Entomopathogenic Fungi in the Soils of China and Their Bioactivity against Striped Flea Beetles Phyllotretastriolata
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection
2.2. Isolation of Fungi from the Soil Samples
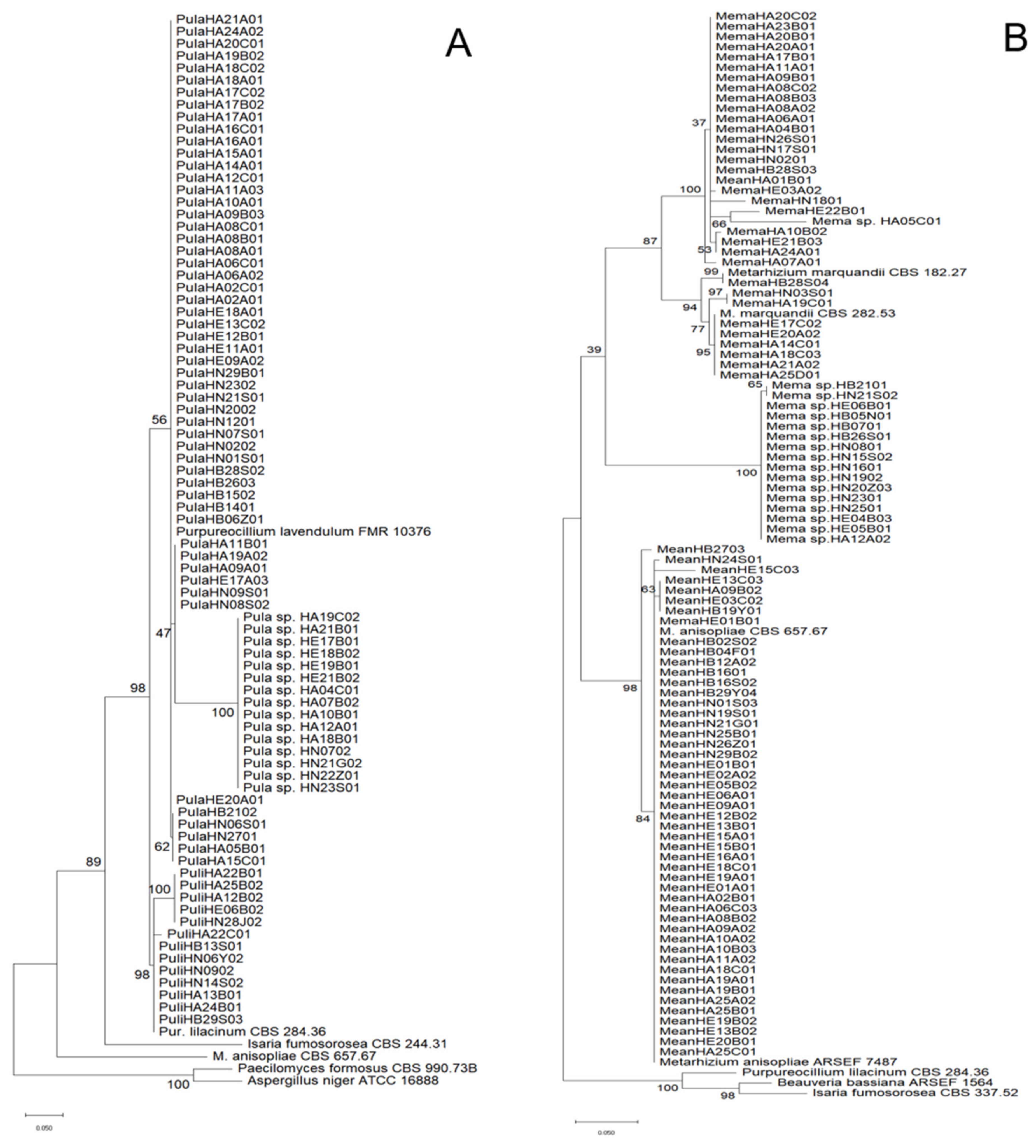
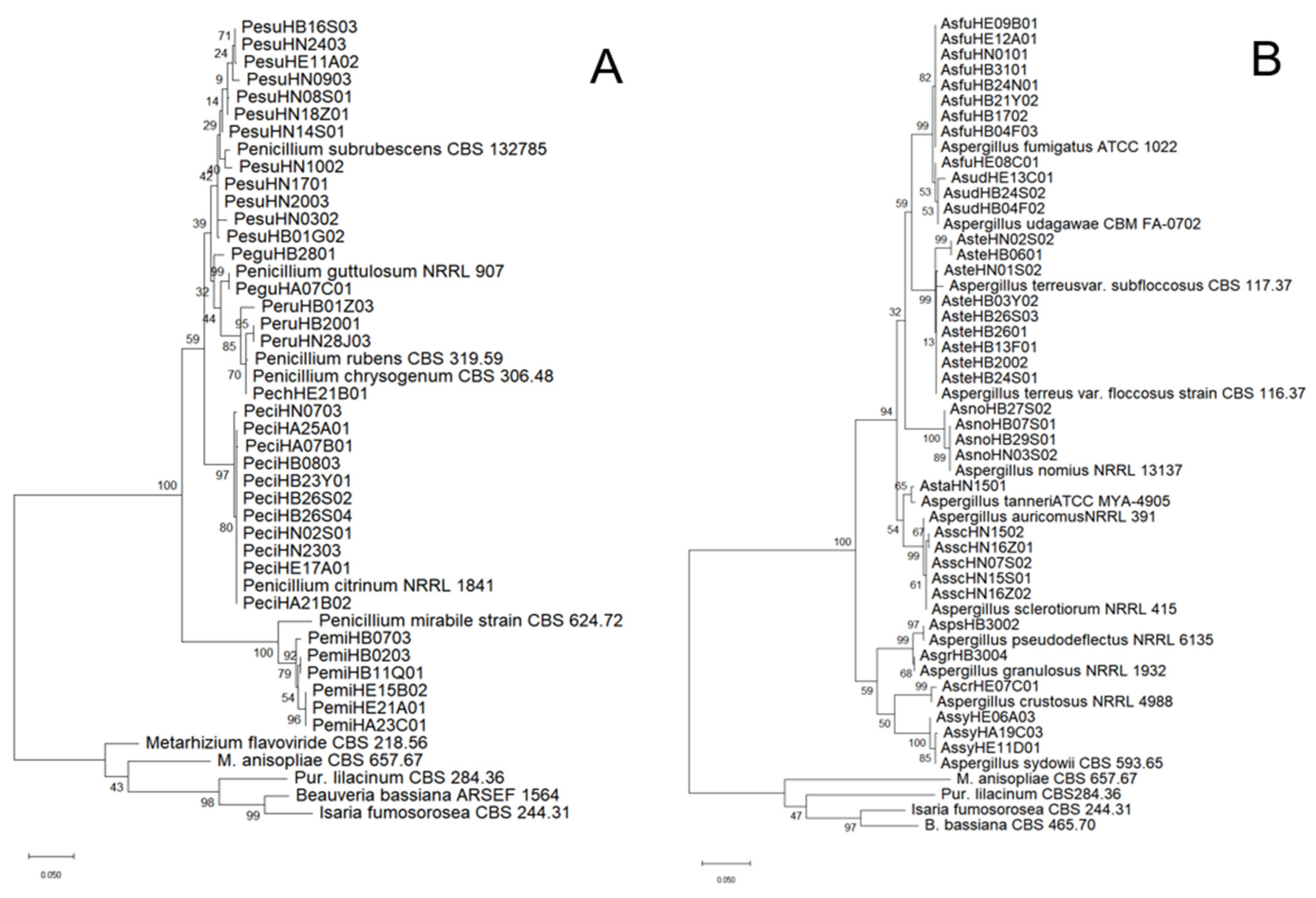
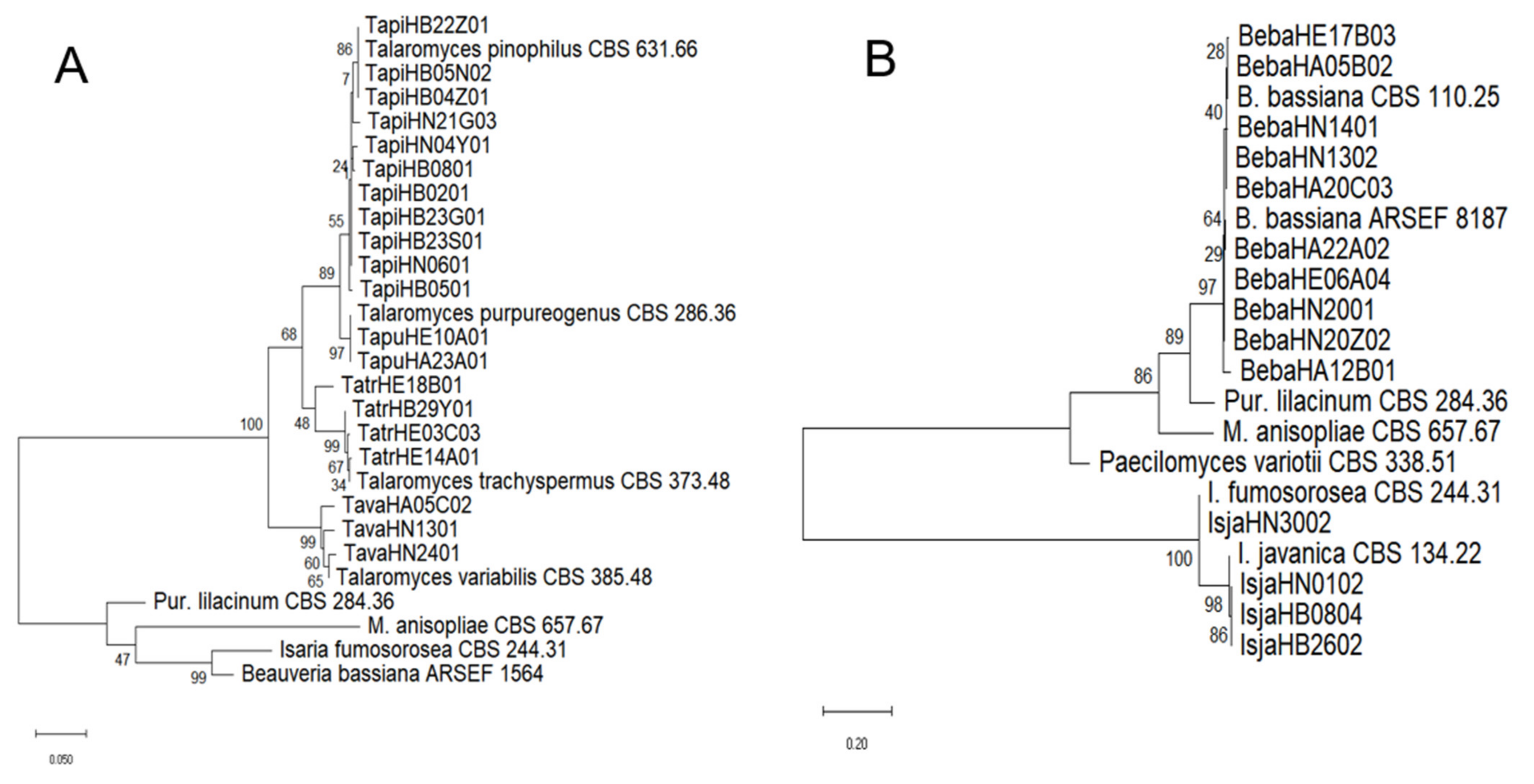
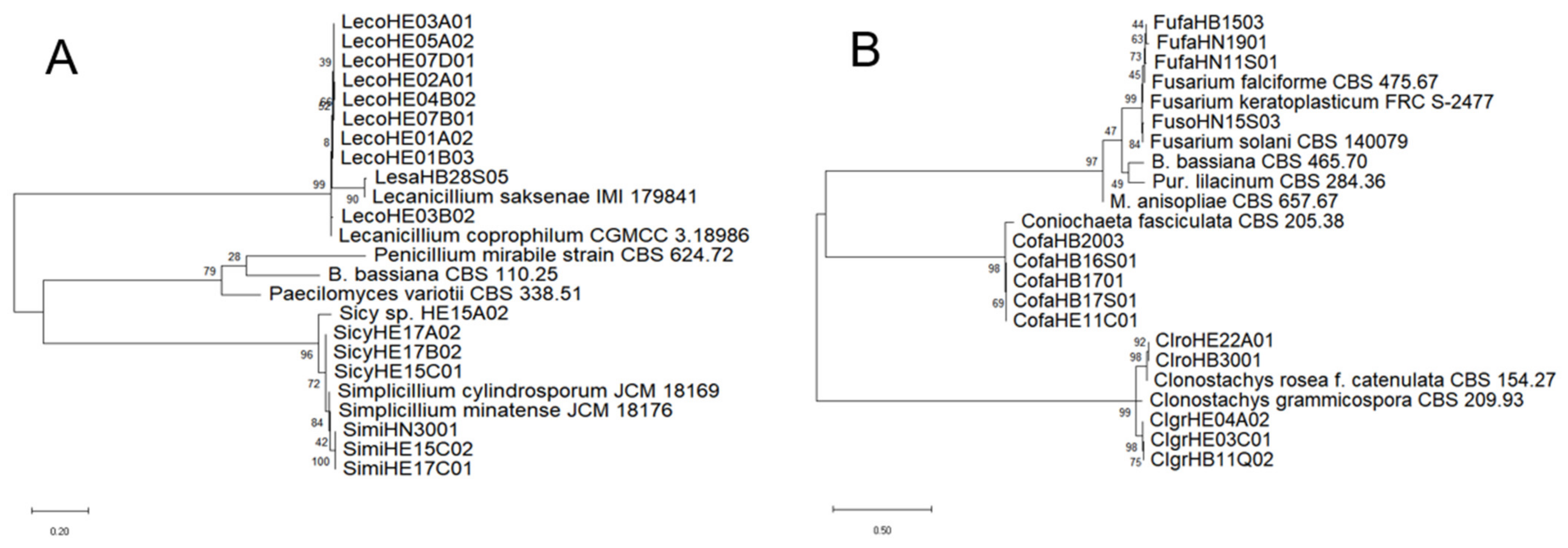
2.3. Identification of Fungal Species and Analysis of Genetic Homology
2.4. Evaluation of the Shannon Evenness Index
2.5. Bioassay of the Fungal Strains against P. striolata
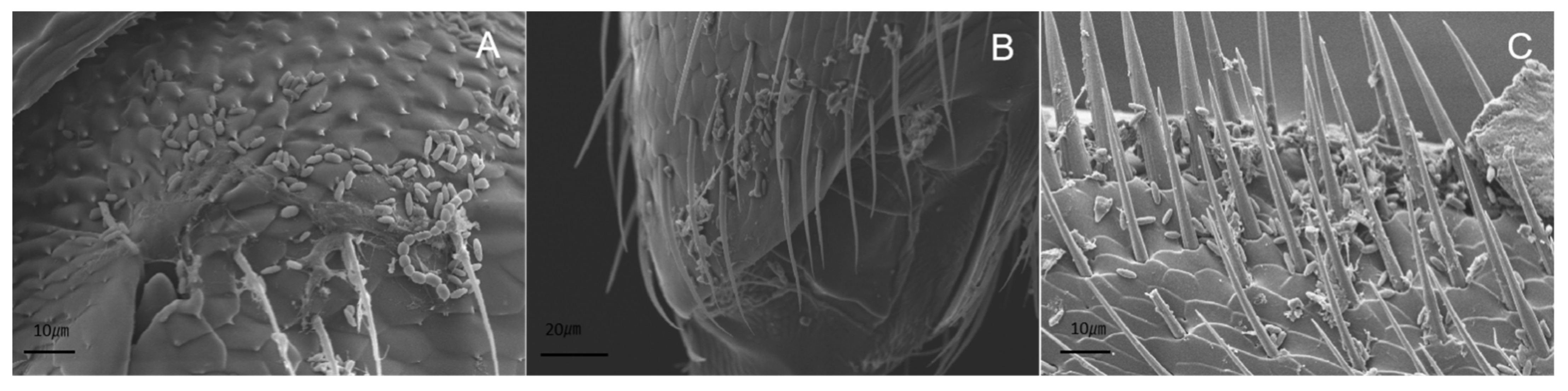
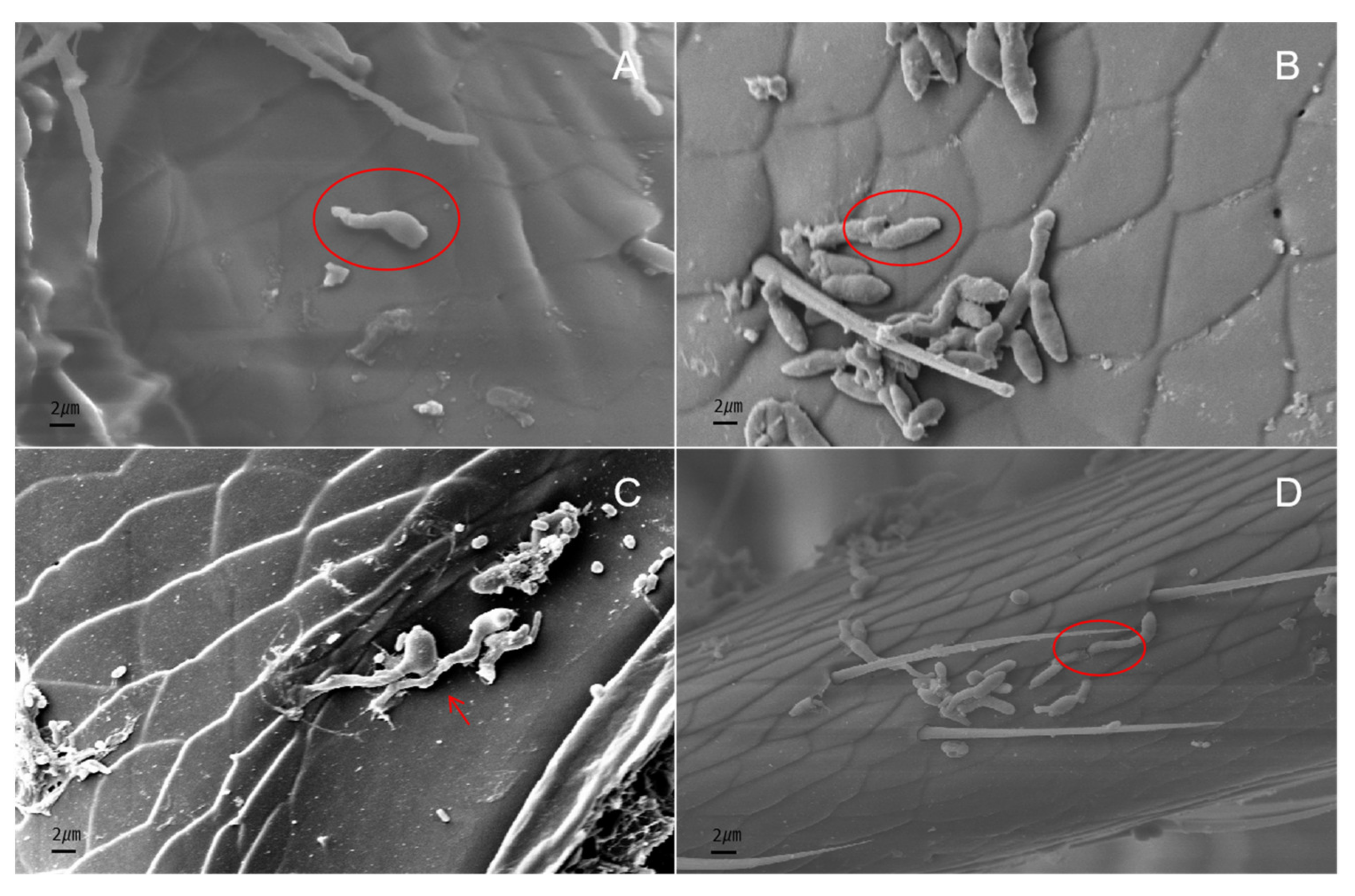
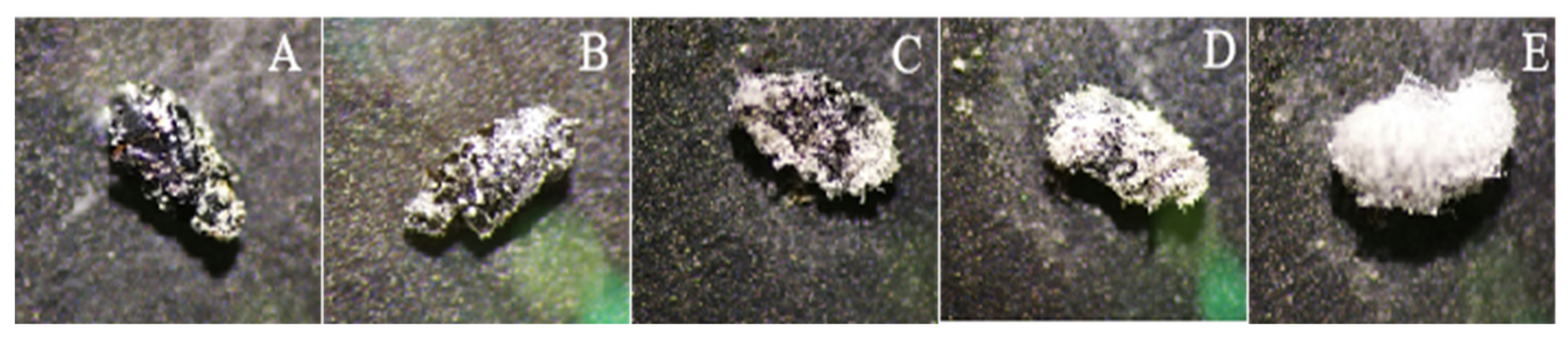
2.6. Scanning Electron Microscopy
2.7. Statistical Analysis
3. Results
3.1. EPF Species Diversity in the Soils of China
3.2. Distribution of Soil EPF in Different Regions
3.3. The Biodiversity of Soil EPF in Different Environments
3.4. The Pathogenicity of Fungal Isolates against P. striolata
3.5. The Pathogenicity of I. javanica against P. striolata
3.6. Scanning Electron Microscopy Observations of Infection Process of I. javanica
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Site | Isolate | GenBank Access No. | Species | |||
|---|---|---|---|---|---|---|
| NO. | Address | Latitude and Longitude | Sample Environment | |||
| HB01 | Xianning, Hubei | 29.267 N, 113.746 E | Fallow land | HB01Z01 | -- | |
| HB01Z02 | -- | |||||
| PeruHB01Z03 | OM372687 | Penicillium rubens | ||||
| HB01Z04 | -- | |||||
| Crop | HB01G01 | -- | ||||
| PesuHB01G02 | OM372688 | Penicillium subrubescens | ||||
| HB02 | Xianning, Hubei | 29.568 N, 114.193 E | Crop | -- | -- | |
| Arbor | HB02S01 | -- | ||||
| MeanHB02S02 | OM372689 | Metarhizium anisopliae | ||||
| Grass | TapiHB0201 | OM372690 | Talaromyces pinophilus | |||
| HB0202 | -- | |||||
| PemiHB0203 | OM372691 | Penicillium mirabile | ||||
| HB03 | Daye, Hubei | 29.973 N, 114.667 E | Crop | HB03Y01 | -- | |
| AsteHB03Y02 | OM372692 | Aspergillus terreus | ||||
| HB03Y03 | -- | |||||
| Grass | HB0301 | -- | ||||
| HB04 | Huanggang, Hubei | 30.372 N, 115.161 E | Fallow land | TapiHB04Z01 | OM372693 | Talaromyces pinophilus |
| Crop | MeanHB04F01 | OM372694 | Metarhizium anisopliae | |||
| AsudHB04F02 | OM372695 | Aspergillus udagawae | ||||
| AsfuHB04F03 | OM372696 | Aspergillus fumigatus | ||||
| HB05 | Xinzhou, Hubei | 30.863 N, 114.881 E | Crop | Mema sp. HB05N01 | OM372697 | Metarhizium marquandii |
| TapiHB05N02 | OM372698 | Talaromyces pinophilus | ||||
| Grass | TapiHB0501 | OM372699 | Talaromyces pinophilus | |||
| HB06 | Huanggang, Hubei | 31.257 N, 115.056 E | Arbor | HB06S01 | -- | |
| Fallow land | PulaHB06Z01 | OM372700 | Purpureocillium lavendulum | |||
| Grass | AsteHB0601 | OM372701 | Aspergillus terreus | |||
| HB07 | Wuhan, Hubei | 30.887 N, 114.462 E | Grass | Mema sp. HB0701 | OM372702 | Metarhizium marquandii |
| HB0702 | -- | |||||
| PemiHB0703 | OM372703 | Penicillium mirabile | ||||
| Arbor | AsnoHB07S01 | OM372704 | Aspergillus nomius | |||
| HB08 | Xiaogan, Hubei | 31.030 N, 113.938 E | Crop | -- | -- | |
| Grass | TapiHB0801 | OM372705 | Talaromyces pinophilus | |||
| HB0802 | -- | |||||
| PeciHB803 | OM372706 | Penicillium citrinum | ||||
| IsjaHB0804 | OM372707 | Isaria javanica | ||||
| HB09 | Xiaogan, Hubei | 31.325 N, 113.580 E | Fallow land | GobuHB0901 | OM372708 | Gongronella butleri |
| HB0902 | -- | |||||
| Suizhou, Hubei | 31.665 N, 113.269 E | Arbor | -- | -- | ||
| Grass | -- | -- | ||||
| HB11 | Xiangyang, Hubei | 31.948 N, 112.929 E | Crop | HB11Y01 | -- | |
| PemiHB11Q01 | OM372709 | Penicillium mirabile | ||||
| ClgrHB11Q02 | OM372710 | Clonostachys grammicospora | ||||
| HB12 | Xiangyang, Hubei | 32.178 N, 112.211 E | Grass | HB12A01 | -- | |
| MeanHB12A02 | OM372711 | Metarhizium anisopliae | ||||
| Arbor | -- | |||||
| HB13 | Xiangyang, Hubei | 32.307 N, 111.614 E | Crop | AsteHB13F01 | OM372712 | Aspergillus terreus |
| HB13F02 | -- | |||||
| Arbor | PuliHB13S01 | OM372713 | Purpureocillium lilacinum | |||
| HB14 | Shiyan, Hubei | 32.502 N, E111.100 E | Grass | PulaHB1401 | OM372714 | Purpureocillium lavendulum |
| Arbor | HB14S01 | -- | ||||
| HB15 | Shiyan, Hubei | 32.020 N, 110.679 E | Fallow land | -- | ||
| Grass | HB1501 | -- | ||||
| PulaHB1502 | OM372715 | Purpureocillium lavendulum | ||||
| FufaHB1503 | OM372716 | Fusarium falciforme | ||||
| HB16 | Shennongjia, Hubei | 31.823 N, 110.508 E | Grass | MeanHB1601 | OM372717 | Metarhizium anisopliae |
| Arbor | CofaHB16S01 | OM372718 | Coniochaeta fasciculata | |||
| MeanHB16S02 | OM372719 | Metarhizium anisopliae | ||||
| PesuHB16S03 | OM372720 | Penicillium subrubescens | ||||
| HB17 | Shennongjia, Hubei | 31.514 N, 110.338 E | Grass | CofaHB1701 | OM372721 | Coniochaeta fasciculata |
| AsfuHB1702 | OM372722 | Aspergillus fumigatus | ||||
| Arbor | CofaHB17S01 | OM372723 | Coniochaeta fasciculata | |||
| HB18 | Yichang, Hubei | 31.266 N, 110.686 E | Grass | HB1801 | -- | |
| HB19 | Enshi, Hubei | 30.007 N, 110.377 E | Grass | HB1901 | -- | |
| HB1902 | -- | |||||
| AcexAB1903 | OM372724 | Acremonium exuviarum | ||||
| Crop | MeanHB20Y01 | OM372725 | Metarhizium anisopliae | |||
| HB20 | Enshi, Hubei | 30.556 N, 109.889 E | Grass | PeruHB2001 | OM372726 | Penicillium rubens |
| AsteHB2002 | OM372727 | Aspergillus terreus | ||||
| CofaHB2003 | OM372728 | Coniochaeta fasciculata | ||||
| Arbor | HB20S01 | -- | ||||
| HB20S02 | -- | |||||
| HB21 | Yichang, Hubei | 30.615 N, 110.513 E | Grass | Mema sp. HB2101 | OM372729 | Metarhizium marquandii |
| PulaHB2102 | OM372730 | Purpureocillium lavendulum | ||||
| Crop | ArkaHB21Y01 | OM372731 | Arthrographis kalrae | |||
| AsfuHB21Y02 | OM372732 | Aspergillus fumigatus | ||||
| HB22 | Yichang, Hubei | 30.582 N, 111.028 E | Fallow land | TapiHB22Z01 | OM372733 | Talaromyces pinophilus |
| Crop | HB22Y01 | -- | ||||
| HB22Y02 | -- | |||||
| HB23 | Yichang, Hubei | 30.688 N, 111.517 E | Crop | PeciHB23Y01 | OM372734 | Penicillium citrinum |
| Orchard | TapiHB23G01 | OM372735 | Talaromyces pinophilus | |||
| Arbor | TapiHB23S01 | OM372736 | Talaromyces pinophilus | |||
| HB24 | Jingmen, Hubei | 30.904 N, 112.185 E | Arbor | AsteHB24S01 | OM372737 | Aspergillus terreus |
| AsudHB24S02 | OM372738 | Aspergillus udagawae | ||||
| Crop | AsfuHB24N01 | OM372739 | Aspergillus fumigatus | |||
| HB24N02 | -- | |||||
| MuelHB24N03 | OM372740 | Mucor ellipsoideus | ||||
| HB24N04 | -- | |||||
| HB25 | Jingmen, Hubei | 30.991 N, 112.854 E | Arbor | HB25S01 | -- | |
| Grass | -- | |||||
| HB26 | Xiaogan, Hubei | 30.868 N, 113.576 E | Arbor | Mema sp.HB26S01 | OM372741 | Metarhizium marquandii |
| PeciHB26S02 | OM372742 | Penicillium citrinum | ||||
| AsteHB26S03 | OM372743 | Aspergillus terreus | ||||
| PeciHB26S04 | OM372744 | Penicillium citrinum | ||||
| Grass | AsteHB2601 | OM372745 | Aspergillus terreus | |||
| IsjaHB2602 | OM372746 | Isaria javanica | ||||
| PulaHB2603 | OM372747 | Purpureocillium lavendulum | ||||
| HB27 | Wuhan, Hubei | 30.478 N, 113.874 E | Arbor | HB27S01 | -- | |
