Mid-Atlantic Big Brown and Eastern Red Bats: Relationships between Acoustic Activity and Reproductive Phenology
Abstract
1. Introduction
2. Materials and Methods
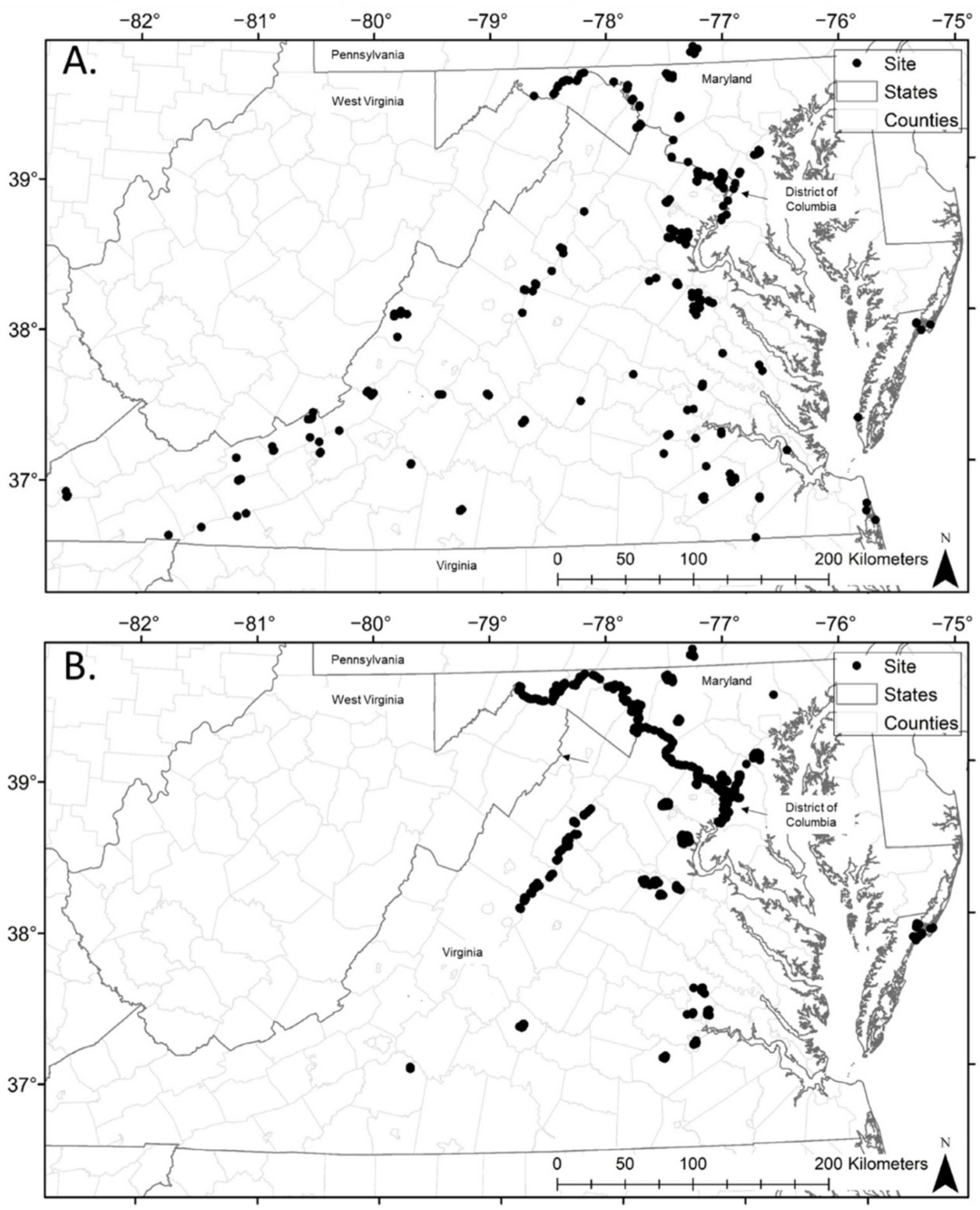
2.1. Study Area
2.2. Methods
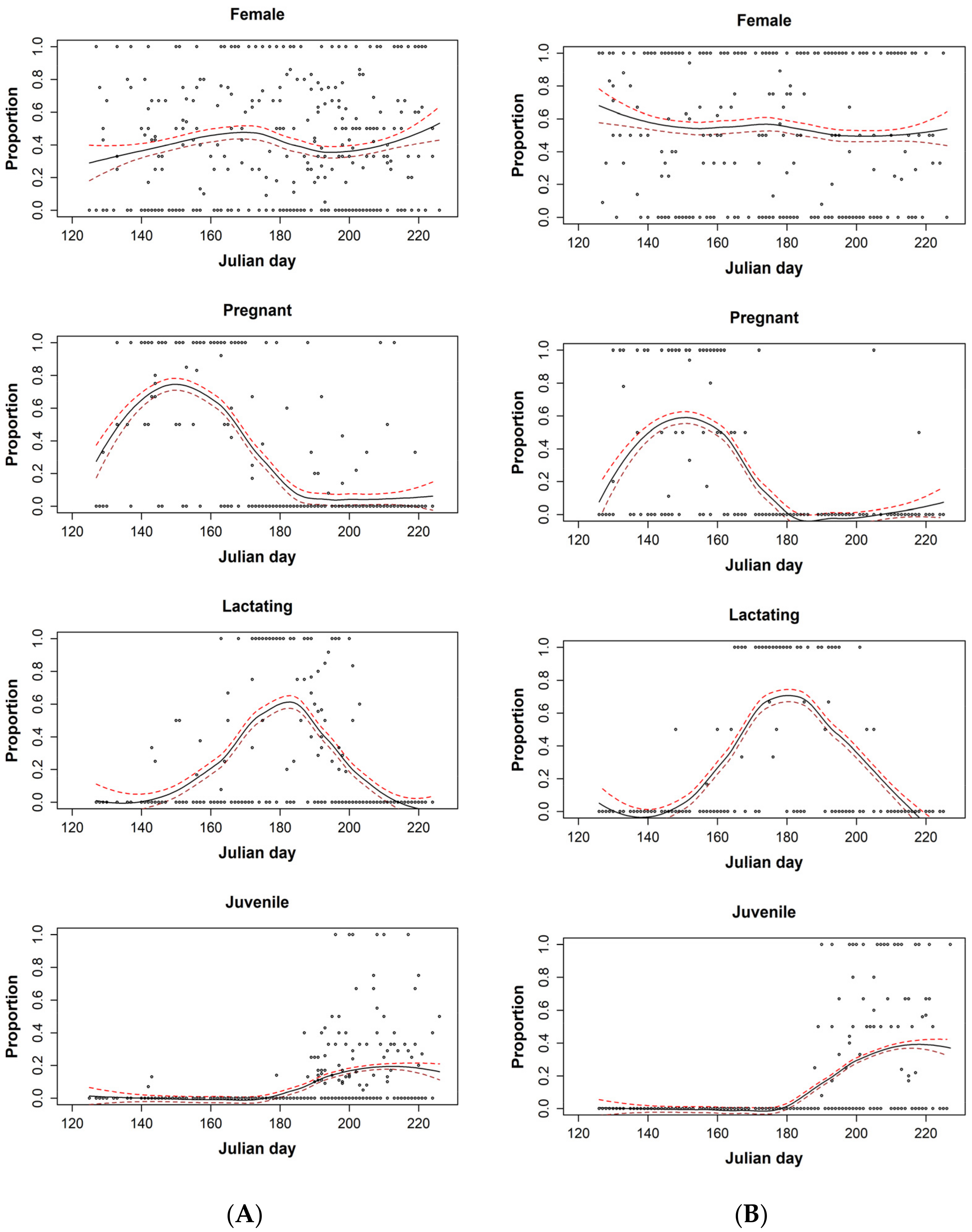
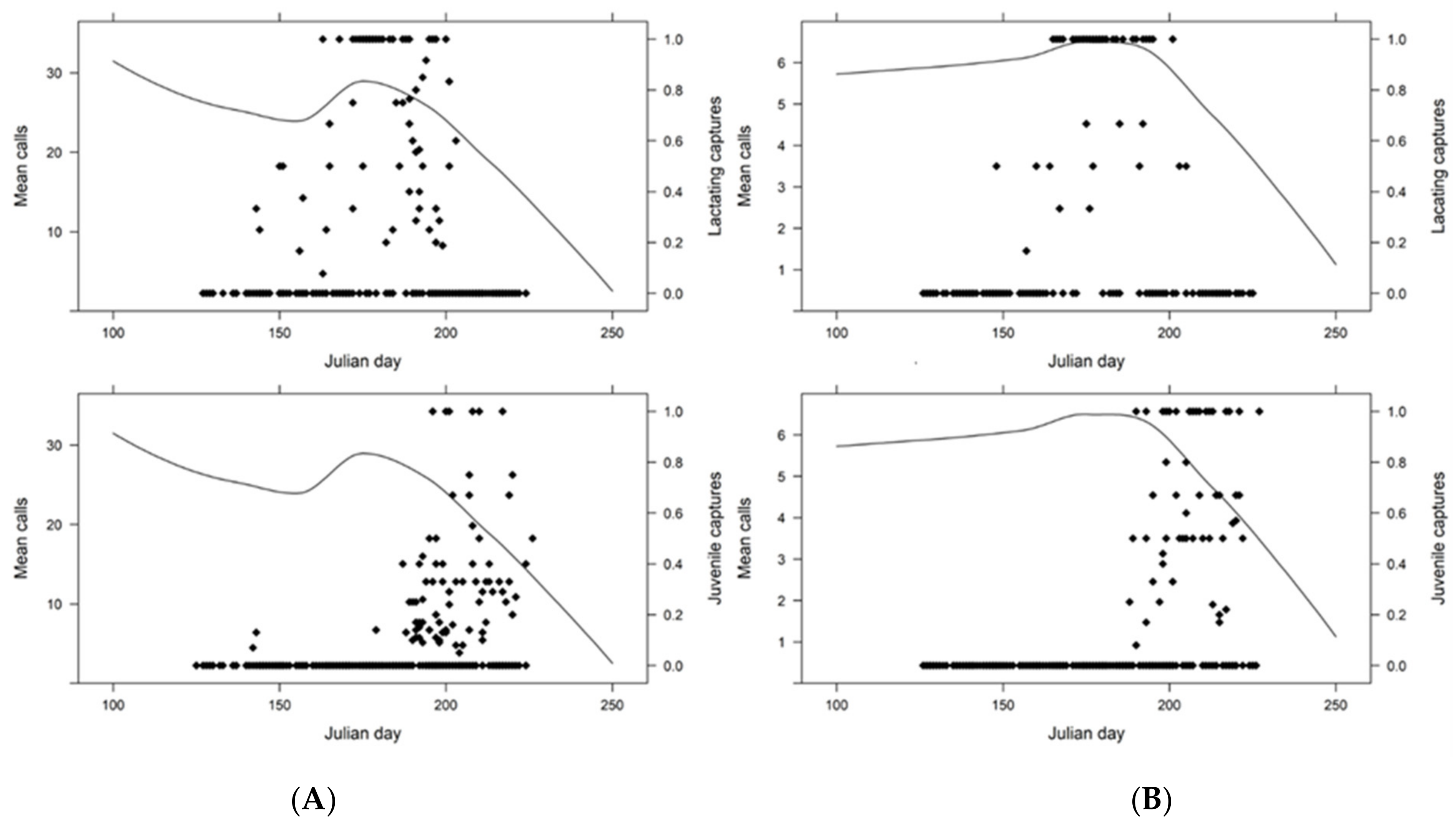
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurta, A.; Murray, S.W. Philopatry and Migration of Banded Indiana Bats (Myotis sodalis) and Effects of Radio Transmitters. J. Mammal. 2002, 83, 585–589. [Google Scholar] [CrossRef]
- McGuire, L.P.; Guglielmo, C.G.; Mackenzie, S.A.; Taylor, P.D. Migratory Stopover in the Long-Distance Migrant Silver-Haired Bat, Lasionycteris noctivagans: Bat Migration Stopover. J. Anim. Ecol. 2012, 81, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Beer, J.R. Survival and Movements of Banded Big Brown Bats. J. Mammal. 1955, 36, 242–248. [Google Scholar] [CrossRef]
- Kurta, A.; Baker, R.H. Eptesicus fuscus . Mamm. Species 1990, 356, 1–10. [Google Scholar] [CrossRef]
- Whitaker, J.O., Jr.; Gummer, S.L. Hibernation of the Big Brown Bat, Eptesicus fuscus, in Buildings. J. Mammal. 1992, 73, 312–316. [Google Scholar] [CrossRef]
