Abstract
The Aviles Canyon System (Northern Atlantic coast of Spain) is one of the ten marine regions studied in the Spanish seas by the LIFE+ INDEMARES project, which aims to identify special areas of conservation within the Natura 2000 Network. This study aims to characterize the composition and distribution of the macrobenthic fauna in order to provide baseline data to obtain a basic knowledge of the environment. Three oceanographic surveys were carried out to investigate species and habitats of this deep-sea ecosystem. The stations were sampled using a box corer, in order to evaluate the distribution and biodiversity of the macroinfauna, and to analyse the granulometric composition and the organic matter content. Sediments were mainly sandy in nature, the finest sediments with the highest organic matter content were found in the deepest areas, while coarser sediments were located in shallow stations. Polychaetes were the best represented group in total number of species and individuals, followed by crustaceans and molluscs. Five major macrobenthic assemblages were determined through multivariate analyses. Bathymetry and sedimentary composition were the main factors structuring the benthic community separating shallow and coarser stations from deeper and finer ones.
1. Introduction
The deep-sea, the largest ecosystem on earth which comprises more than 65% of the surface of the world, is usually defined as deeper than 200 m, where the shelf break begins [1], and where the shallow fauna of the shelf is replaced by the deep-sea fauna of the deep ocean basins. Deep-sea was believed to be faunistically very poor, based on the lack of light, cold temperature and the immense pressure that characterize this area. However, this idea was wrong: this environment, formed by hard and soft bottoms [1], is constituted by a variety of ecosystems and crossed by submarine canyons. Submarine canyons are irregular incisions (“V” or “U” shaped) that cut down the continental slope, connecting continental shelves to deep ocean basins [2]. These complex and heterogeneous systems, that are abundant and ubiquitous along continental margins, are very productive areas [3]. Canyons present different bottom types, are usually recognized as organically enriched environments, and can also be highly unstable due to high sediment loads, internal tides or episodic strong down canyon flows [4,5]. Moreover, these submarine structures are pathways for the transport of sediments and organic matter from the continental shelf to deep basins, often providing high quality food supply [6]. Benthic infaunal communities depend on this allochthonus organic matter, and consequently, canyons maintain higher density of benthic assemblages than open slopes at comparable depths [5,7].
Nowadays, the understanding of the deep-sea and the topographical features that intersect them are changing with the development of new technologies for sampling and study these areas [8]. Submarine canyons are major sources of habitat heterogeneity in continental margin settings [2,9] by change local and regional conditions, and this sediment can be periodically resuspended by natural causes such as internal waves or tidal currents. Consequently, the increased habitat heterogeneity in canyons enhances benthic biodiversity on hard substrata as well as mobile sediments, and they are also often recognized as ‘hotspots’ of biological activity [2].
This work is focused on the study of the Avilés Canyon System (ACS), a complex system of interrelated canyons located in the central Cantabrian Sea (North Iberian continental margin) that was declared SIC (Site of Community Interest) in 2015 after exhaustive sampling campaigns carried out in the area. The ACS is constituted by three main canyons and some other minor tributaries [10]. There is also a marginal platform and a relevant rocky outcrop in the area. The ACS is impacted by a range of natural and anthropogenic activities such as fisheries, intense sea traffic, degradation of the coast due to excessive industrial, urban, and tourism development, etc. Therefore, the managing of the fisheries that take place in the area, and the protection of these marine habitats are very important targets in the ACS. The canyon ecosystems (benthic and pelagic) and the physical processes that support them, together with geology and geophysics, were the focus of a major work program: INDEMARES (LIFE+) project “Inventory and designation of marine Natura 2000 areas in Spanish sea” whose main objective “is to contribute to the protection and sustainable use of the biodiversity in the Spanish seas through the identification of valuable areas for the Natura 2000 Network” (www.indemares.es; accessed on 10 October 2022), and include the study of ten possible new areas that accomplish the necessary requirements for being established as MPAs (Marine Protected Areas). Although the investigation was multidisciplinary, this paper specifically deals with the infaunal macrobenthos of the area, to increase our knowledge on these marine species and communities. The macrofauna are defined as small-sized organisms (0.25–0.50 mm), living buried within the sediment of the ocean floor, and consisting primarily of polychaetes, peracarid crustaceans and bivalve molluscs [1].
In spite of numerous studies conducted in submarine canyons around the world, little is known about the benthic macroinfauna inhabiting these sediments at the present time. Previous ecological works in the submarine canyons areas have been centered on the study of large organisms, such as corals and sponges, or upon fish populations of commercial value and marine top predators (e.g., cetaceans, …), but there are relatively few studies focused upon the community structure of infaunal organisms [9]. Therefore, the objective of this study was to characterize the composition and distribution of the soft-bottom macrofaunal communities of the Aviles Canyon System in order to provide baseline data to contribute to the knowledge of this particular environment. The results of the present research (i) will provide information about endobenthic organisms, (ii) working at the species level will allow us to better understand the interrelationships between the species that structure each community, and (iii) will allow us to describe patterns of biodiversity, abundance and community structure of soft-bottom infaunal macrobenthos of the ACS as well as its currently relationship with environmental variables.
