Abstract
Energy infrastructure is expanding at a global scale and can represent a major threat to wildlife populations. Power lines are one of the main sources of human-induced avian mortality due to electrocution or collision, but many species use electricity pylons as a structure for nesting. Pylon nesting results in human-wildlife conflict because it can cause power outages and structural damage to power lines. The white stork (Ciconia ciconia) is a large-size semicolonial species that increasingly nests on pylons, causing growing operational and economic issues to power companies and energy consumers. In this study, the likelihood of problematic pylon use by nesting storks was predicted using a suite of explanatory variables related to the availability of foraging habitat and human disturbance. During a five-year period (2015–2019), we assessed the distribution of stork nests removed from the highly-risky top part of transmission pylons (220–400 kV) by power company technicians in South western Spain. A total of 839 nests were removed from 11% of the transmission pylons (n = 1196) during the study period. Pylon use intensified on pylons located near to landfills, surrounded by high proportion of grassland, and when close to freshwater sources (water body or river) and other occupied pylons. Human disturbance was unlikely to deter storks from using pylons and pylon use increased in urban areas. The approach used here to predict pylon use by nesting birds has applications for both human-wildlife conflict mitigation and conservation purposes where endangered species use human infrastructure. Power companies may use this kind of information to install anti-nesting devices (to reduce power outages and avian mortality or nesting platforms on suitable pylons (to promote pylons use by endangered species), and to account for the likelihood of conflict-prone use of pylons when siting future power lines.
1. Introduction
Human population growth, and the resulting expansion of anthropogenic infrastructure, including roads, utility corridors, buildings and energy facilities, can pose a major threat to wildlife populations and biodiversity [1,2,3], but see [4]. In order to meet the rising energy demands of modern economies there has been a rapid development of infrastructure associated with energy production [5,6,7], much of which is taking place in ecosystems previously unfragmented by human activities [8]. As of 2017, 4.7 and 96 million km of power transmission and distribution lines, respectively, existed in energy grids around the world, which are increasing in size by approximately 4.5% annually [9]. The presence of these structures in the landscape represents a considerable cause of casualty and mortality for a number of flying bird species, many of which are threatened with extinction [10,11,12,13,14], mainly due to electrocution in distribution power lines (20–32 kV) ([13,14,15]) or collision with transmission lines [10,13,16].
Despite high mortality levels, some bird species can benefit from using these structures as perches for hunting activities [17], as well as nesting [18], which often leads to further human-wildlife conflicts, especially in cases where damage is caused to infrastructure [19]. In fact, the use of electricity pylons for nesting in some species has increased in recent years due to the disappearance of natural nesting structures in favorable breeding areas [20], or to the construction of power lines in open habitats that previously lacked nesting structures, such as natural grasslands [21]. Large birds nesting on pylons are at a high risk of electrocution and collisions, particularly young birds during their maiden flights [22,23]. Additionally, there is some evidence that exposure to electromagnetic radiation may reduce breeding success of birds nesting on pylons compared to those that nest on natural structures, such as trees [24,25].
Power outages, operation difficulties during maintenance works, and alteration of pylon stability, are a common consequence of nesting on power lines, resulting in negative economic consequences for both power companies and energy consumers [26,27,28,29]. Outages occur when nesting materials bridge the gap between the conductors or between the conductor and ground pole [30]. Flashovers due to accumulation of excrement on insulating components and fecal streamers during take-off also result in power outages [30,31]. Nests also attract predators to pylons which can cause further damage to infrastructure [31]. In addition to this, utility companies are often liable for the death of birds by electrocution and collision, and may be subject to large fines and legal action as a result [32]. This has driven utility companies to invest in mitigation measures to reduce electrocution, such as elevated perches, the installation of nesting deterrents, or the translocation of existing nests to nesting platforms [13,26,33,34].
Avian conflicts with infrastructure occur at varying intensities across the landscape, understanding the drivers of interactions between birds and energy infrastructure is therefore paramount for both conservation and economic purposes. Power companies may use this information to invest in mitigation measures in areas sensitive to conflicts, as well as planning future infrastructure developments to minimize damage and costs caused to infrastructure by birds. This information can also be used for conservation purposes. For example, aided by the installation of nesting platforms on safe pylons located in suitable habitat, 22% of the breeding populations of osprey (Pandion haliaetus) in Andalusia (Spain) nest on electricity pylons, playing an important role in the efforts to re-establish the species to the Spanish mainland following a 25-year absence of successful breeding pairs [35].