- Barbour, R.W.; Davis, W.H. Bats of America; University Press of Kentucky: Lexington, KY, USA, 1969; Volume 7. [Google Scholar]
- Menzel, M.A.; Menzel, J.M.; Kilgo, J.C.; Ford, W.M.; Carter, T.C.; Edwards, J.W. Bats of the Savannah River Site and Vicinity; Technical Report; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2003; p. 80. [Google Scholar]
- Shump, K.A.; Shump, A.U. Lasiurus borealis . Mamm. Species 1982, 84, 1–6. [Google Scholar] [CrossRef]
- Altringham, J.D. Bats: From Evolution to Conservation; Oxford University Press: Oxford, UK, 2011; ISBN 0-19-920711-9. [Google Scholar]
- Tuttle, M.D.; Stevenson, D. Growth and Structure of Bats. In Ecology of Bats; Kunz, T.H., Ed.; Plenum Publishing Corporation: New York, NY, USA, 1982; pp. 105–150. [Google Scholar]
- Larsen, R.J.; Boegler, K.A.; Genoways, H.H.; Masefield, W.P.; Kirsch, R.A.; Pedersen, S.C. Mist Netting Bias, Species Accumulation Curves, and the Rediscovery of Two Bats on Montserrat (Lesser Antilles). Acta Chiropterologica 2007, 9, 423–435. [Google Scholar] [CrossRef][Green Version]