2. Materials and Methods
2.1. Study Area
The ACS is a complex canyon and valley system located in the Cantabrian Sea (southern Bay of Biscay) (Figure 1), and is constituted by three major canyons: La Gaviera Canyon, El Corbiro Canyon and Avilés Canyon, and by two conspicuous morphologic features: El Canto Nuevo marginal platform and the Agudo de Fuera rocky outcrop. The Avilés Canyon is located 7 miles from the Spanish coast, from 128 to 4700 m depth, and it is characterized by a sedimentary and V-shaped bottom that is 75 km long. The El Corbiro Canyon is 23 km long, and also has a sedimentary V-shaped floor, while La Gaviera Canyon presents a U-shaped bottom with two narrow differentiated flanks: the east side shows a sedimentary character, whereas the west side is characterized by the incision of different gullies [10]. The continental shelf shows a flat slope with rock outcrops and limited sedimentary zones, showing thin unconsolidated sedimentary cover [10]. These submarine canyons play an important role as high productivity systems transporting sediments and organic matter from the continental shelf to the Biscay Abyssal Plain [10]. Moreover, in this area exists a high diversity of habitats and biological communities resulting from a high gradient of environmental variables, and from the existence of strong hydrodynamic activity associated to the topography [10,11]. Additionally, this canyon system is known to provide Essential Fish Habitats (EFH) for important commercial species, such as hake and monkfish, as well as habitats for sharks, cetaceans and giant squid [12]. The problem in this area is that the intense fishing activity is causing several damaging interactions, as well as the uses of the coast, or the extreme industrial, urban, and tourism expansion are also impacting offshore seas [13].
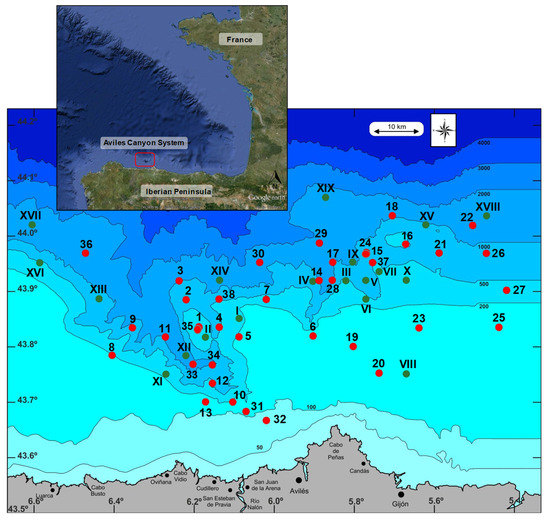
Figure 1.
Location of the ACS, and position of the stations sampled on this study area. Green circles with roman numerals mark the stations where only sediment was collected. Red symbols with cardinal numerals mark the stations where sediment and infauna were collected.
2.2. Sampling Program
Within the framework of the INDEMARES (LIFE+) project, three cruises were carried out in 2010 and 2011 in the ACS to study the infaunal communities of the area, among other multidisciplinary objectives.
During the three surveys, a total of 57 stations (83–1881 m depth) were sampled using an USNEL box corer (sampling area = 0.09 m2) (Figure 1): 57 samples were used to study the sediment characteristics (surveys 0410, 0710 and 0511), and 38 samples were used to study the endobenthic organisms (surveys 0710 and 0511). Previously, sampling stations were selected after confirmation of the presence of soft sediments with the multibeam echosounder (low backscatter values), and a single sample was taken in each station. The stations were not replicated temporally. The infaunal samples were washed carefully through a 0.5 mm mesh size sieve. The retained material was anesthetized with a MgCl2 solution, and then preserved with 8% buffered formaldehyde stained with Rose Bengal solution for later sorting and identification of the fauna. The sorted fauna were classified to the lowest possible taxonomic level and counted, after which they were transferred to a 70% ethanol solution. Additionally, they were assigned to feeding categories (WoRMS, http://www.marinespecies.org, accessed on 10 October 2022), and five trophic groups were considered: carnivores, surface deposit feeders, subsurface deposit-feeders, suspension-feeders and others (the others group includes organisms with very heterogeneous food characteristics such as omnivores, herbivores and scavengers). An additional sediment sample was taken at each station within the box corer to analyze the granulometric composition and the organic matter content of the superficial substratum. These sediment samples were frozen onboard until the later processing in the laboratory. Particle size was analyzed by a combination of dry sieving and sedimentation techniques [14] in order to estimate the median grain size (Q50) and the sorting coefficient (S0). The organic matter content was estimated as the weight loss of dried samples (100 °C, 24 h) after combustion (500 °C, 24 h).
2.3. Data Analysis
Macrofaunal species richness (S), the Shannon–Wiener diversity index (H’, as log2) and Pielou evenness index (J’) were calculated for each sampling station using the community analysis PRIMER v6 software [15]. This software was also used for multivariate analyses. Abundances were calculated and expressed as number of individuals per m2 of sampled area (ind.m−2), and these data were organized into a sample vs. species matrix. Cluster analysis (based on the group-average sorting algorithm) and non-metric multidimensional scaling (MDS) ordination was performed using Bray–Curtis similarity measure, after fourth root transformation of the biological data to classify and order the stations into macrobenthic assemblages. These analyses were carried out to identify the groups of sampling sites that do not differ significantly in the macrofaunal composition. Values of selected abiotic features were further superimposed to visually detect any related pattern in that ordination.
The one-way ANOSIM test (PRIMER) was used to test for differences among samples from different sites with the null hypothesis that there are no significant differences. In addition, the SIMPER (Similarity Percentage) routine was then run to identify species that greatly contributed to the differentiation of station groups. The species present in each group of stations were further classified according to the constancy and fidelity indexes [16,17], which evaluates the constancy and the numerical importance of each species within a group of stations (Supplementary Material, Table S1). Five categories of constancy index were considered according to the number of times the species was found in the total of samples: constant (>76%), very common (51–75%), common (26–50%), uncommon (13–25%) and rare (<12%). According to the ratio between this index and the total constancy in the considered area (or group of stations), the fidelity index classifies the species as accidental (<10%), occasional (11–33%), accessory (34–50%), preferential (51–66%), elective (67–90%) and exclusive (>91%). The BIO-ENV routine of the PRIMER package was used to study the association of environmental factors with species abundances. This was carried out using the species abundances dataset and environmental factors dataset. All variables expressed in percentages were previously transformed by log (x + 1), and all the abiotic variables were standardised. The abiotic variables used in these analyses were water depth (m), total organic matter content (TOM; %), and sediment characteristics, including the weight percentage of coarse sands (>500 μm), fine sands (62–500 μm) and mud (<62 μm), the mean particle diameter (Q50; mm), and the sorting coefficient (S0).
