Abstract
In this study, distyly was clearly confirmed in Polygonum criopolitanum Hance, which exhibited strict self-incompatibility. Unlike other distylous species, style-morph ratios of P. criopolitanum often deviated obviously from 1:1, and many populations were solely composed of long or short stylous flowers; the 1:1 style-morph ratio was occasionally found in very large populations. P. criopolitanum was dimorphic for intrinsic features such as style height and anther height and ancillary features such as pollen size and number. The L-morph flowers produced a significantly smaller and higher number of pollen grains than the S-morph flowers, and the stigma papillae of both morphs were not significantly different. We nearly found no seed sets in most wild populations and very low seed sets occasionally occurred in large populations, which was different from other species of Polygonaceae. Mating experiments showed that P. criopolitanum has a strict self-incompatibility system and clonal propagation was more common than sexual propagation, which was adaptive with the unisexual wild populations. Hygrocolous habitat, 20–60% soil water content, and height gap less than 4 m to the adjacent water were the main limiting factors for the distribution of P. criopolitanum.
1. Introduction
Heterostyly is a genetic polymorphism in which plant populations are composed of 2 (distyly) or 3 (tristyly) floral morphs. The morphs exhibit reciprocal positioning of anthers and stigmas. Flowers with the L-styled morphology have a stigma(s) positioned above the anthers, whereas flowers with the S-styled morphology have anthers placed above the stigma(s) [1,2]. Besides the differences in floral morphology, distyly is often linked to a sporophytically controlled, diallelic incompatibility system that results in intramorph incompatibility [3].
Heterostyly has been documented in at least 193 genera of 30 angiosperm families [2,4]. Some families-genera possess hundreds of heterostylous species, e.g., Oxalidaceae-Oxalis, Primulaceae-Primula, and Rubiaceae [3], and the occurrence of heterostyly has been recently reported in Perovskia [5]. Distyly in Polygonaceae was first described by Hildebrand in Fagopyrum esculentum over 100 years ago [6]; subsequently, many distylous species have been reported for the genera Oxygonum and Aconogonum [7,8,9,10]. The type genus for the family, Polygonum (which has more than 300 species) [11], has a worldwide distribution, but it has been seldom reported to be heterostylous. The first distylous Polygonum chinense was documented in detail by Reddy et al. (1977) [8]. To date, some other distylous species in Polygonum have been reported [12,13,14,15,16,17].
Polygonum criopolitanum Hance is an annual herb with a tufted, prostrate stem at the base that grows to 10–15 cm. Its inflorescence is terminal and capitate, and the perianth is composed of 5-parted purplish-red tepals. The species is always distributed in the sand by riversides and wet ditches. Most of the previous studies on this species focused mainly on its ecology [18], mating system [19], and plant resources [20], and, to the best of our knowledge, the reproductive characters of P. criopolitanum have never been reported in detail.
In this study, we examined variations in floral and reproductive traits and incompatibility systems in natural populations of P. criopolitanum. The aims of this study were as follows: (1) to determine whether P. criopolitanum is typically distylous as other documented distylous species; (2) to record the morph ratios of wild populations of P. criopolitanum; (3) to test the compatibility system after pollen germination, stigma receptivity, and seed set tests; and (4) to discuss the relationship between ecological factors and environmental factors by using canonical correlation analysis (CCA).
2. Materials and Methods
2.1. Study Materials
This study was conducted along the Yangzi River and in Anhui, Jiangxi Province (Figure 1). Herbarium specimens or living materials of P. criopolitanum from Anhui and Jiangxi Province, China, were used for this study. All vouchers were deposited at the herbarium of Anhui Normal University (ANUB), China.
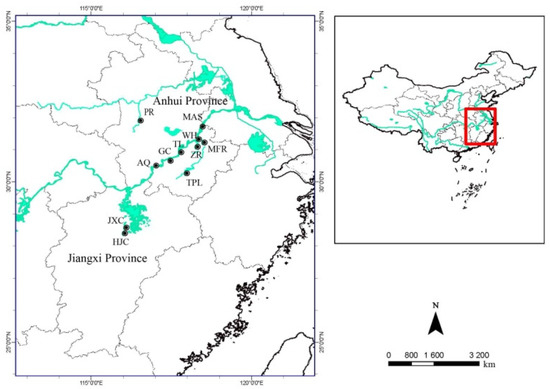
Figure 1.
Study sites of Polygonum criopolitanum (●: Population. JXC = Jinxian County; HJC = Huangjiacun; TPL = Taiping Lake; MFR = Mafeng River; ZR = Zhang River; AQ = Anqing; GC = GuiChi; TL = Tongling; WH = Wuhu; MAS = Maanshan; PR =Pi River).