- Niver, R.A.; King, R.A.; Armstrong, M.P.; Ford, W.M. Methods to Evaluate and Develop Minimum Recommended Summer Survey Effort for Indiana Bats: White Paper; U.S. Fish and Wildlife Service Region 3: St. Paul, MN, USA, 2014. [Google Scholar]
- Ford, W.M.; Britzke, E.R.; Dobony, C.A.; Rodrigue, J.L.; Johnson, J.B. Patterns of Acoustical Activity of Bats Prior to and Following White-Nose Syndrome Occurrence. J. Fish Wildl. Manag. 2011, 2, 125–134. [Google Scholar] [CrossRef]
- Loeb, S.C.; Rodhouse, T.J.; Ellison, L.E.; Lausen, C.L.; Reichard, J.D.; Irvine, K.M.; Coleman, J.T.H.; Thogmartin, W.E.; Sauer, J.R.; Francis, C.M.; et al. A Plan for the North American Bat Monitoring Program (NABat); U.S. Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 2015; p. 100. [Google Scholar]
- White, J.A.; Freeman, P.; Lemen, C.A. Habitat Selection by the Northern Long-Eared Myotis (Myotis septentrionalis) in the Midwestern United States: Life in a Shredded Farmscape. 2017. Available online: https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1515&context=tnas (accessed on 3 October 2019).
- Teets, K.D. Assessment of Data Collection Techniques and White-Nose Syndrome Effects on Bat Communities of South Carolina. Master’s Thesis, Clemson University, Clemson, SC, USA, 2018. [Google Scholar]