3. Results
3.1. Sediments
The soft bottoms sampled in the ACS are dominated by sandy sediments, mainly by fine and very fine sands. The finest sediments with the highest organic matter content are found in the deepest areas of the continental shelf and slope, while coarser sediments are located in the shallower stations of the continental shelf (see Supplementary Material, Table S2 with the main characteristics of the sampling stations and Figure S1 with the PCA analysis representation).
Specifically, the shallowest bottoms of the continental shelf (<500 m depth) are characterized by the coarser sediments of the study area (very coarse, coarse, medium and fine sands). The organic matter content is low (1.2–5%), the sorting coefficient moderate, and the median grain size ranges between 0.12 and 1.15 mm. It is significant to highlight the presence of coarse sand and very coarse sand in the SW region of the continental shelf-break, in front of the Avilés Canyon; at medium depths (500–1000 m depth), fine and very fine sands dominate the sampled bottoms. The medium grain size range between 0.08 and 0.20 mm, the sorting coefficient between poor and moderate, and the organic matter content between 1.8% and 7.6%; finest and muddy sediments are located in the deepest stations (>1000 m depth), and they are characterized by the highest organic matter content (1.8–11.5%), by a lower sorting coefficient (from bad to moderate), and by a median grain size between 0.03 and 0.42 mm.
This general pattern of the spatial distribution of different types of sediment is more or less altered on a smaller scale by the structural complexity of the area.
3.2. Macrofaunal Composition and Structure
A total of 5053 individuals belonging to 505 taxa in 176 families were collected from the infaunal samples. Polychaetes (56.8% of total abundance; 263 species) were by far the most abundant group, followed by Arthropoda (Crustacea Subphyllum: 17.7%; 151 species). Molluscs (13.9%; 60 species), Echinodermata (6.3%; 24 species), and Others group were less common. Figure 2 shows the density (ind/m2) of the major faunal groups ordered by depth. Macrofaunal abundance decrease with depth: the highest abundance values appeared in shallow stations (<500 m depth): sites 27, 25, 6 and 20 (2811, 2511, 2433 and 2311 indv/m2, respectively) at a depth of 457, 157, 168 and 144 m; while the lowest values were recorded at deeper stations: sites 2, 3, and 22 (544, 567 and 733 indv/m2, respectively) at a depth of 637, 1033 and 1184 m. There is also a decrease in the species richness with depth, ranging from 81 species at station 25 (157 m) to 29 species at station 2 (637 m). The highest Shannon H’ diversity index was observed at stations 25 and 37 (H’ = 5.8; medium and fine sand; 157 and 499 m depth, respectively), while the lowest diversity index was found at station 34 (H’ = 4.5; very fine sand; 1017 m depth) (Supplementary Material, Figure S2).
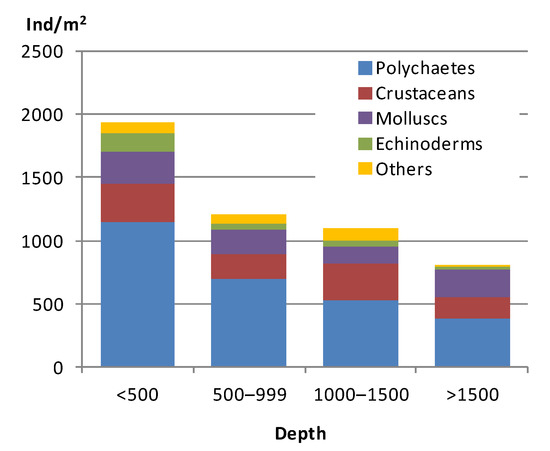
Figure 2.
Abundance indices (indv/m2) of the major macrofauna taxa on the ACS according to depth (m).
Thirteen families (or higher taxonomic level) accounted for more than 50% of all individuals: the polychaetes Spionidae, Paraonidae, Ampharetidae, Sabellidae, Capitellidae, Syllidae, Maldanidae, Onuphidae and Cirratulidae, the bivalves Bivalvia indet. and Thyasiridae, the nemerteans, and the Echinoidea indet. The most speciose families were Syllidae (31 species), Paraonidae (29), Spionidae (22), Ampharetidae (20), Onuphidae (12), Ampeliscidae (10), Lumbrineridae (10) and Terebellidae (10). Regarding the species, the dominant ones were the polychaetes Eclysippe vanelli, Levinsenia flava, Prionospio cirrifera, Aricidea (Aricidea) wassi, Euchone incolor, Glycera lapidum and Prionospio sp., the sipunculid Onchnesoma steenstrupii steenstrupii, and the bivalves Timoclea ovata and Kelliella sp., which accounted for more than 25% of all individuals (Table 1). Six species were present on more than 20 stations: Glycera lapidum, Levinsenia flava, Prionospio sp., Euchone incolor, Onchnesoma steenstrupii steenstrupii, and Notomastus latericeus (Table 1).

Table 1.
Dominant species which accounted for more than 25% of abundance of all individuals, together with species present on more than 20 stations.
Polychaetes were the best represented group in total number of organisms at the three bathymetric levels considered (less than 500 m; 500–1000 m; more than 1000 m). At shallow, medium and deep stations, polychaetes dominated the infaunal community with values of 33.5–80.9%, 39–83.6%, and 28.5–82.4%. The same polychaete families, Spionidae, Paraonidae, and Ampharetidae, were the most abundant ones at these three bathymetric levels. This infaunal group was followed by crustaceans (5.9–39.9%; 6.0–28.6%; 11.8–53.8%), mainly dominated by Ampeliscidae, Melitidae, and Phoxocephalidae amphipods, and by molluscs (3.3–30.7%; 5.6–33.8%; 2.0–26.4%), with Thyasiridae and Veneridae as more abundant families. Echinoderms and the Others group were less abundant.
