Abstract
Biogenic habitats often form hot spots of biodiversity. However, the role of epibiosis and the ‘habitat cascades’ phenomenon in enhancing structural heterogeneity and biodiversity in biogenic habitats in remote and difficult-to-access areas is little known. In this work, we provide the first insight by exploring epibiosis across remote habitats that often support high levels of biodiversity, i.e., cold-water coral reefs and marine caves. The present study acts as a stepping-stone for the further exploration of ‘habitat cascades’ in habitats where scientific knowledge about this phenomenon is limited.
Over the last couple of decades, studies in terrestrial and shallow-water ecosystems (kelp forests and sandy seagrass beds) unraveled and described a novel phenomenon titled ‘habitat cascades’ [1]. Habitat cascades occur when there are indirect positive effects on focal organisms mediated by successive biogenic formation or habitat modification formed by basal and intermediate habitat formers [1]. Habitat cascades are a mechanism that enhances species abundance and diversity [1]. However, apart from kelp forests and seagrass beds, it is largely unknown if habitat cascades are present in other ecosystems, e.g., hard biogenic habitats, and what their role is in biodiversity [2]. This major knowledge gap is even larger for ‘dark habitats’ such as remote deep-sea ecosystems (e.g., cold-water coral reefs) and marine caves, even though these ecosystems are often regarded as hot spots of marine biodiversity [3,4,5].
It was only recently that studies by Kazanidis et al. [6,7] showed that habitat cascades were present in the Mingulay Reef Complex, a cold-water coral reef formed by the scleractinian coral Lophelia pertusa (Linnaeus, 1758), now known as Desmophyllum pertusum (Linnaeus, 1758), which acts as a basal habitat former and hosts intermediate habitat formers [3,4]. Kazanidis et al. [6] showed that a small group of intermediate habitat formers composed of bivalves, tunicates and empty polychaete tubes was colonized by diverse epifaunal communities of focal organisms mainly composed of bryozoans, molluscs and barnacles. Based on these findings, it was suggested that the intermediate habitat formers (bivalves, tunicates and empty polychaete tubes) increased habitat heterogeneity and enhanced biodiversity through habitat cascades [6] (Table 1) in a similar way that epiphytes do in tropical rainforests [8]. At shallower depths, the serpulid polychaete Protula tubularia (Montagu, 1803) has been found to form a core of aggregates, also known as ‘biostalactites’, which so far have been discovered in the marine caves of Italy, Greece and Cyprus in the Mediterranean Sea [9,10,11,12,13]. These biogenic formations act as the basal habitat former and provide the substrate for intermediate habitat formers such as other serpulids and encrusting bryozoans, which in turn can be covered by epibionts (e.g., sponges, brachiopods and foraminiferans). The cavities of the framework formed by the basal and intermediate formers could also be subsequently colonized by focal cavity-dwelling taxa (e.g., sponges and bivalves), which play an active role in the bioconstruction process [12,14,15] (Table 1). Some mobile taxa could also possibly shelter in the microcavities of these frameworks. These bioconstructions represent ideal niches also for heterotrophic bacterial communities (e.g., sulfate-reducing bacteria) which—fed by organic substances produced mostly by metazoans—develop in the microcavities of the primary framework and, through their metabolic activities, induce micrite deposition. This micrite instantly cements and contributes to strengthening the biostructure [11,12,16,17,18,19]. In the general development of biostalactites, bacteria have a dual role in the frame of the habitat cascades; they act both (1) as focal organisms, as their vital functions depend on the organic matter produced by Protula tubularia (representing the basal habitat former) and serpulids, encrusting bryozoans, sponges, brachiopods and foraminiferans (representing the intermediate formers); and (2) as basal habitat-former organisms, as they form cemented substrates for new generations of epibionts.

Table 1.
Examples of hard-substrate habitat cascades in dark marine habitats.
Recent studies have advanced scientific knowledge about the role of habitat cascades and especially the role of heterogeneity within and among co-occurring habitat formers in increasing biodiversity in mangroves, seagrasses, marshes, muds and rocky reefs [20]. There are still, however, major knowledge gaps about the habitat cascades phenomenon in remote environments such as marine caves and the deep sea, which is the largest biome on Earth. These gaps hinder an advanced understanding of habitat cascades on a global scale and especially the mechanisms that shape the structure and functioning of biogenic habitats in remote areas, which are often hot spots of marine biodiversity. Some key questions include the following:
- (1)
- Is the ‘habitat cascades’ phenomenon present in remote areas, and if yes, what is its role in shaping local biodiversity?