2.2. Study Methods
2.2.1. Floral Characters
To document distyly in P. criopolitanum, the height of stigma, height of anther, stigma-anther separation, length of tepal, and flower diameter (sketched in Figure 2) were recorded for 50 or 100 flowers of each morph at random from the Wuhu Mafeng River population (Figure 1), using a vernier caliper with a resolution of 0.01 mm.
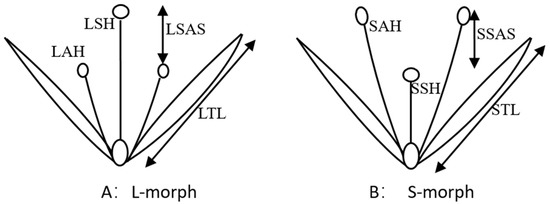
Figure 2.
Pattern diagram of distylous flower of P. criopolitanum ((A): L-morph; (B): S-morph). LTL: tepal length of L-morph; LAH: anther height of L-morph; LSH: stigma height of L-morph; LSAS: stigma-anther separation of L-morph; STL: tepal length of S-morph; SAH: anther height of S-morph; SSH: stigma height of S-morph; SSAS: stigma-anther separation of S-morph.
To count the number of pollen produced, anthers from 100 flowers per morph of the Wuhu populations were placed on individual microscope slides. The numbers of pollen grains per flower were counted using a light microscope, and the diameter of 10 moderate pollen grains from each flower was measured [12,21]. Pollen counts for the style morphs were compared using Student’s t-test.
2.2.2. Scanning Electron Microscopy
According to the standard acetolysis method [22], pollen grains were mounted in glycerin jelly and sealed with paraffin. The size of well-formed pollen grains from each sample was measured. Scanning electron microscopy was performed using acetolyzed pollen grains coated with Au/Pd under a Hitachi S4800 scanning electron microscope (SEM). Fresh stigmas were mounted in a slow-drying glue and observed under the SEM.
2.2.3. Population Structure Survey
To determine the relative frequencies of the 2 style morphs in populations of P. criopolitanum, we surveyed 44 samples to test whether L- and S-morphs occurred at equal frequencies. The chi-square test was performed in R software [23]. We sampled all plants in sporadic populations and 1 × 1 m in patches of populations; for larger samples, and we performed 10 × 10 m sampling.
2.2.4. Environmental and Ecological Factors
Nineteen samples were obtained in Huangshan Taiping Lake, AnqingYingjiang Tower, Guichi Shibasuo, Tongling Shizishan, Yangzi River in Wuhu, Longwo Lake, and Zhang River. Four samples were obtained along the Yangzi River, Longwo Lake, and Zhang River. The first sample was located at the water side, and the other three samples were located along a line drawn perpendicular to the shore at 1-m intervals in altitude up the slope of the riverbank. The number of plants, height, relative coverage, species richness, and presence or absence of P. criopolitanum was recorded. The altitude, height gap to the adjacent water, habitat, annual precipitation, annual average temperature, slope, and canopy density of each sample were also documented, and1 kg of soil from each sample was transferred to the laboratory to test ecological factors such as soil water content, pH, organic matter, total nitrogen (TN), and total phosphorus (TP). The soil water content was measured using the GB9834-88 method; soil TN, Kjeldahl method; and soil TP, Mo-Sb colorimetric method [24].
2.2.5. CCA
CCA was performed using Canoco for Windows 4.5. The graph settings were confirmed using the data for the environmental and ecological factors.
2.2.6. Fluorescent Microscopy
To identify the extent of incompatibility of the breeding system of P. criopolitanum, pollen germination, and pollen tube growth were determined. The style morphs from each population were pollinated; we waited for 12 h to observe growth in the pollen tube, then they were stored in FAA until staining. To estimate the pollen germination, theharvested styles were softened in 8 mol/L NaOH for 24 h rinsed with distilled water. Then, they were stained for 4 h in 0.4 mg/mL aniline blue solution in phosphate buffer (pH 8.0) and gently squashed in a small drop of glycerol mounting medium under a cover slip. The pistil and pollen were scanned with an Olympus (BX61) epifluorescence microscope (420–470 nm excitation, 490–535 nm emission). For each style, pollen germination was detected by the presence of a pollen tube projecting from the grain, and 10 replicates were performed.
