Abstract
In recent years, fungi, particularly lignicolous fungi, have been re-considered as a source for biotechnological and industrial applications. Lignicolous basidiomycetes are the most effective at degrading wood, particularly cellulose, hemicelluloses and lignin, which are among the most resistant biopolymers. This study aims to constitute a research collection of lignicolous fungal strains that are useful for further studies and applications in different production fields. The basidiomata used to isolate the strains in a pure culture were, firstly, identified through macroscopic and microscopic characteristics integrated with ecological data. To obtain pure cultures of dikaryotic mycelia, 96 different strains of Agaricomycetes belonging to 76 different species and related to 51 genera (18 families and 5 orders) were isolated using a malt extract agar (MEA) medium enriched with hydrogen peroxide. The identity of the isolated strains was then confirmed by molecular analysis through the sequencing of the internal transcribed spacer (ITS) region of the ribosomal RNA gene cluster. All the strains are currently conserved using different methods, and their vitality is periodically tested.
1. Introduction
Fungi are an essential, fascinating and useful group of organisms with biotechnological potential for pure and applied research as well as for industrial exploitation [1]. Filamentous fungi have been used for more than a century as versatile and highly productive organisms. Nowadays, fungi, especially basidiomycetes, can be used in many different applications: in the medicinal field as immunostimulants and food supplements; in pharmacology as a source of bioactive compounds against human and animal diseases (e.g., antibacterial antibiotics, antifungals, antiviral agents, anti-cancer agents, anti-diabetes, controllers of cardio-vascular diseases, etc.); in agriculture as biocontrol agents against fungi, insects, nematodes, weeds, etc., as low-impact food and protein sources and to enhance crops and forestry; and for commodities such as cosmetics, preservatives, enzymes and textile dyes [1].
Both scientific research and industrial applications require not only constant material but also simple and fixed conditions. This allows for a better control of the variables that can influence the output of the research, providing an understanding of which parameters can modify the result and how. Being able to reproduce the same experiment is a key principle of scientific research, and it is fundamental for industrial applications to have standard production processes that consistently produce identical products.
Fungal strain diversity represents a genetic resource that should be preserved. For this reason, certified collections have been created. Fungal culture collections are of primary importance in order to deepen our knowledge of the taxonomy, species distribution and officinal properties and to investigate the potential applications of fungi [2]. Moreover, collections can play a role in preserving biodiversity and conserving endangered species ex situ.
Culture collections are a fundamental source for researchers at an international level, and the exchange and availability of quality-guaranteed, authenticated pure cultures are increasingly in demand. The World Federation for Fungal Collections has provided detailed guidelines [3] which aim to ensure collections are of high quality, from the origin to the conservation, and the availability of each strain. In comparison to the past, this is made relatively easy by the increasing accessibility of standardized equipment (including sterile hoods, refrigerators, freezers, etc.) and labware for the isolation, safe culturing and preservation of strains. Nowadays, some major variables make the observation of the culture characteristics reproducible over time and comparable among different work and laboratories. They are: the use of commercial culture media instead of the home-made older ones; the use of conventional Petri dishes, vessels and sealing tools (plastic film or paper adhesive tape alongside the “evergreen” raw cotton for tubes), which differently affect the gas exchange and dehydration; the use of incubators to keep the growth temperature constant or finely tuned.
Some major culture collections around the world include the CBS-KNAW, the All-Russian Collection of Microorganisms (VKM) and the Agro-food & Environmental Fungal Collection (BCCM/MUCL) [4,5,6]. In addition, the Project MIRRI (Microbial Resource Research Infrastructure) is a tool used within the European Union to build a pan-European platform to coordinate the access to individual resources (not only fungal) and promote the above-mentioned quality standards [7].
Besides the well-established fungal culture collections which can afford the requirements for the conformity to WFFC standards, many universities and small research centres all over the world have their own culture collections [8,9,10]. These strains can be considered an important source of biological and genetic material because they are geographically widespread, and their contribution could be significantly representative of the biodiversity of local ecosystems [11,12]. These small collections could thus represent the initial stage in the development of an official collection accessible to the scientific community in the future. This would allow for comparisons among different species, or different strains belonging to the same species, that had been isolated from different substrates, environments or geographical areas. This type of information is often required because the biochemical differences between them could be relevant [13,14,15,16].
The Culture Collection of the University of Pavia (MicUNIPV) has its roots in the former Laboratory of Cryptogamic Botany (the first of its kind in Italy), founded in the 19th century by Santo Garovaglio. Nowadays, the Department of Earth and Environmental Sciences of the University of Pavia is an associated member of the MIRRI Italian Node. By keeping a multi-focus approach, the current Laboratory of Mycology has developed a wide collection of both micromycetes and macromycetes, among which there is a continuously increasing collection of wood decay species [2].
The isolation and study of wood decay species has a particularly strong cultural background in Asia, where the use of these fungi has a long tradition [17,18]. In the Italian landscape, only a few other culture collections have devoted part of their effort to wood decay fungi, namely: MUT—Mycotheca Universitatis Taurinensis; SAF—University of Palermo Mycotheca; PeruMyc—Department of Chemistry, Biology and Biotechnology, University of Perugia; AQUI—University of L’Aquila; ColD-Collection of DISTAV—University of Genova; and BUCC—Bologna University Culture Collection. This list may not be exhaustive, since wood decay fungi have been increasingly gaining the interest of researchers for different base and applied purposes (e.g., FBL—Fungal Biodiversity Lab, Sapienza University of Roma).