| AsnoHB27S02 | OM372748 | Aspergillus nomius | ||||
| Grass | HB2701 | -- | ||||
| HB2702 | -- | |||||
| MeanHB2703 | OM372749 | Metarhizium anisopliae | ||||
| HB28 | Xiantao, Hubei | 30.350 N, 113.424 E | Grass | PeguHB2801 | OM372750 | Penicillium guttulosum |
| Arbor | AcnaHB28S01 | OM372751 | Acrophialophora nainiana | |||
| PulaHB28S02 | OM372752 | Purpureocillium lavendulum | ||||
| MemaHB28S03 | OM372753 | Metarhizium marquandii | ||||
| MemaHB28S04 | OM372754 | Metarhizium marquandii | ||||
| LesaHB28S05 | OM372755 | Lecanicillium saksenae | ||||
| HB29 | Qianjiang, Hubei | 30.373 N, 112.889 E | Crop | TatrHB29Y01 | OM372756 | Talaromyces trachyspermus |
| AcnaHB29Y02 | OM372757 | Acrophialophora nainiana | ||||
| HB29Y03 | -- | |||||
| MeanHB29Y04 | OM372758 | Metarhizium anisopliae | ||||
| Arbor | AsnoHB29S01 | OM372759 | Aspergillus nomius | |||
| HB29S02 | -- | |||||
| PuliHB29S03 | OM372760 | Purpureocillium lilacinum | ||||
| HB29S04 | -- | |||||
| HB30 | Jingzhou, Hubei | 30.352 N, 112.338 E | Grass | ClroHB3001 | OM372761 | Clonostachys rosea |
| AspsHB3002 | OM372762 | Aspergillus pseudodeflectus | ||||
| HB3003 | -- | |||||
| AsgrHB3004 | OM372763 | Aspergillus granulosus | ||||
| Crop | -- | |||||
| HB31 | Jingzhou, Hubei | 30.043 N, 112.158 E | Grass | AsfuHB3101 | OM372764 | Aspergillus fumigatus |
| HB3102 | -- | |||||
| Crop | -- | |||||
| HN01 | Changsha, Hunan | 28.203 N, 113.303 E | Crop | PulaHN01S01 | OM372765 | Purpureocillium lavendulum |
| AsteHN01S02 | OM372766 | Aspergillus terreus | ||||
| MeanHN01S03 | OM372767 | Metarhizium anisopliae | ||||
| Crop | AsfuHN0101 | OM372768 | Aspergillus fumigatus | |||
| IsjaHN0102 | OM372769 | Isaria javanica | ||||
| HN02 | Changde, Hunan | 29.634 N, 111.840 E | Grass | MemaHN0201 | OM372770 | Metarhizium marquandii |
| PulaHN0202 | OM372771 | Purpureocillium lavendulum | ||||
| Arbor | PeciHN02S01 | OM372772 | Penicillium citrinum | |||
| AsteHN02S02 | OM372773 | Aspergillus terreus | ||||
| HN03 | Changde, Hunan | 29.131 N, 111.706 E | Grass | HN0301 | -- | |
| PesuHN0302 | OM372774 | Penicillium subrubescens | ||||
| Arbor | MemaHN03S01 | OM372775 | Metarhizium marquandii | |||
| AsnoHN03S02 | OM372776 | Aspergillus nomius | ||||
| HN04 | Zhangjiajie, Hunan | 29.424 N, 111.163 E | Orchard | TapiHN04Y01 | OM372777 | Talaromyces pinophilus |
| Grass | -- | -- | ||||
| HN05 | Zhangjiajie, Hunan | 29.348 N, 110.568 E | Arbor | HN05S01 | -- | |
| Grass | -- | -- | ||||
| HN06 | Xiangxi, Hunan | 29.034 N, 110.228 E | Arbor | PulaHN06S01 | OM372778 | Purpureocillium lavendulum |
| Grass | TapiHN0601 | OM372779 | Talaromyces pinophilus | |||
| Crop | HN06Y01 | -- | ||||
| PuliHN06Y02 | OM372780 | Purpureocillium lilacinum | ||||
| ChasHN06Y03 | OM372781 | Chloridium aseptatum | ||||
| HN06Y04 | -- | |||||
| HN06Y05 | -- | |||||
| HN07 | Xiangxi, Hunan | 28.623 N, 109.547 E | Grass | HN0701 | -- | |
| Pula sp. HN0702 | OM372782 | Purpureocillium lavendulum | ||||
| PeciHN0703 | OM372783 | Penicillium citrinum | ||||
| Arbor | PulaHN07S01 | OM372784 | Purpureocillium lavendulum | |||
| AsscHN07S02 | OM372785 | Aspergillus sclerotiorum | ||||
| HN08 | Huaihua, Hunan | 26.963 N, 109.747 E | Grass | Mema sp. HN0801 | OM372786 | Metarhizium marquandii |
| Arbor | PesuHN08S01 | OM372787 | Penicillium subrubescens | |||
| PulaHN08S02 | OM372788 | Purpureocillium lavendulum | ||||
| HN09 | Huaihua, Hunan | 26.614 N, 109.671 E | Grass | HataHN0901 | OM372789 | Hawksworthiomyces taylorii |
| PuliHN0902 | OM372790 | Purpureocillium lilacinum | ||||
| PesuHN0903 | OM372791 | Penicillium subrubescens | ||||
| Arbor | PulaHN09S01 | OM372792 | Purpureocillium lavendulum | |||
| HN09S02 | -- | |||||
| HN10 | Yongzhou, Hunan | 26.662 N, 111.493 E | Grass | HN1001 | -- | |
| PesuHN1002 | OM372793 | Penicillium subrubescens | ||||
| HN1003 | -- | |||||
| Arbor | HN10S01 | -- | ||||
| HN11 | Yongzhou, Hunan | 26.063 N, 111.831 E | Arbor | FufaHN11S01 | OM372794 | Fusarium falciforme |
| Fallow land | -- | -- | ||||
| HN12 | Yongzhou, Hunan | 25.528 N, 112.111 E | Grass | PulaHN1201 | OM372795 | Purpureocillium lavendulum |
| CudeHN1202 | OM372796 | Cutaneotrichosporon dermatis | ||||
| HN13 | Chenzhou, Hunan | 25.659 N, 112.729 E | Grass | TavaHN1301 | OM372797 | Talaromyces variabilis |
| BebaHN1302 | OM372798 | Beauveria bassiana | ||||
| HN1303 | -- | |||||
| Arbor | MeteHN13S01 | OM372799 | Melanoctona tectonae | |||
| HN14 | Chenzhou, Hunan | 25.965 N, 113.042 E | Grass | BebaHN1401 | OM372800 | Beauveria bassiana |
| Arbor | PesuHN14S01 | OM372801 | Penicillium subrubescens | |||
| PuliHN14S02 | OM372802 | Purpureocillium lilacinum | ||||
| HN15 | Hengyang, Hunan | 26.426 N, 112.889 E | Grass | AstaHN1501 | OM372803 | Aspergillus tanneri |
| AsscHN1502 | OM372804 | Aspergillus sclerotiorum | ||||
| Arbor | AsscHN15S01 | OM372805 | Aspergillus sclerotiorum | |||
| Mema sp. HN15S02 | OM372806 | Metarhizium marquandii | ||||
| ToalHN15S03 | OM372807 | Tolypocladium album | ||||
| ToalHN15S04 | OM372808 | Tolypocladium album | ||||
| FusoHN15S05 | OM372809 | Fusarium solani | ||||
| HN16 | Hengyang, Hunan | 26.974 N, 112.425 E | Grass | Mema sp. HN1601 | OM372810 | Metarhizium marquandii |
| Orchard | AsscHN16Z01 | OM372811 | Aspergillus sclerotiorum | |||
| AsscHN16Z02 | OM372812 | Aspergillus sclerotiorum | ||||
| HN17 | Loudi, Hunan | 27.440 N, 112.132 E | Grass | PesuHN1701 | OM372813 | Penicillium subrubescens |
| Arbor | MemaHN17S01 | OM372814 | Metarhizium marquandii | |||
| XepiHN17S02 | OM372815 | Xenopolyscytalum pinea | ||||
| CuelHN17S03 | OM372816 | Cunninghamella elegans | ||||
| HN18 | Loudi, Hunan | 27.821 N, 111.763 E | Grass | MemaHN1801 | OM372817 | Metarhizium marquandii |
| HN1802 | ||||||
| Fallow land | PesuHN18Z01 | OM372818 | Penicillium subrubescens | |||
| HN19 | Yiyang, Hunan | 28.264 N, 111.712 E | Arbor | MeanHN19S01 | OM372819 | Metarhizium anisopliae |
| HN19S02 | ||||||
| HN19S03 | ||||||
| Grass | FufaHN1901 | OM372820 | Fusarium falciforme | |||
| Mema sp. HN1902 | OM372821 | Metarhizium marquandii | ||||
| HN20 | Yiyang, Hunan | 28.525 N, 112.045 E | Grass | BebaHN2001 | OM372822 | Beauveria bassiana |
| PulaHN2002 | OM372823 | Purpureocillium lavendulum | ||||
| PesuHN2003 | OM372824 | Penicillium subrubescens | ||||
| Fallow land | HN20Z01 | -- | ||||
| BebaHN20Z02 | OM372825 | Beauveria bassiana | ||||
| Mema sp. HN20Z03 | OM372826 | Metarhizium marquandii | ||||
| HN21 | Changsha, Hunan | 28.222 N, 112.567 E | Crop | MeanHN21G01 | OM372829 | Metarhizium anisopliae |
| Pula sp. HN21G02 | OM372830 | Purpureocillium lavendulum | ||||
| TapiHN21G03 | OM372831 | Talaromyces pinophilus | ||||
| Arbor | PulaHN21S01 | OM372827 | Purpureocillium lavendulum | |||
| Mema sp. HN21S02 | OM372828 | Metarhizium marquandii | ||||
| HN22 | Xiangtan, Hunan | 27.806 N, 112.511 E | Fallow land | Pula sp. HN22Z01 | OM372832 | Purpureocillium lavendulum |
| Arbor | -- | -- | ||||
| HN23 | Xiangtan, Hunan | 27.846 N, 113.017 E | Grass | Mema sp. HN2301 | OM372833 | Metarhizium marquandii |