- Nocera, T.; Ford, W.M.; Silvis, A.; Dobony, C.A. Patterns of Acoustical Activity of Bats Prior to and 10 Years after WNS on Fort Drum Army Installation, New York. Glob. Ecol. Conserv. 2019, 18, e00633. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service. Range-Wide Indiana Bat Survey Guidelines. 2020. Available online: https://www.fws.gov/library/collections/range-wide-indiana-bat-and-northern-long-eared-bat-survey-guidelines (accessed on 1 February 2019).
- USFSMapsandApps. Maps and Apps Growing Degree Days throughout This Century, in the Conterminous U.S. Map. 2018.
- Barr, E. Post-WNS Survey of Bats at NASA Wallops Island Flight Facility: Contract/Grant G16AC00327, 2018 Final Report; U.S. Geological Survey Cooperative Research Units Program: Reston, VA, USA, 2018; p. 8. [Google Scholar]
- Deeley, S.; Freeze, S.; Rohrbaugh, L. Post-White-Nose Syndrome Bat Communities in the National Capital Region; NPS/NCRO/NRR—2021/2319; National Park Service: Fort Collins, CO, USA, 2021. [Google Scholar]
- Deeley, S.; Gorman, K. 2018 Post-WNS Bat Surveys in Gettysburg National Military Park Interim Report, Contract # P17AC01524; Blacksburg, VA, USA, 2018; p. 10.