The aim of this work was to sample as many species as possible within lignicolous Basidiomycota from different environments in northern Italy in order to isolate fungal strains useful for further studies and applications in different fields. A consequential goal of this work was to provide a detailed morphological description of each strain, which can support both applied and pure research.
2. Materials and Methods
2.1. Basidiomata Sampling
The fieldwork was carried out in different geographical areas of northern and central Italy (the Piemonte, Lombardia, Liguria and Lazio regions). In order to collect as many lignicolous species as possible, different environments were investigated (Table 1).

Table 1.
Environments investigated for field sampling in northern Italy. Ecoregional sections and subsections, as in Blasi et al. [19].
In order to obtain the greatest possible species diversity, some areas were more intensively sampled than others; therefore, the effort to collect and isolate strains was not the same for all sites [20].
2.2. Fungal Strains Isolation
In this study, the isolation effort was focused on dykariotic mycelia only; no isolation from basidiospores was attempted.
Only actively growing basidiomata were collected. Where possible, the cleanest samples were completely or partially harvested (depending on their size and local rarity) using a knife and touching them as little as possible.
The collected portion was placed into aluminum foil to keep it clean until the laboratory work.
In order to avoid destroying the basidiomata of Fomitopsis officinalis, its mycelium was directly isolated in the field using a sterilized scalpel and the flame of a lighter. This precaution was taken because this species, whose growth is very slow and mostly restricted to protected areas, was assessed as endangered by the Global Fungal Red List Initiative [21,22].
The protocol generally used to isolate the mycelia from wild basidiomata [23,24,25] was slightly modified according to Rush Wayn [26]. To isolate the fungal strains, Petri dishes of 90 mm diameter were prepared using 2% malt extract agar (MEA) with 6 mL L−1 of a solution of 3% hydrogen peroxide in order to reduce the spore germination of the contaminants.
Based on the references above, the classic withdrawal of a piece of context under sterile conditions was applied for thick-context species (with thicknesses greater than 2 mm). For treating thin-context species, the humid chamber method was applied. To establish a humid environment where the mycelium could regrow for a couple of days, the harvested basidiomata were placed at 10 °C in the dark inside small plastic boxes (humid chambers) on soaked paper. The fresh mycelium that developed in the humid chambers was transferred in sterile conditions under a laminar flow hood into the Petri dishes.
2.3. Basidiomata and Fungal Strains Identification
The identification of the collected basidiomata was carried out by macro- and micro-morphological identification through dichotomous keys [27,28,29,30,31,32,33]. Microscopy was executed using a Paralux monocular microscope.
Furthermore, the main microscopic characteristics of the isolated mycelia were observed based on Stalpers et al. [23], with reference to colony colour, colony edge, mat morphology, clamps and the presence/absence of chlamidospores.
Besides the morphological investigations, the molecular identification of isolates was needed to confirm the species identity. Firstly, to produce a sufficient amount of dry biomass, each strain was put into a 200 mL Erlenmeyer flask containing 50 mL of 2% malt extract (ME) solution and grown for 10 days at 25 °C in the dark and in static. The biomass was then collected with forceps in sterility, placed in glass tubes at −18 °C and freeze-dried (Buchi lyovapor L-200). The DNA was extracted following the NucleoSpin Plant II protocol and then amplified by a Polymerase Chain Reaction (PCR) using the DreamTaq PCR Green Master Mix and the primer pair ITS1 and ITS4. The PCR was performed as follows: denaturation (95 °C) 5 min + 30 s; annealing (50 °C) 45 s; elongation (72 °C) 1 min. All the steps were repeated for 35 cycles, after which the final elongation (72 °C, 10 min) was carried out [2].
The PCR products were purified using the Wizard SV Gel and PCR Clean-Up System (Promega) and sent to Macrogen (The Netherlands) for sequencing. The obtained sequences were assembled, corrected and subsequently analysed by BLAST and Molecular ID searches by respectively using the GenBank (NCBI) [34] and MycoBank (CBS) [35] databases. The taxonomic assignments were based on similarity to reference sequences of these databases.
MycoBank [35] was used as the reference for the taxonomy and systematics.
2.4. Fungal Strain Conservation
The isolated strains were stored under different environments: storage in Petri dishes and tubes with 2% MEA at 4 °C; storage on colonised filter paper discs submerged in sterilised and demineralized water in water vials at 4 °C [36]; and cryopreservation at −80 °C.
2.4.1. Conservation on Paper Discs
For the disc preservation, the sterilised paper filter discs of 5 mm diameter were placed into a 2% MEA Petri dish and consequently colonised by the growing mycelium (Figure 1a,b). The colonised discs were then moved in sterile polypropylene vials containing demineralized water and stored at 4 °C in the dark.
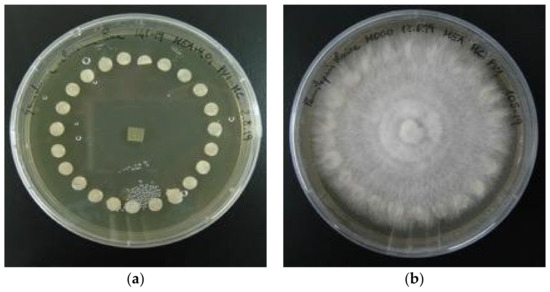
Figure 1.
(a) Petri dish with paper discs before the mycelium growth; (b) the paper discs were colonised by the mycelium and can be moved to vials for storage.
To verify the vitality of the strains, after 18 months, colonised discs were removed from the water under sterile conditions and back-cultured in the MEA Petri dishes at 25 °C in the dark. Analogous back-cultures were set up to test the vitality of the strains in the Petri dishes and tubes at 4 °C. For cultures kept at −80 °C, vitality was tested for random strains only.