3.3. Multivariate Analyses
The ANOSIM test revealed significant differences in faunistic composition between all sites (global R = 0.779, p = 0.001). The dendrogram obtained by cluster analysis showed the presence of four major biological groups of stations at a similarity level of 25% (Figure 3 and Figure 4): group A (140 ± 40 m depth), B (1676 ± 291 m depth), C (161 ± 38 m depth), and D (D1: 489 ± 84 m depth; D2: 970 ± 225 m depth). Stations 3, 10, 11, 18, 21, 22 and 33 did not belong to group A, B, C or D (see Supplementary Material, Table S3 with the results of ANOSIM test for these groups). nMDS ordination (Figure 5) showed similar results to those of the dendrogram (stress: 0.21). The summary of characteristics for each association is shown in Table 2.
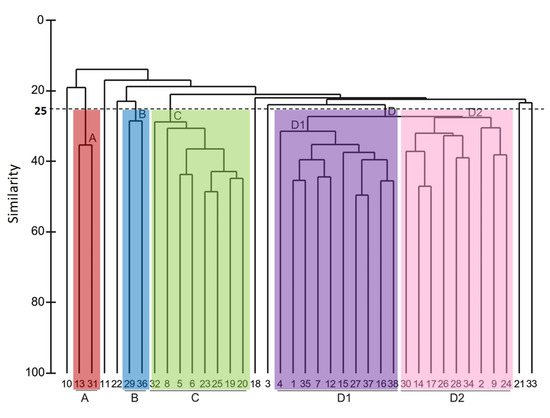
Figure 3.
Hierarchical cluster analysis for benthic macrofauna at the ACS.
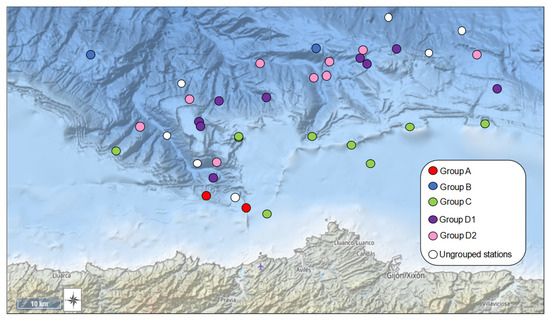
Figure 4.
Location of sampling sites in the ACS showing the groups determined by cluster analysis.
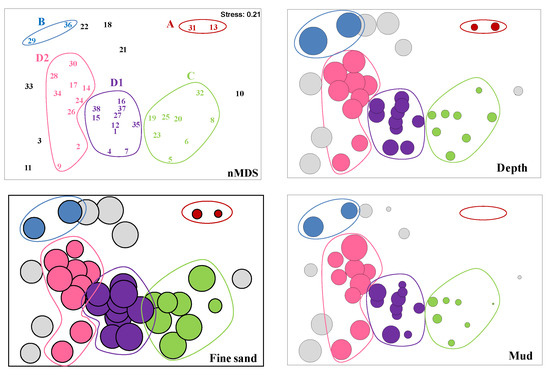
Figure 5.
Non-metric multidimensional scaling (nMDS) ordination of sampling sites showing groups determined by cluster analysis, and ordination of sites with values of some environmental variables superimposed (depth; fine sand; mud).

Table 2.
Abiotic and biotic characteristics of the main infaunal macrobenthic assemblages discriminated by the multivariate analysis of abundance data.
Group A was made up of two shallow stations (medium and very coarse sand), and was characterized by the lowest organic matter content, and the highest number of individuals. The most abundant species of this group (accounting for 25% of all the fauna; Supplementary Material, Table S4) were the bivalves Limatula sp. (constant/elective), the polychaetes Pisione sp. (constant/exclusive), and the amphipods family Aoridae, while the most characteristics species were the constant and exclusive polychaetes Caulleriella bioculata, Goniadella sp., Pisione sp., Sphaerosyllis bulbosa and the bivalve Spisula sp.
Group B comprises the deepest stations of the sampling program. These stations were composed of fine and very fine sediments, with relatively high contents of organic matter. The caprellids (Caprellidae indet.; common/preferential), the polychaetes Aurospio dibranchiata (constant/preferential), and the scaphopods Antalis agilis were the most abundant species of this group (accounting for 25% of all the fauna). The polychaetes Nothria sp. (constant/exclusive), Levinsenia sp. 1 (constant/elective), and Notoproctus sp. (constant/elective), and the isopod Anthuridae sp. (constant/elective) characterized this group.
Group C is defined by the shallowest stations of the study area. This group is composed by very coarse, coarse, medium and fine sands, with low organic matter content. The most abundant species (accounting for 25% of all the fauna) were the nemerteans (Nemertea indet.), the bivalves Bivalvia indet. and Timoclea ovata (constant/preferential), the echinoids Echinoidea indet., and the polychaetes Aricidea (Aricidea) wassi (constant/preferential), Aonides paucibranchiata, Euchone incolor, and Galathowenia oculata. The most characteristic species of this group were the polychaetes Aponuphis bilineata (very common/exclusive), Pista sp. (very common/elective), and Spiochaetopterus sp. 1 (very common/elective), and the decapods (Decapoda indet.; very common/elective).
Group D was further subdivided into group D1 and group D2. Group D1 was made up of fine sand stations located at medium depths. The most abundant taxa of this group (accounting for 25% of all the fauna) were the polychaetes Eclysippe vanelli (constant/preferential), Levinsenia flava, Magelona filiformis, and Prionospio cirrifera, and the bivalves Bivalvia indet., and Kelliella sp., while the most characteristics species were the polychaetes Ophelina abranchiata (constant/elective), the maldanids Maldanidae gen. sp. 2 (very common/elective), and Spiophanes wigleyi (very common/elective). Group D2 comprised sediments deeper than D1, with the highest organic matter content of the study area, and with the finest granulometric fractions (mainly, very fine sands). The polychaetes Levinsenia flava and Aurospio dibranchiata, the caudofoveata (Caudofoveata indet.), the sipunculid Onchnesoma steenstrupii steenstrupii, the bivalves Genaxinus eumyarius (very common/exclusive) and Axinulus croulinensis (very common/preferential), and the nemerteans (Nemertea indet.) were the most abundant taxa of this group (accounted for 25% of all the fauna). The amphipods Metaphoxus simplex (constant/elective) and the polychaetes Paradiopatra sp. 2 (very common/elective) also characterized this group.
