Dormice (Gliridae) in the Diets of Predators in Europe: A Review Broadening Understanding of Dormouse Ecology
Abstract
1. Introduction
- Review the geographic variation in predation on dormice in Europe;
- Identify the most important dormouse predators and compile lists of predators of separate dormouse species;
- Examine cases in which dormice made up noteworthy proportions (>10%) in the diets of predators;
- Evaluate the occurrence of dormice in the winter diets of predators.
2. Materials and Methods
- The numbers of prey items recorded;
- The frequency of occurrence—the percentage of food items found in the total sample;
- The relative frequency of occurrence—the percentage of faeces, pellets or stomach samples in which the particular food item was found;
- The proportion of biomass consumed—the percentage of consumed biomass composed of one type of food item.
3. Results and Discussion
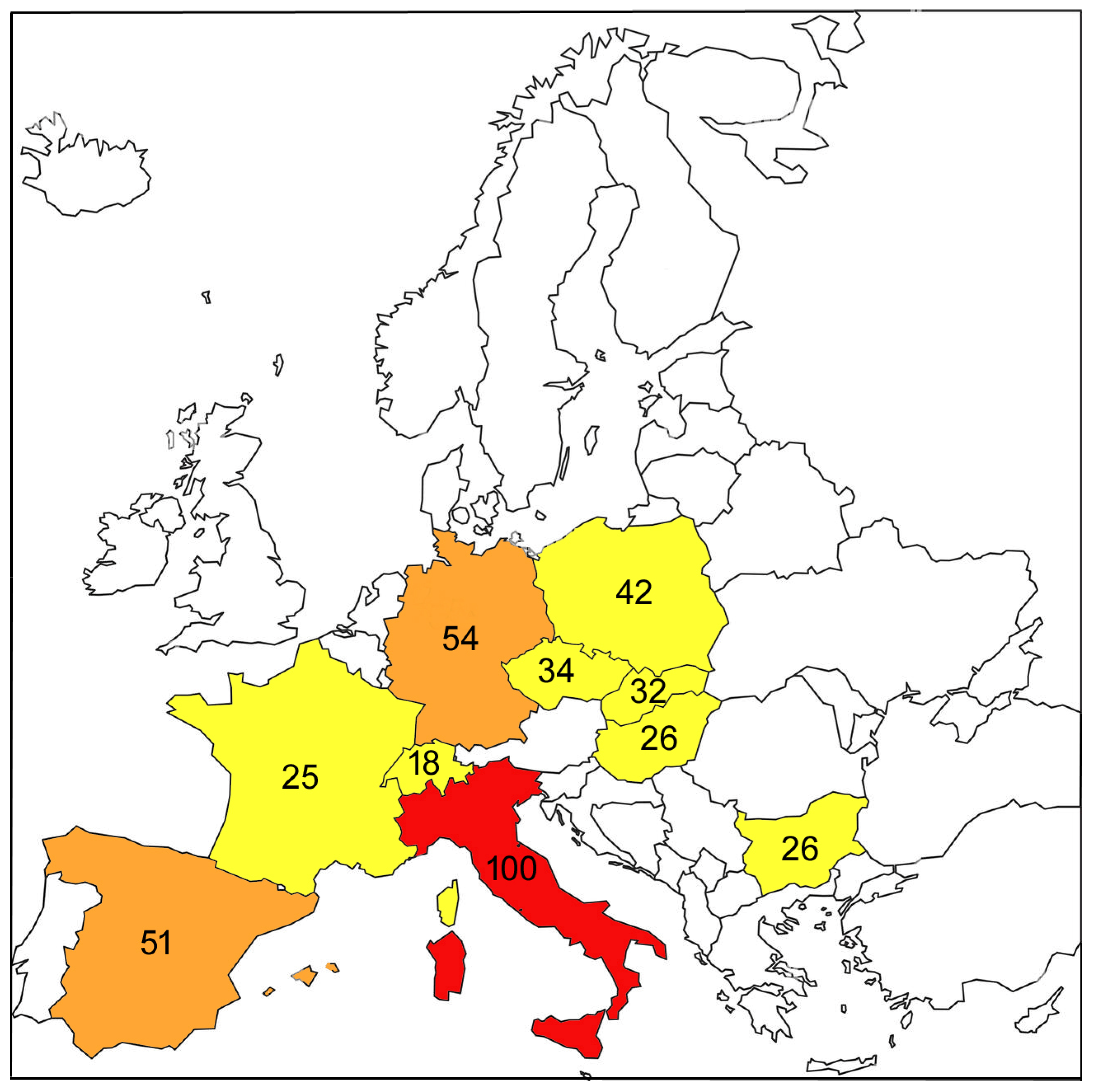
3.1. Geographic Variation in Predation on Dormice in Europe
3.2. The Main Dormouse Predators in Europe
3.3. Noteworthy Proportions of Dormice in Diets of Predators
| Predator Species | Dormouse Species | Proportion in the Diet | Country | Region/Habitat/Season | References | |
|---|---|---|---|---|---|---|
| by No. of Individuals, % | by Biomass, % | |||||
| Strix aluco | G. g. | 13.6 | 71.2 | Italy | Central Italy, beech forest | [50] |
| Strix aluco | G. g. + M. a. | 22.9 | 56 | Italy | Italian Alps, 3 localities | [51] |
| Strix aluco | G. g. | 44 | 81.2 | Italy | Italian Alps, coppice woodland | [24] |
| Strix aluco | G. g. + M. a. | 9.2 | 27.3 | Italy | Italian Alps, broadleaved forest | [24] |
| Strix aluco | G. g. + M. a. + E. q. | 20.9 | 43 | Italy | Italian Alps, mixed high forest | [24] |
| Strix aluco | G. g. + M. a. | 10.7 | 51.2 | Italy | Sicily, beech forest | [52] |
| Strix aluco | G. g. + M. a. | 7.3 | 30.6 | Slovenia | 13 localities | [53] |
| Strix aluco | M. a. + G. g. | 14.97 | Belarus | Brest region, forest | [54] | |
| Strix aluco | M. a. + G. g. | 10.7 | Slovakia | Kremnické vrchy Mts, forest | [55] | |
| Strix aluco | M. a. | 10.24 | Slovakia | Wet cold mountains | [37] | |
| Strix aluco | M. a. | 14.25 | Slovakia | Chočské vrchy Mts. | [37] | |
| Strix aluco | M. a. | 16.24 | Slovakia | Muránska planina Mts. | [37] | |
| Strix aluco | M. a. + G. g. | 10.85 | Slovakia | Veľká Fatra Mts. | [37] | |
| Strix aluco | M. a. + G. g. | 11.04 | Slovakia | Voniaca valley | [37] | |
| Strix aluco | M. a. + G. g. | 17.84 | Romania | Apuseni Mts., 4 localities | [37] | |
| Strix aluco | G. g. | 11.8 | Russia | Caucasus | [37] | |
| Strix aluco | G. g. + M. a. + D. n. | 19.7 | Slovenia | Topla Reber | [37] | |
| Strix aluco | G. g. + M. a. + D. n. | 26.3 | Montenegro | Tara canyon | [37] | |
| Strix aluco | G. g. + M. a. + E. q. | 12.65 | France | Savoy Alps | [37] | |