- (2)
- What is the role of environmental parameters (e.g., depth, habitat type, food availability) in the presence and magnitude of habitat cascades?
- (3)
- Are there common biological traits across the organisms that facilitate habitat cascades across different types of habitats?
In the present study, we provide a series of interesting images showing the habitat cascades phenomenon from two distinct biogenic habitats: the Mingulay cold-water coral reef in Scotland (Figure 1) and marine caves from the Eastern Mediterranean Sea (Figure 2 and Figure 3). The present article is a forerunner and part of a wider examination of habitat cascades’ role in the biodiversity and ecology of hard-substrate biogenic habitats.
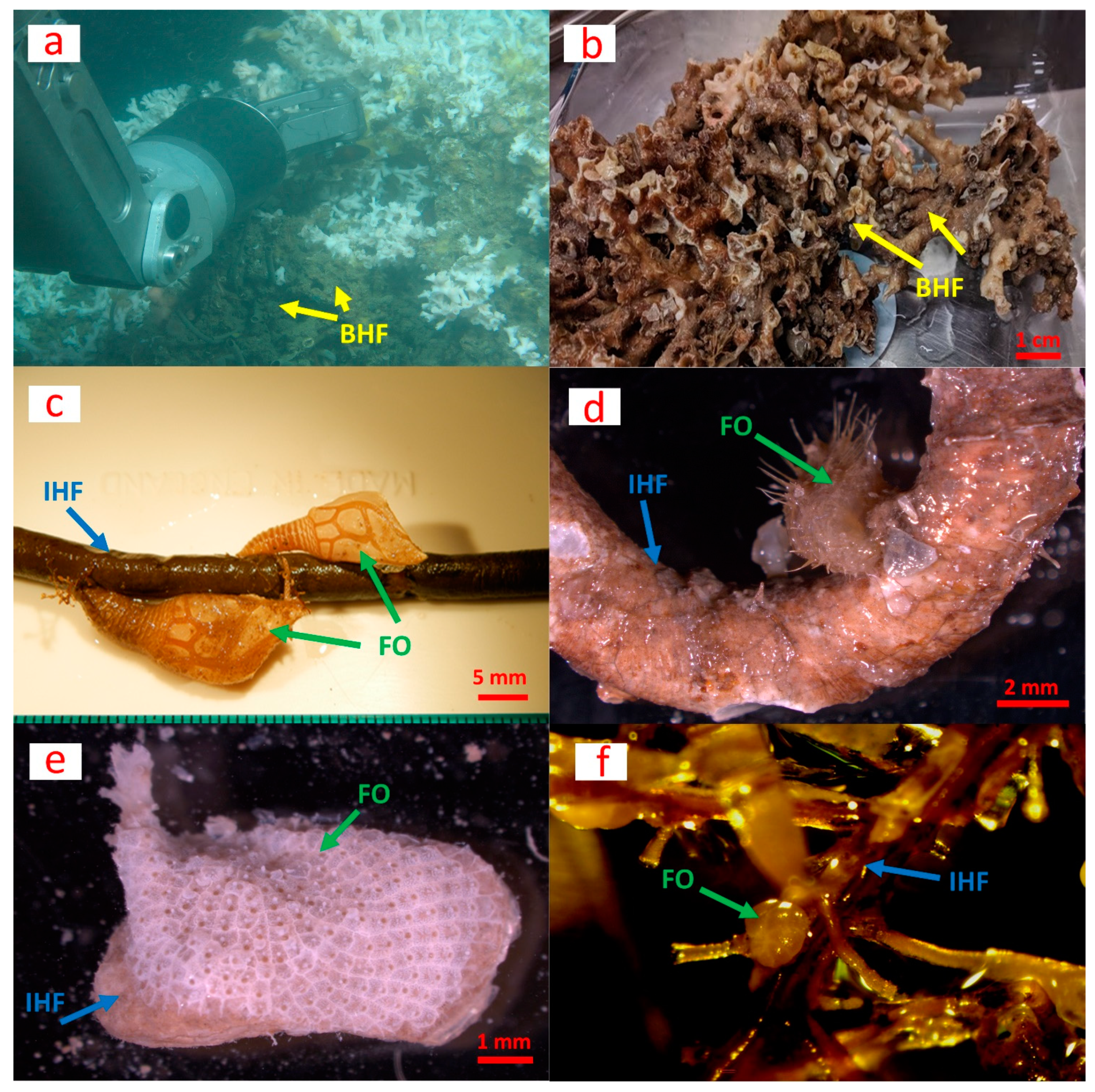
Figure 1.