This study focused on the white stork (Ciconia ciconia), a species which frequently nests on power lines throughout their European breeding range [18,20,36]. The Iberian population of white stork has been rapidly increasing since the 1980s. The last national stork census in Spain showed the population had increased from 6700 pairs in 1984 to 32,217 pairs in 2004 [37]. Similar trends have been observed in Portugal, with a population increase of 660% from 1984–2014, equating to 12,000 breeding pairs, 25% of which now nest on electricity pylons [18]. In addition, a significant proportion of the breeding population has become sedentary in the Iberian Peninsula, and the number of storks staying on their nests over winter has been increasing since the 1980s [38]. The observed population growth and change in migratory habits have been attributed to milder winter temperatures, a year-round abundance of food from landfill sites [38], and the spread of the invasive red swamp crayfish (Procambarus clarkii) through wetlands and rice fields, providing a plentiful food resource. Accordingly, pylon use has been found to intensify where these food resources are available in close proximity to the pylon [18].
Consequently, storks and their nests are a considerable cause of power outages in the Iberian Peninsula, accounting for half of all power outages on distribution lines in Portugal [26]. Their nests, with an average height of 60 ± 28 cm, a diameter of 1401 ± 24 cm, and a weight ranging from 70 to nearly 1400 kg [39], may also cause structural instability of pylons by altering their load distribution and aerodynamics [29]. In response to this, efforts have been made by power companies to dissuade storks from nesting on pylons by installing anti-nesting devices, which are usually a metallic structure located on parts of the pylon frequently used by birds for nest construction. Most designs do not completely exclude nest building, and have been found to be more successful at preventing the establishment of new nests than preventing the reconstruction of previously removed nests [29]. However, recent designs that use a micro electroshock discharge system have been found to deter all stork nesting attempts [26].
White stork can build nests on different parts of pylons with variable risk on operational and structural stability. Nests built on the top part above the pylon waist (e.g., cross-arms, beam, the top-central tower) pose a high risk on sensible parts of pylons, and are thus assessed as problematic and subject to regular intervention (e.g., removal) by power companies. Predicting where nesting is likely to produce potential service alterations may therefore be useful for power companies in implementing mitigation (e.g., anti-nesting devices) before colonization of existing pylons, or to plan optimal power line layouts, and thus reduce the economic burden caused by nesting white storks. The aims of this study were: (i) to characterize the spatial distribution of white stork problematic nests on part of the electricity transmission system of southwestern Spain; (ii) to identify important environmental, ecological and human drivers of use of problematic pylons by nesting white storks; and (iii) to develop a statistical model that can be used by power companies to predict the likelihood of intervention on pylons used by white storks, and the abundance of problematic nests on pylons.
2. Materials and Methods
2.1. Study Area and Data Source
A total of 475 km of power lines and 1196 electricity pylons of the transmission network (220 and 400 kV) in the Spanish provinces of Huelva and Seville (Figure 1) were assessed for the occurrence of white stork nests between the years 2015 and 2019. The data are from nests removed from the top part of pylons by power company technicians and evaluated as potentially problematic due to a perceived risk of structural damage (e.g., high number of nests) or risk of power outage where nests are close to electrical components. During the years 2016–2019, the number of nests removed per pylon was also noted, but for 2015 only the occurrence of nest removal was available. Nests built on non-conflicting parts below the pylon waist may be left on pylons due to lower perceived risk, and therefore would not have been included in this data. Removed nests and pylons from which one or more nests were removed during the study period are hereafter referred to as problematic nests and problematic pylons, respectively.
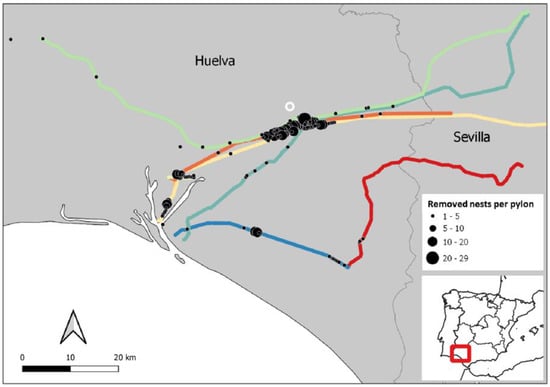
Figure 1.