| PulaHN2302 | OM372834 | Purpureocillium lavendulum | ||||
| PeciHN2303 | OM372835 | Penicillium citrinum | ||||
| Arbor | Pula sp. HN23S01 | OM372836 | Purpureocillium lavendulum | |||
| HN24 | Hengyang, Hunan | 27.229 N, 112.897 E | Grass | TavaHN2401 | OM372837 | Talaromyces variabilis |
| ApcaHN2402 | OM372838 | Apiotrichum cacaoliposimilis | ||||
| PesuHN2403 | OM372839 | Penicillium subrubescens | ||||
| Arbor | MeanHN24S01 | OM372840 | Metarhizium anisopliae | |||
| HN24S02 | -- | |||||
| HN25 | Zhuzhou, Hunan | 26.893 N, 113.374 E | Grass | Mema sp. HN2501 | OM372841 | Metarhizium marquandii |
| Orchard | MeanHN25B01 | OM372842 | Metarhizium anisopliae | |||
| HN26 | Zhuzhou, Hunan | 27.496 N, 113.486 E | Arbor | MemaHN26S01 | OM372843 | Metarhizium marquandii |
| HN26S02 | -- | |||||
| Fallow land | MeanHN26Z01 | OM372844 | Metarhizium anisopliae | |||
| HN27 | Xiangxi, Hunan | 27.914 N, 109.385 E | Grass | PulaHN2701 | OM372845 | Purpureocillium lavendulum |
| PhliHN2702 | OM372846 | Phialophora livistonae | ||||
| HN28 | Huaihua, Hunan | 27.896 N, 109.702 E | Orchard | HN28J01 | -- | |
| PuliHN28J02 | OM372847 | Purpureocillium lilacinum | ||||
| PeruHN28J03 | OM372848 | Penicillium rubens | ||||
| HN29 | Huaihua, Hunan | 27.367 N, 109.935 E | Fallow land | PulaHN29B01 | OM372849 | Purpureocillium lavendulum |
| MeanHN29B02 | OM372850 | Metarhizium anisopliae | ||||
| Grass | ArhiHN2901 | OM372851 | Arthropsis hispanica | |||
| HN30 | Huaihua, Hunan | 27.216 N, 110.420 E | Arbor | SimiHN3001 | OM372852 | Simplicillium minatense |
| IsjaHN3002 | OM372853 | Isaria javanica | ||||
| HN31 | Shaoyang, Hunan | 26.941 N, 110.638 E | Arbor | XepiHN3101 | OM372854 | Xenopolyscytalum pinea |
| HN32 | Shaoyang, Hunan | 26.322 N, 110.837 E | Grass | -- | -- | |
| HE01 | Xingtai, Hebei | 36.905 N, 114.559 E | Crop | MeanHE01A01 | OM372855 | Metarhizium anisopliae |
| LecoHE01A02 | OM372856 | Lecanicillium coprophilum | ||||
| Grass | MeanHE01B01 | OM372857 | Metarhizium anisopliae | |||
| NemaHE01B02 | OM372858 | Nectria mauritiicola | ||||
| LecoHE01B03 | OM372859 | Lecanicillium coprophilum | ||||
| HE02 | Shijiazhuang, Hebei | 35.994 N, 113.758 E | Arbor | LecoHE02A01 | OM372860 | Lecanicillium coprophilum |
| MeanHE02A02 | OM372861 | Metarhizium anisopliae | ||||
| HE02A03 | -- | |||||
| HE03 | Baoding, Hebei | 39.138 N, 115.536 E | Crop | LecoHE03A01 | OM372862 | Lecanicillium coprophilum |
| MemaHE03A02 | OM372863 | Metarhizium marquandii | ||||
| Grass | HE03B01 | -- | ||||
| LecoHE03B02 | OM372864 | Lecanicillium coprophilum | ||||
| Poplar | ClgrHE03C01 | OM372865 | Clonostachys grammicospora | |||
| MeanHE03C02 | OM372866 | Metarhizium anisopliae | ||||
| TatrHE03C03 | OM372867 | Talaromyces trachyspermus | ||||
| HE04 | Zhangjiakou, Hebei | 39.273 N, 115.455 E | Poplar | AualHE04A01 | OM372868 | Auxarthron alboluteum |
| ClgrHE04A02 | OM372869 | Clonostachys grammicospora | ||||
| Crop | HE04B01 | -- | ||||
| LecoHE04B02 | OM372870 | Lecanicillium coprophilum | ||||
| Mema sp. HE04B03 | OM372871 | Metarhizium marquandii | ||||
| HE05 | Zhangjiakou, Hebei | 39.375 N, 114.866 E | Poplar | HE05A01 | -- | |
| LecoHE05A02 | OM372872 | Lecanicillium coprophilum | ||||
| Crop | Mema sp. HE05B01 | OM372873 | Metarhizium marquandii | |||
| MeanHE05B02 | OM372874 | Metarhizium anisopliae | ||||
| HE06 | Zhangjiakou, Hebei | 40.488 N, 114.838 E | Orchard | MeanHE06A01 | OM372875 | Metarhizium anisopliae |
| TrteHE06A02 | OM372876 | Trichurus terrophilus | ||||
| AssyHE06A03 | OM372877 | Aspergillus sydowii | ||||
| BebaHE06A04 | OM372878 | Beauveria bassiana | ||||
| Crop | Mema sp. HE06B01 | OM372879 | Metarhizium marquandii | |||
| PuliHE06B02 | OM372880 | Purpureocillium lilacinum | ||||
| HE07 | Zhangjiakou, Hebei | 41.267 N, 114.785 E | Crop | HE07A01 | -- | |
| AscrHE07C01 | OM372882 | Aspergillus crustosus | ||||
| Grass | LecoHE07B01 | OM372881 | Lecanicillium coprophilum | |||
| Poplar | LecoHE07D01 | OM372883 | Lecanicillium coprophilum | |||
| HE08 | Zhangjiakou, Hebei | 41.073 N, 115.389 E | Grass | HE08A01 | -- | |
| HE08A02 | -- | |||||
| Crop | AualHE08B01 | OM372884 | Auxarthron alboluteum | |||
| AsfuHE08C01 | OM372885 | Aspergillus fumigatus | ||||
| HE09 | Chengde, Hebei | 41.581 N, 116.023 E | Grass | MeanHE09A01 | OM372886 | Metarhizium anisopliae |
| PulaHE09A02 | OM372887 | Purpureocillium lavendulum | ||||
| Elm | AsfuHE09B01 | OM372888 | Aspergillus fumigatus | |||
| Crop | HE09C01 | -- | ||||
| OifuHE09C02 | OM372889 | Oidiodendron fuscum | ||||
| HE10 | Chengde, Hebei | 42.001 N, 116.975 E | Grass | TapuHE10A01 | OM372890 | Talaromyces purpureogenus |
| Crop | -- | -- | ||||
| HE11 | Chengde, Hebei | 42.253 N, 117.143 E | Grass | PulaHE11A01 | OM372891 | Purpureocillium lavendulum |
| PesuHE11A02 | OM372892 | Penicillium subrubescens | ||||
| Pine | CofaHE11C01 | OM372893 | Coniochaeta fasciculata | |||
| Crop | AssyHE11D01 | OM372894 | Aspergillus sydowii | |||
| HE12 | Chengde, Hebei | 41.997 N, 117.655 E | Orchard | AsfuHE12A01 | OM372895 | Aspergillus fumigatus |
| Grass | PulaHE12B01 | OM372896 | Purpureocillium lavendulum | |||
| MeanHE12B02 | OM372897 | Metarhizium anisopliae | ||||
| HE13 | Chengde, Hebei | 41.302 N, 118.038 E | Crop | HE13A01 | -- | |
| MeanHE13B01 | OM372898 | Metarhizium anisopliae | ||||
| MeanHE13B02 | OM372899 | Metarhizium anisopliae | ||||
| Poplar | AsudHE13C01 | OM372900 | Aspergillus udagawae | |||
| PulaHE13C02 | OM372901 | Purpureocillium lavendulum | ||||
| MeanHE13C03 | OM372902 | Metarhizium anisopliae | ||||
| HE14 | Chengde, Hebei | 40.578 N, 117.704 E | Crop | TatrHE14A01 | OM372903 | Talaromyces trachyspermus |
| Grass | -- | -- | ||||
| HE15 | Tangshan, Hebei | 40.108 N, 117.985 E | Crop | MeanHE15A01 | OM372904 | Metarhizium anisopliae |
| Sicy sp. HE15A02 | OM372905 | Simplicillium cylindrosporum | ||||
| Arbor | MeanHE15B01 | OM372906 | Metarhizium anisopliae | |||
| PemiHB15B02 | OM372907 | Penicillium mirabile | ||||
| Grass | SicyHE15C01 | OM372908 | Simplicillium cylindrosporum | |||
| SimiHE15C02 | OM372909 | Simplicillium minatense | ||||
| MeanHE15C03 | OM372910 | Metarhizium anisopliae | ||||
| HE16 | Tangshan, Hebei | 39.584 N, 118.264 E | Grass | MeanHE16A01 | OM372911 | Metarhizium anisopliae |
| HE17 | Tangshan, Hebei | 39.490 N, 118.682 E | Grass | PeciHE17A01 | OM372912 | Penicillium citrinum |
| SicyHE17A02 | OM372913 | Simplicillium cylindrosporum | ||||
| PulaHE17A03 | OM372914 | Purpureocillium lavendulum | ||||
| Orchard | Pula sp. HE17B01 | OM372915 | Purpureocillium lavendulum | |||
| SicyHE17B02 | OM372916 | Simplicillium cylindrosporum | ||||
| BebaHE17B03 | OM372917 | Beauveria bassiana | ||||
| Poplar | SimiHE17C01 | OM372918 | Simplicillium minatense | |||
| MemaHE17C02 | OM372919 | Metarhizium marquandii | ||||
| HE18 | Tangshan, Hebei | 39.408 N, 117.954 E | Crop | PulaHE18A01 | OM372920 | Purpureocillium lavendulum |
| TatrHE18B01 | OM372921 | Talaromyces trachyspermus | ||||