| Strix aluco | G. g. + M. a. + D. n. | 10.8 | Bulgaria | Rocky habitats | [56] | |
| Strix aluco | G. g. + M. a. | 13.5–27.5 | 10–29.5 | Serbia | Three localities | [57] |
| Strix aluco | G. g. + D. n. | 18.3 | 51 | Greece | Axios-Loudias-Aliakmonas National Park | [58] |
| Strix aluco | G. g. + M. a. | 22.7 | Italy | Tuscany, beech forest | [59] | |
| Strix uralensis | G. g. | 58.8 | 93.9 | Slovenia | Post-breeding period | [26] |
| Strix uralensis | Gliridae | 4.9 | 20.6 | Slovenia | Non-breeding period | [60] |
| Bubo bubo | G. g. | 22.4 | 8.5 | Italy | Italian Alps, 3 localities | [51] |
| Bubo bubo | G. g. | 22.8 | Italy | Italian Alps | [61] | |
| Bubo bubo | Gliridae | 28.5 | Italy | Italian Alps | [62] | |
| Bubo bubo | G. g. + E. q. | 15.6 | France | France Alps | [63] | |
| Bubo bubo | G. g. + E. q. | 4.5–14.8 | 1.1–5.7 | France | Alpes Maritimes | [64] |
| Bubo bubo | G. g. | up to 37.7 | Slovenia | Coastal area | [65] | |
| Aegolius funereus | M. a. | 16.3 | Germany | Siegerland, coppiced oak wood | [66] | |
| Aegolius funereus | M. a. | 0–14.6 | Czech Republic | Nine localities | [67] | |
| Aegolius funereus | M. a. | 60.87 | Czech Republic | South Bohemia, one nestbox | [68] | |
| Aegolius funereus | M. a. | 17.5 | Belarus | Brest region | [69] | |
| Aegolius funereus | M. a. | 11.4 | 10.4 | Ukraine | Polesia, 2 localities | [70] |
| Aegolius funereus | M. a. | 12.33 | 10.7 | Serbia | High-mountain coniferous forests | [27] |
| Aegolius funereus | M. a. + D. n. | 12.03 | Slovakia | The whole country | [37] | |
| Aquila chrysaetos | G. g. + M. a. | 11.7 | Italy | Italian Alps | [71] | |
| Aquila chrysaetos | G. g. | 10.5 | France | Provence | [72] | |
| Felis silvestris | G. g. + M. a. + E. q. | 7.1 | 19.5 | Italy | Woodland/open areas | [73] |
| Martes foina | G. g. | 31.43 | 40.26 | Italy | Val Grande National Park | [74] |
| Martes foina | G. g. + M. a. | 17.1 | Italy | Wood Querco-Carpinetum | [75] | |
| Martes foina | G. g.+ M. a.+ Gliridae | 12.8 | 9.8 | Italy | Tuscany region, wood/rural | [76] |
| Martes martes | E. q. | 10.3 | 9.5 | Italy | Sardinia | [77] |
| Martes martes | E. q. | 18.3 | 15.2 | Italy | Minorca, in March–April | [78] |
| Mustela erminea | E. q. | 10.77 | 9.23 | Italy | Italian Alps | [79] |
| Vulpes vulpes | G. g. | 9.9 | Russia | Caucasus | [38] | |
| Vulpes vulpes | M. a. + G. g. | up to 11.8 | Germany | Foothills of Bavarian Alps | [80] | |
| Lynx lynx | G. g. | 18 | 6.9 | SloveniaCroatia | Northern Dinaric Mts. | [25] |
3.4. Dormice in Winter Diets of Predators
4. Conclusions
- Dormice may be an important food source for predators in some regions, especially in autumn;
- Mammalian predators are not a significant factor in dormouse winter mortality;
- Dormice may leave their hibernacula in the winter period;
- Nocturnal dormice may also be active in the daytime.