Geographical location of the 475 km of eight transmission powerlines (220–400 kV; color lines) included in this study, and number of nests removed per intervened pylons (black dots) of the lines during the period 2016–2019, in the Spanish provinces of Huelva and Seville (Spain). Location of the Buenavista landfill is also included (white circle).
Exact locations of all pylons were incorporated into a geographical information system [40] and were used to derive a suite of environmental predictors of problematic occupation of pylons and abundance of removed nests. Predictors were related to foraging habitats and human disturbance, according to relevant scientific literature. Two variables expressing distance to important foraging areas were assessed: distance to landfill (km) and distance to freshwater marshland (km). All freshwater marshes that occurred in the study area were >20 ha in area. Salt marshes and sea shores were not included as they are not relevant to breeding white storks [41]. Rice fields have been identified as important foraging habitat for white storks, but were scarce in the study area and did not occur within the foraging range of breeding storks from the nest (≤5 km [38,42]). For these reasons, this variable was not included as a candidate predictor in modelling.
In order to assess the proportion of suitable habitat surrounding the pylons, we calculated the proportional area of foraging habitats and the average value of night-time light intensity within 1 km radius buffers centered on pylons (n = 1196). The buffer radius of 1 km was chosen to reflect the core foraging range of this species around the nest, which averages 1–2 km [38,42,43]. Habitat categories were derived from the Corine Land Cover (CLC) inventory for 2018 (available at: https://land.copernicus.eu/pan-european/corine-land-cover; accessed on 15 April 2021). The 44 land use classes available were pooled to create 9 classes of potentially suitable foraging habitat for white storks (Table 1) and one class of unsuitable habitat, which was excluded from analyses. An inclusion criterion of 10 or more intervened pylons (i.e., nest removal) within each land use/cover type considered was used to exclude habitats poorly represented in the study area and unlikely to be meaningful in later analyses. Four variables that expressed the availability of suitable foraging habitat were finally included in the analyses (Table 1). The occurrence of freshwater (rivers and water bodies) in the buffer was also recorded, since white storks are a species closely linked to the presence of rivers, lakes, and ponds [44,45].

Table 1.
List of candidate predictor variables used to model the distribution of white stork (Ciconia ciconia) problematic nests on transmission pylons in the Spanish provinces of Huelva and Seville. Mean distances shown here are calculated from the pylon to the nearest boundary of the specified habitat, and habitat proportions represent the relative amount of habitat within 1 km radius buffers centred on the pylons.
The nighttime light intensity was used as a proxy variable to express human disturbance around the pylons. This variable represents the mean light intensity value of pixels (15 arc second resolution) which intersected the buffers and has been described as a good indicator of human activity and infrastructures [46,47]. Night-time lights were sourced from weather satellite recordings processed by the National Oceanographic and Atmospheric Administration’s (NOAA) Earth Observations Group (EOG) as an annual, cloud free, composite of radiance values for the year of 2016 [48]. Pylon features and the presence of anti-nesting devices were not included as this information was only available for a limited number of pylons.
2.2. Data Analysis
We modeled the effect of habitat and human predictors, estimated on 1 km buffers around pylons, on the probability of occurrence of problematic pylons and abundance of problematic nests per pylon (n = 1196) of white storks. For all models, collinearity between predictor variables was examined using variance inflation factors (VIFs > 3) and Pearson’s correlation coefficients (r > 0.7). Spatial autocorrelation in the response variables and model residuals was assessed using Moran’s Index (Moran’s I) with a Monte-Carlo simulation to derive p values using the spdep package in R studio [49].