2.4.2. Cryoconservation
The cryoconservation protocol has an initial step in which the strains are inoculated in a flask with a liquid medium (ME 2%). After 7 days, or after good mycelium production, the biomass can be stored. Operating under sterile conditions, the mycelium was withdrawn from the flask and placed in a 10 mL tube containing a 15% solution of glycerol. The mycelial suspension in the glycerol solution was then homogenised by vortexing at 3000 rpm for 30 s. Mycelium homogeneous cutting was obtained by adding broken microscopy cover slides which had previously been autoclaved. Then, 1 mL of the suspension was placed in 1.5 mL sterile cryotubes. For each fungal strain, four replicates were stored at −80 °C.
All the strains are currently maintained in the research fungal collections of Mogu S.r.l (MRFC) and of the University of Pavia (MicUNIPV).
2.5. Morphological Description of Pure Cultures
The mycelia of each strain were described based on MEA cultures in 90 mm Petri dishes, incubated at 25 °C in the dark and checked every 48 h. The inoculum came from a 10-day-old mother colony and was placed at the edge of the plate to allow the colony to expand over the whole dish diameter [37]. The radius was measured using a calliper (0.1 mm resolution). The growth rate (mm day−1) was calculated for each strain on day 7 of growth and reported as the average of the three replicates. The uncertainty from random error (the absolute uncertainties of the individual measures) was calculated according to Harris [38].
Besides the basic visual inspection of macromorphology, the main micromorphological characters were examined on day 7 of growth (or day 15 for very slow growing strains) by a Zeiss Stemi 2000-C stereoscope and by a Nikon LABOPHOT-2 microscope. The mycelia were mounted in lactophenol cotton blue or lactophenol-acid fuchsin for optical microscopy.
3. Results
3.1. Basidiomata Sampling
The main purpose of this study was not to carry out a blind scan of different ecosystems but, instead, to precisely identify certain species known to grow in a particular habitat type or in a specific place. This was possible thanks to long-term data which have been collected and registered by a local mycological group concerned with mushroom species growing in the Varese province (Italy) since 1990 [39].
The principal types of habitats that constitute the landscapes in northern Italy, and those present in the province of Varese, were investigated during specific sampling campaigns. Fresh broadleaf forests are the most represented habitat, and 38 strains (40%) were isolated from the species collected there. Urban and suburban environments also contained many lignicolous species, leading to the isolation of 27 strains (28.4%) (Figure 2). Even if there are fewer trees in urban areas than in natural environments, many species of lignicolous basidiomycetes grow in urban settings. Trees in public parks, private gardens and along roads can host fungi as they are older and generally in poor health due to the low-quality growing conditions, over-pruning and wounds caused by cars or root cutting for excavations, etc. Furthermore, in these urban areas, the species of tree that usually grow in different environments can coexist.
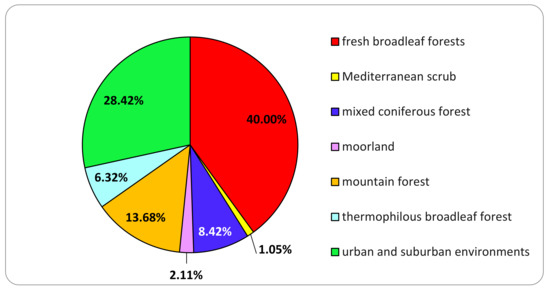
Figure 2.
Distribution of the isolates among the explored habitats where the basidiomata originated.
In total, 26 genera of trees on which the fungal species were growing could be identified (Figure 3). In particular, the majority of the collected basidiomata were growing on Quercus spp. (12%).
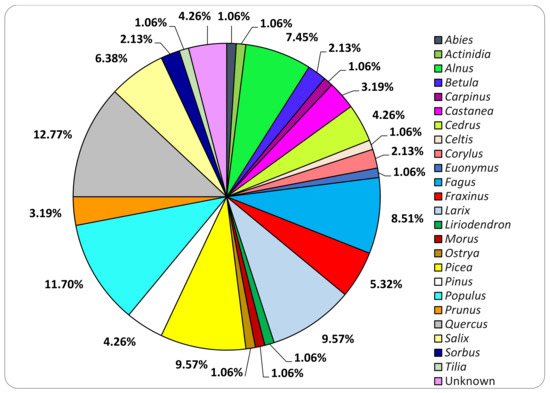
Figure 3.
Tree genera hosting the original basidiomata from which the strains were isolated.
3.2. Fungal Strains Collection
The mycelium in pure culture has been successfully isolated from 96 out of the 103 basidiomata collected (93.2%). The molecular confirmation of the morphological identification showed that all the isolated strains belong to Agaricomycetes, namely, 76 different species from 51 genera, 18 families and 5 orders (Agaricales, Gloeophyllales, Hymenochaetales, Polyporales and Russulales) (Table 2).

Table 2.
Taxonomy of the isolated strains and reference to the code within the Mogu S.r.l research fungal collection (MRFC). Taxonomy relies on MycoBank [35].
As reported above, MycoBank [35] was used as the only reference in this study. However, in comparison with Index Fungorum [40] and part of the literature, nomenclatural issues are still being debated, mainly for the following species: Polyporus badius (Pers.) Schwein., also known as Picipes badius (Pers.) Zmitr. & Kovalenko, and Polyporus squamosus (Huds.) Fr., also known as Cerioporus squamosus (Huds.) Quél according to Mycobank and Bernicchia & Gorjon (2020) [28].
