [Google Scholar] [CrossRef]
- Duarte-Moreno, A.N.; Villamizar-Escalante, D.; Rondón-González, F. PCR Detection of Haemoproteus archilochus in Rufous-Tailed Hummingbird Amazilia tzacatl (Trochilidae) in Colombia. Acta Biológica Colomb. 2022, 27, 140–143. [Google Scholar] [CrossRef]
- Manwell, R.D. How Many Species of Avian Malaria Parasites Are There? Am. J. Trop. Med. 1935, 15, 265–282. [Google Scholar] [CrossRef]
- Chagas, C.R.F.; Valkiūnas, G.; Nery, C.V.C.; Henrique, P.C.; Gonzalez, I.H.L.; Monteiro, E.F.; de Oliveira Guimarães, L.; Romano, C.M.; Kirchgatter, K. Plasmodium (Novyella) nucleophilum from an Egyptian Goose in São Paulo Zoo, Brazil: Microscopic Confirmation and Molecular Characterization. Int. J. Parasitol. Parasites Wildl. 2013, 2, 286–291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Williams, N.A.; Bennett, G.F.; Mahrt, J. Avian Haemoproteidae. 6. Description of Haemoproteus caprimulgi Sp. Nov., and a Review of the Haemoproteids of the Family Caprimulgidae. Can. J. Zool. 1975, 53, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.F. Avian Haemoproteidae. 10. The Haemoproteids of the Avian Family Scolopacidae. Can. J. Zool. 1979, 57, 901–907. [Google Scholar] [CrossRef]
- Kruse, W. Ueber Blutparasiten. Arch. Für Pathol. Anat. Und Physiol. Und Für Klin. Med. 1890, 120, 541–560. [Google Scholar]
- Valkiūnas, G.; Iezhova, T.A.; Evans, E.; Carlson, J.S.; Martínez-Gómez, J.E.; Sehgal, R.N. Two New Haemoproteus Species (Haemosporida: Haemoproteidae) from Columbiform Birds. J. Parasitol. 2013, 99, 513–521. [Google Scholar] [CrossRef]
- Cepeda, A.S.; Lotta-Arévalo, I.A.; Pinto-Osorio, D.F.; Macías-Zacipa, J.; Valkiūnas, G.; Barato, P.; Matta, N.E. The Experimental Characterization of Complete Life Cycle of Haemoproteus columbae, with Description of Natural Host-Parasite System to Study This Infection. Int. J. Parasitol. 2019, 49, 975–984. [Google Scholar] [CrossRef]
- Gabaldon, A.; Ulloa, G. Plasmodium (Haemamoeba) tejerai Sp. n. of the Domestic Turkey (Meleagris gallopavo) of Venezuela. Boletín De La Dir. De Malariol. Y Saneam. Ambient. 1977, 17, 255–273. [Google Scholar]
- Silveira, P.; Belo, N.O.; Lacorte, G.A.; Kolesnikovas, C.K.; Vanstreels, R.E.; Steindel, M.; Catão-Dias, J.L.; Valkiūnas, G.; Braga, E.M. Parasitological and New Molecular-Phylogenetic Characterization of the Malaria Parasite Plasmodium tejerai in South American Penguins. Parasitol. Int. 2013, 62, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Vanstreels, R.E.T.; Kolesnikovas, C.K.; Sandri, S.; Silveira, P.; Belo, N.O.; Ferreira Junior, F.C.; Epiphanio, S.; Steindel, M.; Braga, E.M.; Catao-Dias, J.L. Outbreak of Avian Malaria Associated to Multiple Species of Plasmodium in Magellanic Penguins Undergoing Rehabilitation in Southern Brazil. PLoS ONE 2014, 9, e94994. [Google Scholar] [CrossRef]
- Fecchio, A.; Bell, J.A.; Pinheiro, R.B.; Cueto, V.R.; Gorosito, C.A.; Lutz, H.L.; Gaiotti, M.G.; Paiva, L.V.; França, L.F.; Toledo-Lima, G. Avian Host Composition, Local Speciation and Dispersal Drive the Regional Assembly of Avian Malaria Parasites in South American Birds. Mol. Ecol. 2019, 28, 2681–2693. [Google Scholar] [CrossRef]