Basal habitat former (BHF), intermediate habitat formers (IHF) and focal organisms (FO) in the Mingulay cold-water coral reef (north-east Atlantic). (a,b) Dead coral framework largely consisting of Lophelia pertusa (now known as Desmophyllum pertusum) serving as the basal habitat former (BHF) for intermediate habitat formers (IHF) such as polychaetes, bivalves and hydroids (some depicted in (c–f)); (c) organic tube of the polychaete Bispira volutacornis (Montagu, 1804) (IHF) hosting the velvet goose barnacle Scalpellum scalpellum (Linnaeus, 1767) as a focal organism (FO); (d) carbonate tube of a serpulid (IHF) hosting the bivalve Modiolula phaseolina (Philippi, 1844) (FO); (e) the bivalve Anomia ephippium (Linnaeus, 1758) (IHF) colonized by the encrusting bryozoan Schizomavella (Schizomavella) linearis (Hassall, 1841) (FO); (f) the hydroid Halecium sp. (IHF) hosting foraminifera (FO). Photos by the Changing Oceans Expedition (RRS James Cook 073)/University of Edinburgh.
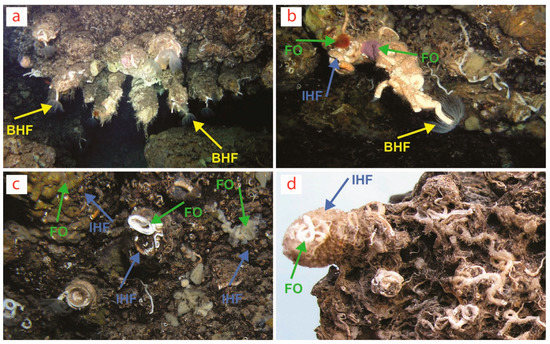
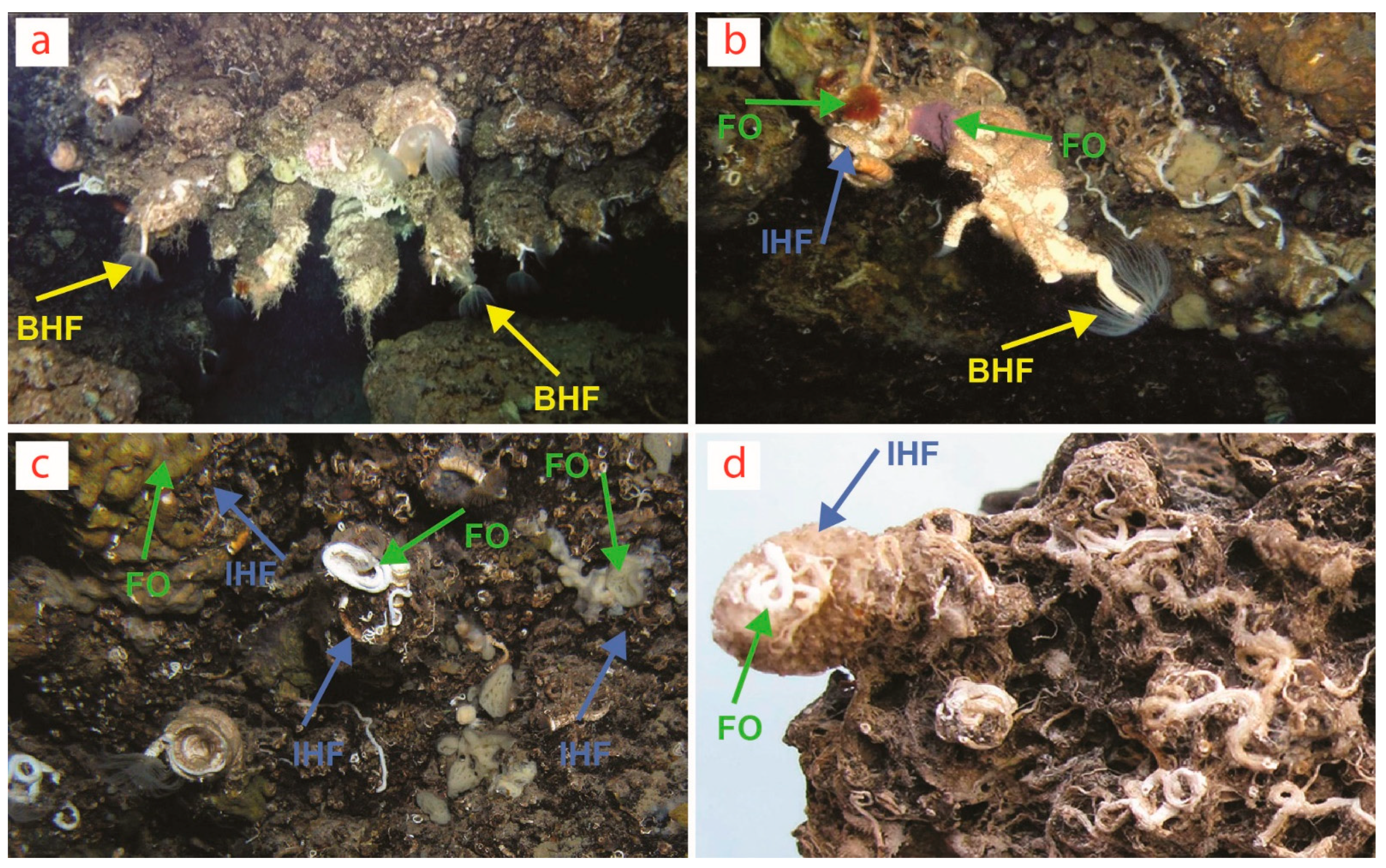
Figure 2.
Biostalactites built by the serpulid polychaete Protula tubularia (Montagu, 1803) in marine caves of (a) Crete and (b,c) Lesvos Island in the Aegean Sea (Greece, Eastern Mediterranean) and (d) Sicily (Plemmirio Marine Protected Area, Ionian Sea, Italy, Central Mediterranean). Yellow arrows point to the basal habitat former (BHF) P. tubularia constituting the core of the biostalactites, usually better visible at their tips. Blue arrows point to the intermediate habitat formers (IHF) (i.e., mostly other serpulids and encrusting bryozoans). Green arrows point to focal organisms (FO) (i.e., mostly sponges and also some serpulids and bryozoans). Further endolithic FO (i.e., boring bivalves, sponges, brachiopods, foraminiferans and heterotrophic bacterial communities) are not visible. BHF are not visible in (c,d) because they are completely covered by IHF and FO. Photos by V. Gerovasileiou (a–c) and A. Rosso (d).