The family of Polyporaceae is the most represented in the collection: almost 50% of the isolated species belong to it (Figure 4). For orders, Polyporales is by far the most represented: 55.6% of the isolated families belong to it (Figure 5).
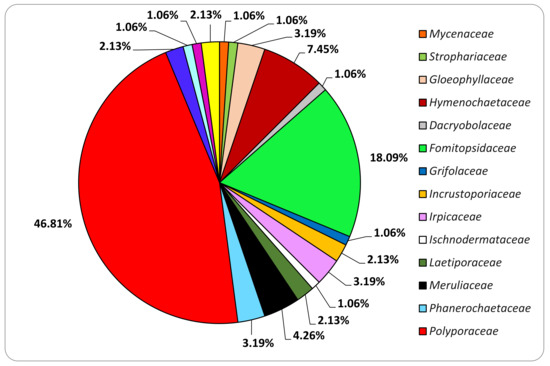
Figure 4.
Families represented in the set of isolated strains included in this study.
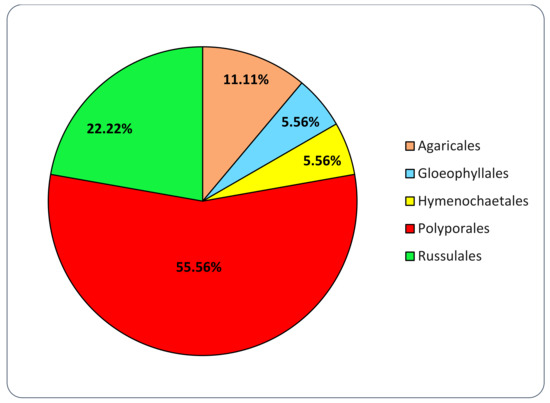
Figure 5.
Orders represented in the set of isolated strains included in this study.
Among all of the species listed in Table 2, 80% are considered white rot agents, while 20% are brown rot.
3.3. Basidiomata and Fungal Strains Identification
The molecular identification of the strains confirmed the morphological identification of the collected basidiomata and disentangled the uncertain identity of poorly differentiated samples (primordia of Fomitiporia contigua 085-19, Ganoderma adspersum 007-18, Daedaleopsis tricolor 028-18) or species that are very similar to each other (Fomitiporia mediterranea, Cyanosporus alni, Postia tephroleuca, Dichomitus squalens, Porostereum spadiceum).
The identifications of two strains (Antrodia sp. and Antrodia cfr. alpina) were still uncertain, and these could not be unequivocally identified. In particular, the basidioma macrocharacteristics and the host tree suggest that the strain was A. alpina (e.g., its change to red in KOH and its growth on Larix decidua), but the molecular analysis on the ITS region does not exclude A. xantha. Therefore, the strain 134-19 is referred to as Antrodia cfr. alpina. Further molecular markers (e.g., factor 1-α and LSU) are needed to confirm the identities of these strains [41].
The molecular analysis was important for Fomitiporia mediterranea, which can only be distinguished from Fomitiporia punctata using ITS sequences, since all the microscopical and macroscopical elements are the same. MycoBank reported the Laetiporus genus in Fomitopsidaceae because Laetiporaceae is considered invalid. Nevertheless, Phaeolus is placed in Laetiporaceae. We decided to follow Justo et al. (2017) [42] and consider Laetiporaceae Julic 1989 [33] valid, so Laetiporus could be included in this family along with Phaeolus.
The genus Ganoderma P. Karst. is a taxonomical group whose strains are among the most difficult to distinguish [27]. Nevertheless, the reported strains in Table 2 have been unequivocally identified. In particular, the morphological discrimination between G. applanatum and G. adspersum basidiomata could be difficult if the collected specimens are too young. However, G. adspersum could be identified from mycelium layers among tube strates, but if the basidioma is less than two years old, this feature cannot be observed. In this case, molecular analyses are always required to be sure of their exact identification. Ganoderma resinaceum, when grown on Salix or Alnus close to water, sometimes has long stipes and presents a thinner context when compared to the specimens usually growing on Quercus spp. This can mean that it resembles G. lucidum morphologically, but these two species are well separated by molecular analysis. On the other hand, for the identification of G. carnosum, it was more critical to use molecular methods over morphological identification because different but equally supported (>97%) identification alternatives were produced by the comparative analysis of the ITS sequences in both Mycobank [35] and NCBI [34]. The molecular analysis failed to discriminate the isolated strain from Ganoderma valesiacum (which has a white context and grows on Larix decidua only) as well as from Ganoderma oregonense and Ganoderma tsugae, (two North American species) [43,44]. In addition, G. valesiacum and G. carnosum present quite different mycelia on MEA: the first pigments quite quickly and forms a thin layer of hyphae, whereas the second forms a white, thicker and faster growing mycelium. Further studies are ongoing to clarify whether they can be treated as a single species or whether they should be considered different entities
Finally, for Cyclocybe cylindracea, the identification of the taxonomic situation is still uncertain. As shown in Vizzini et al. [45], two well supported clades exist. Furthermore, two names are accepted: Cyclocybe aegerita (V. Brig.) Vizzini and Cyclocybe cylindracea (DC.) Angelini & Vizzini, but no typus is assigned to them (according to personal communication with Vizzini). It is probable that the two accepted names will be assigned to the two existing clades of this collective species.
As it can be observed in Table 2, only a small number of corticioid strains have been isolated, even though they are abundant and occur frequently in nature. It is particularly difficult to isolate them properly since they not only have a very thin context but are also rarely found clean and actively growing. Among the corticioid species, the mycelia of Terana caerulea and Porostereum spadiceum were isolated with success. The basidiomata of these two species are quite common in nature, but they are thin and close to the ground, so the strains in pure culture are not so common. It is difficult to maintain these two species on artificial media. Storage using paper-filter disks at 4 °C has proven to be effective, as the two species were able to regrow after 18 months of storage.