- Pacheco, M.A.; García-Amado, M.A.; Manzano, J.; Matta, N.E.; Escalante, A.A. Blood Parasites Infecting the Hoatzin (Opisthocomus hoazin), a Unique Neotropical Folivorous Bird. PeerJ 2019, 7, e6361. [Google Scholar] [CrossRef] [PubMed]
- Huff, C.G. Plasmodium elongatum n. Sp., an Avian Malarial Organism with an Elongate Gametocyte. Am. J. Hyg. 1930, 11, 385–391. [Google Scholar] [CrossRef]
- Palinauskas, V.; Žiegytė, R.; Iezhova, T.A.; Ilgūnas, M.; Bernotienė, R.; Valkiūnas, G. Description, Molecular Characterisation, Diagnostics and Life Cycle of Plasmodium elongatum (Lineage PERIRUB01), the Virulent Avian Malaria Parasite. Int. J. Parasitol. 2016, 46, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Harl, J.; Himmel, T.; Valkiūnas, G.; Ilgūnas, M.; Nedorost, N.; Matt, J.; Kübber-Heiss, A.; Alic, A.; Konicek, C.; Weissenböck, H. Avian Haemosporidian Parasites of Accipitriform Raptors. Malar. J. 2022, 21, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Barino, G.T.M.; Rossi, M.F.; de Oliveira, L.; Reis Junior, J.L.; D’Agosto, M.; Dias, R.J.P. Haemoproteus syrnii (Haemosporida: Haemoproteidae) in Owls from Brazil: Morphological and Molecular Characterization, Potential Cryptic Species, and Exo-Erythrocytic Stages. Parasitol. Res. 2021, 120, 243–255. [Google Scholar] [CrossRef]
- DeBrock, S.; Cohen, E.; Balasubramanian, S.; Marra, P.P.; Hamer, S.A. Characterization of the Plasmodium and Haemoproteus Parasite Community in Temperate-Tropical Birds during Spring Migration. Int. J. Parasitol. Parasites Wildl. 2021, 15, 12–21. [Google Scholar] [CrossRef]
- Ellis, V.A.; Fecchio, A.; Ricklefs, R.E. Haemosporidian Parasites of Neotropical Birds: Causes and Consequences of Infection. Auk 2020, 137, ukaa055. [Google Scholar] [CrossRef]
- Bernal, R.; Gradstein, S.; Celis, M. Catalogue of Plants and Lichens of Colombia; JRS Biodiversity Foundation: Arlington, VA, USA, 2015. [Google Scholar]
- Matoso, R.V.; Cedrola, F.; Barino, G.T.M.; Dias, R.J.P.; Rossi, M.F.; D’Agosto, M. New Morphological and Molecular Data for Haemoproteus (H.) paramultipigmentatus in the Atlantic Forest of Brazil. Parasitol. Int. 2021, 84, 102375. [Google Scholar] [CrossRef] [PubMed]
- LaPointe, D.A.; Goff, M.L.; Atkinson, C.T. Thermal Constraints to the Sporogonic Development and Altitudinal Distribution of Avian Malaria Plasmodium relictum in Hawai’i. J. Parasitol. 2010, 96, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.M.; Ferreira, F.C.; Araújo, A.C.; Hirano, L.Q.L.; Paludo, G.R.; Braga, É.M. Molecular Detection of Leucocytozoon in Red-Legged Seriemas (Cariama cristata), a Non-Migratory Bird Species in the Brazilian Cerrado. Vet. Parasitol. Reg. Stud. Rep. 2022, 31, 100652. [Google Scholar] [CrossRef] [PubMed]
- Fecchio, A.; Silveira, P.; Weckstein, J.; Dispoto, J.; Anciães, M.; Bosholn, M.; Tkach, V.; Bell, J. First Record of Leucocytozoon (Haemosporida: Leucocytozoidae) in Amazonia: Evidence for Rarity in Neotropical Lowlands or Lack of Sampling for This Parasite Genus? J. Parasitol. 2018, 104, 168–172. [Google Scholar] [CrossRef]
- Rodríguez, O.A.; Moya, H.; Matta, N.E. Avian Blood Parasites in the National Natural Park Chingaza: High Andes of Colombia. Hornero 2009, 24, 1–6. [Google Scholar]