SIMPER analysis showed that the bivalve Limatula sp., and the polychaetes Pisione sp., Protodorvillea kefersteini and Prionospio cirrifera explained most of the dissimilarity between groups A and C (average dissimilarity = 80.1%), while the bivalves Limatula sp. and Tellina sp. together with the polychaete Pisione sp., contributed greatly to the differentiation of A from D1 (average dissimilarity = 86.9%). The bivalve Timoclea ovata, and the polychaetes Eclysippe vanelli, Ophelina abranchiata, and Levinsenia gracilis differentiated group C from D1 (average dissimilarity = 73.4%). Group D1 differed from D2 (average dissimilarity = 72.8%) due to the polychaetes Eclysippe vanelli, Prionospio cirrifera, and Aricidea (Aricidea) wassi, whereas the polychaetes Nothria sp., Levinsenia sp. 1, and the cirratulids (Cirratulidae indet.), the sipunculid Onchnesoma steenstrupii steenstrupii, and the cumaceans (Cumacea indet.) greatly contributed to separating D2 from B (average dissimilarity = 77.0%).
3.4. Relationship between Biotic and Environmental Variables
The BIO-ENV procedure analysis suggests that the variables depth, fine sand and mud content are the major structuring factors of the benthic community (ρ = 0.671). Depth and mud content show the highest correlation values if they are considered separately (ρw = 0.500 and 0.473, respectively).
The nMDS ordination of sites with superimposed values of these three abiotic variables showed that stations appeared distributed from left to right following decreasing values of depth, mud and fine sand content (Figure 5).
3.5. Trophic Structure
The macrofaunal community of the ACS was dominated by deposit feeders, specifically by surface deposit feeders which represent more than 40% of total abundance on average. At most stations (33 stations), surface deposit feeders predominated and represented between 25.6% and 54.7% of the populations. The second most strongly represented trophic group were the subsurface deposit feeders which accounted for more than 25% of total abundance on average (from 4.2 to 53.2%); this is the major feeding mode at four stations. Suspensivores (0–31.6%) dominated only in one station, while Carnivores and the Others group did not dominate any station. Moreover, all trophic groups showed the same pattern with depth, decreasing their abundances at greater bathymetries. However, the pattern of the proportion of each trophic group is different (Figure 6). In this case, carnivores and surface deposit feeders decrease their proportion with depth, whereas subsurface deposit feeders and the Others group increase this proportion. Suspensivores show a light decrease with depth.
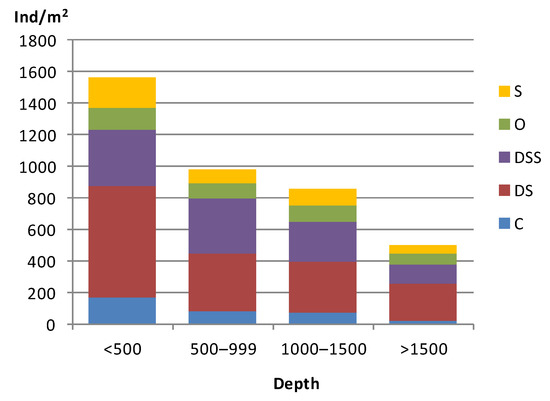
Figure 6.
Relative abundance (%) of macrofaunal feeding types at each strata. O: Others group; C: carnivores; S: suspensivores; DSS: subsurface deposit feeders; DS: surface deposit feeders.
4. Discussion
Previously to this study, [11] characterized the habitat of the deep-water coral reefs in La Gaviera Canyon (ACS), and [10] described the geomorphology of the ACS. However, the only preliminary descriptions focused on the species composition of the benthic assemblages of the ACS were made by [18] using an unpublished historical dataset obtained in 1987–1988, and by [19], with the data of this study but only considering the family taxonomic level. [18] described the macrobenthic community assemblages in the ACS (N Iberian Shelf) on the basis of 42 anchor dredge and/or epibenthic sledge collected in 1987–1988, sieved on a 2 mm mesh, collected at water depths of 31–1400 m. They identified 810 macrofaunal taxa belonging to eleven Phyla, and, in the present work, were identified 505 taxa and six Phyla (38 stations from 83 to 1881 m). Other [18] work focused on larger animals (megafaunal communities) than this study does, such as epifauna settling on big stones, corals or pennatulaceans, and comprised phyla not considered in the present study, such as Cnidarians, Brachiopods or Bryozoans. Therefore, these factors, together with the different sampling methods used in both studies (gears, sieves, etc.), make comparisons difficult, and reveal that this work adds new information with actualized data about the ACS, more focused in the infaunal community (gear: box corer; sieve: 0.5 mm; maximum depth: 1881 m). In spite of that, there are general patterns that can be observed in both works, such as the decrease in sediment grain size with depth. As was stated in [19], median grain-size decreased and sediment organic matter content increased with increasing water depth in the ACS. The coarser sediments (very coarse, coarse and medium sands) are located at the shallowest stations (continental shelf), while fine and very fine sands prevail below 300 m. This gradient in grain size from the shallowest sites down to the deeper areas has also been recorded in other deep-sea areas [1,20,21]. Moreover, [18] suggested depth as the major structuring agent of the benthic community, and that sediment characteristics were also an important factor influencing this community structure. The same results are shown in this work, where the statistical analyses select depth, fine sand and mud as the major structuring agents of the community, and where the cluster analyses also discriminated between shallow from deep stations.