| Pula sp. HE18B02 | OM372922 | Purpureocillium lavendulum | ||||
| TrteHE18B03 | OM372923 | Trichurus terrophilus | ||||
| Poplar | MeanHE18C01 | OM372924 | Metarhizium anisopliae | |||
| HE19 | Tianjin, Hebei | 38.768 N, 117.184 E | Crop | MeanHE19A01 | OM372925 | Metarhizium anisopliae |
| NemaHE19A02 | OM372926 | Nectria mauritiicola | ||||
| Orchard | Pula sp. HE19B01 | OM372927 | Purpureocillium lavendulum | |||
| MeanHE19B02 | OM372928 | Metarhizium anisopliae | ||||
| HE20 | Cangzhou, Hebei | 38.151 N, 115.740 E | Crop | PulaHE20A01 | OM372929 | Purpureocillium lavendulum |
| MemaHE20A02 | OM372930 | Metarhizium marquandii | ||||
| Grass | MeanHE20B01 | OM372931 | Metarhizium anisopliae | |||
| HE21 | Hengshui, Hebei | 37.719 N, 115.193 E | Crop | PemiHB21A01 | OM372932 | Penicillium mirabile |
| Grass | PechHE21B01 | OM372933 | Penicillium chrysogenum | |||
| Pula sp. HE21B02 | OM372934 | Purpureocillium lavendulum | ||||
| MemaHE21B03 | OM372935 | Metarhizium marquandii | ||||
| HE22 | Handan, Hebei | 36.804 N, 115.193 E | Crop | ClroHE22A01 | OM372936 | Clonostachys rosea |
| MemaHe22B01 | OM372937 | Metarhizium marquandii | ||||
| HA01 | Xinxiang, Henan | 35.268 N, 113.974 E | Orchard | HA01A01 | -- | |
| Crop | MemaHA01B01 | OM372938 | Metarhizium marquandii | |||
| Grass | -- | -- | ||||
| HA02 | Linzhou, Henan | 35.994 N, 113.758 N | Crop | PulaHA02A01 | OM372939 | Purpureocillium lavendulum |
| MeanHA02B01 | OM372940 | Metarhizium anisopliae | ||||
| Arbor | PulaHA02C01 | OM372941 | Purpureocillium lavendulum | |||
| HA03 | Linzhou, Henan | 35.928 N, 113.655 E | Crop | -- | -- | |
| HA04 | Puyang, Henan | 36.090 N, 115.124 E | Crop | TrteHA04A01 | OM372942 | Trichurus terrophilus |
| MemaHA04B01 | OM372943 | Metarhizium marquandii | ||||
| HA04B02 | -- | |||||
| Grass | Pula sp. HA04C01 | OM372944 | Purpureocillium lavendulum | |||
| HA05 | Kaifeng, Henan | 34.790 N, 114.485 E | Crop | -- | -- | |
| Grass | PulaHA05B01 | OM372945 | Purpureocillium lavendulum | |||
| BebaHA05B02 | OM372946 | Beauveria bassiana | ||||
| Crop | Mema sp. HA05C01 | OM372947 | Metarhizium marquandii | |||
| TavaHA05C02 | OM372948 | Talaromyces variabilis | ||||
| HA06 | Kaifeng, Henan | 34.895 N, 114.328 E | Crop | MemaHA06A01 | OM372949 | Metarhizium marquandii |
| PulaHA06A02 | OM372950 | Purpureocillium lavendulum | ||||
| HA06A03 | -- | |||||
| Poplar | OifuHA06B01 | OM372951 | Oidiodendron fuscum | |||
| HA06B02 | -- | |||||
| Grass | PulaHA06C01 | OM372952 | Purpureocillium lavendulum | |||
| ChloHA06C02 | OM372953 | Chrysosporium lobatum | ||||
| MeanHA06C03 | OM372954 | Metarhizium anisopliae | ||||
| HA07 | Zhengzhou, Henan | 34.481 N, 113.030 E | Arbor | MemaHA07A01 | OM372955 | Metarhizium marquandii |
| Orchard | PeciHA07B01 | OM372956 | Penicillium citrinum | |||
| Pula sp. HA07B02 | OM372957 | Purpureocillium lavendulum | ||||
| Crop | PeguHA07C01 | OM372958 | Penicillium guttulosum | |||
| HA08 | Luoyang, Henan | 34.555 N, 112.873 E | Crop | PulaHA08A01 | OM372959 | Purpureocillium lavendulum |
| MemaHA08A02 | OM372960 | Metarhizium marquandii | ||||
| PulaHA08B01 | OM372961 | Purpureocillium lavendulum | ||||
| MeanHA08B02 | OM372962 | Metarhizium anisopliae | ||||
| MemaHA08B03 | OM372963 | Metarhizium marquandii | ||||
| PulaHA08C01 | OM372964 | Purpureocillium lavendulum | ||||
| MemaHA08C02 | OM372965 | Metarhizium marquandii | ||||
| HA09 | Luoyang, Henan | 34.768 N, 112.093 E | Crop | PulaHA09A01 | OM372966 | Purpureocillium lavendulum |
| MeanHA09A02 | OM372967 | Metarhizium anisopliae | ||||
| Poplar | MemaHA09B01 | OM372968 | Metarhizium marquandii | |||
| MeanHA09B02 | OM372969 | Metarhizium anisopliae | ||||
| PulaHA09B03 | OM372970 | Purpureocillium lavendulum | ||||
| HA10 | Sanmenxia, Henan | 34.797 N, 111.243 E | Crop | PulaHA10A01 | OM372971 | Purpureocillium lavendulum |
| MeanHA10A02 | OM372972 | Metarhizium anisopliae | ||||
| Pula sp. HA10B01 | OM372973 | Purpureocillium lavendulum | ||||
| MemaHA10B02 | OM372974 | Metarhizium marquandii | ||||
| MeanHA10B03 | OM372975 | Metarhizium anisopliae | ||||
| HA11 | Sanmenxia, Henan | 34.626 N, 110.914 E | Crop | MemaHA11A01 | OM372976 | Metarhizium marquandii |
| MeanHA11A02 | OM372977 | Metarhizium anisopliae | ||||
| PulaHA11A03 | OM372978 | Purpureocillium lavendulum | ||||
| PulaHA11B01 | OM372979 | Purpureocillium lavendulum | ||||
| Grass | -- | -- | ||||
| HA12 | Nanyang, Henan | 33.566 N, 111.185 E | Crop | Pula sp. HA12A01 | OM372980 | Purpureocillium lavendulum |
| Mema sp. HA12A02 | OM372981 | Metarhizium marquandii | ||||
| BebaHA12B01 | OM372982 | Beauveria bassiana | ||||
| PuliHA12B02 | OM372983 | Purpureocillium lilacinum | ||||
| TrteHA12B03 | OM372984 | Trichurus terrophilus | ||||
| Grass | PulaHA12C01 | OM372985 | Purpureocillium lavendulum | |||
| HA13 | Nanyang, Henan | 33.072 N, 111.792 E | Crop | -- | -- | -- |
| PuliHA13B01 | OM372986 | Purpureocillium lilacinum | ||||
| HA13B02 | -- | -- | ||||
| HA14 | Nanyang, Henan | 32.780 N, 112.707 E | Crop | PulaHA14A01 | OM372987 | Purpureocillium lavendulum |
| NemaHA14B01 | OM372988 | Nectria mauritiicola | ||||
| Grass | MemaHA14C01 | OM372989 | Metarhizium marquandii | |||
| HA15 | Xinyang, Henan | 32.401 N, 113.931 E | Crop | PulaHA15A01 | OM372990 | Purpureocillium lavendulum |
| ChasHA15A02 | OM372991 | Chloridium aseptatum | ||||
| Grass | PulaHA15C01 | OM372992 | Purpureocillium lavendulum | |||
| HA16 | Xinyang, Henan | 32.338 N, 114.128 E | Crop | PulaHA16A01 | OM372993 | Purpureocillium lavendulum |
| MaauHA16A02 | OM372994 | Malbranchea aurantiaca | ||||
| Grass | PulaHA16C01 | OM372995 | Purpureocillium lavendulum | |||
| HA17 | Zhumadian, Henan | 32.707 N, 114.109 E | Crop | PulaHA17A01 | OM372996 | Purpureocillium lavendulum |
| MemaHA17B01 | OM372997 | Metarhizium marquandii | ||||
| PulaHA17B02 | OM372998 | Purpureocillium lavendulum | ||||
| Grass | HA17C01 | -- | -- | |||
| PulaHA17C02 | OM372999 | Purpureocillium lavendulum | ||||
| HA18 | Luohe, Henan | 33.510 N, 113.980 E | Crop | PulaHA18A01 | OM373000 | Purpureocillium lavendulum |
| Pula sp. HA18B01 | OM373001 | Purpureocillium lavendulum | ||||
| HA18B02 | -- | |||||
| Grass | MeanHA18C01 | OM373002 | Metarhizium anisopliae | |||
| PulaHA18C02 | OM373003 | Purpureocillium lavendulum | ||||
| MemaHA18C03 | OM373004 | Metarhizium marquandii | ||||
| HA19 | Pingdingshan, Henan | 33.652 N, 113.370 E | Crop | MeanHA19A01 | OM373005 | Metarhizium anisopliae |
| PulaHA19A02 | OM373006 | Purpureocillium lavendulum | ||||
| MeanHA19B01 | OM373007 | Metarhizium anisopliae | ||||
| PulaHA19B02 | OM373008 | Purpureocillium lavendulum | ||||
| Grass | MemaHA19C01 | OM373009 | Metarhizium marquandii | |||
| Pula sp. HA19C02 | OM373010 | Purpureocillium lavendulum | ||||
| AssyHA19C03 | OM373011 | Aspergillus sydowii | ||||
| HA20 | Xuchang, Henan | 34.052 N, 113.709 E | Crop | MemaHA20A01 | OM373012 | Metarhizium marquandii |
| MemaHA20B01 | OM373013 | Metarhizium marquandii | ||||