- Fecchio, A.; Ellis, V.A.; Bell, J.A.; Andretti, C.B.; D’horta, F.M.; Silva, A.M.; Tkach, V.V.; Weckstein, J.D. Avian Malaria, Ecological Host Traits and Mosquito Abundance in Southeastern Amazonia. Parasitology 2017, 144, 1117–1132. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Swanson, B.L.; Fallon, S.M.; MartÍnez-AbraÍn, A.; Scheuerlein, A.; Gray, J.; Latta, S.C. Community Relationships of Avian Malaria Parasites in Southern Missouri. Ecol. Monogr. 2005, 75, 543–559. [Google Scholar] [CrossRef]
- Smith, R.B.; Greiner, E.C.; Wolf, B.O. Migratory Movements of Sharp-Shinned Hawks (Accipiter striatus) Captured in New Mexico in Relation to Prevalence, Intensity, and Biogeography of Avian Hematozoa. Auk 2004, 121, 837–846. [Google Scholar] [CrossRef]
- Ramey, A.M.; Reed, J.A.; Walther, P.; Link, P.; Schmutz, J.A.; Douglas, D.C.; Stallknecht, D.E.; Soos, C. Evidence for the Exchange of Blood Parasites between North America and the Neotropics in Blue-Winged Teal (Anas discors). Parasitol. Res. 2016, 115, 3923–3939. [Google Scholar] [CrossRef]
- Boal, C.W.; Hudelson, K.S.; Mannan, R.W.; Estabrook, T.S. Hematology and Hematozoa of Adult and Nestling Cooper’s Hawks in Arizona. J. Raptor Res. 1998, 32, 281–285. [Google Scholar]
- Reeves, A.B.; Smith, M.M.; Meixell, B.W.; Fleskes, J.P.; Ramey, A.M. Genetic Diversity and Host Specificity Varies across Three Genera of Blood Parasites in Ducks of the Pacific Americas Flyway. PLoS ONE 2015, 10, e0116661. [Google Scholar] [CrossRef] [PubMed]
- Pigeault, R.; Vézilier, J.; Cornet, S.; Zélé, F.; Nicot, A.; Perret, P.; Gandon, S.; Rivero, A. Avian Malaria: A New Lease of Life for an Old Experimental Model to Study the Evolutionary Ecology of Plasmodium. Philos. Trans. R. Soc. B 2015, 370, 20140300. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C.R.F.; Valkiūnas, G.; de Oliveira Guimarães, L.; Monteiro, E.F.; Guida, F.J.V.; Simões, R.F.; Rodrigues, P.T.; de Albuquerque Luna, E.J.; Kirchgatter, K. Diversity and Distribution of Avian Malaria and Related Haemosporidian Parasites in Captive Birds from a Brazilian Megalopolis. Malar. J. 2017, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cocumelli, C.; Iurescia, M.; Diaconu, E.L.; Galietta, V.; Raso, C.; Buccella, C.; Stravino, F.; Grande, F.; Fiorucci, L.; De Liberato, C. Plasmodium matutinum Causing Avian Malaria in Lovebirds (Agapornis roseicollis) Hosted in an Italian Zoo. Microorganisms 2021, 9, 1356. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Huang, X.; Valkiūnas, G.; Yang, M.; Zheng, C.; Pu, T.; Zhang, Y.; Dong, L.; Suo, X.; Zhang, C. Malaria Parasites and Related Haemosporidians Cause Mortality in Cranes: A Study on the Parasites Diversity, Prevalence and Distribution in Beijing Zoo. Malar. J. 2018, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vanstreels, R.E.T.; de Angeli Dutra, D.; Ferreira-Junior, F.C.; Hurtado, R.; Egert, L.; Mayorga, L.F.S.; Bhering, R.C.; Braga, É.M.; Catão-Dias, J.L. Epidemiology, Hematology, and Unusual Morphological Characteristics of Plasmodium during an Avian Malaria Outbreak in Penguins in Brazil. Parasitol. Res. 2019, 118, 3497–3508. [Google Scholar] [CrossRef]