The distribution and diversity of deep-sea infauna have mainly been related to depth and sediment [6], and canyons systems are extremely variable in the terms of sediment and organic matter [5]. The presence of three canyons and rocky outcrops on the continental shelf has a strong influence on the sedimentary processes, generating the different deposits found [10]. Different sediment types can allow the settlement of different benthic fauna, and moreover, sediment composition changes with depth, and this fact limits the distribution of some species [1]. The depth distribution of a species may also be controlled by food availability [22]: the abundances of the macrofaunal organisms decrease to the abyss because of the reduction in nutrient input [23]. Furthermore, there are other factors besides sediment type and nutrient input that vary with depth and that can also influence infaunal organisms’ distribution, such as temperature, hydrostatic pressure, light intensity or current dynamics besides biological interactions (competition, predation) [23]. Therefore, macrofaunal abundance decreases with depth in many deep-sea environments [1], and the results of this work agree with this pattern that has already been recorded in other deep-sea areas ([24] in the Gay Head-Bermuda transect; [25] in the tropical Northeast Atlantic; [20] in the Goban Spur in the NE Atlantic Ocean; [21] in the Northwest Atlantic; [1] in the Gulf of Mexico; [5] in three Portuguese submarine canyons in the NE Atlantic).
According to the studies that appear in [23], species richness pattern follows a parabolic curve along depth gradient: diversity increases to intermediate depths but then decreases toward the abyss. In the ACS this pattern does not take place because the existence of the submarine canyons could affect this pattern, and in this work the species richness decreases regularly with depth, giving rise to a similar decrease in the diversity index according to depth. One possible explanation for this pattern is that species diversity may also be related to the sediment particle diversity that provides great habitat complexity, so if the particle diversity decreases with depth, the same is going to happen with the species diversity [23].
Polychaetes are the numerically dominant macrofaunal taxon in the deep-sea [26,27]. In the ACS, these annelids accounted for 56.8% of the total macrofaunal abundance, attaining similar relative abundances to other deep-sea areas [5,18,21,26,28]. The most numerous families of this faunistic group were mostly the same as have already been recorded in these other studies, where Spionidae, Paraonidae and Cirratulidae stand out among the rest of the families of these deep-sea areas. Our results also show that the next most abundant faunistic group of deep-sea macrofauna are the peracarid crustaceans, followed by the molluscs, as also found by [26].
Regarding the trophic groups, deposit feeders species are largely dominant. Deposit feeders ingest sediment so they rely on sediments for nutrition [23]. They comprise the majority of species, and specifically in this work, surface deposit feeders, that feed from the sediment surface, are more numerous than subsurface deposit feeders, that feed as they burrow through the sediment, at most of the stations in the ACS. Deposit feeding is the dominant feeding mode in the deep-sea [5,6], with surface deposit feeders as the most abundant macrofaunal feeding mode in deep-sea sediments [26,29]. The dominance of deposit feeding may arise because the habitat is unsuitable for suspension feeders, and also due to a possible limitation of resources and prey for macrophagous feeders. Moreover, the proportion of surface deposit feeders and carnivores, the latter typically more abundant at shallow stations [5,30,31], tended to decrease with water depth, whereas the proportion of subsurface deposit feeders and the Others group tended to increase. Suspension feeders, which feed on material they collect from the water column, decrease in overall importance with increasing water depth [27] given the low suspended-particle in the deep sea. Therefore, in this work suspension feeding is rare [23,26].
Matches with Habitat Characterization
During the cruises carried out in the INDEMARES project, the sea floor of the margin was mapped to locate and map possible Vulnerable Marine Ecosystems, including continental shelf, continental slope and a narrow band of abyssal plain attached to the base of the continental slope [10]. The investigation was multidisciplinary, involving geology–geophysics, biology (benthic and pelagic), ecology and physical oceanography. The INDEMARES surveys used multidisciplinary sampling gear in an attempt to reach these objectives, such as otter trawl and beam trawl for communities that inhabit sedimentary areas; ROV, benthic photolander platform and photogrammetric sled for complex and vulnerable areas; box-corer to study sedimentological characteristics, organic content data and infaunal communities (this work); multibeam echosounder, high-resolution seismic profiles (TOPAS system), etc. Thus, the study of the geomorphology and shallow structure of the area will provide important information about the configuration of the sea floor, which is essential in understanding the distribution of benthic communities and bottom circulation patterns.
The results obtained in those studies shown that the particular habitats found in the area do not seem to match with the criteria for the classification of the habitats according to EUNIS (discrepancies in their design, the same habitat can be classified into different levels, etc.) [32]. However, there are 24 habitats identified already in the ACS after the study carried out by the INDEMARES (LIFE+) project [33], which collected different kinds of data: bathymetry and geomorphology, substratum, hydrography and dynamics, biological samples, and fishery impacts. Some of these benthic habitats identified match with the infaunal groups determined in this work through the multivariate analyses. Thus, group A is located on the “Faunal communities in Atlantic offshore circalittoral coarse sediment” (MD321), group C on the “Faunal communities in Atlantic offshore circalittoral sand” (MD521), group D1 mainly on the “Sparse communities on Atlantic upper bathyal sand” (ME521) and group D2 on the “Atlantic upper bathyal sand” (ME52). Some of these habitats, particularly the habitats located in sedimentary grounds, and their biological communities can be altered and disturbed by fishing activities [13].