| Grass | PulaHA20C01 | OM373014 | Purpureocillium lavendulum | |||
| MemaHA20C02 | OM373015 | Metarhizium marquandii | ||||
| BebaHA20C03 | OM373016 | Beauveria bassiana | ||||
| HA21 | Zhoukou, Henan | 33.978 N, 114.867 E | Crop | PulaHA21A01 | OM373017 | Purpureocillium lavendulum |
| MemaHA2102 | OM373018 | Metarhizium marquandii | ||||
| Grass | Pula sp. HA21B01 | OM373019 | Purpureocillium lavendulum | |||
| PeciHA21B02 | OM373020 | Penicillium citrinum | ||||
| HA22 | Shangqiu, Henan | 34.350 N, 115.572 E | Crop | HA22A01 | -- | |
| BebaHA22A02 | OM373021 | Beauveria bassiana | ||||
| PuliHA22B01 | OM373022 | Purpureocillium lilacinum | ||||
| Grass | PuliHA22C01 | OM373023 | Purpureocillium lilacinum | |||
| HA23 | Shangqiu, Henan | 34.596 N, 115.109 E | Crop | TapuHA23A01 | OM373024 | Talaromyces purpureogenus |
| MemaHA23B01 | OM373025 | Metarhizium marquandii | ||||
| HA23B02 | -- | |||||
| Orchard | PemiHA23C01 | OM373026 | Penicillium mirabile | |||
| HA24 | Kaifeng, Henan | 34.771 N, 114.806 E | Crop | MemaHA24A01 | OM373027 | Metarhizium marquandii |
| PulaHA24A02 | OM373028 | Purpureocillium lavendulum | ||||
| PuliHA24B01 | OM373029 | Purpureocillium lilacinum | ||||
| HA25 | Zhengzhou, Henan | 34.838 N, 114.036 E | Crop | PeciHA25A01 | OM373030 | Penicillium citrinum |
| MeanHA25A02 | OM373031 | Metarhizium anisopliae | ||||
| Grass | MeanHA25B01 | OM373032 | Metarhizium anisopliae | |||
| PuliHA25B02 | OM373033 | Purpureocillium lilacinum | ||||
| Crop | MeanHA25C01 | OM373034 | Metarhizium anisopliae | |||
| Poplar | MemaHA25D01 | OM373035 | Metarhizium marquandii | |||
| HA25D02 | -- | |||||
References
- Assadi, B.H.; Chouikhi, S.; Ettaib, R.; M’Hamdi, N.B.; Belkadhi, M.S. Effect of the native strain of the predator Nesidiocoris tenuis Reuter and the entomopathogenic fungi Beauveria bassiana and Lecanicillium muscarium against Bemisia tabaci (Genn.) under greenhouse conditions in Tunisia. Egypt. J. Biol. Pest Control 2021, 31, 47. [Google Scholar] [CrossRef]
- Ni, C.C. Current situation and prospect of using entomopathogenic microorganisms to control pests. World Pestic. 2005, 27, 35–37. [Google Scholar]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Q.; Liang, W.L.; Huang, L.P.; Shen, B.B. Identification of 29 strains of entomogenous fungi and their toxicity to Bemisia tabaci. J. South China Agric. Univ. 2020, 41, 57–67. [Google Scholar] [CrossRef]
- Lian, T.; Qin, C.S.; Jie, Y.Z.; Xu, J.Z.; Zhao, D.Y.; Qiu, H.L.; Yang, H.; Lai, G.D. Biological characteristics of six strains of entomophytic fungi and theirpathogenicity against Curculio chinensis (Coleoptera: Curculionidae). J. Environ. Entomol. 2019, 41, 642–649. [Google Scholar] [CrossRef]
- Wang, J.X.; Ma, J.L. Application of entomogenous fungi in biological control of agriculture and forestry pests. J. Zhejiang For. Colg. 2009, 26, 286–291. [Google Scholar] [CrossRef]
- Song, X.B.; Peng, A.T.; Cheng, B.P.; Ling, J.F.; Chen, X.; Zhang, L.H. Isolation of identification of a Beauveria bassiana strain infecting Diaphorina citri. Plant Prot. 2017, 43, 139–144. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Lopes, R.B.; Delalibera, I., Jr.; Fernandes, E.; Luz, C.; Faria, M. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. J. Invertebr. Pathol. 2019, 165, 46–53. [Google Scholar] [CrossRef]
- Zec-Vojinovic, M.; Hokkanen, H.M.T.; Büchs, W.; Klukowski, Z.; Luik, A.; Nilsson, C.; Ulber, B.; Williams, I.H. Natural occurrence of pathogens of oilseed rape pests in agricultural fields in Europe. In Proceedings of the International Symposium of Integrated Pest Management in Oilseed Rape, Gottingen, Germany, 3–5 April 2006. [Google Scholar]
- Li, Z.Z. Application status of entomogenous fungi in pest control. J. Anhui Agric. Colg. 1987, 14, 59–66. [Google Scholar]
- Wu, Y.Y.; Tang, Y.L.; Gu, N.C.; Li, T.T.; Bao, J.L.; Li, T.; Li, C.F.; Wei, J.H.; Pan, G.Q.; Zhou, Z.Y. Isolation and identification of three Beauveria bassiana isolates in Chongqing area. J. Southwest Univ. 2019, 41, 14–19. [Google Scholar] [CrossRef]
- Soroka, J.; Grenkow, L.; Otani, J.; Gavloski, J.; Olfert, O. Flea beetle (Coleoptera: Chrysomelidae) species in canola (Brassicaceae) on the northern Great Plains of North America. Can. Èntomol. 2018, 150, 100–115. [Google Scholar] [CrossRef]
- Cao, C.X.; Huang, D.Y.; Yao, J.W.; Zhu, Z.G.; Zheng, J.L.; Zhou, R.H.; Wang, K. Field application techniques for control of Phyllotreta striolata with microbial insecticides on radish. Chin. J. Biol. Control. 2020, 36, 987–991. [Google Scholar] [CrossRef]
- Noosidum, A.; Mangtab, S.; Lewis, E.E. Biological control potential of entomopathogenic nematodes against the striped flea beetle, Phyllotreta sinuata Stephens (Coleoptera: Chrysomelidae). Crop Prot. 2020, 141, 105448. [Google Scholar] [CrossRef]
- Gao, X.J.; Zhang, M.M.; Wang, H.L.; Zu, J.H.; Yang, W.J.; Qiao, Y.S. The combined effect and safety of bifenthrin-acetamiprid on Phyllotreta striolata. Agrochem 2022, 61, 57–60. [Google Scholar] [CrossRef]
- Yan, X.; Han, R.; Moens, M.; Chen, S.; De Clercq, P. Field evaluation of entomopathogenic nematodes for biological control of striped flea beetle, Phyllotreta striolata (Coleoptera: Chrysomelidae). BioControl 2012, 58, 247–256. [Google Scholar] [CrossRef]
- Andersen, C.L.; Hazzard, R.; Vandriesche, R.; Mangan, F.X. Alternative Management Tactics for Control of Phyllotreta cruciferae and Phyllotreta striolata (Coleoptera: Chrysomelidae) on Brassica rapa in Massachusetts. J. Econ. Èntomol. 2006, 99, 803–810. [Google Scholar] [CrossRef]
- Mason, J.; Alford, A.M.; Kuhar, T.P. Flea Beetle (Coleoptera: Chrysomelidae) Populations, Effects of Feeding Injury, and Efficacy of Insecticide Treatments on Eggplant and Cabbage in Southwest Virginia. J. Econ. Èntomol. 2019, 113, 887–895. [Google Scholar] [CrossRef]
- Hoarau, C.; Campbell, H.; Prince, G.; Chandler, D.; Pope, T. Biological control agents against the cabbage stem flea beetle in oilseed rape crops. Biol. Control 2022, 167, 104844. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Pereira-Junior, R.A.; Fernandes, E.; Quintela, E.D.; Dunlap, C.A.; Arthurs, S.P. Phenotype responses to abiotic stresses, asexual reproduction and virulence among isolates of the entomopathogenic fungus Cordyceps javanica (Hypocreales: Cordycipitaceae). Microbiol. Res. 2018, 216, 12–22. [Google Scholar] [CrossRef]
- Fang, W.; Lu, H.-L.; King, G.; Leger, R.J.S. Construction of a Hypervirulent and Specific Mycoinsecticide for Locust Control. Sci. Rep. 2014, 4, 7345. [Google Scholar] [CrossRef]
- Kuang, Z.B.; Lv, L.H.; Feng, X.; Chen, H.Y.; Wu, Y.J.; He, Y.R. Pathogenicity of Beauveria bassiana isolate to cruciferous vegetable insect pests. Chin. Bull. Entomol. 2005, 42, 673–676. [Google Scholar] [CrossRef]
- He, Y.C.; Chen, J.; Shi, M.Z.; Li, J.Y.; Wang, T.; Fu, J.W.; Wu, M.X. Screening and culture of a strain of Metarhizium highly pathogenic to Phyllotreta striolata, Fujian. J. Agric. Sci. 2017, 32, 189–194. [Google Scholar] [CrossRef]