- Kalen, N.; Ford, W.M.; Silvis, A.; Powers, K.E.; Keifer, T. Post-White-Nose Syndrome Assessment of Bat Species Occupancy at Catoctin Mountain Park and Harpers Ferry National Historical Park—Interim Report 2015; National Park Service U.S. Department of the Interior: Blacksburg, VA, USA, 2015. [Google Scholar]
- Muthersbaugh, M.S. Seasonal Activity Patterns of Bats in the Central Appalachians. Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2018. [Google Scholar]
- Silvis, A.; Kniowski, A.B.; Ford, W.M. Distribution of Indiana Bats (Myotis sodalis) and Northern Long-Eared Bats (M. Septentrionalis) in Virginia; Virginia Field Office: Glouchester, VA, USA, 2017; p. 61. [Google Scholar]
- St. Germain, M.J., Jr.; Kniowski, A.B.; Silvis, A.; Ford, W.M. Who Knew? First Myotis sodalis (Indiana Bat) Maternity Colony in the Coastal Plain of Virginia. Northeast. Nat. 2017, 24, N5–N10. [Google Scholar] [CrossRef]
- Menzel, M.A.; Menzel, J.M.; Castleberry, S.B.; Ozier, J.; Ford, W.M.; Edwards, J.W. Illustrated Key to Skins and Skulls of Bats in the Southeastern and Mid-Atlantic States; USDA Forest Service, Northeastern Research Station: Newton Square, PA, USA, 2002; p. 18. [Google Scholar]
- Francl, K.E.; Ford, W.M.; Sparks, D.W.; Brack, V. Capture and Reproductive Trends in Summer Bat Communities in West Virginia: Assessing the Impact of White-Nose Syndrome. J. Fish Wildl. Manag. 2012, 3, 33–42. [Google Scholar] [CrossRef]
- Anthony, E.L.P. Age Determination in Bats. In Ecological and Behavioral Methods for the Study of Bats; Kunz, T.H., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1988; pp. 47–58. [Google Scholar]
- Racey, P.A. Reproductive Assessment in Bats. In Ecological and Behavioral Methods for the Study of Bats; Kunz, T.H., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1988; pp. 31–43. [Google Scholar]
- Deeley, S. Ecology of Mid-Atlantic Bats after White-Nose Syndrome: Communities, Reproduction, and Diet within an Urban-to-Rural Gradient. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2019. [Google Scholar]
- Ford, W.M. Kaleidoscope 4.2.0 Subsequent Test on Expanded Data; Virginia Cooperative Fish and Wildlife Research Unit: Blacksburg, VA, USA, 2017; p. 4. [Google Scholar]
- U.S. Fish and Wildlife Service. Indiana Bat Summer Survey Guidance—Automated Acoustic Bat ID Software Programs. Available online: http://www.fws.gov/midwest/Endangered/mammals/inba/surveys/inbaAcousticSoftware.html (accessed on 18 August 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Timpone, J.; Francl, K.E.; Sparks, D.; Brack, V.; Beverly, J. Bats of the Cumberland Plateau and Ridge and Valley Provinces, Virginia. Southeast. Nat. 2011, 10, 515–528. [Google Scholar] [CrossRef]
- Racey, P.A.; Swift, S.M. Variations in Gestation Length in a Colony of Pipistrelle Bats (Pipistrellus pipistrellus) from Year to Year. J. Reprod. Fertil. 1981, 61, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, I.M.; Barclay, R.M.R. Diets of Juvenile, Yearling, and Adult Big Brown Bats (Eptesicus fuscus) in Southeastern Alberta. J. Mammal. 1998, 79, 764–771. [Google Scholar] [CrossRef][Green Version]
- Barclay, R.M.R. The Effect of Reproductive Condition on the Foraging Behavior of Female Hoary Bats, Lasiurus cinereus. Behav. Ecol. Sociobiol. 1989, 24, 31–37. [Google Scholar] [CrossRef]