- Ishtiaq, F.; Rao, M.; Huang, X.; Bensch, S. Estimating Prevalence of Avian Haemosporidians in Natural Populations: A Comparative Study on Screening Protocols. Parasites Vectors 2017, 10, 127. [Google Scholar] [CrossRef]
- Almeida, G.G.; Costa, P.A.C.; da Silva Araujo, M.; Gomes, G.R.; Carvalho, A.F.; Figueiredo, M.M.; Pereira, D.B.; Tada, M.S.; Medeiros, J.F.; da Silva Soares, I. Asymptomatic Plasmodium vivax Malaria in the Brazilian Amazon: Submicroscopic Parasitemic Blood Infects Nyssorhynchus darlingi. PLoS Negl. Trop. Dis. 2021, 15, e0009077. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T.A.; Loiseau, C.; Sehgal, R.N. Nested Cytochrome b Polymerase Chain Reaction Diagnostics Detect Sporozoites of Hemosporidian Parasites in Peripheral Blood of Naturally Infected Birds. J. Parasitol. 2009, 95, 1512–1515. [Google Scholar] [CrossRef]
- Gadalla, A.A.; Siciliano, G.; Farid, R.; Alano, P.; Ranford-Cartwright, L.; McCarthy, J.S.; Thompson, J.; Babiker, H.A. Real-Time PCR Assays for Detection and Quantification of Early P. falciparum Gametocyte Stages. Sci. Rep. 2021, 11, 19118. [Google Scholar] [CrossRef]
- Bertram, M.R.; Hamer, S.A.; Hartup, B.K.; Snowden, K.F.; Medeiros, M.C.; Outlaw, D.C.; Hamer, G.L. A Novel Haemosporida Clade at the Rank of Genus in North American Cranes (Aves: Gruiformes). Mol. Phylogenet. Evol. 2017, 109, 73–79. [Google Scholar] [CrossRef]
- Yabsley, M.J.; Vanstreels, R.E.; Martinsen, E.S.; Wickson, A.G.; Holland, A.E.; Hernandez, S.M.; Thompson, A.T.; Perkins, S.L.; West, C.J.; Bryan, A.L. Parasitaemia Data and Molecular Characterization of Haemoproteus catharti from New World Vultures (Cathartidae) Reveals a Novel Clade of Haemosporida. Malar. J. 2018, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lotta, I.A.; Valkiūnas, G.; Pacheco, M.A.; Escalante, A.A.; Hernández, S.R.; Matta, N.E. Disentangling Leucocytozoon Parasite Diversity in the Neotropics: Descriptions of Two New Species and Shortcomings of Molecular Diagnostics for Leucocytozoids. Int. J. Parasitol. Parasites Wildl. 2019, 9, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, A.; de la Puente, J.; Onrubia, A.; Pérez-Tris, J. Molecular Characterization of Haemosporidian Parasites from Kites of the Genus Milvus (Aves: Accipitridae). Int. J. Parasitol. 2013, 43, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Honjo, Y.; Fukumoto, S.; Sakamoto, H.; Hikosaka, K. New PCR Primers Targeting on a Cytochrome b Gene Reveal Diversity of Leucocytozoon Lineages in an Individual Host. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Wandeler, P.; Hoeck, P.E.; Keller, L.F. Back to the Future: Museum Specimens in Population Genetics. Trends Ecol. Evol. 2007, 22, 634–642. [Google Scholar] [CrossRef]
- Galen, S.C.; Nunes, R.; Sweet, P.R.; Perkins, S.L. Integrating Coalescent Species Delimitation with Analysis of Host Specificity Reveals Extensive Cryptic Diversity despite Minimal Mitochondrial Divergence in the Malaria Parasite Genus Leucocytozoon. BMC Evol. Biol. 2018, 18, 128. [Google Scholar] [CrossRef]
- Lepage, D.; Warnier, J. The Peters’ Check-List of the Birds of the World (1931–1987) Database. Available online: https://avibase.bsc-eoc.org/peterschecklist.jsp (accessed on 30 July 2022).