2.2.7. Seed Set Experiments
The seed set experiments were performed using natural populations and controlled pollination populations. For the natural populations, we set 14 1 × 1 m samples in Taiping Lake (2 samples), Luan Pi River (1), Guichi (1), Anqing (1), Tongling (1), Wuhu (5), and Jinxian (3). We calculated the style-morph ratio and then obtained all infructescences of one plant to count the number of seeds (n = 45).
The hybrid experiments were conducted in the laboratory. Legitimate and illegitimate pollination (including selfing) were performed; the seed sets were recorded in every treatment mode: emasculation and bagging, selfing, illegitimate (intramorph) pollination, and legitimate (intermorph) pollination. Each mode was conducted using 100 flowers.
2.2.8. Seed Germination Experiments
Normally developed seeds were soaked in water for 48 h and then disinfected in 75% ethyl alcohol for 30 s. Thirty treated seeds were placed in a culture dish in an illumination incubator to observe the seed germination, with 3 replicates.
3. Results
3.1. Floral Biology
P. criopolitanum is an annual herb with terminal and capitate inflorescence. The peduncle is covered by dense glandular hair, and each bract is 1-flowered. The pattern of floral variation in the wild populations demonstrates that P. criopolitanum has conventional distylous floral syndrome (Figure 3A–D). The tepal lengths of the long stylous flower (hereafter L-morph) and short stylous flower (hereafter S-morph) were 2.53 ± 0.44 and 2.47 ± 0.29 mm, respectively, and the tepal diameters of the L-morph and S-morph were 6.58 ± 0.47 and 6.42 ± 0.25 mm, respectively. No significant differences were observed between the tepal lengths as well tepal diameters of the L-morph and S-morph (Table 1, p > 0.05). The anthers and stigma are reciprocally positioned in the flowers of the two morphs (Figure 4 and Figure 5). Five stamens are situated between the base of adjacent tepals, and the anthers are purple. Styles 2, seldom 3, connate at the middle-upper part (Figure 5A–D). In addition, the two morphs have five nectaries arranged at the base of each ovary (Figure 3C,D). The stigma and anther heights of the L-morph were 4.02 ± 0.66 and 2.19 ± 0.42 mm, respectively, and the stigma and anther heights of the S-morph were3.90 ± 0.42 and 1.83 ± 0.55 mm, respectively; significant differences were observed between the heights of the stigma and anther of both morphs (p < 0.05, Table 1). We found that the stigma-anther separation of the L-morph (2.06 ± 0.39) was longer than that of the S-morph (1.83 ± 0.55; p < 0.05, Table 1).

Figure 3.
Flowers of the L- and S-morphs of P. criopolitanum ((A,C): L-morph; (B,D): S-morph).

Table 1.
Morphological features of the long- and short-morph flowers of P.criopolitanum. Differences between the means were analyzed using one-way analysis of variance (ANOVA; mean ± standard error).
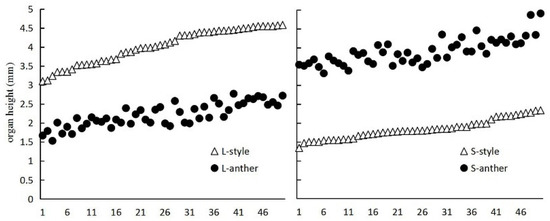
Figure 4.
Style and anther length of P.criopolitanum (ranked by style length to illustrate the reciprocal correspondence of stigma and anther positions in the long- and short-styled morphs. Positions of the stigmas and anthers are indicated by open triangle (△) and solid circle (●), respectively).
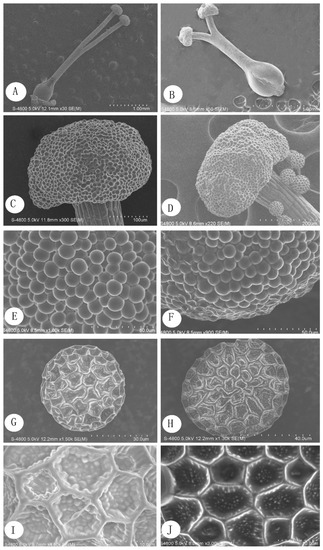
Figure 5.
Micromorphological characteristics of P. criopolitanum (SEM). (A) pistil of L-morph; (B) pistil of S-morph; (C) stigma of L-morph; (D) stigma of S-morph; (E) stigma papillae of L-morph; (F) stigma papillae of S-morph; (G) pollen of L-morph; (H) pollen of S-morph; (I) pollen epidermal ornamentation of L-morph; (J) pollen epidermal ornamentation of S-morph.