We first ran a Generalized Linear Model (GLM) with binomial errors distribution to model the presence/absence of problematic nests on pylons as the response binary variable. This model was expected to be highly affected by spatial autocorrelation (SAC) due to the overlapping of 1 km radius buffers around pylons and the spatial aggregation of occupied pylons (average inter-pylon distance of 356 m). The Moran’s I test showed a significant SAC in model residuals, so a residual autocovariate (RAC) term with a neighborhood distance of 1 km was added to account for SAC in the model [50]. In comparison to the standard autologistic approach, the RAC method allows explanatory variables to be fitted and account for some of the SAC that exists in the dependent variable before the autocovariate is derived from model residuals [50]. Both the standard autologistic approach and the RAC method were compared, and in all cases the inclusion of a RAC term in the model resulted in improved model fit and reduced Moran’s I compared to the standard autologistic approach.
Next, we used a GLM with a negative binomial distribution to model the abundance of problematic nests removed from pylons between 2016 and 2019, hereafter referred to as nest abundance. This variable was over dispersed (i.e., zero inflated) and therefore did not fit a Poisson distribution. SAC was also present in nest abundance and remained in model residuals after the model was fitted. To account for this, a RAC with a 1 km spatial neighborhood size was again included in this model.
2.3. Model Selection
Model selection was performed using a backwards stepwise regression procedure based on AIC values. Starting with the full model, candidate predictors were iteratively removed according to their relative contribution to the model AIC, until the final model with the lowest AIC was achieved. The variable P_grassland was arcsine-transformed in all models to avoid effect size overestimation due to large variance. The variables P_non_irrigated_arable, Dist_wetland_km, and P_perm_irrigated_crops were dropped during the model selection process.
The amount of deviance explained by each model was assessed by calculating the adjusted D2 with the mod EvA package in R studio [51,52], which can be considered as an analogue to R2 in linear regression [53].
3. Results
A total of 134 (11.2%) of the 1196 pylons in the study had one or more white stork nests removed between 2015–2019, and a total of 839 nests were removed between 2016 and 2019 (number of removed nests was not available for 2015). The percentage of problematic pylons intervened annually (2015–2019) ranged from 6–8% (M ± SD = 7% ± 0.7). Removed nests from problematic pylons ranged from 1–9 (M ± SD = 2.4 ± 0.25). Problematic pylons were mostly spatially aggregated (Figure 1 and Figure 2), showing a positive spatial autocorrelation in the occurrence of problematic nests on pylons up to 12 km, with the highest values occurring up to 5 km. A similar pattern was observed for the number of nests removed per pylon, with positive spatial autocorrelation occurring up to 9 km (Figure 2).
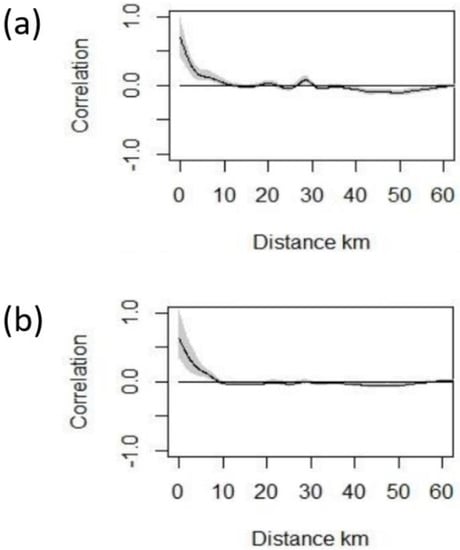
Figure 2.
Spline correlograms of spatial autocorrelation (SAC) based on Moran’ I statistic for: (a) presence/absence of problematic transmission pylons and (b) abundance of problematic nests on transmission pylons, in the provinces of Huelva and Seville (southern Spain) for the period 2015–2019. Moran’s I informs on the distribution pattern of observations and ranges from −1 (regular) to +1 (complete clustered), with value 0 showing random distribution.
3.1. Predictors of Occurrence of Problematic Pylons
The probability of a transmission pylon being occupied by at least one problematic nest of white storks was found to increase with increasing proportion of grassland, the presence of freshwater (waterbody or river), increasing night-time light intensity, and with decreasing distance to a landfill site (Table 2). Significant positive spatial autocorrelation was present in model residuals due to the strong attraction of storks to pylons already occupied, or near other stork nests, even after accounting for habitat variables. The inclusion of a RAC term to account for SAC resulted in improved model fit, with an increase of 34% in explained model deviance. The RAC term was actually the most significant predictor in the models where it was included.