- Clark, B.S.; Leslie, D.M.; Carter, T.S. Foraging Activity of Adult Female Ozark Big-Eared Bats (Plecotus townsendii ingens) in Summer. J. Mammal. 1993, 74, 422–427. [Google Scholar] [CrossRef]
- Rintoul, J.L.P.; Brigham, R.M. The Influence of Reproductive Condition and Concurrent Environmental Factors on Torpor and Foraging Patterns in Female Big Brown Bats (Eptesicus fuscus). J. Comp. Physiol. B 2014, 184, 777–787. [Google Scholar] [CrossRef]
- Rydell, J. Variation in Foraging Activity of an Aerial Insectivorous Bat during Reproduction. J. Mammal. 1993, 74, 503–509. [Google Scholar] [CrossRef]
- Kurta, A.; Kunz, T.H.; Nagy, K.A. Energetics and Water Flux of Free-Ranging Big Brown Bats (Eptesicus fuscus) during Pregnancy and Lactation. J. Mammal. 1990, 71, 59–65. [Google Scholar] [CrossRef]
- Kurta, A.; Bell, G.P.; Nagy, K.A.; Kunz, T.H. Energetics of Pregnancy and Lactation in Free-Ranging Little Brown Bats (Myotis lucifugus). Physiol. Zool. 1989, 62, 804–818. [Google Scholar] [CrossRef]
- Walters, B.L.; Sparks, D.W.; Whitaker, J.O., Jr.; Ritzi, C.M. Timing of Migration by Eastern Red Bats (Lasiurus borealis) through Central Indiana. Acta Chiropterologica 2006, 8, 259–263. [Google Scholar] [CrossRef]
- Jonasson, K.A.; Guglielmo, C.G. Sex Differences in Spring Migration Timing and Body Composition of Silver-Haired Bats Lasionycteris noctivagans. J. Mammal. 2016, 97, 1535–1542. [Google Scholar] [CrossRef]
- Muthersbaugh, M.S.; Ford, W.M.; Powers, K.E.; Silvis, A. Activity Patterns of Bats during the Fall and Spring along Ridgelines in the Central Appalachians. J. Fish Wildl. Manag. 2019, 10, 180–195. [Google Scholar] [CrossRef]
- True, M.C.; Reynolds, R.J.; Ford, W.M. Monitoring and Modeling Tree Bat (Genera: Lasiurus, Lasionycteris) Occurrence Using Acoustics on Structures off the Mid-Atlantic Coast—Implications for Offshore Wind Development. Animals 2021, 11, 3146. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deeley, S.; Ford, W.M.; Kalen, N.J.; Freeze, S.R.; St. Germain, M.; Muthersbaugh, M.; Barr, E.; Kniowski, A.; Silvis, A.; De La Cruz, J. Mid-Atlantic Big Brown and Eastern Red Bats: Relationships between Acoustic Activity and Reproductive Phenology. Diversity 2022, 14, 319. https://doi.org/10.3390/d14050319
Deeley S, Ford WM, Kalen NJ, Freeze SR, St. Germain M, Muthersbaugh M, Barr E, Kniowski A, Silvis A, De La Cruz J. Mid-Atlantic Big Brown and Eastern Red Bats: Relationships between Acoustic Activity and Reproductive Phenology. Diversity. 2022; 14(5):319. https://doi.org/10.3390/d14050319
Chicago/Turabian StyleDeeley, Sabrina, W. Mark Ford, Nicholas J. Kalen, Samuel R. Freeze, Michael St. Germain, Michael Muthersbaugh, Elaine Barr, Andrew Kniowski, Alexander Silvis, and Jesse De La Cruz. 2022. "Mid-Atlantic Big Brown and Eastern Red Bats: Relationships between Acoustic Activity and Reproductive Phenology" Diversity 14, no. 5: 319. https://doi.org/10.3390/d14050319
APA StyleDeeley, S., Ford, W. M., Kalen, N. J., Freeze, S. R., St. Germain, M., Muthersbaugh, M., Barr, E., Kniowski, A., Silvis, A., & De La Cruz, J. (2022). Mid-Atlantic Big Brown and Eastern Red Bats: Relationships between Acoustic Activity and Reproductive Phenology. Diversity, 14(5), 319. https://doi.org/10.3390/d14050319