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holden-Musser, M.E.; Juškaitis, R.; Musser, G.M. Family Gliridae (Dormice). In Handbook of the Mammals of the World. Lagomorphs and Rodents I; Wilson, D.E., Lacher, T.E., Jr., Mittermeier, R.A., Eds.; Lynx Edicions: Barselona, Spain, 2016; Volume 6, pp. 838–889. [Google Scholar]
- Airapetyants, A.E. Dormice; Leningrad University Press: Leningrad, Russia, 1983; 192p. (In Russian) [Google Scholar]
- Rossolimo, O.L.; Potapova, E.G.; Pavlinov, I.Y.; Kruskop, S.V.; Voltzit, O.V. Dormice (Myoxidae) of the World; Moscow University Press: Moscow, Russia, 2001; 229p. (In Russian) [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. 2022. Available online: http://www.iucnredlist.org/ (accessed on 15 November 2022).
- Kryštufek, B. Glis glis (Rodentia: Gliridae). Mamm. Species 2010, 42, 195–206. [Google Scholar] [CrossRef]
- Juškaitis, R. The Common Dormouse Muscardinus avellanarius: Ecology, Population Structure and Dynamics, 2nd ed.; Nature Research Centre Publishers: Vilnius, Lithuania, 2014; 196p. [Google Scholar]
- Morris, P. Dormice: A Tail of Two Species, 2nd ed.; Whittet Books: Stansted, UK, 2011; 144p. [Google Scholar]
- Montecchio, L.; Scattolin, L.; De Battisti, R. Dormouse injuries predispose beech to infection by Neonectria ditissima. For. Pathol. 2011, 41, 114–119. [Google Scholar] [CrossRef]
- Büchner, S.; Trout, R.; Adamík, P. Conflicts with Glis glis and Eliomys quercinus in households: A practical guideline for sufferers (Rodentia: Gliridae). Lynx 2018, 49, 19–26. [Google Scholar] [CrossRef]
- Andreychev, A. Distribution and population density forest dormouse (Dryomys nitedula, Rodentia, Gliridae) in a region of the Middle Volga, Russia. Ecol. Environ. Conserv. 2021, 27, 369–373. [Google Scholar]
- Carpaneto, G.M.; Cristaldi, M. Dormice and man: A review of past and present relations. Hystrix 1994, 6, 303–330. [Google Scholar]
- Juškaitis, R. Interactions between dormice (Gliridae) and hole-nesting birds in nestboxes. Folia Zool. 2006, 55, 225–236. [Google Scholar]
- Adamík, P.; Král, M. Nest losses of cavity nesting birds caused by dormice (Gliridae, Rodentia). Acta Theriol. 2008, 53, 185–192. [Google Scholar] [CrossRef]
- Czeszczewik, D.; Walankiewicz, W.; Stanska, M. Small mammals in nests of cavity-nesting birds: Why should ornithologists study rodents? Can. J. Zool. 2008, 86, 286–293. [Google Scholar] [CrossRef]
- Kuipers, L.; Scholten, J.; Thissen, J.B.M.; Bekkers, L.; Geertsma, M.; Pulles, R.; Siepel, H.; van Turnhout, L. The diet of the garden dormouse (Eliomys quercinus) in the Netherlands in summer and autumn. Lutra 2012, 55, 17–27. [Google Scholar]
- Juškaitis, R.; Baltrūnaitė, L. Feeding on the edge: The diet of the hazel dormouse Muscardinus avellanarius (Linnaeus 1758) on the northern periphery of its distributional range. Mammalia 2013, 77, 149–155. [Google Scholar] [CrossRef]
- Juškaitis, R.; Baltrūnaitė, L. Seasonal variability in the diet of the forest dormouse, Dryomys nitedula, on the north-western edge of its distributional range. Folia Zool. 2013, 62, 311–318. [Google Scholar] [CrossRef]
- Juškaitis, R.; Baltrūnaitė, L.; Augutė, V. Diet of the fat dormouse (Glis glis) on the northern periphery of its distributional range. Mammal Res. 2015, 60, 155–161. [Google Scholar] [CrossRef]
- Vekhnik, V.A. Nutrition of the edible dormouse (Glis glis Linnaeus, 1766) across the distributional range. J. Wildl. Biodivers. 2022, 6, 1–23. [Google Scholar]
- Jentzsch, M. Zur Verbreitung der Haselmaus (Muscardinus avellanarius Linnaeus, 1758) in Sachsen-Anhalt. Herzynia N. F. 2004, 37, 127–135. [Google Scholar]