The stigma, which is capitate or spherical with globular papillae on the stigma surface, is similar in the L-morph and S-morph. No significant differences were observed in the size and shape of the stigma papillae (Figure 5C–F).
The pollen grains of P. criopolitanum are spheroidal in both morphs, and the pollen grain surface is covered with reticulate exine structures that are pentagonal or hexagonal. Each reticular mesh in the pollen contains many smooth papillae (Figure 5G–J).
The pollen size and number of the two morphs of P. criopolitanum showed significant differences. The mean pollen diameters of the L-morph and S-morph were 51 ± 1.92 μm and 62 ± 2.51 μm, respectively, and the mean pollen number per flower of the L-morph and S-morph was 647 ± 40 and 526 ± 38, respectively (Table 1). Although an overlap was detected in the pollen sizes of both morphs, flowers of the L-morph produced significantly more and smaller pollen grains than the ones of the S-morph (Table 1, p < 0.01).
3.2. Style-Morph Ratios
The chi-square test is a measure of whether the style-morph ratios deviate from 1:1. The survey results showed that the style-morph ratios of P. criopolitanum obviously deviated from 1:1, and parthenogenetic populations were always detected in the wild populations. We found that many populations were solely composed of long or short stylous flowers (e.g., ZhengfengTower or Jinxian County populations), whereas the 1:1 morph ratio was seldom found in larger populations (Jinxian County, Taiping Lake, and Mafeng River populations; Table 2).

Table 2.
Style-morph ratios in 44 natural populations of P. criopolitanum.
3.3. CCA Results
3.3.1. Characteristics of the Environmental Factors
The environmental factor indices of 19 samples are listed in Table 3.

Table 3.
Characteristics of the environmental factors of P. criopolitanum.
3.3.2. Characteristics of Ecological Factors
The ecological factor indices of 19 samples are listed in Table 4.

Table 4.
Characteristics of ecological factors of P. criopolitanum.
3.3.3. CCA of P. criopolitanum Samples
The relationship between ecological factors and environmental factors is shown in Figure 6.
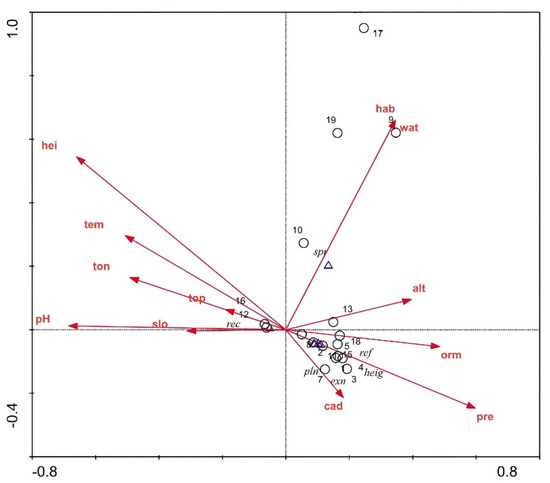
Figure 6.
CCA ordination diagram of P. criopolitanum. Note: alt:altitude; hei: height gap; wat: water; hab: habitat; pre: precipitation; tem: temperature; slo: slope; cad: canopy density; pH: pH; orm: organic matter; ton: total nitrogen; top: total phosphorus. Positions of the samples and environmental factors are indicated by open circle (○) and open triangle (△), respectively.
In the CCA ordination diagram, the red lines and arrowheads refer to the environmental factors, and the length of the segment refers to the relationship degree between the sample distribution and environmental factors. The angle between the ordination axes and arrowhead connecting the line indicates the correlation degree between the environmental factor and its ordination axes, and the quadrant where the arrowhead is distributed indicates the positive or negative relationship between the environmental factor and its ordination axes. Figure 6 shows the major ecological factors that affect the population distribution of P. criopolitanum: habitat, soil water content, and height gap to the adjacent water. Hygrocolous habitat, 20–60% soil water content, and height gap less than 4 m to the adjacent water were the main limiting factors for the distribution of P. criopolitanum. Annual precipitation maydirectly influence the soil water content and hygrocoloushabitat. The population distribution of P. criopolitanum had no obvious relationship with the slope, pH, altitude, organic matter, TN, and TP. The axes showed that the soil water content increased progressively from top to bottom.
3.4. Mating System Relationships
The fluorescent experiments showed that the rate of pollen germination in the style of the intermorph was obviously higher than that of the self- and intramorph (Figure 7A–F). The statistics showed that the pollen germination rate of pin style × thrum pollen was 72.0 ± 4.0% (n = 10) and that of thrum style × pin pollen was 73.0 ± 3.5% (n = 10). During intramorph pollination, the pollen germination rate of pin style × pin pollen was 43 ± 2.6% (n = 10) and that of thrum style × thrum pollen was 42.0 ± 3.1% (n = 10).