Table 2.
Results of reduced binomial and negative binomial GLMs modeling the probability of transmission pylons being occupied by problematic white stork (Ciconia ciconia) nests and abundance of problematic nests, respectively, in the study area (southwestern Spain). Both models are based on individual towers with 1 km radius buffers (n = 1196) plus a residual auto covariate RAC term with a 1 km spatial neighborhood size. Coefficient estimates (β) ± standard error (SE), and odds ratios (OR) are reported for reduced model with the lowest AIC score. Significant terms are highlighted: * p < 0.05, ** p < 0.01, *** p < 0.001.
Pylon occupation with problematic nests was only intense around one of the two landfill sites present in the study area (Figure 1). The predicted probabilities showed that even when close to a landfill site, the probability of occurrence of problematic nests on a pylon was still dependent on the land cover within the 1 km buffer area around the pylon.
3.2. Drivers of Abundance of Problematic Nests
The number of nests removed from pylons between 2016 and 2019 increased with increasing proportion of grassland, increased night-time light intensity, and decreasing distance to a landfill site. Number of nests removed per pylons also decreased when there was a high proportion of agroforestry or permanently irrigated agricultural land in the 1 km radius buffer (Table 2). Inclusion of the RAC resulted in a 36% increase in the amount of deviance explained by the model. However, spatial autocorrelation was still present in the model residuals, showing that even after the explanatory variables were accounted for the storks chose to nest on pylons that already contained other problematic nests.
Unlike the occurrence models, the presence of freshwater (water body or river) in the 1 km buffer was not an important predictor of nest abundance. Mean night-time light intensity and distance to landfill remained significant, and grassland was the only natural foraging habitat which positively influenced nest abundance, showing that overall, storks nested in higher densities close to anthropogenic habitats, and areas with higher levels of human activity for feeding, which may be linked to availability of food from human waste in urban areas.
4. Discussion
This study builds upon previous research on the drivers of pylon use by white storks in the Iberian Peninsula in demonstrating the role of foraging habitat availability in nest site selection by breeding birds. In addition, the influence of human disturbance on pylon occupation was illustrated. With increased amounts of urbanization and growing human populations, it is important to consider the implications of human disturbance in species distribution models. The findings of this study have particular implications for reducing the economic burden of pylon use by nesting birds in Southern Spain, but the same approach is applicable to other areas, and for use in the conservation of endangered species that use human infrastructures.
4.1. Spatial Distribution of Pylon Use
The percentage of occupied pylons with problematic nests (11% from 2015–2019, annual mean of 7%) was higher than the pylon occupation rate reported in a previous study in Spain, where 5% of 4366 pylons surveyed in the north western Spanish plateau contained white stork nests [54]. We can consider this observed increase as conservative since values reported in this study are likely underestimated, as not all nests were removed by the power company and therefore some occupied pylons were not included in the data. In this sense, the level of pylon occupancy found in a subsequent study carried out in western Andalusia in 2014 was of 11% [55]. This increase is consistent with the proportion of problematic pylons intervened in the present study and could be the result of a regional abundance in white storks, but also reflects the increasing population levels and increasing proportion of white stork pairs that nest on transmission pylons in the Iberian Peninsula [18].
The majority of problematic pylons in this study were spatially aggregated (Figure 1), which is partly a result of storks choosing nest sites close to favorable foraging habitats [18,56,57]. However, spatial autocorrelation was still detected in the model residuals after habitat variables had been accounted for. This shows the importance of preexisting nests as an important social driver of pylon use due to the semi-colonial breeding behavior of this species in southwestern Europe [58,59,60]. White storks exhibit also high levels of philopatry [61], and thus subsequent generations returning to their natal territories to breed may also play a role in the formation of large aggregations of nests on sections of power line, even long after the pylons are first colonized.