- Nedyalkov, N.; Popgeorgiev, G.; Staneva, A. Updated distribution of the elusive Roach’s mouse-tailed dormouse, Myomimus roachi Bate, 1937 (Mammalia: Rodentia: Gliridae) in Bulgaria. Hist. Nat. Bulg. 2018, 29, 3–8. [Google Scholar] [CrossRef]
- Bertolino, S. Distribution and status of the declining garden dormouse Eliomys quercinus. Mammal Rev. 2017, 47, 133–147. [Google Scholar] [CrossRef]
- Fedyń, I.; Figarski, T.; Kajtoch, Ł. Overview of the impact of forest habitats quality and landscape disturbances on the ecology and conservation of dormice species. Eur. J. For. Res. 2021, 140, 511–526. [Google Scholar] [CrossRef]
- Marchesi, L.; Sergio, F.; Pedrini, P. Implications of temporal changes in forest dynamics on density, nest-site selection, diet and productivity of tawny owls Strix aluco in the Alps. Bird Study 2006, 53, 310–318. [Google Scholar] [CrossRef][Green Version]
- Krofel, M.; Huber, D.; Kos, I. Diet of Eurasian lynx Lynx lynx in the northern Dinaric Mountains (Slovenia and Croatia): Importance of edible dormouse Glis glis as alternative prey. Acta Theriol. 2011, 56, 315–322. [Google Scholar] [CrossRef]
- Vrezec, A.; Mihelič, D. The Ural owl, Strix uralensis macroura, in Slovenia: An overview of current knowledge on species ecology. Riv. Ital. Ornitol. 2013, 82, 30–37. [Google Scholar] [CrossRef]
- Rajković, D.Z. Diet composition and prey diversity of Tengmalm’s owl Aegolius funereus (Linnaeus, 1758; Aves: Strigidae) in central Serbia during breeding. Turk. J. Zool. 2018, 42, 346–351. [Google Scholar]
- Juškaitis, R. Winter mortality of the common dormouse (Muscardinus avellanarius) in Lithuania. Folia Zool. 1999, 48, 11–16. [Google Scholar]
- Rozycka, D.; Lim, J.M.; Trout, R.C.; Brooks, S. Have feral boar significantly impacted hazel dormouse populations in Sussex, England? Folia Zool. 2015, 64, 337–341. [Google Scholar] [CrossRef]
- Scaravelli, D.; Aloise, G. Predation on dormice in Italy. Hystrix 1994, 6, 245–255. [Google Scholar]
- März, R. Nachweise von Schläfern aus Gewöllen. Beitr. Vogelkunde 1963, 8, 388–396. [Google Scholar]
- Obuch, J. Dormice in the diet of owls in Slovakia. Lynx 1998, 29, 31–41, (In Slovak with English Summary). [Google Scholar]
- Obuch, J. Dormice in the diet of owls in the Middle East. Trak. Univ. J. Sci. Res. 2001, 2, 145–150. [Google Scholar]
- Moreno, S. Lirón careto–Eliomys quercinus. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Barja, I., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2017; Available online: http://www.vertebradosibericos.org/ (accessed on 15 November 2022).
- Janossy, D.; Schmidt, E. Die Nahrung des Uhus (Bubo bubo). Regionale und erdzeitliche Änderungen. Bonn. Zool. Beitr. 1970, 21, 25–51. [Google Scholar]
- Capizzi, D. Diet shifts of the tawny owl Strix aluco in central and northern Italy. Ital. J. Zool. 2000, 67, 73–79. [Google Scholar] [CrossRef]
- Obuch, J. Spatial and temporal diversity of the diet of the tawny owl (Strix aluco). Slovak Raptor J. 2011, 5, 1–120. [Google Scholar] [CrossRef]
- Donaurov, S.S.; Popov, V.C.; Khonjakina, Z.P. The dormouse (Glis glis caspicus (Sat.) in the Caucasian reservation territory. Proc. Cauc. State Reserve 1938, 1, 227–280, (In Russian with English Summary). [Google Scholar]
- Ognev, S.I. Mammals of the USSR and Adjacent Countries. Rodents; Academy of Science of the USSR Press: Moscow–Leningrad, Russia, 1947; Volume 5, 809p. (In Russian) [Google Scholar]
- Lozan, M.; Belik, L.; Samarskij, S. Dormice (Gliridae) of the South-West USSR; Shtiintsa: Kishinev, Moldova, 1990; 147p. (In Russian) [Google Scholar]
- Hanski, I.P.; Henttonen, H. Garden Dormouse Disappeared from Finland? Luomus. 2014. Available online: https://www.luomus.fi/en/garden-dormouse-disappeared-finland (accessed on 15 November 2022).