The present study provides an important insight into infaunal assemblages of the ACS. Benthic infauna are mostly sedentary, so they are frequently examined to track the impact of disturbance on marine environments. Exploration and investigation on canyons macrobenthic assemblages provide important information for understanding the ecosystem processes in those environments. This work based on the ACS and its ecosystems tries to address the actual condition of the canyons system as well as to gain important environmental baseline information, because there is a lack of studies in deep infaunal communities as well as a taxonomic bias toward larger animals [34]. Over the last years, significant progress on deep sea macroecology has been made, but much still remains to be done. Therefore, future studies should investigate the distribution of individual species in the deep sea, as well as to test specific hypotheses and patterns, and to increase our information of specific taxa, basins and oceans [35].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15010053/s1, Figure S1: Principal component analysis (PCA) showing sampling sites and the environmental variables for the ACS (Q50: median grain size; S0: sorting coefficient; TOM: total organic matter content); Figure S2: Density (ind/m2), richness (numer of taxa) and Shannon index (H’, log2) of macroinfauna vs. depth; Table S1: Constancy and fidelity indices; Table S2: Depth, location, sedimentary type and sorting coefficient (S0) of sampling stations in the ACS; Table S3: Results of the ANOSIM test; Table S4: Constancy and Fidelity indices of the more abundant species of each group of stations.
Author Contributions
Conceptualization, A.L., S.P. and F.S.; methodology, A.L., S.P. and F.S.; software, A.L.; validation, A.L., S.P. and F.S.; formal analysis, A.L.; investigation, A.L., S.P. and F.S.; resources, A.L., S.P. and F.S.; data curation, A.L., S.P. and F.S.; writing—original draft preparation, A.L., S.P. and F.S.; writing—review and editing, A.L., S.P. and F.S.; visualization, A.L., S.P. and F.S.; supervision, A.L., S.P. and F.S.; project administration, F.S.; funding acquisition, F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially funded by the European Commission LIFE+ ‘Nature and Biodiversity’ (INDEMARES Project, 07/NAT/E/000732) and by the Spanish Science and Technology Ministry (ECOMARG3 Project).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank all the participants of the oceanographic surveys, and the crew of the R/V Vizconde de Eza and Thalassa.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thistle, D. The deep-sea floor: An overview. In Ecosystems of the Deep Oceans. Ecosystems of the World; Tyler, P.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 28, pp. 5–37. [Google Scholar]
- De Leo, F.C.; Smith, C.R.; Rowden, A.A.; Bowden, D.A.; Clark, M.R. Submarine canyons: Hotspots of benthic biomass and productivity in the deep sea. Proc. R. Soc. Lond. 2010, 277, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Coelho, H.; Villarreal, M.R.; Leitão, P.C.; del Río, G.D. The low frequency circulation over Aviles canyon: Observations and modeling. Geophys. Res. Abstr. 2004, 6, 07206. [Google Scholar]
- Schlacher, T.A.; Schlacher-Hoenlinger, M.A.; Williams, A.; Althaus, F.; Hooper, J.N.A.; Kloser, R. Richness and distribution of sponge megabenthos in continental margin canyons off southeastern Australia. Mar. Ecol. Prog. Ser. 2007, 340, 73–88. [Google Scholar] [CrossRef]
- Cunha, M.R.; Paterson, G.L.J.; Amaro, T.; Blackbird, S.; Stigter, H.C.; Ferreira, C.; Glover, A.; Hilario, A.; Kiriakoulakis, K.; Neal, L.; et al. Biodiversity of macrofaunal assemblages from three Portuguese submarine canyons (NE Atlantic). Deep Sea Res. II 2011, 58, 2433–2447. [Google Scholar] [CrossRef]
- Mamouridis, V.; Cartes, J.E.; Parra, S.; Fanelli, E.; Salinas, J.I.S. A temporal analysis on the dynamics of deep-sea macrofauna: Influence of environmental variability off Catalonia coasts (western Mediterranean). Deep Sea Res. Part I 2011, 58, 323–337. [Google Scholar] [CrossRef]
- Paterson, G.L.J.; Glover, A.G.; Cunha, M.R.; Neal, L.; de Stitger, H.; Kiriakoulakis, K.; Billett, D.S.M.; Wolff, G.; Tiago, A.; Ravara, A.; et al. Disturbance, productivity and diversity in deep-sea canyons: A worm’s eye view. Deep Sea Res. II 2011, 58, 2448–2460. [Google Scholar] [CrossRef]
- Tyler, P.A. Ecosystems of the Deep Oceans. Ecosystems of the World; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- De Leo, F.C.; Vetter, E.W.; Smith, C.R.; Rowden, A.A.; McGranaghan, M. Spatial scale-dependent hábitat heterogeneity influences submarine canyon macrofaunal abundance and diversity off the Main and Northwest Hawaiian Islands. Deep Sea Res. II 2014, 104, 267–290. [Google Scholar] [CrossRef]
- Gómez-Ballesteros, M.; Druet, M.; Muñoz, A.; Arrese, B.; Rivera, J.; Sánchez, F.; Cristobo, J.; Parra, S.; García-Alegre, A.; González-Pola, C.; et al. Geomorphology of the Avilés Canyon System, Cantabrian Sea (Bay of Biscay). Deep Sea Res. II 2014, 106, 99–117. [Google Scholar] [CrossRef]
- Sánchez, F.; González-Pola, C.; Druet, M.; García-Alegre, A.; Acosta, J.; Cristobo, J.; Parra, S.; Ríos, P.; Altuna, A.; Gómez-Ballesteros, M.; et al. Habitat characterization of deep-water coral reefs in La Gaviera Canyon (Avilés Canyon System, Cantabrian Sea). Deep Sea Res. Part II 2014, 106, 118–140. [Google Scholar] [CrossRef]