- Meyling, N.V.; Eilenberg, J. Occurrence and distribution of soil borne entomopathogenic fungi within a single organic agroecosystem. Agric. Ecosyst. Environ. 2006, 113, 336–341. [Google Scholar] [CrossRef]
- Masoudi, A.; Koprowski, J.L.; Bhattarai, U.R.; Wang, D. Elevational distribution and morphological attributes of the entomopathogenic fungi from forests of the Qinling Mountains in China. Appl. Microbiol. Biotechnol. 2017, 102, 1483–1499. [Google Scholar] [CrossRef]
- Niu, X.; Xie, W.; Zhang, J.; Hu, Q. Biodiversity of Entomopathogenic Fungi in the Soils of South China. Microorganisms 2019, 7, 311. [Google Scholar] [CrossRef]
- Dong, T.; Zhang, B.; Jiang, Y.; Hu, Q. Isolation and Classification of Fungal Whitefly Entomopathogens from Soils of Qinghai-Tibet Plateau and Gansu Corridor in China. PLoS ONE 2016, 11, e0156087. [Google Scholar] [CrossRef]
- Hu, Q.-B.; Ren, S.-X.; Wu, J.-H.; Chang, J.-M.; Musa, P.D. Investigation of destruxin A and B from 80 Metarhizium strains in China, and the optimization of cultural conditions for the strain MaQ10. Toxicon 2006, 48, 491–498. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Schoch, C.L.; Robbertse, B.; Robert, V.; Vu, D.; Cardinali, G.; Irinyi, L.; Meyer, W.; Nilsson, R.H.; Hughes, K.; Miller, A.N.; et al. Finding needles in haystacks: Linking scientific names, reference specimens and molecular data for Fungi. Database 2014, 2014, bau061. [Google Scholar] [CrossRef]
- Gujjari, P.; Suh, S.-O.; Coumes, K.; Zhou, J.J. Characterization of oleaginous yeasts revealed two novel species: Trichosporon cacaoliposimilis sp. nov. and Trichosporon oleaginosus sp. nov. Mycologia 2011, 103, 1110–1118. [Google Scholar] [CrossRef]
- Sugiura, Y.; Hironaga, M. Arthrographis kalrae, a rare causal agent of onychomycosis, and its occurrence in natural and commercially available soils. Med. Mycol. 2010, 48, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, A.; Sutton, D.A.; Gené, J.; Fothergill, A.W.; Cano-Lira, J.F.; Guarro, J. Rare Arthroconidial Fungi in Clinical Samples: Scytalidium cuboideum and Arthropsis hispanica. Mycopathologia 2012, 175, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.W. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 2008, 100, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.; Peterson, S.; Frisvad, J.; Varga, J. New species in Aspergillus section Terrei. Stud. Mycol. 2011, 69, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.; Groenewald, M.; De Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-J.; Zhang, H.; Dong, W.; Boonmee, S.; Zhang, D. Introducing Dictyochaeta aquatica sp. nov. and two new species of Chloridium (Chaetosphaeriaceae, Sordariomycetes) from aquatic habitats. Phytotaxa 2018, 362, 187–199. [Google Scholar] [CrossRef]
- Schroers, H.J. A monograph of Bionectria (Ascomycota, Hypocreales, Bionectriaceae) and its Clonostachys anamorphs. Stud. Mycol. 2001, 17, 1–214. [Google Scholar] [CrossRef]
- Liu, X.Y.; Huang, H.; Zheng, R.Y. Relationships within Cunninghamella based on sequence analysis of ITS rDNA. Mycotaxon 2001, 80, 77–95. [Google Scholar]
- Sandoval-Denis, M.; Crous, P. Removing chaos from confusion: Assigning names to common human and animal pathogens in Neocosmospora. Pers. Mol. Phylogeny Evol. Fungi 2018, 41, 109–129. [Google Scholar] [CrossRef]
- Short, D.P.G.; O’Donnell, K.; Zhang, N.; Juba, J.H.; Geiser, D.M. Widespread Occurrence of Diverse Human Pathogenic Types of the Fungus Fusarium Detected in Plumbing Drains. J. Clin. Microbiol. 2011, 49, 4264–4272. [Google Scholar] [CrossRef]
- Schroers, H.-J.; Samuels, G.J.; Zhang, N.; Short, D.P.; Juba, J.; Geiser, D.M. Epitypification of Fusisporium (Fusarium) solani and its assignment to a common phylogenetic species in the Fusarium solani species complex. Mycologia 2016, 108, 806–819. [Google Scholar] [CrossRef]
- Walther, G.; Pawłowska, J.; Alastruey-Izquierdo, A.; Wrzosek, M.; Rodriguez-Tudela, J.; Dolatabadi, S.; Chakrabarti, A.; de Hoog, G. DNA barcoding in Mucorales: An inventory of biodiversity. Pers. Mol. Phylogeny Evol. Fungi 2013, 30, 11–47. [Google Scholar] [CrossRef]
- De Beer, Z.W.; Marincowitz, S.; Duong, T.A.; Kim, J.-J.; Rodrigues, A.; Wingfield, M.J. Hawksworthiomyces gen. nov. (Ophiostomatales), illustrates the urgency for a decision on how to name novel taxa known only from environmental nucleic acid sequences (ENAS). Fungal Biol. 2016, 120, 1323–1340. [Google Scholar] [CrossRef]
- Humber, R.A.; Rocha, L.F.; Inglis, P.W.; Kipnis, A.; Luz, C. Morphology and molecular taxonomy of Evlachovaea-like fungi, and the status of this unusual conidial genus. Fungal Biol. 2013, 117, 1–12. [Google Scholar] [CrossRef]
- Rehner, S.A.; Minnis, A.M.; Sung, G.-H.; Luangsa-Ard, J.J.; Devotto, L.; Humber, R.A. Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 2011, 103, 1055–1073. [Google Scholar] [CrossRef]
- Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Manoch, L.; Samson, R.A. On the relationships of Paecilomyces sect. Isarioidea species. Mycol. Res. 2005, 109, 581–589. [Google Scholar] [CrossRef]
- Inglis, P.W.; Tigano, M.S. Identification and taxonomy of some entomopathogenic Paecilomyces spp. (Ascomycota) isolates using rDNA-ITS Sequences. Genet. Mol. Biol. 2006, 29, 132–136. [Google Scholar] [CrossRef]
- Cabanillas, H.E.; de León, J.H.; Humber, R.A.; Murray, K.D.; Jones, W.A. Isaria poprawskii sp. nov. (Hypocreales: Cordycipitaceae), a new entomopathogenic fungus from Texas affecting sweet potato whitefly. Mycoscience 2013, 54, 158–169. [Google Scholar] [CrossRef]
- Ayala-Zermeño, M.; Gallou, A.; Berlanga-Padilla, A.; Serna-Domínguez, M.; Arredondo-Bernal, H.; Montesinos-Matías, R. Characterisation of entomopathogenic fungi used in the biological control programme of Diaphorina citri in Mexico. Biocontrol Sci. Technol. 2015, 25, 1192–1207. [Google Scholar] [CrossRef]
- Su, L.; Zhu, H.; Guo, Y.; Du, X.; Guo, J.; Zhang, L.; Qin, C. Lecanicillium coprophilum (Cordycipitaceae, Hypocreales), a new species of fungus from the feces of Marmota monax in China. Phytotaxa 2019, 387, 55–62. [Google Scholar] [CrossRef]
- Sugiyama, M.; Mikawa, T. Phylogenetic analysis of the non-pathogenic genus Spiromastix (Onygenaceae) and related onygenalean taxa based on large subunit ribosomal DNA sequences. Mycoscience 2001, 42, 413–421. [Google Scholar] [CrossRef]
- Koehn, F.E.; Kirsch, D.R.; Feng, X.; Janso, J.; Young, M. A Cell Wall-Active Lipopeptide from the Fungus Pochonia bulbillosa. J. Nat. Prod. 2008, 71, 2045–2048. [Google Scholar] [CrossRef]
- Oxelman, B.; Lidén, M.; Rabeler, R.K.; Popp, M. A revised generic classification of the tribe Sileneae (Caryophyllaceae). Nord. J. Bot. 2000, 20, 743–748. [Google Scholar] [CrossRef]
- Nonaka, K.; Ōmura, S.; Masuma, R.; Kaifuchi, S. Three new Pochonia taxa (Clavicipitaceae) from soils in Japan. Mycologia 2013, 105, 1202–1218. [Google Scholar] [CrossRef]
- Samson, R.; Houbraken, J.; Varga, J.; Frisvad, J. Polyphasic taxonomy of the heat resistant ascomycete genus Byssochlamys and its Paecilomyces anamorphs. Pers. Mol. Phylogeny Evol. Fungi 2009, 22, 14–27. [Google Scholar] [CrossRef]