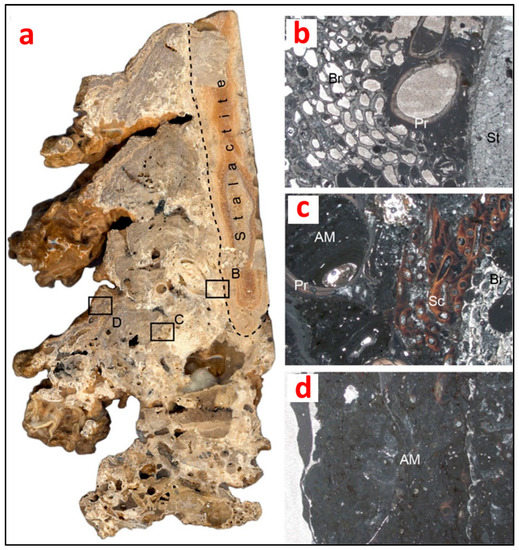
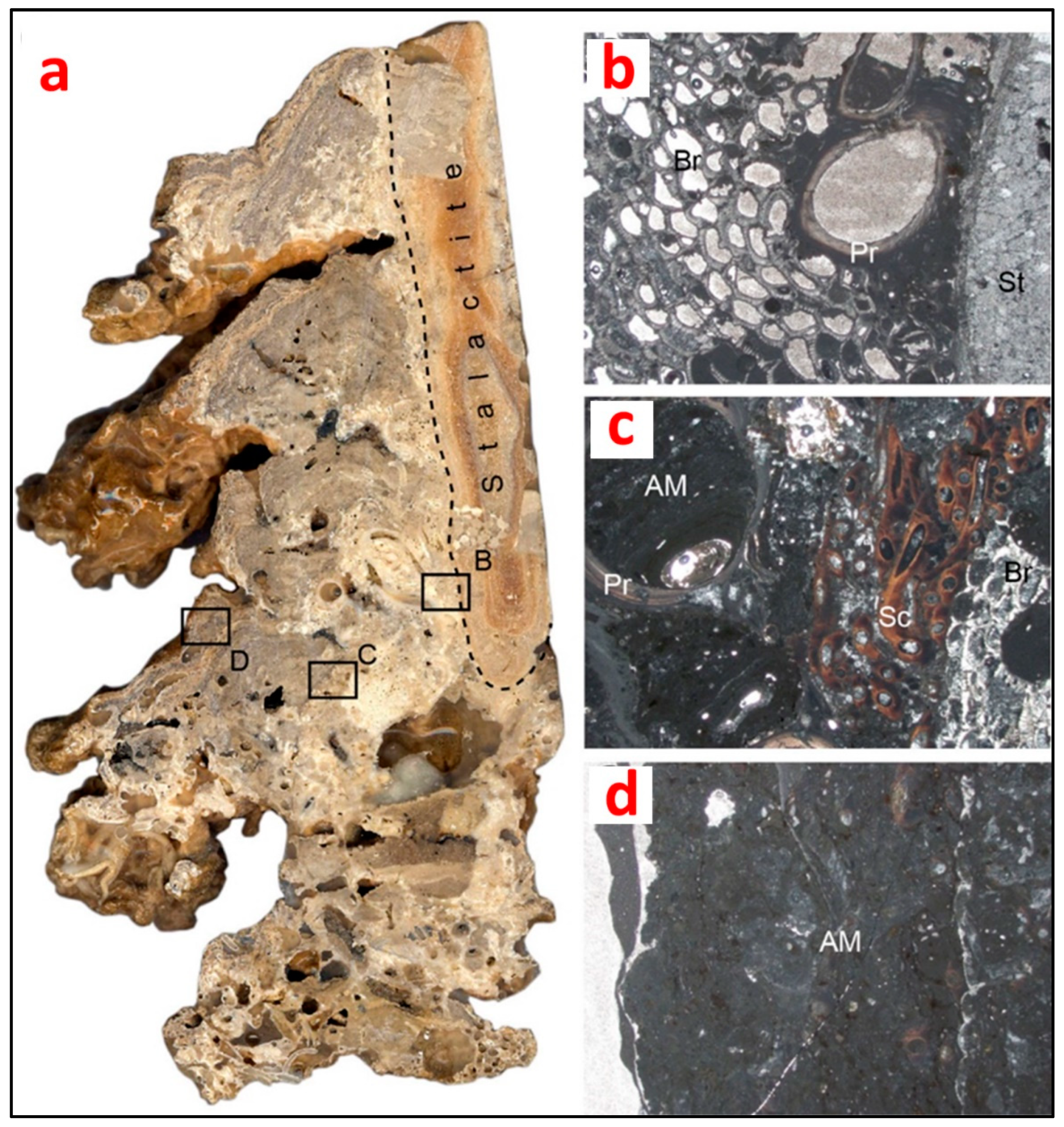
Figure 3.