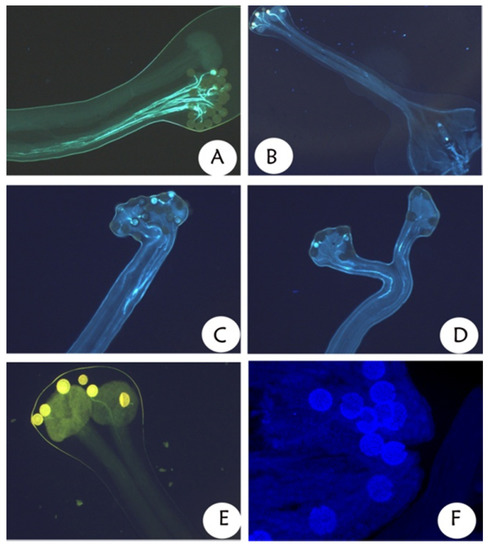
Figure 7.
Fluorescent experiments of pollen germination of P. criopolitanum. (A) L-morph intermorph pollinations; (B) S-morph legitimate pollinations; (C) L-morph illegitimate pollination; (D) S-morph illegitimate pollination. (E) Selfing of L-morph; (F) Selfing of S-morph.
During self-pollination, the rate of pollen germination of the pin self was 9.0 ± 0.7%, whereas no pollen germination was identified in the case of the thrum self.
3.5. Seed Sets
The statistics showed that the seed sets of P. criopolitanum were very low in the wild populations; moreover, the seed sets in the population were often 0, and, even in larger populations with both L- and S-morph, the seed sets were always less than 10% (Table 5).

Table 5.
Seed sets of P.criopolitanum in different populations under natural conditions.
The statistics showed that the seed set rate was 0 under emasculation, selfing, and illegitimate pollination conditions. Moreover, the seed set rate was low under legitimate pollination (Table 6).

Table 6.
Seed set rate under different treatment conditions.
3.6. Seed Germination
The statistics showed that the seed germination of L-morph and S-morph was 13.33 ± 3.87% and 16.67 ± 3.87%, respectively, with no significant differences between the seed germination of L-morph and S-morph (p < 0.01). We found widespread asexual clonal reproduction phenomena in the fields.
4. Discussion
This study revealed that P. criopolitanum has all the polymorphic intrinsic features with respect to style and stamen heights and accessorial characteristics with respect to pollen size and number. The polymorphic density of stigma papillae has recently been reported for the tristylous Lythrumsalicaria [25] and distylous Polygonum jucundum [12]. However, in this study, we found no significant differences between the L-morph and S-morph of P. criopolitanum, including tepal size; thus, P. criopolitanum is typically distylous in intrinsic features and not in all ancillary features.
Flower morph frequencies have always received much attention [26], especially with respect to the consequences for inbreeding [27] or long-term population persistence [28]. In addition, discrepancies in the morph ratio have been found to be much higher in small rather than large populations of Primula veris [29] and Primula elatior [30], but populations solely composed of the L-morph or S-morph were seldom reported. Interestingly, we observed that the monotypic populations of P. criopolitanum were always found in wild populations, which perfectly accounted for the particularly low seed sets of the species.
Fluorescent microscopy showed that pin × pin crosses do occur at a low rate, and no pollen germination was identified in the case of the thrum self, which can explain slight deviations from the 1:1 ratio in a large population. Very common clonal growth in P. criopolitanum can explain why the populations fixed for the L-morph or S-morph were always distributed in the fields.
In distylous species, different taxa havebreeding systems with different compatibility levels. Most heterostylous species have a mating system with strict self-incompatibility; for example, a diallelic self-incompatibility system was reported in Tylosema esculentum through in vivo and in vitro diallel crossing experiments. The major site of pollen tube inhibition in the intramorph crosses was found to be in style [31]. Arnebia szechenyi has also been recorded to show heteromorphic self-incompatibility, which was further supported by the fact that no fruit was produced by flowers subjected to self-pollination or intramorph pollination [32]. In contrast, some species do not exhibit a strict self-compatibility system. In many species of Primula, selfing or crossing with a plant of the same morph will also produce a small numberof seeds [33]; for example, the fertility of a legitimate crossof Primula merrilliana was high, whereas the fertility of an illegitimate cross waslow [34], and Pulmonaria officinalis and Ceratostigma willmottianum were found to bepartially self-compatible [35,36]. Unlike most heterostylous species, Primula oreodoxa was found to be fully self-compatible under controlled self- and cross-pollinations [37]; flowers of Psychotria carthagenensis were also self-compatible [38], and atypical distylous Psychotria goyazensis was proven to be an intramorph self-compatible species [39]. In Europe, Armeria maritima is completely self-incompatible, but it lost its self-incompatibility during its migration to the New World through the Arctic regions [40]. Seed production is thought to be more sensitive to habitat fragmentation in heterostylous plants than in plants with other breeding systems because potential mating partners are more limited [41].