Among the isolated strains, Dichomitus squalens, Fomitopsis iberica, Niveoporofomes spraguei, Ganoderma carnosum, Ganoderma valesiacum, Fomitopsis officinalis, Polyporus corylinus and Sarcoporia polyspora are considered uncommon or rare species with a scattered distribution, at least in Italy, as reported in the Checklist of Italian fungi—Basidiomycota [46] and in Bernicchia & Gorjon [29].
Some species are host-specific, such as G. valesiacum and F. officinalis, which are strictly associated with Larix decidua. On the contrary, other strains, even if not common, showed a very large spectrum of hosts: in particular, F. iberica could grow on both angiosperms and gymnosperms. Notably, this species was found exclusively in urban parks. Other species that grow preferably in urban areas are F. mediterranea, P. fraxinea, G. adspersum and G. resinaceum. Ganoderma carnosum, which usually grows in Abies alba forests, was found in two different public parks on decayed coniferous stumps.
Due to the decision to isolate the mycelia from fresh and actively growing basidiomata, only a small number of collected samples could not be isolated: Fistulina hepatica, Pleurotus dryinus, Serpula himantioides, Meruliopsis taxicola, Dendropolyporus umbellatus, Rigidoporus sanguinolentus, Neofavolus suavissimus, Favolaschia calocera and Hericium cirrhatum. This was because molds or bacterial contaminations were always present, overgrowing the target mycelium even with the addition of hydrogen peroxide.
3.4. Morphological Description of Pure Cultures
The main characteristics of the mycelia in pure culture are reported in Table 3.

Table 3.
Morphological description of the strains in pure culture and the average growth rate of the three replicates calculated at 7 days after inoculation (absolute uncertainty from random error ± 0.17 mm).
Besides the morphological characteristics reported in Table 3, some additional features could be observed later (i.e., when the colonies were over 15 days old) and are reported as follows:
- -
- Phylloporia ribis showed thin, up to 1 cm long, crystals. The nature of these peculiar structures is unknown and could be worthy of further investigation (Figure 6);
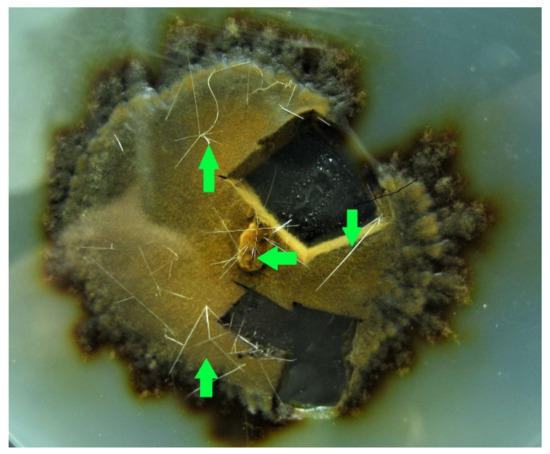 Figure 6. Pure culture of Phylloporia ribis showing white 1 cm-long crystals, a number of which are indicated by the green arrows.
Figure 6. Pure culture of Phylloporia ribis showing white 1 cm-long crystals, a number of which are indicated by the green arrows. - -
- The brown rot agents Gloeophyllum odoratum, Neolentinus lepideus, Fomitopsis officinalis, Antrodia cfr. Alpina and Fomitopsis iberica and the white rot agents Fuscoporia contigua and Polyporus squamosus produced a non-localized MEA colour change to darker hues;
- -
- Neolentinus lepideus pure cultures developed a strong and pleasant anisate smell, similar to the basidiomata;
- -
- Abortiporus biennis and Peniophora quercina produced dark-reddish exudates.
Exudates are recurrent in A. biennis and P. quercina according to both the literature [23] and the authors’ previous experience.
Regarding the mycelium characteristics, all of the strains related to the Ganoderma genus had a very compact and thin-layered mycelium. On the other hand, Agaricales had a fluffy and inconsistent mycelium when compared to Polyporales.
The strains belonging to Hymenochaetaceae (Fuscoporia, Phylloporia, Fomitiporia, Inonotus) produced a coloured mycelium in the Petri dish: Fuscoporia and Fomitiporia showed a brownish, thick mycelium, whereas Inonotus and Phylloporia presented a thin, yellowish mycelium with extrusions in agar dark-brown compounds. A number of other strains present a coloured mycelium: Antrodia cfr. alpina has a sulphur-yellow mycelium; Stereum hirsutum has a mycelium that is light orange; Terana caerulea has a mycelium that starts out white before becoming an intense blue colour; and Pycnoporus cinnabarinus has an orange-reddish mycelium reflecting the colour of its basidiomata.
The important characteristics for the biotechnological application of fungal strains are the consistency of the mycelium production and the growth rate in culture. A few strains presented thin (or transparent), inconsistent mycelia (I. latemarginatus, Polyporus squamosus, Stereum sanguinolentum, Terana caerulea). Others showed a very slow growth rate: Antrodia cfr. alpina, Fomitopsis officinalis, Osteina obducta and Cyanosporus alni among brown rot agents; and Fuscoporia torulosa, Inonotus radiatus, Phylloporia ribis and Skeletocutis amorpha among white rot agents.
The cultures of Laetiporus sulphureus and Fomitopsis officinalis have a dusty surface due to the production of asexual spores.