4.2. Environmental/Ecological Drivers of Pylon Nesting
Landfill, grassland, and fresh water (water body or river) were the most important foraging habitats that positively influenced pylon occupation by problematic nests of white storks. Other habitat types that have previously been identified as preferred foraging habitat—such as agroforestry, irrigated crops, non-irrigated arable, and wetlands [18,42,56]—were not significant in this study, or were negatively associated with the presence of problematic pylons and abundance of problematic nests (Table 2). This may be the result of a strong reliance on landfill for feeding, since pairs nesting close to a landfill site are reported to have smaller non-landfill foraging ranges and show a higher dependence on landfill for feeding, travelling up to 28 km to feed at a landfill during the breeding season [38]. Of all problematic pylons managed during the study period, 75% were located within 28 km of a landfill, which may explain the lower importance of some non-landfill foraging habitats considered. The abundance of nests removed between 2016 and 2019 was also significantly higher near landfill, with 59% more nests on pylons within 28 km of a landfill than those farther away. This could also explain why the abundance of removed nests decreased when the proportion of agroforestry increased, as this habitat was mostly distributed far from the landfill site used by storks. Pylon intervention did not occur in equal intensity at both landfill sites present in the study area, and predicted probabilities of problematic pylons were varied for pylons close to a landfill, particularly Burguillos (Figure 1). Most of the non-landfill foraging of storks nesting close to landfill takes place in close proximity to the nest [38]. Therefore, land cover surrounding pylons remains an important driver of pylon use, even when pylons are close to landfill. In any case, the results showed the important role of landfills for nesting site selection in the white stork, which is consistent with previous studies, e.g., [62,63,64].
The variable that represented permanently irrigated agricultural land (P_perm_irrigated_crops) was associated with large areas of polytunnel and fruit crops that were present in parts of study area (pers.obs.), which have not been identified as suitable foraging habitat for white storks. Additionally, the likelihood of nest site use by white stork has been reported to decrease in areas of intensive and permanent agriculture [56]. The negative effect of this variable on abundance of intervened nests could therefore reflect the lack of suitability of this habitat for breeding storks.
White storks use a variety of fresh water habitats, where they feed on amphibians, fish, and aquatic invertebrates [65]. Accordingly, the presence of water bodies and other freshwater habitats, such as wetlands, have been recognized as important drivers of nest site selection in previous studies [18,56,61,66]. In this study, the significant effect of water bodies and/or rivers within the buffer area may have been associated with the presence of areas of shallow water and small wetlands, where preys are more accessible to storks and where the invasive red-clawed crayfish is abundant, which can make up as much as 80% of the prey items in the stork’s diet [67]. However, this variable was not a significant driver of abundance of problematic nests removed, suggesting that storks selected pylons close to water but did not form large aggregations around this habitat.
Grasslands, such as meadows and pastures, are one of the principal habitats used by this species for foraging [68,69,70], and several studies identify proximity to grassland as among the most important habitat drivers of nest site selection [42,45,66,71]. In Spain storks have been shown to selectively use tall grass pasture over other available habitats such as cereal sown land, and wooded areas, due to comparatively high densities of arthropod prey found in this habitat [42]. In the same study, storks only visited arable land sporadically, usually after ploughing, which might explain the lack of importance of the non-irrigated arable variable in this study. In northern France the proportion of grasslands had the greatest positive effect on storks occupying a nest site, and occupancy was highest when more than 50% the habitat within a 2 km radius of the nest site was grassland [56]. These findings are consistent with the use of grassland in this study, where pylons with a high proportion of grassland were more likely to be occupied with problematic nests, and more likely to contain multiple problematic nests.
The night-time light intensity variable was not strongly correlated with any other variable included in the models and therefore largely reflected urban areas, where night-time light intensity is highest [46,47]. This proxy variable was included to assess the potential impacts of human disturbance from urban areas and other areas of intense human activity on the presence of problematic pylons, which increased with increasing night-time light intensity. Breeding storks may therefore be undeterred by human disturbance and favor pylons close to urban areas. This species frequently nests in close proximity to humans in towns and cities, where they are attracted by availability of nest structures and food from urban waste [36,68]. There is only limited evidence to support lower occupancy of nest sites near urban areas and roads [56], despite a potential reduction in breeding performance due to human disturbance [58].