In this study, we nearly found no seed sets in most wild populations and very low seed sets occasionally occurred in large populations, which was different from other species of Polygonaceae; for example, the seed set of Polygonum perfoliatum has a high seed set rate (even up to 84%) [42], and bagging experiments have shown that 47% flowers of Polygonum thunbergii are self-pollinated because of no pollinator visits. Despite a high probability of cross-pollination, the probability of fruit set within the ramet was 0.30 because of resource limitations [43]. What interested us the most was that we found a similar scenario in Polygonum viviparum. The fruit set of P. viviparum has never been observed in North American populations, and sexual reproduction is clearly a rare event in this species [44]. The lack of viable seed production in P. viviparum has no single developmental explanation. A similar adaptive reproduction mechanism may exist in these species. On the basis of a common monotypic distribution, pollen germination during the stigma experiments, and the absence of seed sets in the wild populations, we inferred that P. criopolitanum has a strict self-incompatibility system.
Previous surveys of the incompatibility status of island flora such as the flora of New Zealand, Hawaii, and the Galápagos have shown a deficit in taxa with heteromorphic incompatibility when compared with continental areas [45]. We found that the dependence on water and environmental characteristics of the hydro-fluctuation belt may be important factors for the establishment of thereproduction system of P. criopolitanum. On the basis of the seed sets (natural and artificial conditions) and shoreside distribution along the Yangzi River or other lakes, the strict self-incompatibility, as well as the sex reproduction efficiency, of P. criopolitanum was less dominant than asexual clonal reproduction and uniparental reproduction occurred by clone and not by self-compatible sex reproduction. Baker (1955) referred mainly to self-fertilization as a trait that would confer reproductive assurance during colonization, but we found a different scenario in P.criopolitanum [40]. Somes pecies have been reported to abandon sexual reproduction for some form of clonal reproduction, at least in some habitats or parts of their geographic range [46,47].
In conclusion, P. criopolitanum was typical distylous species with a strict self-incompatibility reproductive system. The wild population of P. criopolitanum deviated obviously from 1:1, and 1:1 style-morph ratios were occasionally found in very large populations. The strict self-incompatibility reproductive system, main environmental factors stress, and common monomorphic populations resulted in the low seed sets, which can explain the general asexual clonal reproduction instead of sexual reproduction in the species. To understand the molecular adaptation mechanism of P. criopolitanum, further studies on pollen flow and gene flow at the molecular level need to be performed.
Author Contributions
Project administration and writing, M.-L.C.; investigation, M.-Y.Q.; data curation, B.-B.B. and X.H. All authors have read and agreed to the published version of the manuscript.