- Uttendörfer, O. Die Ernährung der deutschen Raubvögel und Eulen und ihre Bedeutung in der heimischen Natur, 2 Aufl; Aula Verlag: Wiesbaden, Germany, 1997; 412p. [Google Scholar]
- Pukinskij, J.B. The Life of Owls; Leningrad University Press: Leningrad, Russia, 1977; 240p. (In Russian) [Google Scholar]
- Galeotti, P. Strix aluco Tawny Owl. In Birds of Western Palearctic, Update 3; Oxford University Press: Oxford, UK, 2001; pp. 43–77. [Google Scholar]
- Glue, D.E. Food of the barn owl in Britain and Ireland. Bird Study 1997, 21, 200–210. [Google Scholar] [CrossRef]
- Bright, P.W.; Morris, P.A. Why are dormice rare? A case study in conservation biology. Mammal Rev. 1996, 26, 157–187. [Google Scholar] [CrossRef]
- Andreychev, A.V.; Lapshin, A.S.; Kuznetsov, V.A. Food spectrum of the eagle owl (Bubo bubo) in the Republic of Mordovia. Zool. Zhurnal 2014, 93, 248–258. [Google Scholar]
- Capizzi, D.; Luiselli, L. The diet of the four-lined snake (Elaphe quatuorlineata) in mediterranean central Italy. Herpetol. J. 1997, 7, 1–5. [Google Scholar]
- Kryštufek, B.; Hudoklin, A.; Pavlin, D. Population biology of the edible dormouse Glis glis in mixed montane forest in central Slovenia over three years. Acta Zool. Hung. 2003, 49 (Suppl. 1), 85–97. [Google Scholar]
- Manganaro, A.; Ranazzi, L.; Salvati, L. The diet of Tawny Owls (Strix aluco) breeding in different woodlands of Central Italy. Buteo 2000, 11, 115–124. [Google Scholar]
- Sergio, F.; Marchesi, L.; Pedrini, P.; Peneteriani, V. Coexistence of a generalist owl with its intraguild predator: Distance-sensitive or habitat-mediated avoidance? Anim. Behav. 2007, 74, 1607–1616. [Google Scholar] [CrossRef]
- Sarà, M.; Zanca, L. The feeding habits of the tawny owl Strix aluco in Sicily and in Aspromonte (Calabria). Avocetta 1989, 13, 31–39, (In Italian with English Summary). [Google Scholar]
- Kuhar, B.; Kalan, G.; Janžekovič, F. Diet of the Tawny Owl Strix aluco in the Kozjansko region (E Slovenia). Acrocephalus 2006, 27, 147–154, (In Slovenian with English Summary). [Google Scholar]
- Demianchyk, V. Long-term dynamics of wood species of Micromammalia on the Vygonoshchi wood-marsh file. Sci. J. Lesya Ukr. Volyn Natl. Univ. 2009, 2, 234–238, (In Russian with English Summary). [Google Scholar]
- Poláček, M.; Baláž, M.; Obuch, J. Diet of the tawny owl (Strix aluco) in urban and forest environment. Tichodroma 2012, 24, 29–39, (In Slovak with English Summary). [Google Scholar]
- Obuch, J.; Benda, P. Contribution to the feeding ecology of Strix aluco and Bubo bubo (Aves: Strigiformes) in southwestern Bulgaria. Acta Soc. Zool. Bohem. 1996, 60, 43–49. [Google Scholar]
- Jovanovic-Grove, T.; Šćiban, M.; Ružić, M. Study of Tawny Owl Strix aluco Diet from Pellet Samples Collected in Serbia. The Owl Pages. 2008. Available online: https://www.owlpages.com/owls/articles.php?a=58 (accessed on 15 November 2022).
- Vavylis, D. Diet Analysis of Three Owl Species Tyto alba, Strix aluco and Asio otus. Bachelor’s Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2012. (In Greece). [Google Scholar]
- Turini, R. Preliminary data on the micromammals of the “Orrrido di Botri Natural Reserve” from Tawny Owl pellets and fox and weasel scat analysis. Atti della Societa Toscana di Scienze Naturali Mem. Ser. B 1994, 101, 101–105, (In Italian with English Summary). [Google Scholar]
- Vrezec, A. The ecology of the Ural Owl at south-western border of its distribution (Slovenia). Raptors Conserv. 2016, 32, 8–20. [Google Scholar] [CrossRef]
- Marchesi, L.; Sergio, F.; Pedrini, P. Costs and benefits of breeding in human-altered landscapes for the eagle owl Bubo bubo. Ibis 2002, 144, 164–177. [Google Scholar] [CrossRef]
- Marchesi, L.; Pedrini, P.; Sergio, F. Biases associated with diet study methods in the Eagle Owl. J. Raptor Res. 2002, 36, 11–16. [Google Scholar]
- Bayle, P. Regime alimentaire du grand-duc d’Europe Bubo bubo en periode de reproduction dans le Parc National du Mercantour et ses environs Alpes-Maritimes et Alpes-de-Haute-Provence, France. Avocetta 1996, 201, 12–25. [Google Scholar]
- Rathgeber, C.; Bayle, P. Régime alimentaire du grand duc d’Europe, Bubo bubo, en période de reproduction, dans la région de menton (Alpes-Maritimes, France). Avocetta 1997, 20, 12–25. [Google Scholar]