Some other species, such as Abortiporus biennis, Coriolopsis gallica and C. trogii, Daedaleopsis confragosa, Fomes fomentarius, Fomitopsis iberica and F. pinicola, Ganoderma carnosum and G. lucidum, Irpex lacteus, Irpiciporus pachyodon, Lenzites betulinus, Polyporus alveolaris, Stereum hirsutum, Trametes gibbosa, T. hirsute and T. suaveolens presented a fast-growing and homogeneous tough colony.
3.5. Fungal Strains Conservation
To date, all of the isolated strains have resulted in successful conservation thanks to the application of combined storage methods.
Based on the back-cultures, all of the strains are maintained alive after the classic storage in MEA (Petri dish or tube) at 4 °C.
It has been demonstrated that all the isolated strains are maintained alive for at least 18 months in water vials on paper-filter discs at 4 °C, but not all regrew immediately when transferred to a new MEA Petri dish. Of particular note is the case of Osteina undosa on 162-19 colonized filter paper discs. After 18 months of storage in water vials at 4 °C, the discs were placed on MEA Petri dishes for strain refreshment. The mycelium started to grow again only after 7 months of total inactivity at 25 °C.
All the randomly back-cultured strains removed from −80 °C were able to regrow on MEA.
4. Conclusions
Strains isolated in pure culture from lignicolous fungi are a powerful tool for both pure and applied research.
The successful isolation ratio was very high in the developed method. In total, only 9 out of 103 strains could not be isolated (less than 10% of the total).
From the perspective of taxonomy and systematics, this work has achieved a remarkable stock of new strains from both common and rare species; such strains will be available for future studies and collaborations. The main outcome to be highlighted is therefore the possibility to fill a geographic gap by introducing strains from northern Italy in such future studies; this is particularly true for the rare/uncommon species that are often excluded or poorly represented in the experimental sets due to the lack of strains in pure culture.
Among the many possible applications of fungi, the characteristics of the mycelia are particularly important in the case of the formation of myco-materials. Mycenaceae, Strophariaceae, Dacryobolaceae, Laetiporaceae and Bondartzewiaceae suggest their inadequacy for producing materials based on fungi, as their colonies on artificial media are thin, slow-growing and formed of an inconsistent mycelium. The strains belonging to the Fomitopsidaceae, Hymenochaetaceae, Irpicaceae, Meruliaceae, Phanaerochaetaceae, Polyporaceae and Stereaceae fungal families seem to be the most suitable for myco-materials due to the high growth rates, homogeneity and stiffness of their mycelial colony.
This study provides the first step for further work on the selection of suitable fungal strains in order to obtain pure fungal materials or biocomposites based on fungi. Consistent with the results of this study, 21 different strains belonging to 20 species were selected from the strain set described above and examined as described in Cartabia et al. [38].
Author Contributions
Conceptualization, E.S. and M.C.; methodology, M.C., R.M.B., S.B. (Simone Buratti) and C.E.G.; validation, A.B.; investigation, M.C., S.B. (Simone Buratti) and R.M.B.; resources, S.B. (Stefano Babbini) and E.S.; data curation, M.C., C.E.G. and S.B. (Simone Buratti); writing—original draft preparation, M.C., R.M.B. and S.B. (Simone Buratti); writing—review and editing, C.E.G., E.S., S.B. (Simone Buratti), R.M.B. and M.C.; supervision, A.B., S.B. (Stefano Babbini) and E.S.; project administration, E.S. and R.M.B.; funding acquisition, S.B. (Stefano Babbini) and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Fondazione Cariplo and Regione Lombardia, Grant No. 2018-1765, project entitled ‘MYCO-ADVANCED LEATHER MATERIALS (MATER)’.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data are available in the article.
Acknowledgments
The authors wish to thank the Associazione Micologica Bresadola, Group of Varese (Italy) and, in particular, Mario Cervini and Nino Macchi for all their support and suggestions. The authors are very grateful to Alfredo Vizzini (University of Turin, Italy) for his help with some considered taxonomical issues. The authors are very grateful to Sergio Pérez Gorjón for his wise suggestions during the revision to improve the paper structure. The authors are also grateful to Daniele Dondi (University of Pavia, Italy), the principal investigator of the project MATER.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef] [Green Version]
- Girometta, C.E.; Bernicchia, A.; Baiguera, R.M.; Bracco, F.; Buratti, S.; Cartabia, M.; Picco, A.M.; Savino, E. An Italian Research Culture Collection of Wood Decay Fungi. Diversity 2020, 12, 58. [Google Scholar] [CrossRef] [Green Version]
- WFCC—World Federation for Culture Collections. Available online: www.wfcc.info (accessed on 29 December 2021).
- CBS-KNAW Collections. Available online: www.cbs.knaw.nl (accessed on 29 December 2021).
- All Russian Collection of Microorganisms—VKM. Available online: www.vkm.ru (accessed on 29 December 2021).
- MIRRI—Microbial Resorurce Research Infrastructure. Available online: www.mirri.org (accessed on 29 December 2021).
