Integrative Taxonomy Supports Two New Species of Rhodiola (Crassulaceae) in Xizang, China
Abstract
:1. Introduction
2. Materials and Methods
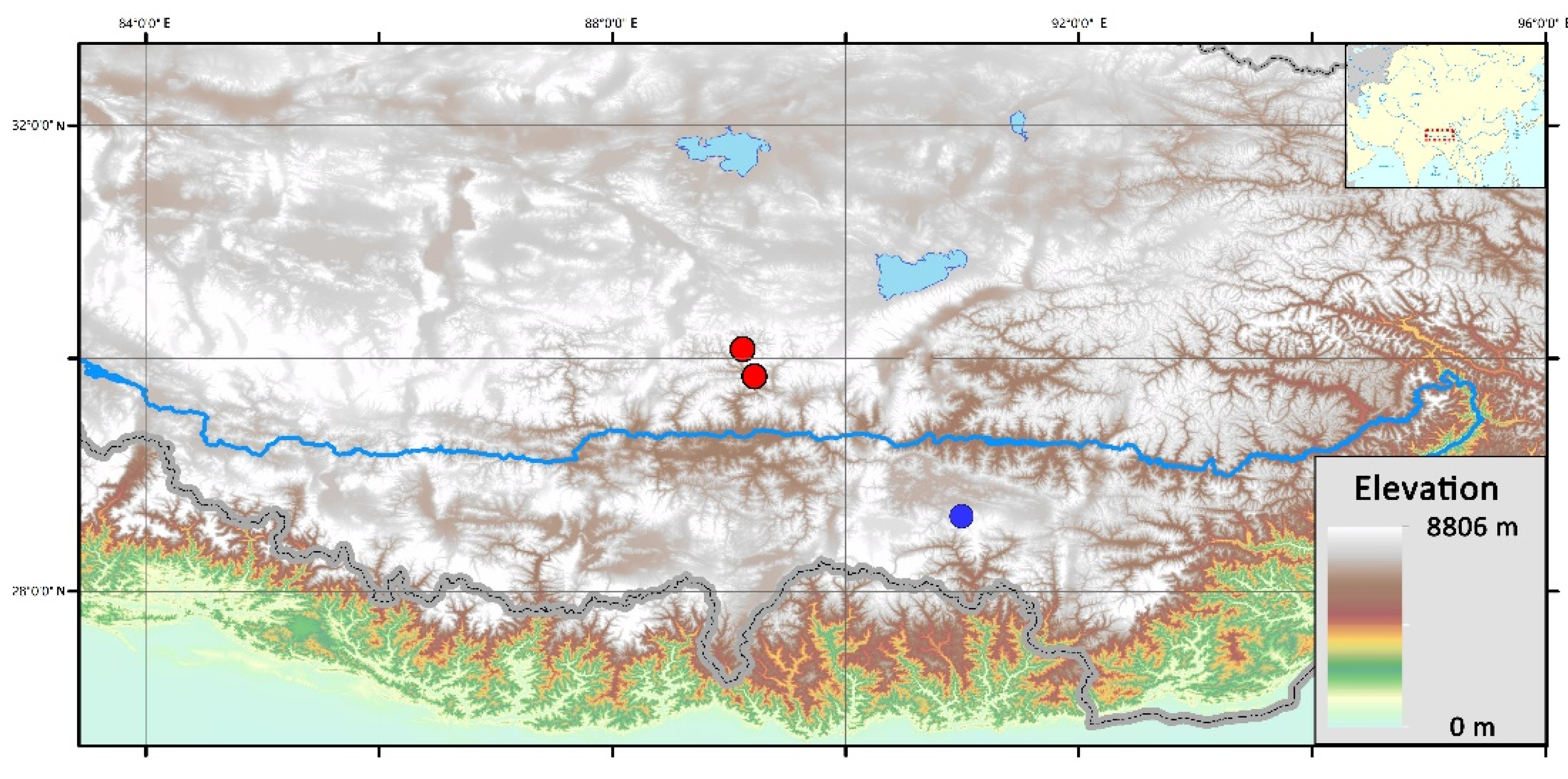
2.1. Materials Collection and Field Investigation
2.2. Morphological Analysis
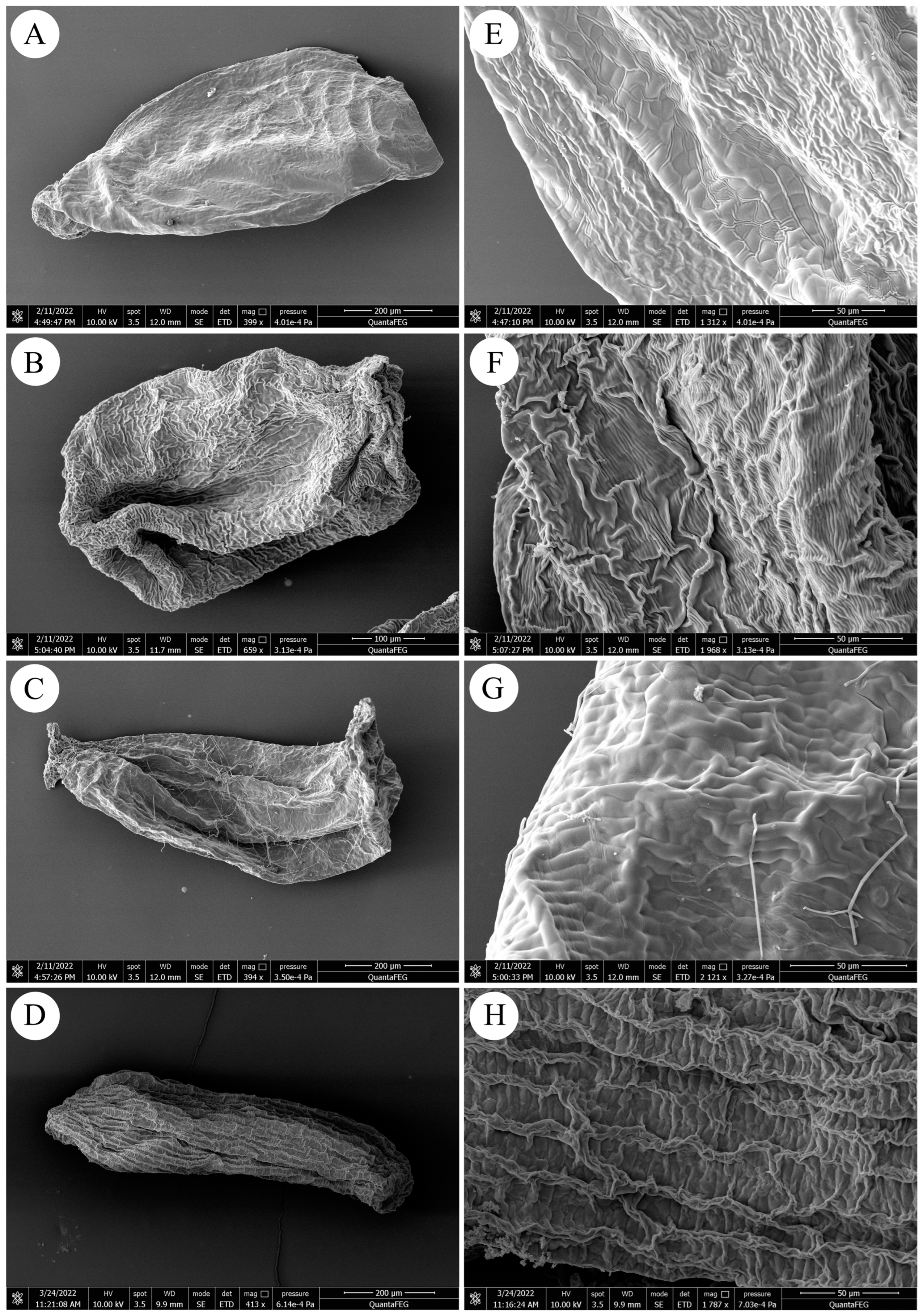
2.3. Scanning Electron Microscopy (SEM) Analysis
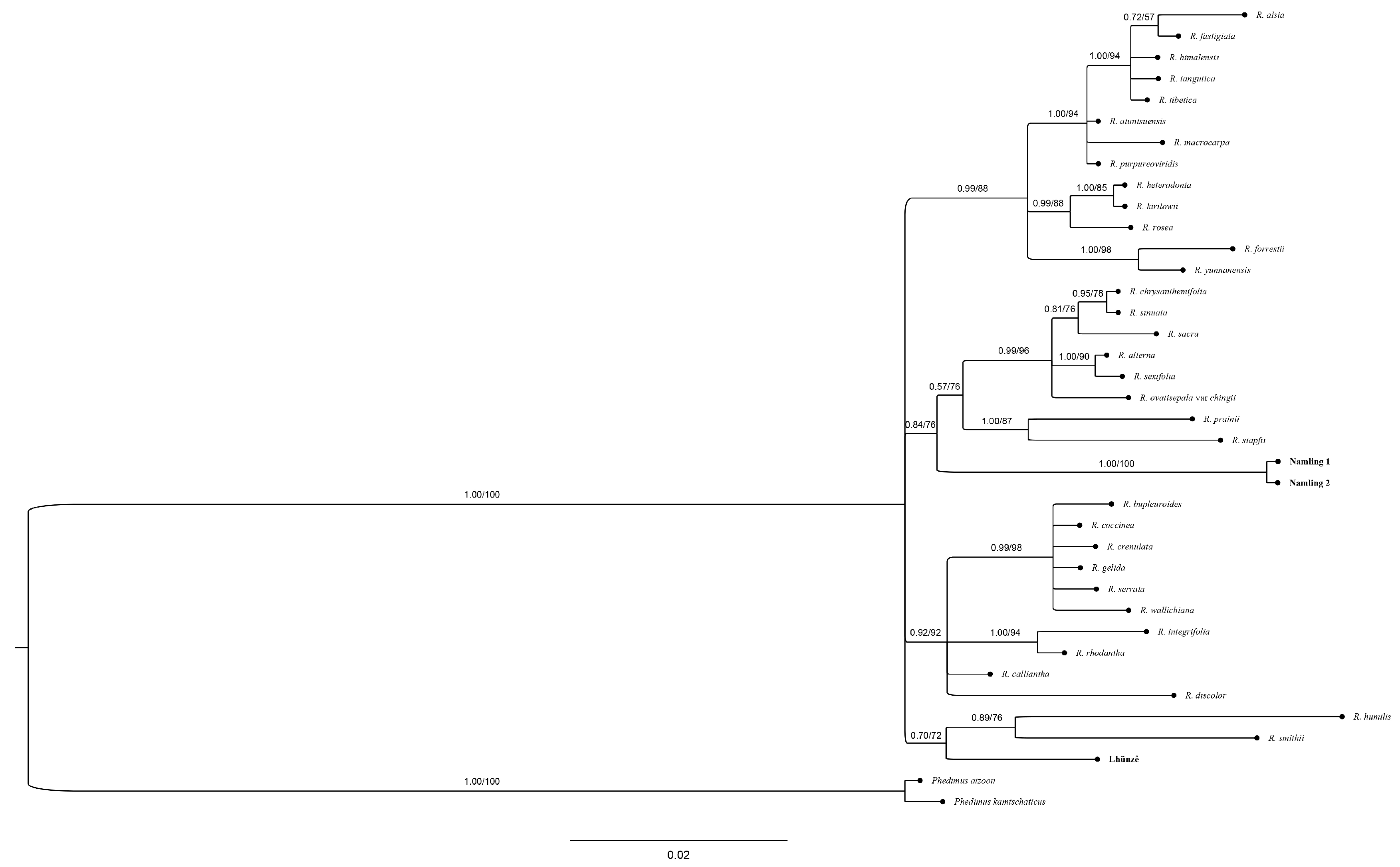
2.4. Phylogenetic Analysis
3. Results
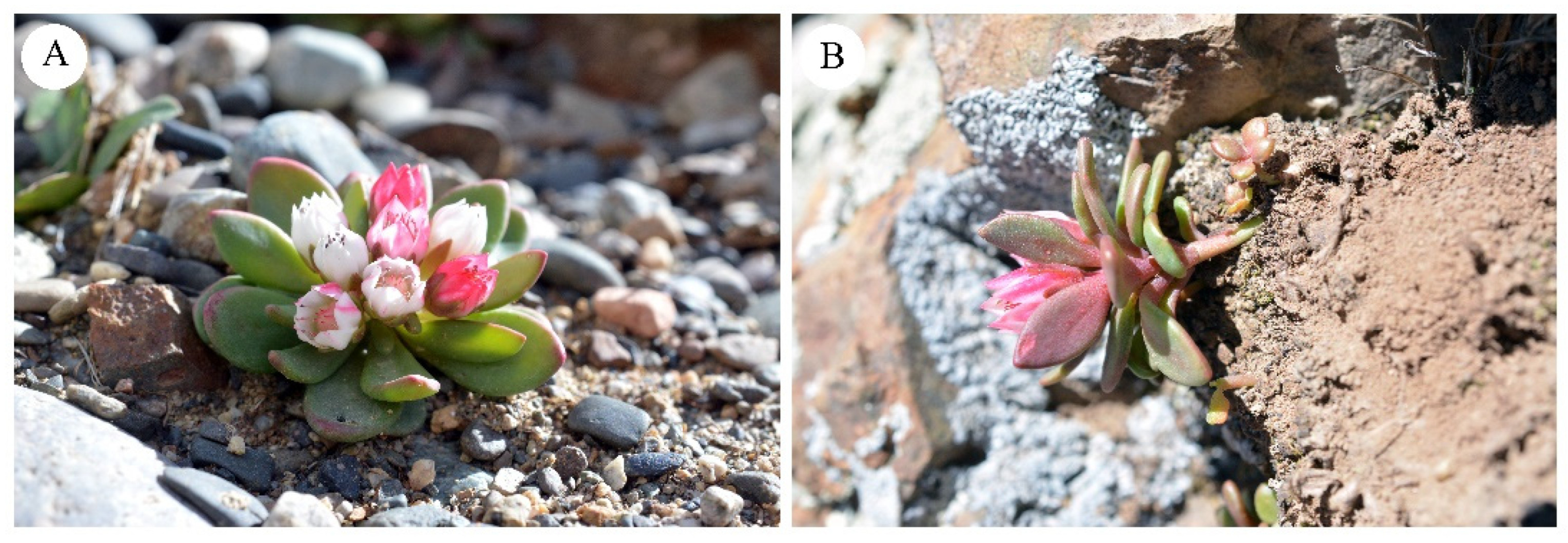
3.1. Habitat
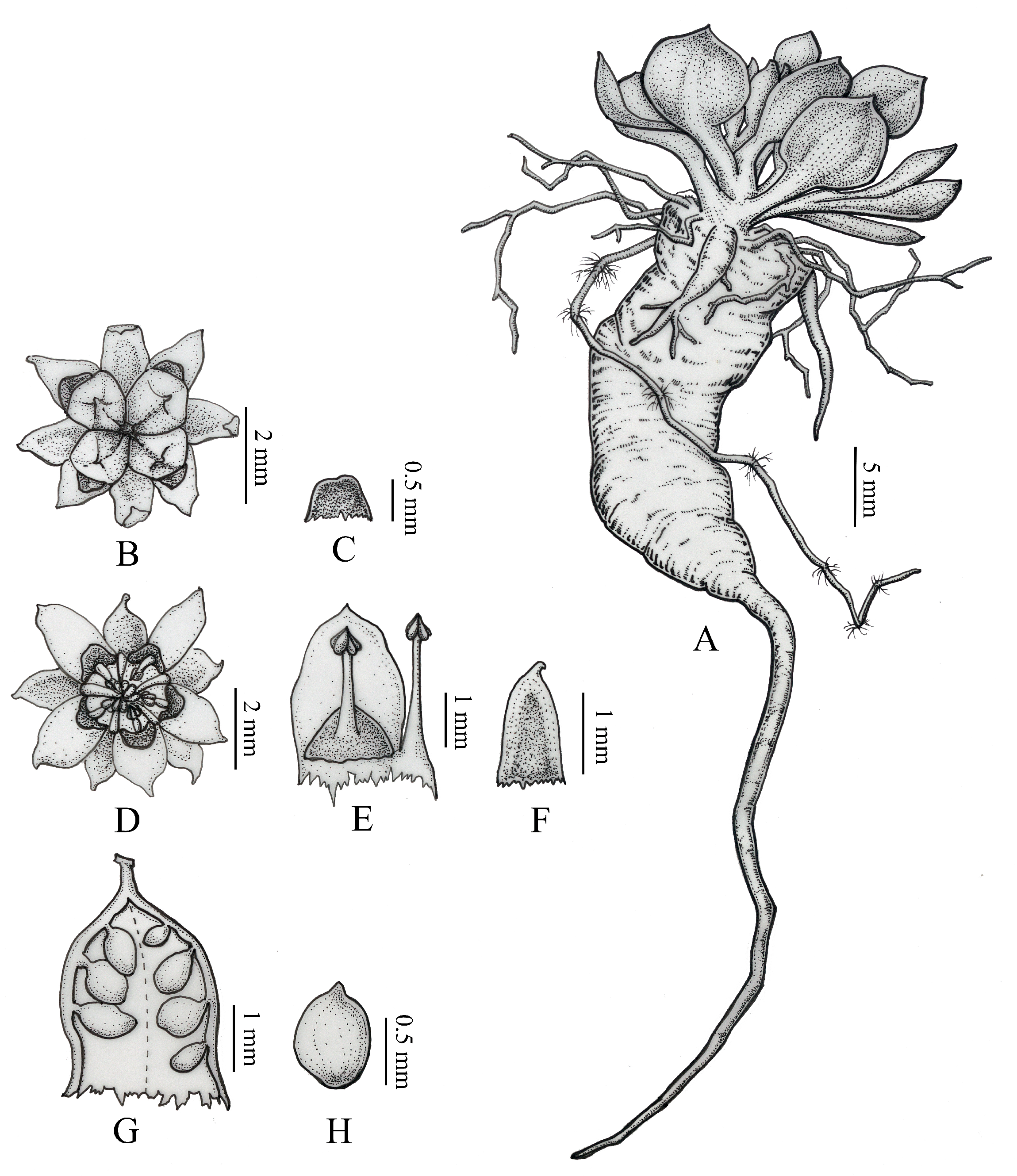
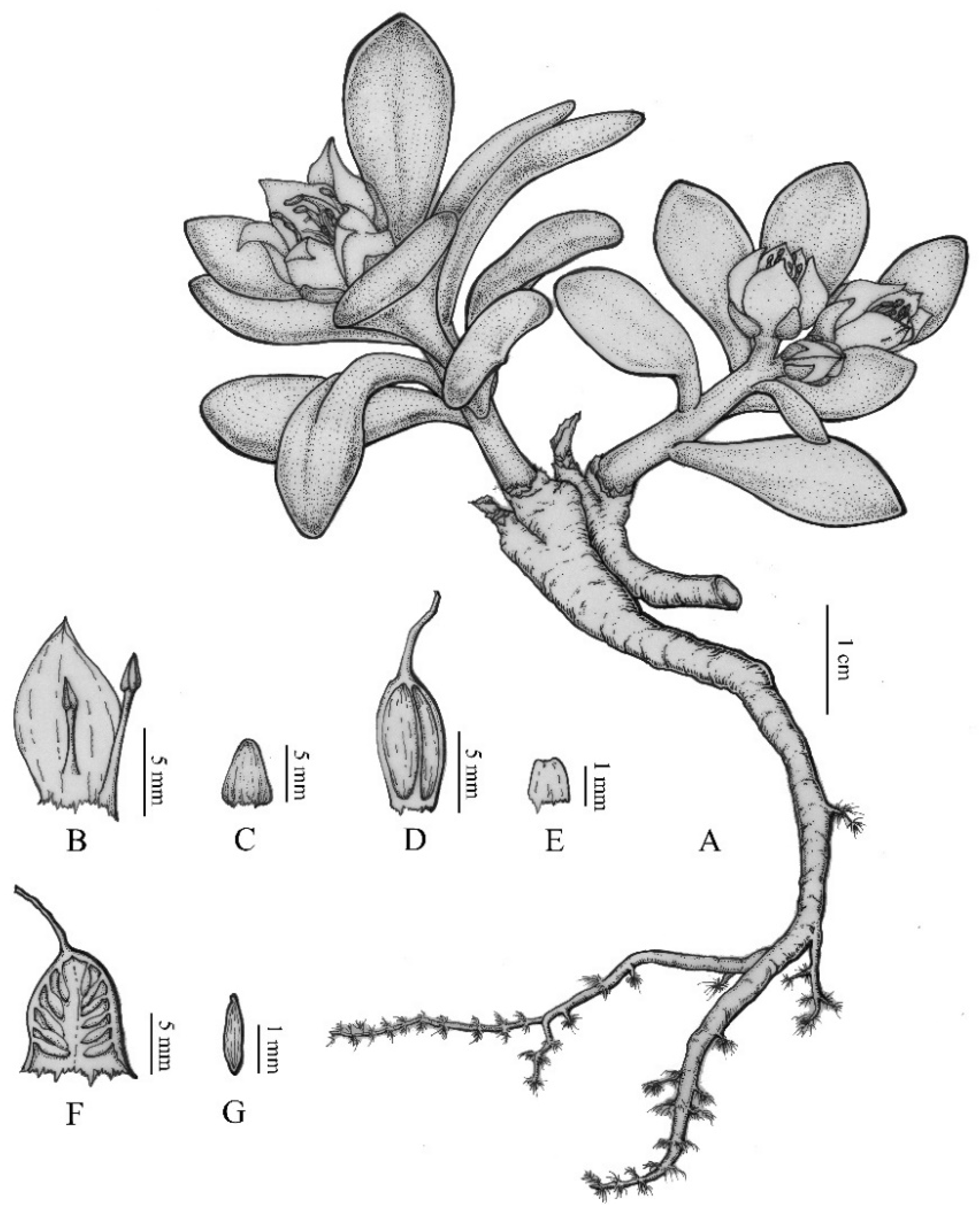
3.2. Morphological Analysis
3.3. SEM Analysis
3.4. Molecular Analyses
4. Discussion
5. Description of the New Species
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.Y. Hengduan Mountain flora and her significance. J. Jpn. Bot. 1988, 63, 297–311. [Google Scholar]
- Wu, S.G.; Yang, Y.P.; Fei, Y. On the flora of the alpine region in the Qinghai-Xizang (Tibet) plateau. Acta Bot. Yunnan 1995, 17, 233–250. [Google Scholar]