- Instituto de Hidrología. Meteorología y Estudios Ambientales Atlas Climatológico de Colombia. Available online: http://atlas.ideam.gov.co/visorAtlasClimatologico.html (accessed on 12 July 2022).
- Gómez Mercado, J.M.; Martínez Hincapie, Y.; Mosalvo, A.; Carpio, E.; Goyeneche, S.; Lubo, H.; Herran, V.; Gutierrez, W.; Frias, R.; Deluque, A. Plan de Desarrollo Municipal 2016–2019 Manaure Equidad, Efectividad y Ciudadanía. Available online: http://www.manaureguajira.gov.co/Documents/plandedesarrollo.pdf (accessed on 14 July 2022).
- Ministerio de Ambiente, Vivienda y Desarrollo Territorial; Corporación Autónoma Regional del Magdalena (Corpamag); Instituto de Investigacines Marinas y Costeras, «José Benito Vives de Andreis, Invemar» Plan de Manejo Santuario de Flora y Fauna de La Ciénaga Grande de Santa Marta. Available online: https://www.corpamag.gov.co/archivos/PMA/PlanManejoRBRamsar.pdf (accessed on 30 July 2022).
- Valle, A.G.; Osorno-Arango, A.M.; Gil-Agudelo, D.L. Estructura y Regeneración Del Bosque de Manglar de La Ciénaga de Cholón, Isla Barú, Parque Nacional Natural Corales Del Rosario y San Bernardo, Caribe Colombiano. Boletín De Investig. Mar. Y Costeras-INVEMAR 2011, 40, 115–130. [Google Scholar]
- Gil-Ospina, R.F.; Bedoya-Zuluaga, F.A.; Castaño-Villa, G.J. Tendencias Poblacionales En Algunas Especies de Aves Acuáticas En La Laguna Del Otún Entre 1998 y 2007. Bol. Cient. Mus. Hist. Nat. 2010, 14, 92–98. [Google Scholar]
- Rivera, D. Páramos de Colombia. Banco De Occidente. I/M Editores. Imprelibros Santiago De Cali Colomb. 2001. [Google Scholar]
- Cadena, D.; Estacio, H.; Erazo, L. Plan de Desarrollo “Es Tiempo de Avanzar”. Available online: http://www.pupiales-narino.gov.co/apc-aaaafiles/33663063366461626161306634386632/plan-de-desarrollopupiales-2012-2015.pdf (accessed on 14 July 2022).
- Vargas Ríos, O.; Pedraza, P. El Parque Nacional Natural Chingaza; Universidad Nacional de Colombia, Facultad de Ciencias, Departamento de Biología: Bogotá, Colombia, 2004; ISBN 958-33-6824-5. [Google Scholar]
- Ramsar Sites Information Service Annotated List of Wetlands of International Importance Colombia. Available online: https://rsis.ramsar.org/sites/default/files/rsiswp_search/exports/Ramsar-Sites-annotated-summary-Colombia.pdf?1658250317 (accessed on 14 July 2022).
- Department of Geosciences Universidad Nacional de Colombia Boletín Del Análisis de Las Variables Obtenidas En La Estación Meteorológica de La Universidad Nacional. Available online: http://www.geociencias.unal.edu.co/?niv=not¬=545&dep=12 (accessed on 30 April 2013).
- Lentijo, G.; Kattan, G. Estratificación Vertical de Las Aves En Una Plantación Mono Específica y En Bosque Nativo En La Cordillera Central de Colombia. Ornitol. Colomb. 2005, 3, 51–61. [Google Scholar]
- Usma, J.; Trujillo, F. Biodiversidad Del Casanare: Ecosistemas Estratégicos Del Departamento; Gobernación del Casanare-WWF Colombia: Bogotá, Colombia, 2011. [Google Scholar]
- Corporacion Autonoma del Valle del Cauca Plan de Manejo Ambiental Del Complejo de Humedales Del Alto Río Cauca Asociado a La Laguna de Sonso—Designado Como Sitio RAMSAR (Valle Del Cauca). Available online: https://www.cvc.gov.co/sites/default/files/2019-02/PMA%20Sitio%20Ramsar_VF_web.pdf (accessed on 19 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).