(a) Polished longitudinal section of a biostalactite from the Mazzere cave (Plemmirio Marine Protected Area, Sicily, Italy). Note the internal framework of the bioconstruction formed of skeletons mixed with fine autochthonous micrite derived from microbial metabolic activity (rectangles represent the location of the microfacies showed in b, c and d); (b) inner part of the bioconstruction formed by skeletons of the serpulid Protula tubularia, acting as basal habitat former, encrusted by bryozoans, here acting as intermediate habitat formers; (c) intermediate part of the bioconstruction showing the superimposition of skeletons of encrusting organisms (intermediate habitat formers) and the microbial-derived autochthonous micrite-filling microcavities (bacteria act in this part of the bioconstruction as focal organisms); (d) near-surface part of the bioconstruction forming the substrate for a new generation of epifaunal communities. It is mainly composed of microbial-derived autochthonous micrite (bacteria act in this part of the bioconstruction as basal habitat formers). St: stalactite; Pr: Protula tubularia; Br: bryozoan colony; Sc: Semivermilia crenata (O.G. Costa, 1861); AM: autochthonous micrite. Photos by A. Guido.
Author Contributions
Conceptualization, G.K.; methodology, G.K. and V.G.; formal analysis, G.K., A.G., A.R., R.S., J.M.R. and V.G.; investigation, G.K., A.G., A.R., R.S., V.G. and J.M.R.; writing—original draft preparation, G.K. and V.G.; writing—review and editing, G.K., A.G., A.R., R.S., J.M.R. and V.G.; funding acquisition, J.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
MUR grants (ex 60% 2021 A.G., University of Calabria). A.R. and R.S. have received funding from the University of Catania through “PiaCeRi–Piano Incentivi per la Ricerca di Ateneo 2020–22 linea di intervento 2”. This study was also funded by the UK Ocean Acidification programme (NE/H017305/1 to J.M.R.) and European Union’s Horizon 2020 research and innovation programme under grant agreement no. 678760 (ATLAS) and no. 818123 (iAtlantic) to J.M.R. This output reflects only the authors’ view, and the European Union cannot be held responsible for any use that may be made of the information contained therein.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data from the Mingulay Reef Complex have been deposited to the online archive PANGAEA (DOI: https://doi.pangaea.de/10.1594/PANGAEA.915974) (Accessed on 10 February 2022).
Acknowledgments
The authors would like to thank the following persons for providing valuable guidance in the identification of the specimens from the Mingulay Reef Complex: Anna Holmes (Bivalvia), Graham Oliver (Bivalvia) and Jennifer Loxton (Bryozoa). This is the Catania Paleontological Research Group contribution n. 483.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thomsen, M.S.; Wernberg, T.; Altieri, A.; Tuya, F.; Gulbransen, D.; McGlathery, K.J.; Holmer, M.; Silliman, B.R. Habitat cascades: The conceptual context and global relevance of facilitation cascades via habitat formation and modification. Integr. Comp. Biol. 2010, 50, 158–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, M.S.; Altieri, A.H.; Angelini, C.; Bishop, M.J.; Gribben, P.E.; Lear, G.; He, Q.; Schiel, D.R.; Silliman, B.R.; South, P.M.; et al. Secondary foundation species enhance biodiversity. Nat. Ecol. Evol. 2018, 2, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Wheeler, A.J.; Freiwald, A. Reefs of the deep: The biology and geology of cold-water coral ecosystems. Science 2006, 312, 543–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, L.-A.; Roberts, J.M. Global biodiversity in cold-water coral reef ecosystems. In Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Rossi, S., Bramanti, L., Gori, A., Orejas, C., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 1–21. [Google Scholar]
- Gerovasileiou, V.; Bianchi, C.N. Mediterranean marine caves: A synthesis of current knowledge. Oceanogr. Mar. Biol. Annu. Rev. 2021, 59, 1–88. [Google Scholar]