- Stackebrandt, E.; Schüngel, M.; Martin, D.; Smith, D. The microbial resource research infrastructure MIRRI: Strength through coordination. Microorganisms 2015, 3, 890–902. [Google Scholar] [CrossRef] [Green Version]
- Gargano, M.L. Mycotheca of edible and medicinal mushrooms at herbarium SAF as a potential source of nutraceuticals and cultivated mushrooms. Int. J. Med. Mushrooms 2018, 20, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.; Persiani, A.M.; Ambrosio, E.; Vizzini, A.; Venturella, G.; Donnini, D.; Angelini, P.; Di Piazza, S.; Pavarino, M.; Lunghini, D.; et al. Macrofungi as ecosystem resources: Conservation versus exploitation. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2013, 147, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Varese, G.C.; Angelini, P.; Bencivenga, M.; Buzzini, P.; Donnini, D.; Gargano, M.L.; Maggi, O.; Pecoraro, L.; Persiani, A.M.; Savino, E.; et al. Ex situ conservation and exploitation of fungi in Italy. Plant Biosyst. 2011, 145, 997–1005. [Google Scholar] [CrossRef]
- Savino, E.; Girometta, C.E.; Chinaglia, S.; Guglielminetti, M.; Rodolfi, M.; Bernicchia, A.; Perini, C.; Salerni, E.; Picco, A.M. Medicinal mushrooms in Italy and their ex situ conservation through culture collection. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products (ICMBMP8), New Delhi, India, 19–22 November 2014. [Google Scholar]
- Doria, E.; Altobelli, E.; Girometta, C.E.; Nielsen, E.; Zhang, T.; Savino, E. Evaluation of lignocellulolytic activities of ten fungal species able to degrade poplar wood. Int. Biodeterior. Biodegrad. 2014, 94, 160–166. [Google Scholar] [CrossRef]
- Baldrian, P.; Gabriel, J. Intraspecific variability in growth response to cadmium of the wood-rotting fungus Piptoporus betulinus. Mycologia 2002, 94, 428–436. [Google Scholar] [CrossRef]
- Pawlik, A.; Janusz, G.; Dębska, I.; Siwulski, M.; Frąc, M.; Rogalski, J. Genetic and metabolic intraspecific biodiversity of Ganoderma lucidum. BioMed Res. Int. 2015, 2015, 726149. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Sun, H.; Vainio, E.J.; Raffaello, T.; Kovalchuk, A.; Morin, E.; Duplessis, S.; Asiegbu, F.O. Intraspecific comparative genomics of isolates of the Norway spruce pathogen (Heterobasidion parviporum) and identification of its potential virulence factors. BMC Genom. 2018, 19, 220. [Google Scholar] [CrossRef] [Green Version]
- Corana, F.; Cesaroni, V.; Mannucci, B.; Baiguera, R.M.; Picco, A.M.; Savino, E.; Ratto, D.; Perini, C.; Kawagishi, H.; Girometta, C.E.; et al. Array of Metabolites in Italian Hericium erinaceus Mycelium, Primordium, and Sporophore. Molecules 2019, 24, 3511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.C.; Yang, Z.L.; Cui, B.K.; Yu, C.J.; Zhou, L.W. Species diversity and utilization of medicinal mushrooms and fungi in China. Int. J. Med. Mushrooms 2009, 11, 287–302. [Google Scholar] [CrossRef]
- Vaidya, J.G.; Lamrood, P.Y. Traditional medicinal mushrooms and fungi of India. Int. J. Med. Mushrooms 2000, 2, 209–214. [Google Scholar] [CrossRef]
- Blasi, C.; Capotorti, G.; Copiz, R.; Guida, D.; Mollo, B.; Smiraglia, D.; Zavattero, L. Classification and mapping of the ecoregions of Italy. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2014, 148, 1255–1345. [Google Scholar] [CrossRef]
- Sutherland, W.J. Ecological Census Techniques: A Handbook; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Girometta, C.E.; Rovelli, L.; Bracco, F.; Brescia, F.; Baiguera, R.M.; Chiatante, G.; Picco, A.M.; Savino, E. The Medicinal Wood-Decay Species Laricifomes officinalis in the Alpe Veglia–Alpe Devero Natural Park (Italian Alps): Spatial Analysis and Growth Tests of Pure Cultures. Acta Mycol. 2021, 56, 569. [Google Scholar] [CrossRef]
- Global Fungal Red List Initiative. Available online: http://iucn.ekoo.se/iucn/species_view/297501 (accessed on 2 January 2022).
- Stalpers, J.A. Identification of wood-inhabiting fungi in pure culture. Stud. Mycol. 1978, 16, 1–248. [Google Scholar]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms, 3rd ed.; Ten Speed Press: Berkeley, CA, USA, 2000. [Google Scholar]
- Sturini, M.; Girometta, C.E.; Maraschi, F.; Savino, E.; Profumo, A. A preliminary investigation on Metal Bioaccumulation by Perenniporia fraxinea. Bull. Environ. Contam. Toxicol. 2017, 98, 508–512. [Google Scholar] [CrossRef]
- Rush Wayn, R. Growing Mushrooms—The easy way. In Home Mushroom Cultivation with Hydrogen Peroxide Volume I, 3rd ed.; 2001; Available online: https://pdfcoffee.com/2-growing-mushrooms-the-easy-way-home-mushroom-cultivation-with-hydrogen-peroxidepdf-pdf-free.html (accessed on 29 December 2021).
- Ryvarden, L.; Melo, I. Poroid Fungi of Europe, 2nd ed.; Fungiflora: Oslo, Norway, 2017. [Google Scholar]
- Bernicchia, A.; Gorjón, S.P. Corticiaceae; Candusso: Alassio, Italy, 2006. [Google Scholar]
- Bernicchia, A.; Gorjon, S.P. Polypores of the Mediterranean Region; Romar: Segrate, Italy, 2020. [Google Scholar]
- Rivoire, B. Polypores des France et d’Europe; MYCOPOLYDEV: Orliénas, France, 2020. [Google Scholar]
- Janh, H. Pilze die an Holz Waksen, 1st ed.; Busse: Herford, Germany, 1979. [Google Scholar]
- Breitenbach, J.; Kranzlin, F. Champignons de Suisse T2; Mykologia: Lucerne, Switzerland, 1986. [Google Scholar]
- Julich, W. Guida alla Determinazione dei Funghi Vol. 2, Aphyllophorales, Heterobasidiomycetes, Gasteromycetes, 1st ed.; Saturnia: Roncafort di Trento, Italy, 1989. [Google Scholar]
- Genebank NCBI. Available online: https://blast.ncbi.nlm.nih.gov (accessed on 2 January 2022).