- Hughes, C.E.; Atchison, G.W. The ubiquity of alpine plant radiations: From the Andes to the Hengduan Mountains. New Phytol. 2015, 207, 275–282. [Google Scholar] [CrossRef]
- Xia, X.M.; Yang, M.Q.; Li, C.L.; Huang, S.X.; Jin, W.T.; Shen, T.T.; Wang, F.; Li, X.H.; Yoichi, W.; Zhang, L.H.; et al. Spatiotemporal Evolution of the Global Species Diversity of Rhododendron. Mol. Biol. Evol. 2022, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Meng, S.Y.; Wen, J.; Rao, G.Y. Phylogenetic relationships and character evolution of Rhodiola (Crassulaceae) based on nuclear ribosomal ITS and plastid trnL-F and psbA-trnH sequences. Syst. Bot. 2014, 39, 441–451. [Google Scholar] [CrossRef]
- Yu, W.B.; Liu, M.L.; Wang, H.; Mill, R.R.; Ree, R.H.; Yang, J.B.; Li, D.Z. Towards a comprehensive phylogeny of the large temperate genus Pedicularis (Orobanchaceae), with an emphasis on species from the Himalaya-Hengduan Mountains. BMC Plant Biol. 2015, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ouchon, C.H.P.; Ernández, A.N.F.; Assar, J.A.M.N.; Oyer, F.R.B.; Ubert, S.E.A. Phylogenomic analysis of the explosive adaptive radiation of the Espeletia complex (Asteraceae) in the tropical Andes. Syst. Biol. 2018, 67, 1041–1060. [Google Scholar] [CrossRef]
- Carter, K.A.; Liston, A.; Bassil, N.V.; Alice, L.A.; Bushakra, J.M.; Sutherland, B.L.; Mockler, T.C.; Bryant, D.W.; Hummer, K.E. Target Capture Sequencing Unravels Rubus Evolution. Front. Plant Sci. 2019, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Naciri, Y.; Christe, C.; Bétrisey, S.; Song, Y.G.; Deng, M.; Garfì, G.; Kozlowski, G. Species delimitation in the East Asian species of the relict tree genus Zelkova (Ulmaceae): A complex history of diversification and admixture among species. Mol. Phylogenet. Evol. 2019, 134, 172–185. [Google Scholar] [CrossRef]