Funding
The Natural Science Foundation of Anhui Province (1808085MC76) and the opening fund for provincial key laboratory and key discipline of Colleges of Life Sciences in AHNU, China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Population data and environmental and ecological factors data were gotten in 2021. Morphological data were gotten from February 2019 to September 2021. The data from the study is available upon request from authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ganders, E. The biology of heterostyly. N. Z. J. Bot. 1979, 17, 607–635. [Google Scholar] [CrossRef]
- Lloyd, D.G.; Webb, C.J. The evolution of heterostyly. In Evolution and Function of Heterostyly; Barrett, S.C.H., Ed.; Springer: Berlin, Germany, 1992; pp. 151–178. [Google Scholar]
- Barrett, S.C.H.; Jesson, L.K.; Baker, A.M. The evolution and function of stylar polymorphisms in flowering plants. Ann. Bot. 2000, 85 (Suppl. A), 253–265. [Google Scholar] [CrossRef]
- Chen, M.L.; You, Y.L.; Zhang, X.P. Advances in the research of heterostyly. Acta Pratacult. Sin. 2013, 19, 226–239. [Google Scholar]
- Moon, H.K. Confirmation of distyly in Perovskia and floral dimorphism of P. abrotanoides (Salviinae: Lamiaceae). Flora 2021, 283, 151905. [Google Scholar] [CrossRef]
- Darwin, C. The Different Forms of Flowers on Plants of the Same Species; Appleton & Co.: New York, NY, USA, 1877. [Google Scholar]
- Graham, R.A. Polygonaceae. In Flora of Tropical East; Turril, W.B., Redhead, E.M., Eds.; Africa Crown Agents: London, UK, 1958; pp. 1–40. [Google Scholar]
- Reddy, N.P.; Bahadur, B.; Kumar, P.V. Heterostyly in Polygonum chinense. J. Genet. 1977, 63, 79–82. [Google Scholar] [CrossRef]
- Hong, S.P. The dimorphic heterostyly in Aconogonon campanulatum (Polygonaceae). Plant Syst. Evol. 1991, 176, 125–131. [Google Scholar] [CrossRef]
- Hong, S.P. Pollen dimorphism in heterostylous species of Oxygonum. Syst. Geogr. Plants 1999, 68, 245–252. [Google Scholar] [CrossRef]
- Li, A.J.; Bao, B.J. Polygonaceae. In Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2003; Volume 5, pp. 277–350. [Google Scholar]
- Chen, M.L.; Zhang, X.P. Distyly in Polygonum jucundum Meisn. Plant Syst. Evol. 2010, 288, 139–148. [Google Scholar] [CrossRef]
- Chen, M.L. Floral morphology and breeding system in Polygonum hastato-sagittatum Mak. (Polygonaceae). Flora 2012, 207, 365–371. [Google Scholar] [CrossRef]
- Lv, F.J.; Cui, M.C.; Chen, M.L. Reproductive biology of Polygonum japonicum. Acta Pratacult. Sin. 2013, 22, 196–203. [Google Scholar]
- Wen, H.H.; Chen, M.L.; Zhang, Z.S. Reproductive biology in the distylous species of Polygonum orientale. Acta Pratacult. Sin. 2015, 24, 155–162. [Google Scholar]
- Wang, C.H.; Du, W.; Wang, X.F. Reproductive investment in a cleistogamous morph of Polygonum jucundum (Polygonaceae). PlantSyst. Evol. 2017, 303, 559–563. [Google Scholar] [CrossRef]
- Guo, Y.N.; Chen, S.F.; Chen, M.L.; Li, B. Persicariajucunda var. rotunda (Polygonaceae, Persicarieae), a distinct distylous taxa raised to specific rank. PhytoKeys 2019, 126, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.W. Reproductive Ecology of Polygonum criopolitanum; Jiangxi Agricultural University: Nanchang, China, 2019. [Google Scholar]
- Fang, Y.B.; Shao, J.W.; Wei, Y.; Zhang, X.P. Pilot study on the breeding system of seven species of Polygonum. J. Biol. 2009, 26, 38–40. [Google Scholar]
- Xu, S.Q. Studies on Polygonum resources and utilization in Cixi. Chin. Wild Plant Resour. 2013, 32, 32–36. [Google Scholar]
- Karise, R.; Mänd, M.; Ivask, M.; Koskor, E.; Bender, A. The effect of pollen amount and its caloric value in hybrid lucerne (Medicago xvaria) on its attractiveness to bumble bees (Bombus terrestris). Agron. Res. 2006, 4, 211–216. [Google Scholar]
- Erdtman, G. The acetolysis technique: A revised description. Sven. Bot. Tisdkr. 1960, 54, 561–564. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, Vienna. 2019. Available online: https://www.R-Project.org (accessed on 15 October 2021).