- Peterson, S.W.; Orchard, S.S.; Menon, S. Penicillium menonorum, a new species related to P. pimiteouiense. IMA Fungus 2011, 2, 121–125. [Google Scholar] [CrossRef]
- Samson, R.; Yilmaz, N.; Houbraken, J.; Spierenburg, H.; Seifert, K.; Peterson, S.; Varga, J.; Frisvad, J. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol. 2011, 70, 159–183. [Google Scholar] [CrossRef]
- Pan, X.; Richardson, M.D.; Deng, S.; Kremer, R.J.; English, J.T.; Mihail, J.D.; Sams, C.E.; Scharf, P.C.; Veum, K.S.; Xiong, X. Effect of Organic Amendment and Cultural Practice on Large Patch Occurrence and Soil Microbial Community. Crop Sci. 2017, 57, 2263–2272. [Google Scholar] [CrossRef]
- Dung, J.K.; Kaur, N.; Walenta, D.L.; Alderman, S.C.; Frost, K.E.; Hamm, P.B. Reducing Claviceps purpurea sclerotia germination with soil-applied fungicides. Crop Prot. 2018, 106, 146–149. [Google Scholar] [CrossRef]
- Dash, C.K.; Bamisile, B.S.; Keppanan, R.; Qasim, M.; Lin, Y.; Islam, S.U.; Hussain, M.; Wang, L. Endophytic entomopathogenic fungi enhance the growth of Phaseolus vulgaris L. (Fabaceae) and negatively affect the development and reproduction of Tetranychus urticae Koch (Acari: Tetranychidae). Microb. Pathog. 2018, 125, 385–392. [Google Scholar] [CrossRef]
- De Azevedo, A.G.C.; Stuart, R.M.; Sigsgaard, L. Presence of a generalist entomopathogenic fungus influences the oviposition behaviour of an aphid-specific predator. BioControl 2018, 63, 655–664. [Google Scholar] [CrossRef]
- Sangbaramou, R.; Camara, I.; Huang, X.-Z.; Shen, J.; Tan, S.-Q.; Shi, W.-P. Behavioral thermoregulation in Locusta migratoria manilensis (Orthoptera: Acrididae) in response to the entomopathogenic fungus, Beauveria bassiana. PLoS ONE 2018, 13, e0206816. [Google Scholar] [CrossRef] [PubMed]
- Canassa, F.; Tall, S.; Moral, R.A.; de Lara, I.A.; Delalibera, I.; Meyling, N.V. Effects of bean seed treatment by the entomopathogenic fungi Metarhizium robertsii and Beauveria bassiana on plant growth, spider mite populations and behavior of predatory mites. Biol. Control 2019, 132, 199–208. [Google Scholar] [CrossRef]
- Hou, C.X.; Qin, G.X.; Liu, T.; Guo, X.J. Advances in defense mechanism of insects against pathogenic fungi. J. Anhui Agric. Sci. 2012, 40, 11649–11652. [Google Scholar] [CrossRef]
- Horikawa, M.; Shimazu, M.; Aibe, M.; Kaku, H.; Inai, M.; Tsunoda, T. A role of uroleuconaphins, polyketide red pigments in aphid, as a chemopreventor in the host defense system against infection with entomopathogenic fungi. J. Antibiot. 2018, 71, 992–999. [Google Scholar] [CrossRef]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001, 7, 2982–2992. [Google Scholar] [CrossRef]
- Alem, M.A.S.; Oteef, M.D.Y.; Flowers, T.H.; Douglas, L.J. Production of Tyrosol by Candida albicans Biofilms and Its Role in Quorum Sensing and Biofilm Development. Eukaryot. Cell 2006, 5, 1770–1779. [Google Scholar] [CrossRef]
- Baert, K.; Devlieghere, F.; Bo, L.; Debevere, J.; De Meulenaer, B. The effect of inoculum size on the growth of Penicillium expansum in apples. Food Microbiol. 2008, 25, 212–217. [Google Scholar] [CrossRef]
- Li, C.Y.; Liang, Z.H.; Huang, K.L. Research progress on quorum sensing of Aspergilus flavus. J. Food Saf. Qual. 2015, 6, 3205–3210. [Google Scholar] [CrossRef]
- Ke, H.; Niu, Y.; Gu, D.; Wu, J.; Chen, Q. Quorum sensing molecule, farnesol and its action mechanism in fungi. J. Food Saf. Qual. 2017, 8, 862–868. [Google Scholar] [CrossRef]
- Chen, H.; Fujita, M.; Feng, Q.; Clardy, J.; Fink, G.R. Tyrosol is a quorum-sensing molecule in Candida albicans. Proc. Natl. Acad. Sci. USA 2004, 101, 5048–5052. [Google Scholar] [CrossRef]
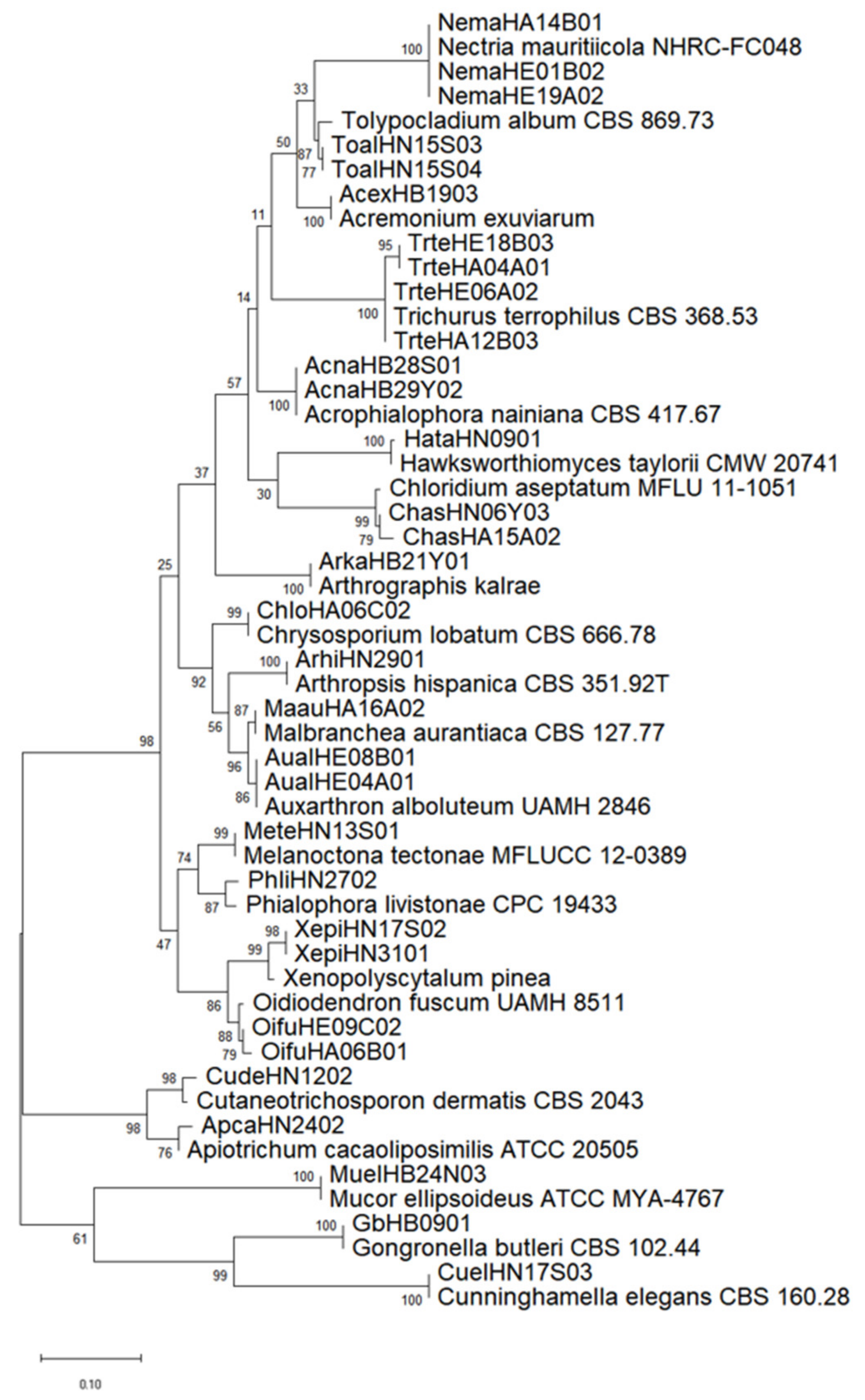

| Strain/Voucher | GenBank Accession Number | Geographic Origin | Reference |
|---|---|---|---|
| Acremonium exuviarum | NR_077167 | Canada | [30] |
| Acrophialophora nainiana CBS 417.67 | MK926894 | China | Unpublished |
| Apiotrichum cacaoliposimilis ATCC 20505 | NR_154671 | USA | [31] |
| Arthrographis kalrae | AB506810 | Japan | [32] |
| Arthropsis hispanica CBS 351.92T | HE965759 | Spain | [33] |
| Aspergillus auricomus NRRL 391 | NR_135388 | USA | [34] |
| Aspergillus crustosus NRRL 4988 | NR_135366 | USA | [34] |
| Aspergillus fumigatus ATCC 1022 | NR_121481 | USA | [30] |
| Aspergillus granulosus NRRL 1932 | NR_135348 | USA | [30] |
| Aspergillus niger ATCC 16888 | NR_111348 | USA | [30] |
| Aspergillus nomius NRRL 13137 | NR_121218 | USA | [30] |
| Aspergillus pseudodeflectus NRRL 6135 | NR_135372 | USA | [34] |
| Aspergillus sclerotiorum NRRL 415 | NR_131294 | USA | [34] |
| Aspergillus sydowii CBS 593.65 | NR_131259 | Japan | Unpublished |
| Aspergillus tanneri ATCC MYA-4905 | NR_111840 | USA | [30] |
| Aspergillus terreus var. subfloccosus CBS 117.37 | NR_149331 | Netherlands | [35] |
| Aspergillus udagawae CBM FA-0702 | NR_137442 | Japan | Unpublished |
| Auxarthron alboluteum UAMH 2846 | NR_111137 | Canada | [30] |
| Beauveria bassiana ARSEF 1564 | NR_111594 | USA | [30] |
| Beauveria bassiana ARSEF 8187 | HQ444271 | Canada | [30] |
| Beauveria bassiana CBS 465.70 | MH859798 | Netherlands | [36] |
| Beauveria bassiana CBS 110.25 | MH854802 | Sri Lanka | [36] |
| Beauveria pseudobassiana ARSEF 3405 | NR_111598 | USA | [30] |
| Chloridium aseptatum MFLU 11-1051 | NR_158365 | China | [37] |
| Chrysosporium lobatum CBS 666.78 | NR_111087 | Spain | [30] |
| Clonostachys grammicospora CBS 209.93 | NR_137650 | Netherlands | [38] |
| Clonostachys rosea f. catenulata CBS 154.27 | NR_145021 | Netherlands | [38] |
| Coniochaeta fasciculata CBS 205.38 | NR_154770 | Spain | Unpublished |
| Cordyceps cateniannulata CBS 152.83 | NR_111169 | Thailand | [30] |
| Cunninghamella elegans CBS 160.28 | NR_154747 | China | [39] |
| Cutaneotrichosporon dermatis CBS 2043 | NR_130667 | USA | Unpublished |
| Fusarium falciforme CBS 475.67 | NR_164424 | Netherlands | [40] |
| Fusarium keratoplasticum FRC S-2477 | NR_130690 | USA | [41] |
| Fusarium solani CBS 140079 | NR_163531 | Slovenia | [42] |
| Gongronella butleri CBS 102.44 | JN206284 | Netherlands | [43] |
| Gongronella butleri CBS 157.25 | JN206607 | Netherlands | [43] |
| Hawksworthiomyces taylorii CMW 20741 | NR_155176 | South Africa | [44] |
| Isaria cateniannulata ARSEF 6242 | GU734760 | Brazil | [45] |
| Isaria farinosa ARSEF 4029 | HQ880828 | USA | [46] |
| Isaria farinosa CBS 262.58 | AY624179 | Thailand | [47] |
| Isaria fumosorosea ARSEF 887 | EU553334 | Brazil | [48] |
| Isaria fumosorosea CBS 244.31 | AY624182 | Thailand | [47] |
| Isaria fumosorosea CBS 337.52 | EF411219 | Thailand | Unpublished |
| Isaria javanica CBS 134.22 | DQ403723 | USA | [49] |
| Isaria javanica CHE-CNRCB 303/2 | KM234213 | Mexico | [50] |
| Lecanicillium coprophilum CGMCC 3.18986 | NR_163303 | China | [51] |
| Lecanicillium saksenae IMI 179841 | NR_111102 | United Kingdom | [30] |
| Malbranchea aurantiaca CBS 127.77 | AB040704 | Japan | [52] |
| Melanoctona tectonae MFLUCC 12-0389 | NR_154194 | Thailand | Unpublished |
| Metarhizium anisopliae CBS 657.67 | MH859066 | Netherlands | [36] |