Alternatively, storks may be attracted to nocturnal lighting. Although they are a diurnal species that forages almost exclusively during the day time, there are some records of nocturnal feeding, especially on moonlit nights [41,72]. In Poland, in 2004 storks were recorded for the first time foraging close to artificial lighting at night, catching insects under streetlamps in an urban environment [73]. It is therefore possible that storks were attracted to artificial lights for nocturnal feeding. However, there is currently no record of this behavior in Spain in the literature, and it is unlikely to be an important factor in nest site selection when other abundant food resources are available.
4.3. Potential Limitations
The main source of bias in this study was that pylon use was represented by the removal of nests on sensitive structures of occupied pylons, instead of a complete census of the transmission system. Consequently, pylon use is likely to be underestimated as some occupied pylons without problematic nests were not recorded, and some non-problematic nests were left on intervened pylons. As a result, some habitat drivers that may trigger early pylon use at low intensities during colonization might not be represented in the model, and the expected attraction effect by non-removed nests was not fully accounted for. Nonetheless, nest removal occurs on sections of lines occupied by storks, and therefore the spatial distribution of removed nests closely reflects the spatial distribution of nests on the transmission system, particularly where pylon use is most intense. The same applies to nest abundance, so pylons that had more nests removed from them are likely to have contained more nests. This information is still valuable to the power company as areas of higher abundance of problematic nests are where structural damage and outages are most likely to occur.
Pylon structural features and the presence of anti-nesting devices on pylons were not included due to lack of available information for all pylons. Studies have shown that pylon use varies between pylons with different structural design [18,54]. However, structural features have a relatively low importance in predicting pylon use compared to land cover [18].
Habitat selection by storks is influenced by regional population density. Habitats which are initially less favorable to storks during the colonization process may be selected more frequently as density increases [56]. Pylon use has also been reported to increase at very high population densities, probably due to increased competition for nest sites [18]. In this study, information regarding actual population density or non-pylon nests was not available. However, the social effect of occupied pylons was considered in the study, and only a weak influence of population density on pylon use has been reported [18].
Finally, a static land cover map from the year 2018 was used to characterize the distribution of suitable foraging habitats. Land cover categories in this database are broadly classified and may not capture potentially important variations within a given habitat type. For example, not all grassland habitats are of equal importance to white storks, and preferences may vary depending on agricultural practices, the presence of grazing animals, or the amount of flooding [42,69,74]. However, the intention of this study was to provide a generalizable method of identifying habitat drivers of conflict-prone use of pylons at a landscape scale, by a species which utilizes relatively large areas and range habitats for foraging.
5. Conclusions
This study demonstrated that the availability of foraging habitat, social attraction, and human activity are important factors that play a part in the occupation pattern of pylons by nesting birds, which pose a risk to the operation, maintenance, and structural stability of pylons. This information can therefore be used to reduce human-wildlife conflicts on energy infrastructure by reducing associated costs and disturbances to customers and companies, as well as promoting conservation by: (1) Identifying existing pylons with a high likelihood of problematic use by nesting birds to pre-emptively install anti-nesting devices; (2) using predictive models during planning stages to identify areas to site new power lines that minimize potential human-wildlife conflicts; and (3) installing nesting platforms/boxes to promote nesting in areas of suitable habitat for species of conservation value. Promoting pylon use for nesting may increase the risk of electrocution and collision, particularly in areas of suitable habitat [75], so further mitigation should be taken on these sections of power lines to make pylon use safer. In conclusion, the approaches used here provided information appropriate for mitigating the effects of pylon nesting by white storks in southwestern Spain, particularly on pylons close to landfills, with a high proportion of grassland in the surroundings, near to water bodies, close to urban areas, and on previously occupied line sections. This same approach may be adapted to a plethora of different bird species that nest on human infrastructures around the world. Minimizing wildlife conflicts with energy infrastructure is essential for both the economic development of an expanding human population, and to safeguard the future of declining species living alongside infrastructures, in the face of growing human pressures.