- Sánchez, F.; Olaso, I. Effects of fisheries on the Cantabrian Sea shelf ecosystem. Ecol. Model. 2004, 172, 151–174. [Google Scholar] [CrossRef]
- Punzón, A.; Arronte, J.C.; Sánchez, F.; García-Alegre, A. Spatial characterization of the fisheries in the Avilés canyon system (Cantabrian Sea, Spain). Cienc. Mar. 2016, 42, 237–260. [Google Scholar] [CrossRef]
- Buchanan, J.B. Sediment analysis. In Methods for the Study of Marine Benthos; Holme, N.A., McIntyre, A.D., Eds.; Blackwell Scientific Publications: Oxford, UK, 1984; pp. 41–65. [Google Scholar]
- Clarke, K.; Gorley, R. PRIMER v6: User Manual/Tutorial; Primer-E Ltd.: Plymouth, UK, 2006. [Google Scholar]
- Glémarec, M. Bionomie benthique de la partie orientale du Golfe de Morbihan. Cah. Biol. Mar. 1964, 5, 33–96. [Google Scholar]
- Cabioch, L. Contribution a la connaissance des peuplements benthiques de la Manche occidentale. Cah. Biol. Mar. 1968, 9, 493–720. [Google Scholar]
- Louzao, M.; Anadon, N.; Arrontes, J.; Alvarez-Claudio, C.; Fuente, D.M.; Ocharan, F.; Anadon, A.; Acuna, J.L. Historical macrobenthic community assemblages in the Avilés Canyon, N Iberian Shelf: Baseline biodiversity information for a marine protected area. J. Mar. Syst. 2010, 80, 47–56. [Google Scholar] [CrossRef]
- Lourido, A.; Parra, S.; Sánchez, F. A comparative study of the macrobenthic infauna of two bathyal Cantabrian Sea areas: The Le Danois Bank and the Avilés Canyon System (S Bay of Biscay). Deep Sea Res. II 2014, 106, 141–150. [Google Scholar] [CrossRef]
- Flach, E.; Muthumbi, A.; Heip, C. Meiofauna and macrofauna community structure in relation to sediment composition at the Iberian margin compared to the Goban Spur (NE Atlantic). Prog. Oceanogr. 2002, 52, 433–457. [Google Scholar] [CrossRef]
- Levin, L.A.; Gooday, A.J. The deep Atlantic Ocean. In Ecosystems of the Deep Oceans. Ecosystems of the World; Tyler, P.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 28, pp. 111–178. [Google Scholar]
- Gage, J.D. Food inputs, utilization, carbon flow and energetics. In Ecosystems of the Deep Oceans. Ecosystems of the World; Tyler, P.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 28, pp. 315–380. [Google Scholar]
- Stuart, C.T.; Rex, M.A.; Etter, R.J. Large-scale spatial and temporal patterns of deep-sea benthic species diversity. In Ecosystems of the Deep Oceans. Ecosystems of the World; Tyler, P.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 28, pp. 295–311. [Google Scholar]
- Hessler, R.R.; Sanders, H.L. Faunal diversity in the deep sea. Deep Sea Res. 1967, 14, 65–78. [Google Scholar] [CrossRef]
- Galeron, J.; Sibuet, M.; Mahaut, M.; Dinet, A. Variation in structure and biomass of the benthic communities at three contrasting sites in the tropical Northeast Atlantic. Mar. Ecol. Prog. Ser. 2000, 197, 121–137. [Google Scholar] [CrossRef]
- Gage, J.D.; Tyler, P.A. Deep-Sea Biology. A Natural History of Organisms at the Deep-Sea Floor; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Ramirez-Llodra, E.; Brandt, A.; Danovaro, R.; De Mol, B.; Escobar, E.; German, C.R.; Levin, L.A.; Martinez Arbizu, P.; Menot, L.; Buhl-Mortensen, P.; et al. Deep, diverse and definitely different: Unique attributes of the world’s largest ecosystem. Biogeosciences 2010, 7, 2851–2899. [Google Scholar] [CrossRef]
- Escobar-Briones, E.; Estrada-Santillán, E.L.; Legendre, P. Macrofaunal density and biomass in the Campeche canyon, southwestern Gulf of Mexico. Tropical studies in oceanography. The deep Gulf of Mexico benthos Program. Deep-Sea Res. II 2008, 55, 2679–2685. [Google Scholar] [CrossRef]
- Shields, M.A.; Blanco-Perez, R. Polychaete abundance, biomass and diversity patterns at the Mid-Atlantic Ridge, North Atlantic Ocean. Deep Sea Res. II 2013, 98B, 315–325. [Google Scholar] [CrossRef]
- Stora, G.; Bourcier, M.; Arnoux, A.; Gerino, M.; Le Campion, J.; Gilbert, F.; Durbec, J.P. The deep-sea macrobenthos on the continental slope of the northwestern Mediterranean Sea: A quantitative approach. Deep Sea Res. I 1999, 46, 1339–1368. [Google Scholar] [CrossRef][Green Version]
- Probert, P.K.; Glasby, C.J.; Grove, S.L.; Paavo, B.L. Bathyal polychaete assemblages in the region of the Subtropical Front, Chatham Rise, New Zealand. N. Z. J. Mar. 2009, 43, 1121–1135. [Google Scholar] [CrossRef][Green Version]
- Davies, C.E.; Moss, D.; Hill, M.O. EUNIS Habitat Classification Revised 2004; Report of European Environment Agency (EEA); European Nature Information System (EUNIS): Copenhagen, Denmark, 2004.
- Sánchez, F.; Gómez-Ballesteros, M.; González-Pola, C.; Punzón, A. Sistema de Cañones Submarinos de Avilés. Proyecto LIFE +INDEMARES; Fundación Biodiversidad del Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2014. [Google Scholar]
- Clark, M.R.; Rowden, A.A.; Schlacher, T.; Williams, A.; Consalvey, M.; Stocks, K.I.; Rogers, A.D.; O’Hara, T.D.; White, M.; Shank, T.M.; et al. The ecology of seamounts: Structure, function, and human impacts. Ann. Rev. Mar. Sci. 2010, 2, 253–278. [Google Scholar] [CrossRef] [PubMed]
- McClain, C.R.; Rex, M.A.; Etter, R.J. Patterns in deep-sea macroecology. In Marine Macroecology; Witman, J.D., Roy, K., Eds.; The University of Chicago Press: Chicago, IL, USA, 2009; Chapter 3. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).