- Lipej, L.; Kryštufek, B. Dormice as prey of owls: Evidence from Slovenia. In Abstract Book, Proceedings of the IVth International Conference on Dormice (Rodentia, Gliridae), Edirne, Turkey, 13–16 September 1999; Trakya University Press: Edirne, Turkey, 1999; p. 26. [Google Scholar]
- Klaas, C. Zur Verbreitung des Rauhfuβkauzes. Nat. und Mus. 1971, 101, 467–471. [Google Scholar]
- Kloubec, B.; Vacík, R. Outline of food ecology of Tengmalm‘s owl (Aegolius funereus L.) in Czechoslovakia. Tichodroma 1990, 3, 103–125, (In Czech with English Summary). [Google Scholar]
- Mráz, L. Small mammals in the food of owls on the territory of south Bohemia. Lynx 1987, 23, 63–74, (In Czech with English Summary). [Google Scholar]
- Kitel’, D.A. Pecularities of Breeding of Some Owl Species in the Brest Oblast‘. Master’s Thesis, Pushkin University, Brest, Belarus, 2010. (In Russian). [Google Scholar]
- Kuzmenko, Y.; Mishta, A. Diet of Tengmalm’s owl (Aegolius funereus) and great grey owl (Strix nebulosa) in northern Ukraine. Proc. Zool. Mus. 2014, 45, 65–69, (In Ukrainian with English and Russian Summaries). [Google Scholar]
- Pedrini, P.; Sergio, F. Density, productivity, diet, and human persecution of golden eagles (Aquila chrysaetos) in the central-eastern Italian Alps. J. Raptor Res. 2001, 35, 40–48. [Google Scholar]
- Iborra, O.; Arthur, C.; Bayle, P. Importance trophique du lapin de garenne pour les grands rapaces provençaux. Vie et Milieu 1990, 40, 177–188. [Google Scholar]
- Apostolico, F.; Vercillo, F.; La Porta, G.; Ragni, B. Long-term changes in diet and trophic niche of the European wildcat (Felis silvestris silvestris) in Italy. Mammal Res. 2016, 61, 109–119. [Google Scholar] [CrossRef]
- Balestrieri, A.; Mosini, A.; Saino, N. Distribuzione ed Ecologia di Martora e Faina nel Parco Nazionale della Val Grande 2015–2016; Technical Report; University of Milan: Milan, Italy, 2016; 47p. [Google Scholar]
- Bertolino, S.; Dore, B. Food habits of the stone marten Martes foina in “La Mandria“ Regional Park (Piedmont region, north-western Italy). Hystrix 1995, 7, 105–111. [Google Scholar]
- Genovesi, P.; Secchi, M.; Boitani, L. Diet of stone martens: An example of ecological flexibility. J Zool. 1996, 238, 545–555. [Google Scholar] [CrossRef]
- Lombardini, M.; Murru, M.; Repossi, A.; Cinerari, C.E.; Vidus Rosin, A.; Mazzoleni, L.; Meriggi, A. Spring diet of the pine marten in Sardinia, Italy. Anim. Biodivers. Conserv. 2015, 38, 183–190. [Google Scholar] [CrossRef]
- Clevenger, A.P. Spring and summer food habits and habitat use of the European pine marten (Martes martes) on the island of Minorca, Spain. J. Zool. 1993, 229, 153–161. [Google Scholar] [CrossRef]
- Martinoli, A.; Preatoni, D.G.; Chiarenzi, B.; Wauters, L.A.; Tosi, G. Diet of stoats (Mustela erminea) in an Alpine habitat: The importance of fruit consumption in summer. Acta Oecol. 2001, 22, 45–53. [Google Scholar] [CrossRef]
- Storch, I.; Kleine, C. Zur Nahrungswahl des Fuchses in den Voralpen. Z. Jagdwiss. 1991, 37, 267–270. [Google Scholar] [CrossRef]
- Milchev, B.; Georgiev, V. Food spectrum and predominant prey in the diet of the eagle owl Bubo bubo population in Southeastern Bulgaria. For. Ideas 2019, 25, 56–69. [Google Scholar]
- Baudvin, H.; Jouaire, S. Le régime alimentaire d’une population forestière de Chouettes hulottes (Strix aluco) en Bourgogne. Rev. Sci. Bourgogne-Nat. 2006, 4, 85–89. [Google Scholar]
- Hecker, K.; Bakó, B.; Csorba, G. New data on the distribution of the Hungarian dormouse species (Gliridae). Állattani Közlemények 2003, 88, 57–67, (In Hungarian with English Summary). [Google Scholar]
- Balčiauskienė, L.; Balčiauskas, L. Common dormouse as a prey item of breeding tawny owls in five districts of Lithuania. Acta Zool. Lituan. 2008, 18, 58–62. [Google Scholar] [CrossRef]
- Juškaitis, R. Summer mortality in the hazel dormouse (Muscardinus avellanarius) and its effect on population dynamics. Acta Theriol. 2014, 59, 311–316. [Google Scholar] [CrossRef]
- Verbeylen, G.; Andre, A.; Desmet, A.; Manzanares, L.; Mels, B.; Pulles, R.; Swinnen, K.; Vanseuningen, I.; Vermeiren, M. et al. Nest Site Selection and Use of Other Habitats by the Hazel Dormouse Muscardinus Avellanarius in Voeren (Flanders); Report Natuurpunt studie 2017/3; Natuurpunt Research Department (Mammal Working Group): Mechelen, Belgium, 2017; 851p. [Google Scholar]