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Hermann, B.P.; Mal, T.K.; Williams, R.J.; Dollahon, N.R. Quantitative evaluation of stigma polymorphism in a tristylous weed, Lythrumsalicaria (Lythraceae). Am. J. Bot. 1999, 86, 1121–1129. [Google Scholar] [CrossRef]
- Endels, P.; Jacquemyn, H.; Brys, R.; Hermy, M.; De Blust, G. Temporal changes (1986–1999) in populations of primrose (Primula vulgaris Huds.) in an agricultural landscape and implications for conservation. Biol. Conserv. 2002, 105, 11–25. [Google Scholar] [CrossRef]
- Goodwillie, C. Inbreeding depression and mating systems in two species of Linanthus (Polemoniaceae). Heredity 2000, 84, 283–293. [Google Scholar] [CrossRef]
- Byers, D.L.; Meagher, T.R. Mate availability in small populations of plant species with homomorphic sporophitic self-incompatibility. Heredity 1992, 68, 353–359. [Google Scholar] [CrossRef]
- Kéry, M.; Matthies, D.; Spillmann, H.H. Reduced fecundity and offspring performance in small populations of the declining grassland plants Primula veris and Gentiana lutea. J. Ecol. 2000, 88, 17–30. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R.; Hermy, M. Patch occupancy, population size and reproductive success of a forest herb (Primula elatior) in a fragmented landscape. Oecologia 2002, 130, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Hartley, M.L.; Tshamekeng, E.; Thomas, S.M. Functional heterostyly in Tylosema esculentum (Caesalpinioideae). Ann. Bot. 2002, 89, 67–76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, C.; Wang, L.L.; Lan, D.; Yang, Y.P.; Duan, Y.W. Pollination ecology of Arnebiaszechenyi(Boraginaceae), a Chinese endemic perennial characterized by distyly and heteromorphic self-incompatibility. Ann. Bot. Fenn. 2014, 51, 297–304. [Google Scholar] [CrossRef]
- Arnold, E.S.; Richards, A.J. On the occurrence of unilateral imcompatibility in Primula section Aleuritia Duby and the origin of Primula scotica Hook. Bot. J. Linn. Soc. 1998, 128, 359–368. [Google Scholar]
- Chen, M.L. Comparative reproductive biology of Primula merrilliana Schltr. and P. cicutariifolia Pax. Plant Syst. Evol. 2009, 278, 23–32. [Google Scholar] [CrossRef]
- Brys, R.; Jacquemyn, H.; Beeckman, T. Morph-ratio variation, population size and female reproductive success in distylous Pulmonaria officinalis (Boraginaceae). J. Evol. Biol. 2008, 21, 1281–1289. [Google Scholar] [CrossRef]
- Gao, S.P.; Li, W.J.; Hong, M.T.; Lei, T.; Shen, P.; Li, J.N.; Jiang, M.Y.; Duan, Y.F.; Shi, L.S. The nonreciprocal heterostyly and heterotypic self-incompatibility of Ceratostigma willmottianum. J. Plant Res. 2021, 134, 543–557. [Google Scholar] [CrossRef]
- Yuan, S.; Barrett, S.C.H.; Zhang, D.X. Genetics of distyly and homostyly in a self-compatible Primula. Heredity 2019, 122, 110–119. [Google Scholar] [CrossRef]
- Faria, R.R.; Ferrero, V.; Navarro, L.; Araujo, A.C. Flexible mating system in distylous populations of Psychotria carthagenensis Jacq. (Rubiaceae) in Brazilian Cerrado. Plant Syst. Evol. 2012, 298, 619–627. [Google Scholar] [CrossRef]
- Rodrigues, E.B.; Consolaro, H. Atypical distyly in Psychotria goyazensis Mull. Arg. (Rubiaceae), an intramorph self-compatible species. Acta Bot. Bras. 2013, 27, 155–161. [Google Scholar] [CrossRef][Green Version]
- Baker, H.G. Self-compatibility and establishment after “long-distance” dispersal. Evolution 1955, 9, 347–348. [Google Scholar]
- Ågren, J. Population size, pollinator limitation, and seed set in the self-incompatible herb Lythrumsalicaria. Ecology 1996, 77, 1779–1790. [Google Scholar] [CrossRef]
- Smith, J.R.; Hough-Goldstein, J.; Lake, E.C. Variable Seed Viability of Mile-a-Minute Weed (Devil’s Tearthumb, Persicaria perfoliata). Invasive Plant Sci. Manag. 2014, 7, 107–112. [Google Scholar] [CrossRef]
- Momose, K.; Inoue, T. Pollination and factors limiting fruit set of chasmogamous flowers of an amphicarpic annual, Polygonum thunbergii (Polygonaceae). Res. Popul. Ecol. 1993, 35, 79–93. [Google Scholar] [CrossRef]
- Diggle, P.K.; Meixner, M.A.; Carroll, A.B.; Aschwanden, C.F. Barriers to sexual reproduction in Polygonum viviparum: A comparative developmental analysis of P. viviparum and P. bistortoides. Ann. Bot. 2002, 89, 145–156. [Google Scholar] [CrossRef]
- Barrett, S.C.H. The reproductive biology and genetics of island plants. Philos. Trans. R. Soc. Lond. B 1996, 351, 725–733. [Google Scholar]
- Sculthorpe, C.D. The Biology of Aquatic Vascular Plants; Edward Arnold: London, UK, 1967. [Google Scholar]
- Philbrick, C.T.; Les, D.H. Evolution of aquatic angiosperm reproductive system. BioScience 1996, 46, 815–826. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).