- Mycobank CBS. Available online: www.mycobank.org (accessed on 2 January 2022).
- García-García, M.; Rocha-Zavaleta, L.; Valdez-Cruz, N.A.; Trujillo-Roldan, M.A. Conservation of the mycelia of the medicinal mushroom Humphreya coffeata (Berk.) Stey. in sterile distilled water. MethodsX 2014, 1, 19–22. [Google Scholar] [CrossRef]
- Cartabia, M.; Girometta, C.E.; Milanese, C.; Baiguera, R.M.; Buratti, S.; Branciforti, D.S.; Vadivel, D.; Girella, A.; Babbini, S.; Savino, E.; et al. Collection and Characterization of Wood Decay Fungal Strains for Developing Pure Mycelium Mats. J. Fungi 2021, 7, 1008. [Google Scholar] [CrossRef]
- Harris, D.C. Quantitative Chemical Analysis, 7th ed.; W. H. Freeman and Company: New York, NY, USA, 2007. [Google Scholar]
- Cervini, M.; Gabba, G.; Macchi, P. Censimento dei Funghi Della Provincia di Varese; Nicolini Editore: Varese, Italy, 2000. [Google Scholar]
- Index Fungorum. Available online: http://www.indexfungorum.org/ (accessed on 2 January 2022).
- Spirin, V.; Runnel, K.; Vlasák, J.; Miettinen, O.; Põldmaa, K. Species diversity in the Antrodia crassa group (Polyporales, Basidiomycota). Fungal Biol. 2015, 119, 1291–1310. [Google Scholar] [CrossRef] [Green Version]
- Justo, A.; Miettinen, O.; Floudas, D.; Ortiz-Santana, B.; Sjökvist, E.; Lindner, D.; Nakasone, K.; Niemelä, T.; Larsson, K.H.; Ryvarden, L.; et al. A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biol. 2017, 121, 798–824. [Google Scholar] [CrossRef] [PubMed]
- Moncalvo, J.M.; Wang, H.F.; Hseu, R.S. Gene phylogeny of the Ganoderma lucidum complex based on ribosomal DNA sequences. Comparison with traditional taxonomic characters. Mycol. Res. 1995, 99, 1489–1499. [Google Scholar] [CrossRef]
- Hong, S.G.; Jung, H.S. Phylogenetic analysis of Ganoderma based on nearly complete mitochondrial small-subunit ribosomal DNA sequences. Mycologia 2004, 96, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Vizzini, A.; Angelini, C.; Ercole, E. Le sezioni Velatae e Aporus di Agrocybe sottogenere Aporus: Rivalutazione del genere Cyclocybe Velen. ed una nuova specie. RMR 2014, 92, 21–38. [Google Scholar]
- Onofri, S. Checklist of Italian Fungi–Basidiomycota; Carlo Delfino Editore: Sassari, Italy, 2005. [Google Scholar]
- Landingin, H.R.R.; Francisco, B.E.; Dulay, R.M.R.; Kalaw, S.; Reyes, R. Optimization of culture conditions for mycelial growth and basidiocarp production of Cyclocybe cylindracea (Maire). CLSU Int. J. Sci. Technol. 2020, 4, 1–17. [Google Scholar] [CrossRef]
- Nobles, M.K. Studies in forest pathology: VI. Identification of cultures of wood-rotting fungi. Can. J. Res. 1948, 26, 281–431. [Google Scholar] [CrossRef]
- Petre, C.V.; Tanase, C. Culture characteristics of 20 lignicolous basidiomycetes species that synthesize volatile organic compounds. Analele Stiintifice ale Universitatii" Al. I. Cuza" din Iasi 2013, 59, 37. [Google Scholar]
- Badalyan, S.M.; Shnyreva, A.V.; Iotti, M.; Zambonelli, A. Genetic resources and mycelial characteristics of several medicinal polypore mushrooms (Polyporales, Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 371–384. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M. A new wood-decaying basidiomycete species associated with esca of grapevine: Fomitiporia mediterranea (Hymenochaetales). Mycol. Prog. 2002, 1, 315–324. [Google Scholar] [CrossRef]
- Balaeş, T.; Tănase, C. Description of in vitro cultures for some spontaneous lignicolous basidiomycetes species. Analele Ştiinţifice ale Universităţii “Al.I.Cuza” Iaşi s.II a Biologie Vegetală 2012, 58, 19–29. [Google Scholar]
- Buchalo, A.; Mykchaylova, O.; Lomberg, M.; Wasser, S.P. Microstructures of Vegetative Mycelium of Macromycetes in Pure Cultures; Alterpress: Kiev, Ukraine, 2009. [Google Scholar]
- Dresch, P.; Rosam, K.; Grienke, U.; Rollinger, J.M.; Peintner, U. Fungal strain matters: Colony growth and bioactivity of the European medicinal polypores Fomes fomentarius, Fomitopsis pinicola and Piptoporus betulinus. AMB Express 2015, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petre, C.V.; Tănase, C. Description of the culture characteristics of some lignicolous basidiomycetes species grown on three synthetic media. J. Plant Dev. 2013, 20, 105–114. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).