- Juškaitis, R. Local impact of the tawny owls (Strix aluco) on the common dormice (Muscardinus avellanarius) in Lithuania. Ekológia 2004, 23, 305–309. [Google Scholar]
- Żmihorski, M.; Gryz, J.; Krauze-Gryz, D.; Olczyk, A.; Osojca, G. The tawny owl Strix aluco as a material collector in faunistic investigations: The case study of small mammals in NE Poland. Acta Zool. Lituan. 2011, 21, 185–191. [Google Scholar] [CrossRef]
- Iannarilli, F.; Melcore, I.; Sozio, G.; Roviani, D.; Mortelliti, A. Long-term colonization and extinction patterns of a forest-dependent rodent (Muscardinus avellanarius) in highly fragmented landscapes. Hystrix 2017, 28, 73–77. [Google Scholar]
- Juškaitis, R. Forest dormouse Dryomys nitedula (Pallas, 1778). In Red Data Book of Lithuania. Animals, Plants, Fungi; Rašomavičius, V., Ed.; Lututė: Vilnius, Lithuania, 2021; 300p, (In Lithuanian with English Summary). [Google Scholar]
- Goethe, F. Die Säugetiere des Teutoburger Waldes und des Lipperlandes. Abh. Landesmus. Nat. Münster Westfal. 1955, 17, 5–195. [Google Scholar]
- Ćirović, D.; Penezić, A.; Milenković, M.; Paunović, M. Winter diet composition of the golden jackal (Canis aureus L., 1758) in Serbia. Mamm. Biol. 2014, 79, 132–137. [Google Scholar] [CrossRef]
- Romanowski, J.; Żmihorski, M. Seasonal and habitat variation in the diet of the tawny owl (Strix aluco) in Central Poland during unusually warm years. Biologia 2009, 64, 365–369. [Google Scholar] [CrossRef]
- Southern, H.N. Tawny owls and their prey. Ibis 1954, 96, 384–410. [Google Scholar] [CrossRef]
- Vogel, P.; Frey, H. L’hibernation du muscardin Muscardinus avellanarius (Gliridae, Rodentia) en nature: Nids, fréquence des reveils et température corporelle. Bull. Soc. Vaudoise Sci. Nat. 1995, 83, 217–230. [Google Scholar]
- Gubert, L.; McDonald, R.A.; Wilson, R.J.; Chanin, P.; Bennie, J.J.; Mathews, F. The elusive winter engineers: Structure and materials of hazel dormouse hibernation nests. J. Zool. 2022, 316, 81–91. [Google Scholar] [CrossRef]
- Tulis, F. The Influence of Land Use to Diet of Long-Eared Owl (Asio otus). Ph.D. Thesis, Constantine the Philosopher University, Nitra, Slovakia, 2013. (In Slovakian with English Summary). [Google Scholar]
- Tulis, F. (Constantine the Philosopher University, Nitra, Slovakia). Personal communication, 2022.
- Ruf, T.; Bieber, C. Why hibernate? Predator avoidance in the edible dormouse. Mammal Res. 2022. [Google Scholar] [CrossRef]
- Polak, S. The use of caves by the edible dormouse (Myoxus glis) in the Slovenian karst. Nat. Croat. 1997, 6, 313–321. [Google Scholar]
- Vietinghoff-Riesch, A. Der Siebenschläfer (Glis glis L.); VEB Gustav Fischer Verlag: Jena, Germany, 1960; 196p. [Google Scholar]
- Drenik, K. Diet of Wild Boar (Sus scrofa) in Kočevje Region. Master’s Thesis, University of Ljubljana, Slovenia, 2007. (In Slovenian with English Summary). [Google Scholar]
- Schley, L.; Roper, T.J. Diet of wild boar Sus scrofa in Western Europe, with particular reference to consumption of agricultural crops. Mammal. Rev. 2003, 33, 43–56. [Google Scholar] [CrossRef]
- Ballari, S.A.; Barrios-García, M.N. A review of wild boar Sus scrofa diet. Mammal Rev. 2014, 44, 124–134. [Google Scholar] [CrossRef]
- Singer, F.J.; Swank, W.T.; Clebsh, E.E.C. Effects of wild pig rooting in a deciduous forest. J. Wildlife Manag. 1984, 48, 464–473. [Google Scholar] [CrossRef]
- Mori, E.; Ferretti, F.; Lagrotteria, A.; La Greca, L.; Solano, E.; Fattorini, N. Impact of wild boar rooting on small forest-dwelling rodents. Ecol. Res. 2020, 35, 675–681. [Google Scholar] [CrossRef]
- Büchner, S. (Büro für ökologische Studien, Markersdorf, Germany). Personal communication, 2022.
- Panchetti, F.; Amori, G.; Carpaneto, G.M.; Sorace, A. Activity patterns of the common dormouse (Muscardinus avellanarius) in different Mediterranean ecosystems. J. Zool. 2004, 262, 289–294. [Google Scholar] [CrossRef]
- Likhachev, G.N. Population structure of the common dormouse (Muscardinus avellanarius). Byull. Moskovsk. Obshch. Isp. Prir. Otd. Biol. 1966, 71, 18–29, (In Russian with English Summary). [Google Scholar]
- Bieber, C.; Juškaitis, R.; Turbill, C.; Ruf, T. High survival during hibernation affects onset and timing of reproduction. Oecologia 2012, 169, 155–166. [Google Scholar] [CrossRef]
| Predator Species | Dormouse Species | ||||
|---|---|---|---|---|---|