| Metarhizium flavoviride CBS 218.56 | MH857590 | Czech | [36] |
| Metarhizium marquandii CBS 282.53 | MH857200 | Netherlands | [36] |
| Metarhizium marquandii CBS 182.27 | NR_131994 | Thailand | [47] |
| Metarhizium carneum CBS 239.32 | NR_131993 | Thailand | [47] |
| Metapochonia bulbillosa 38G272 | EU999952 | USA | [53] |
| Metapochonia bulbillosa CBS 145.70 | AJ292397 | UK | [54] |
| Metapochonia bulbillosa FKI-4395 | AB709836 | Japan | [55] |
| Mucor ellipsoideus ATCC MYA-4767 | NR_111683 | USA | [55] |
| Nectria mauritiicola NHRC-FC048 | AJ558115 | Russia | Unpublished |
| Oidiodendron fuscum UAMH 8511 | NR_111035 | Canada | [30] |
| Paecilomyces formosus CBS 990.73B | NR_149329 | Netherlands | [56] |
| Paecilomyces variotii CBS 338.51 | FJ389930 | Netherlands | [56] |
| Penicillium chrysogenum CBS 306.48 | NR_077145 | USA | [30] |
| Penicillium subrubescens CBS 132785 | NR_111863 | Netherlands | [30] |
| Penicillium rubens CBS 319.59 | MH857874 | Netherlands | [30] |
| Penicillium rubens CBS 129667 | NR_111815 | Netherlands | [30] |
| Penicillium guttulosum NRRL 907 | NR_144820 | USA | [57] |
| Penicillium citrinum NRRL 1841 | NR_121224 | USA | [30] |
| Penicillium mirabile CBS 624.72 | JN899322 | Netherlands | [58] |
| Phialophora livistonae CPC 19433 | NR_111824 | Netherlands | [30] |
| Purpureocillium lilacinum CBS 284.36 | NR_111432 | USA | [30] |
| Purpureocillium lavendulum FMR 10376 | NR_111433 | Spain | [30] |
| Simplicillium cylindrosporum JCM 18169 | NR_111023 | Japan | [30] |
| Simplicillium minatense JCM 18176 | NR_111025 | Japan | [30] |
| Talaromyces pinophilus CBS 631.66 | NR_111691 | Netherlands | [30] |
| Talaromyces purpureogenus CBS 286.36 | NR_121529 | Netherlands | [30] |
| Talaromyces trachyspermus CBS 373.48 | NR_147425 | Netherlands | [30] |
| Talaromyces variabilis CBS 385.48 | NR_103670 | Netherlands | [30] |
| Tolypocladium album CBS 869.73 | NR_155018 | Japan | Unpublished |
| Trichurus terrophilus CBS 368.53 | LN850976 | Spain | Unpublished |
| Region | Soil Sample Numbers | Isolate Number | Isolation Rate (%) | EPF Species | SHEI | ||
|---|---|---|---|---|---|---|---|
| Fungi | EPF | Fungi | EPF | ||||
| Hunan | 54 | 97 | 39 | 85.19 | 62.96 | 9 | 0.87 |
| Hubei | 50 | 58 | 24 | 80.00 | 40.00 | 8 | 0.88 |
| Henan | 63 | 73 | 83 | 98.41 | 90.48 | 7 | 0.84 |
| Hebei | 59 | 74 | 42 | 71.19 | 54.24 | 8 | 0.78 |
| Total | 226 | 302 | 188 | 83.70 * | 61.92 * | 11 | -- |
| Region | Soil Sample Numbers | Isolate Number | Isolation Rate (%) | EPF Species | SHEI | ||
|---|---|---|---|---|---|---|---|
| Fungi | EPF | Fungi | EPF | ||||
| Arbor | 64 | 79 | 45 | 79.69 | 59.38 | 9 | 0.86 |
| Crop | 85 | 114 | 83 | 84.71 | 69.41 | 9 | 0.76 |
| Fallow land | 9 | 12 | 6 | 100.00 | 55.56 | 6 | 1.00 |
| Grass | 68 | 97 | 54 | 85.29 | 60.29 | 9 | 0.81 |
| Total | 226 | 302 | 188 | 87.42 * | 61.16 * | 11 | -- |
| Isolated Strain | Species | Adjusted Mortality (%) |
|---|---|---|
| ApcaHN2402 | Apiotrichum cacaoliposimilis | 12.96 ± 0.56 |
| AsgrHB3004 | Aspergillus granulosus | 26.19 ± 0.24 |
| AsnoHB27S02 | Aspergillus nomius | 21.05 ± 0.72 |
| AsnoHN03S02 | Aspergillus nomius | 12.96 ± 0.57 |
| AspsHB3002 | Aspergillus pseudodeflectus | 21.88 ± 0.85 |
| AsscHN1502 | Aspergillus sclerotiorum | 7.55 ± 0.35 |
| AssyHE06A03 | Aspergillus sydowii | 19.30 ± 0.38 |
| AstaHN1501 | Aspergillus tanneri | 45.24 ± 0.39 |
| AsteHN01S02 | Aspergillus terreus | 15.71 ± 0.73 |
| AsudHE13C01 | Aspergillus udagawae | 16.00 ± 0.44 |
| BebaHA22A02 | Beauveria bassiana | 10.70 ± 0.19 |
| ChasHA15A02 | Chloridium aseptatum | 1.28 ± 0.51 |
| CudeHN1202 | Cutaneotrichosporon dermatis | 2.83 ± 0.10 |
| FufaHN1901 | Fusarium falciforme | 28.07 ± 0.68 |
| IsjaHB2602 | Isaria javanica | 9.52 ± 0.30 |
| IsjaHN3002 | Isaria javanica | 67.86 ± 0.61 |
| LecoHE07B01 | Lecanicillium coprophilum | 18.18 ± 0.33 |
| LesaHB28S05 | Lecanicillium saksenae | 26.32 ± 0.45 |
| MeanHE15B01 | Metarhizium anisopliae | 23.56 ± 0.37 |
| MeanHE20B01 | Metarhizium anisopliae | 19.62 ± 0.45 |
| MemaHA24A01 | Metarhizium marquandii | 4.55 ± 0.42 |
| MemaHN26S01 | Metarhizium marquandii | 22.81 ± 0.91 |
| Mema sp. HN2501 | Metarhizium marquandii | 5.63 ± 0.41 |
| MeteHN13S01 | Melanoctona tectonae | 15.00 ± 1.12 |
| MuelHB24N03 | Mucor ellipsoideus | 15.00 ± 0.60 |
| NemaHA14B01 | Nectria mauritiicola | 16.33 ± 0.30 |
| OifuHA06B01 | Oidiodendron fuscum | 1.85 ± 0.19 |
| PeciHA25A01 | Penicillium citrinum | 9.74 ± 0.29 |
| PesuHN1002 | Penicillium subrubescens | 6.67 ± 0.27 |
| PhliHN2702 | Phialophora livistonae | 16.33 ± 0.57 |
| PulaHA08C01 | Purpureocillium lavendulum | 3.92 ± 0.30 |
| SicyHE17A02 | Simplicillium cylindrosporum | 15.00 ± 0.27 |
| SimiHE17C01 | Simplicillium minatense | 8.16 ± 0.23 |
| TapiHB23G01 | Talaromyces pinophilus | 7.02 ± 0.16 |
| TapiHB23S01 | Talaromyces pinophilus | 31.58 ± 0.32 |
| TatrHE03C03 | Talaromyces trachyspermus | 11.11 ± 0.20 |
| TatrHE14A01 | Talaromyces trachyspermus | 6.56 ± 0.44 |
| TatrHE18B01 | Talaromyces trachyspermus | 8.33 ± 0.21 |
| ToalHN15S03 | Tolypocladium album | 11.67 ± 0.65 |
| HA13B02 | – | 2.27 ± 0.31 |
| HA17C01 | – | 11.43 ± 0.18 |
| HB3003 | – | 23.81 ± 0.34 |
| HB3102 | – | 8.87 ± 0.69 |
| HE07A01 | – | 14.81 ± 0.32 |
| HN06Y05 | – | 14.81 ± 0.41 |
| HN20Z01 | – | 7.41 ± 0.23 |
| HN28J01 | – | 1.96 ± 0.22 |
| Control | – | 3.33 ± 0.45 |
| Concentration (Spores/mL) | Accumulated Mortality (%) | Muscardine Cadaver Rate (%) | ||||
|---|---|---|---|---|---|---|
| 1 d | 3 d | 7 d | 1 d | 3 d | 7 d | |
| CK | 3.33 ± 2.36 c | 6.67 ± 2.36 c | 10.00 ± 4.08 d | 0 | 0 | 0 |
| 1.0 × 104 | 10.00 ± 4.08 bc | 18.33 ± 2.36 b | 40.00 ± 4.08 c | 0 | 0 | 6.67 ± 4.71 c |
| 1.0 × 105 | 15.00 ± 0 ab | 23.33 ± 2.36 b | 41.67 ± 2.36 c | 0 | 0 | 11.67 ± 4.71 c |
| 1.0 × 106 | 13.33 ± 6.23 abc | 20.00 ± 4.08 b | 53.33 ± 4.71 b | 0 | 1.67 ± 2.36 b | 21.67 ± 6.23 b |
| 1.0 × 107 | 21.67 ± 8.5 a | 33.33 ± 6.23 a | 61.67 ± 4.71 b | 0 | 3.33 ± 2.36 b | 26.67 ± 2.36 b |
| 1.0 × 108 | 23.33 ± 2.36 a | 36.67 ± 4.71 a | 80.00 ± 4.08 a | 0 | 8.33 ± 2.36 a | 41.67 ± 2.36 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Zhang, X.; Hu, Q.; Weng, Q. Entomopathogenic Fungi in the Soils of China and Their Bioactivity against Striped Flea Beetles Phyllotretastriolata. Diversity 2022, 14, 464. https://doi.org/10.3390/d14060464
Zhang K, Zhang X, Hu Q, Weng Q. Entomopathogenic Fungi in the Soils of China and Their Bioactivity against Striped Flea Beetles Phyllotretastriolata. Diversity. 2022; 14(6):464. https://doi.org/10.3390/d14060464
Chicago/Turabian StyleZhang, Ke, Xiaofeng Zhang, Qiongbo Hu, and Qunfang Weng. 2022. "Entomopathogenic Fungi in the Soils of China and Their Bioactivity against Striped Flea Beetles Phyllotretastriolata" Diversity 14, no. 6: 464. https://doi.org/10.3390/d14060464
APA StyleZhang, K., Zhang, X., Hu, Q., & Weng, Q. (2022). Entomopathogenic Fungi in the Soils of China and Their Bioactivity against Striped Flea Beetles Phyllotretastriolata. Diversity, 14(6), 464. https://doi.org/10.3390/d14060464