- Kazanidis, G.; Henry, L.-A.; Roberts, J.M. Hidden structural heterogeneity enhances marine hotspots’ biodiversity. Coral Reefs 2021, 40, 1615–1630. [Google Scholar] [CrossRef]
- Kazanidis, G.; Henry, L.-A.; Vad, J.; Johnson, C.; De Clippele, L.H.; Roberts, J.M. ATLAS Mingulay Reef Complex Macrobenthos and Environmental Data; Pangaea: Bremen, Germany, 2020. [Google Scholar] [CrossRef]
- Nakamura, A.; Kitching, R.L.; Cao, M.; Creedy, T.J.; Fayle, T.M.; Freiberg, M.; Hewitt, C.N.; Itioka, T.; Pin Koh, L.; Ma, K.; et al. Forests and their canopies: Achievements and horizons in canopy science. Trends Ecol. Evol. 2017, 32, 438–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmonte, G.; Ingrosso, G.; Poto, M.; Quarta, G.; D’Elia, M.; Onorato, R.; Calcagnile, L. Biogenic stalactites in submarine caves at the Cape of Otranto (SE Italy): Dating and hypothesis on their formation. Mar. Ecol. 2009, 30, 376–382. [Google Scholar] [CrossRef]
- Belmonte, G.; Guido, A.; Mastandrea, A.; Onorato, R.; Rosso, A.; Sanfilippo, R. Animal forests in submarine caves. In Perspectives on the Marine Animal Forests of the World; Rossi, S., Bramanti, L., Eds.; Springer: Cham, Switzerland, 2020; pp. 129–145. [Google Scholar]
- Guido, A.; Jimenez, C.; Achilleos, K.; Rosso, A.; Sanfilippo, R.; Hadjioannou, L.; Petrou, A.; Russo, F.; Mastandrea, A. Cryptic serpulid-microbialite bioconstructions in the Kakoskali submarine cave (Cyprus, Eastern Mediterranean). Facies 2017, 63, 21. [Google Scholar] [CrossRef]
- Guido, A.; Gerovasileiou, V.; Russo, F.; Rosso, A.; Sanfilippo, R.; Voultsiadou, E.; Mastandrea, A. Composition and biostratinomy of sponge-rich biogenic crusts in submarine caves (Aegean Sea, Eastern Mediterranean). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 534, 109338. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Rosso, A.; Guido, A.; Gerovasileiou, V. Serpulid communities from two marine caves in the Aegean Sea, eastern Mediterranean. J. Mar. Biol. Assoc. UK 2017, 97, 1059–1068. [Google Scholar] [CrossRef] [Green Version]
- Sanfilippo, R.; Rosso, A.; Guido, A.; Mastandrea, A.; Russo, F.; Riding, R.; Taddei Ruggiero, E. Metazoan/microbial biostalactites from present-day submarine caves in the Mediterranean Sea. Mar. Ecol. 2015, 36, 1277–1293. [Google Scholar] [CrossRef]
- Rosso, A.; Sanfilippo, R.; Guido, A.; Gerovasileiou, V.; Taddei Ruggiero, E.; Belmonte, G. Colonisers of the dark: Biostalactite-associated metazoans from “lu Lampiùne” submarine cave (Apulia, Mediterranean Sea). Mar. Ecol. 2021, 42, e12634. [Google Scholar] [CrossRef]
- Guido, A.; Heindel, K.; Birgel, D.; Rosso, A.; Mastandrea, A.; Sanfilippo, R.; Russo, F.; Peckmann, J. Pendant bioconstructions cemented by microbial carbonate in submerged marine caves (Holocene, SE Sicily). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 388, 166–180. [Google Scholar] [CrossRef]
- Guido, A.; Rosso, A.; Sanfilippo, R.; Russo, F.; Mastandrea, A. Frutexites from microbial/metazoan bioconstructions of recent and Pleistocene marine caves (Sicily, Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 453, 127–138. [Google Scholar] [CrossRef]
- Gischler, E.; Heindel, K.; Birgel, D.; Brunner, B.; Reitner, J.; Peckmann, J. Cryptic biostalactites in a submerged karst cave of the Belize Barrier Reef revisited: Pendant bioconstructions cemented by microbial micrite. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 278, 34–51. [Google Scholar] [CrossRef]
- Gischler, E.; Birgel, D.; Brunner, B.; Eisenhauer, A.; Meyer, G.; Buhre, S.; Peckmann, J. A giant underwater stalactite from the Blue Hole, Belize, revisited: A complex history of massive carbonate accretion under changing meteoric and marine conditions. J. Sediment. Res. 2017, 87, 1260–1284. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Altieri, A.H.; Angelini, C.; Bishop, M.J.; Bulleri, F.; Farhan, R.; Frühling, V.M.M.; Gribben, P.E.; Harrison, S.B.; He, Q.; et al. Heterogeneity within and among co-occurring foundation species increases biodiversity. Nat. Commun. 2022, 13, 581. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).