| Muscardinus avellanarius | Glis glis | Dryomys nitedula | Eliomys quercinus | Myomimus roachi | |
| Mammals | |||||
| Brown rat Rattus norvegicus | 1 | ||||
| House rat Rattus rattus | 1 | ||||
| Brown bear Ursus arctos | 2 | ||||
| Golden jackal Canis aureus | 5 | 2 | 1 | ||
| Grey wolf Canis lupus | 3 | 2 | 2 | ||
| Domestic dog Canis familiaris | 1 | ||||
| Red fox Vulpes vulpes | 15 | 14 | 1 | 6 | |
| Eurasian otter Lutra lutra | 2 | ||||
| Beech marten Martes foina | 7 | 15 | 6 | ||
| Pine marten Martes martes | 7 | 14 | 1 | 10 | |
| Eurasian badger Meles meles | 2 | 3 | 1 | 2 | |
| Stoat Mustela erminea | 1 | 1 | |||
| Least weasel Mustela nivalis | 5 | 3 | |||
| Western polecat Mustela putorius | 1 | 1 | |||
| American mink Neovison vison | 2 | ||||
| Common genet Genetta genetta | 1 | 7 | |||
| Egyptian mongoose Herpestes ichneumon | 3 | ||||
| European wildcat Felis silvestris | 8 | 13 | 3 | 6 | |
| Domestic cat Felis catus | 8 | 1 | 3 | ||
| Eurasian lynx Lynx lynx | 4 | 1 | |||
| Iberian lynx Lynx pardinus | 2 | ||||
| Northern racoon Procyon lotor | 2 | 1 | |||
| Wild boar Sus scrofa | 1 | 3 | |||
| Owls | |||||
| Common barn owl Tyto alba | 71 | 26 | 5 | 26 | 5 |
| Eurasian scops owl Otus scops | 2 | 2 | |||
| Eurasian eagle owl Bubo bubo | 16 | 44 | 18 | 28 | |
| Tawny owl Strix aluco | 102 | 66 | 27 | 22 | |
| Ural owl Strix uralensis | 7 | 9 | 4 | ||
| Great grey owl Strix nebulosa | 1 | ||||
| Eurasian pygmy owl Glaucidium passerinum | 9 | ||||
| Little owl Athene noctua | 3 | 1 | 2 | 1 | |
| Boreal owl Aegolius funereus | 58 | 3 | 10 | 16 | |
| Northern long-eared owl Asio otus | 47 | 12 | 3 | 5 | |
| Short-eared owl Asio flammeus | 1 | ||||
| Diurnal birds | |||||
| Lesser spotted eagle Clanga pomarina | 1 | 1 | 1 | ||
| Spanish imperial eagle Aquila adalberti | 1 | ||||
| Eastern imperial eagle Aquila heliaca | 2 | 2 | |||
| Golden eagle Aquila chrysaetos | 1 | 4 | 1 | ||
| Bonelli’s eagle Aquila fasciata | 2 | ||||
| Eurasian sparrowhawk Accipiter nisus | 1 | 1 | 2 | ||
| Northern goshawk Accipiter gentilis | 2 | ||||
| Red kite Milvus milvus | 2 | ||||
| Black kite Milvus migrans | 2 | ||||
| Eurasian buzzard Buteo buteo | 2 | 1 | 1 | 1 | |
| Lanner falcon Falco biarmicus | 2 | 1 | |||
| Saker falcon Falco cherrug | 1 | ||||
| Eurasian jay Garrulus glandarius | 1 | ||||
| Eurasian magpie Pica pica | 1 | ||||
| Common raven Corvus corax | 2 | 1 | 1 | 1 | |
| Common pheasant Phasianus colchicus | 1 | ||||
| Reptiles | |||||
| Four-lined snake Elaphe quatuorlineata | 2 | ||||
| Aesculapian ratsnake Zamenis longissimus | 1 | 6 | |||
| Adder Vipera berus | 1 | 1 | |||
| Asp viper Vipera aspis | 4 | 1 | |||
| Total number of predator species | 37 | 34 | 16 | 31 | 2 |
| Total number of sources | 404 | 263 | 80 | 165 | 5 |
| Predator Species | Number of Sources | Total Number of Sources | ||||
|---|---|---|---|---|---|---|
| Muscardinus avellanarius | Glis glis | Dryomys nitedula | Eliomys quercinus | Total Dormice | ||
| Owls | ||||||
| Tyto alba | 9 | 1 | 10 | 100 | ||
| Strix aluco | 1 | 1 | 1 | 3 | 137 | |
| Asio otus | 19 | 1 | 20 | 53 | ||
| Mammals | ||||||
| Canis aureus | 1 | 1 | 1 | 1 * | 10 | |
| Vulpes vulpes | 3 | 2 | 5 | 24 | ||
| Martes foina | 3 | 3 | 22 | |||
| Martes martes | 2 | 2 | 25 | |||
| Martes sp. | 1 | 1 | 2 | |||
| Felis silvestris | 1 | 1 | 17 | |||
| Lynx lynx | 1 | 1 | 6 | |||
| Procyon lotor | 1 | 1 | 2 | |||
| Sus scrofa | 1 | 1 | 2 | 3 | ||
| Total | 37 | 12 | 3 | 1 | 53 | 400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juškaitis, R. Dormice (Gliridae) in the Diets of Predators in Europe: A Review Broadening Understanding of Dormouse Ecology. Diversity 2023, 15, 52. https://doi.org/10.3390/d15010052
Juškaitis R. Dormice (Gliridae) in the Diets of Predators in Europe: A Review Broadening Understanding of Dormouse Ecology. Diversity. 2023; 15(1):52. https://doi.org/10.3390/d15010052
Chicago/Turabian StyleJuškaitis, Rimvydas. 2023. "Dormice (Gliridae) in the Diets of Predators in Europe: A Review Broadening Understanding of Dormouse Ecology" Diversity 15, no. 1: 52. https://doi.org/10.3390/d15010052
APA StyleJuškaitis, R. (2023). Dormice (Gliridae) in the Diets of Predators in Europe: A Review Broadening Understanding of Dormouse Ecology. Diversity, 15(1), 52. https://doi.org/10.3390/d15010052