- Du, C.; Liao, S.; Boufford, D.E.; Ma, J. Twenty years of Chinese vascular plant novelties, 2000 through 2019. Plant Divers. 2020, 42, 393–398. [Google Scholar] [CrossRef]
- Fu, K.T.; Ohba, H. Crassulaceae. In Flora of China; Wu, C.Y., Raven, P.H., Eds.; Science Press: Beijing, China, 2001; Volume 8, pp. 202–268. [Google Scholar]
- Ohba, H. Crassulaceae. In Illustrated Handbook of Succulent Plants; Eggli, U., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2003; Volume 14, pp. 210–227. [Google Scholar]
- Thiede, J.; Eggli, U. Crassulaceae. In The Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007; Volume 9, pp. 83–118. [Google Scholar]
- Fu, S.H.; Fu, K.T. Crassulaceae. In Flora Reipublicae Popularis Sinicae; Chen, W.Q., Ruan, Y.Z., Eds.; Science Press: Beijing, China, 1984; Volume 34, pp. 33–216. [Google Scholar]
- Zhang, J.Q.; Meng, S.Y.; Allen, G.A.; Wen, J.; Rao, G.Y. Rapid radiation and dispersal out of the qinghai-tibetan plateau of an alpine plant lineage Rhodiola (Crassulaceae). Mol. Phylogenet. Evol. 2014, 77, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Wen, J.; Ren, Y.; Zhang, J.Q. From seven to three: Integrative species delimitation supports major reduction in species number in Rhodiola section Trifida (Crassulaceae) on the Qinghai-Tibetan Plateau. Taxon 2019, 68, 268–279. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Meng, S.Y.; Rao, G.Y. Two new species of Rhodiola (Crassulaceae) from the Qinghai-Tibetan Plateau. Phytotaxa 2015, 224, 159–172. [Google Scholar] [CrossRef]
- Gontcharova, S.B.; Gontcharov, A.A.; Yakubov, V.V.; Kondo, K. Seed surface morphology in some representatives of the Genus Rhodiola sect. Rhodiola (Crassulaceae) in the Russian Far East. Flora 2009, 204, 17–24. [Google Scholar]
- Zhang, J.Q.; Meng, S.Y.; Wen, J.; Rao, G.Y. DNA barcoding of Rhodiola (Crassulaceae): A case study on a group of recently diversified medicinal plants from the Qinghai-Tibetan Plateau. PLoS ONE 2015, 10, e0119921. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.N.; Ren, C.Q.; Zhang, J.Q. Can plastome data resolve recent radiations? Rhodiola (Crassulaceae) as a case study. Bot. J. Linn. Soc. 2021, 197, 513–526. [Google Scholar] [CrossRef]
- Bywater, M. Observations on seeds of Crassula sect. Rosulares. Kew Bull. 1980, 35, 401–402. [Google Scholar] [CrossRef]
- Weng, M.L.; Kuo-Huang, L.-L. Comparative Anatomy and Histochemical Study of the Seeds of Sedum formosanum N. E. br. And Sedum morrisonense Hayata. Taiwania 1998, 43, 307–315. [Google Scholar]
- Wickens, G.E.; Bywater, M. Seed studies in Crassula subgen. Disporocarpa. Kew Bull. 1980, 34, 629–637. [Google Scholar] [CrossRef]
- Zhu, R.W.; Li, Y.C.; Zhong, D.L.; Zhang, J.Q. Establishment of the most comprehensive ITS2 barcode database to date of the traditional medicinal plant Rhodiola (Crassulacaee). Sci. Rep. 2017, 7, 1–9. [Google Scholar]
- IUCN. Guidelines for Using the IUCN Red List Categories and Criteria; Version 15. 2022. Available online: http://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 2 April 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, S.; Wang, Z.; Ye, L. Integrative Taxonomy Supports Two New Species of Rhodiola (Crassulaceae) in Xizang, China. Diversity 2022, 14, 289. https://doi.org/10.3390/d14040289
Meng S, Wang Z, Ye L. Integrative Taxonomy Supports Two New Species of Rhodiola (Crassulaceae) in Xizang, China. Diversity. 2022; 14(4):289. https://doi.org/10.3390/d14040289
Chicago/Turabian StyleMeng, Shiyong, Zimeng Wang, and Lv Ye. 2022. "Integrative Taxonomy Supports Two New Species of Rhodiola (Crassulaceae) in Xizang, China" Diversity 14, no. 4: 289. https://doi.org/10.3390/d14040289
APA StyleMeng, S., Wang, Z., & Ye, L. (2022). Integrative Taxonomy Supports Two New Species of Rhodiola (Crassulaceae) in Xizang, China. Diversity, 14(4), 289. https://doi.org/10.3390/d14040289