Author Contributions
Conceptualization, E.M.B., M.F. and R.M.; methodology, E.M.B. and R.M.; formal analysis, E.M.B., R.M.; investigation, R.M. and M.F.; data curation, R.M.; writing—original draft preparation, E.M.B.; writing—review and editing, R.M., M.K., V.M. and M.F.; supervision, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Red Eléctrica de España (REE) through a technical supporting project. CSIC project reference #060401190046.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank A. Franco of the School of Environmental Sciences, University of East Anglia for her academic support and advice throughout the manuscript. To Red Eléctrica de España, for collecting information on nest removal and information about their pylons.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sanderson, E.W.; Jaiteh, M.; Levy, M.A.; Redford, K.H.; Wannebo, A.V.; Woolmer, G. The Human Footprint and the Last of the Wild. BioScience 2002, 52, 891. [Google Scholar] [CrossRef]
- Butchart, S.H.M. Global Biodiversity: Indicators of recent decline. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Hovick, T.J.; Elmore, R.D.; Dahlgren, D.K.; Fuhlendorf, S.D.; Engle, D.M.; Hill, M. Evidence of negative effects of anthropogenic structures on wildlife: A review of grouse survival and behavior. J. Appl. Ecol. 2014, 51, 1680–1689. [Google Scholar] [CrossRef]
- Ferrer, M.; De Lucas, M.; Hinojosa, E.; Morandini, V. Transporting Biodiversity Using Transmission Power Lines as Stepping-Stones? Diversity 2020, 12, 439. [Google Scholar] [CrossRef]
- De Lucas, M.; Janss, G.; Whitfield, P.; Ferrer, M. Collision fatality of raptors in wind farms does not depend on raptor abundance. J. Appl. Ecol. 2008, 45, 1695–1703. [Google Scholar] [CrossRef]
- Martins, R.C.; Ascensa, F.; Amico, M.D. Bird.On the wire: Landscape planning considering costs and benefits for bird populations coexisting with power lines. Ambio 2018, 47, 650–656. [Google Scholar] [CrossRef]
- Ferrer, M.; Morandini, V.; Baumbush, R.; Muriel, R.; De Lucas, M.; Calabuig, C. Efficacy of different types of “bird flight diverter” in reducing bird mortality due to collision with transmission power lines. Glob. Ecol. Conserv. 2020, 23, e01130. [Google Scholar] [CrossRef]
- Lior, N. Energy resources and use: The present situation and possible paths to the future. Energy 2008, 33, 842–857. [Google Scholar] [CrossRef]
- Kalt, G.; Thunshirn, P.; Haberl, H. A global inventory of electricity infrastructures from 1980 to 2017: Country-level data on power plants, grids and transformers. Data Brief 2021, 38, e107351. [Google Scholar] [CrossRef]
- Jenkins, A.R.; Smallie, J.J.; Diamond, M. Avian collisions with power lines: A global review of causes and mitigation with a South African perspective. Bird Conserv. Int. 2010, 20, 263–278. [Google Scholar] [CrossRef]
- Ferrer, M.; Hiraldo, F. Man-induced sex-biased mortality in the Spanish imperial eagle. Biol. Conserv. 1992, 60, 57–60. [Google Scholar] [CrossRef]
- Ferrer, M.; Janss, G. Birds and Power Lines; Editorial Quercus: Madrid, Spain, 1999. [Google Scholar]
- Ferrer, M. Birds and Power Lines: From Conflict to the Solution; Migres Foundation: Seville, Spain, 2012. [Google Scholar]
- Ferrer, M.; De La Riva, M.; Castroviejo, J. Electrocution of raptors on power lines in Southern Spain. J. Field Ornithol. 1991, 62, 181–190. [Google Scholar]
- Bevanger, K. Biological and conservation aspects of bird mortality caused by electricity power lines: A review. Biol. Conserv. 1989, 86, 67–76. [Google Scholar] [CrossRef]
- Silva, J.P.; Manuel, J.; Alcazar, R.; Correia, R.; Delgado, A.; Moreira, F. A spatially explicit approach to assess the collision risk between birds and overhead power lines: A case study with the little bustard. Biol. Conserv. 2014, 170, 256–263. [Google Scholar] [CrossRef]
- Morelli, F.; Beim, M.; Jerzak, L.; Jones, D.; Tryjanowski, P. Can roads, railways and related structures have positive effects on birds?—A review. Transp. Res. Part D Transp. Environ. 2014, 30, 21–31. [Google Scholar] [CrossRef]
- Moreira, F.; Martins, R.C.; Catry, I.; D’Amico, M. Drivers of power line use by white storks: A case study of birds nesting on anthropogenic structures. J. Appl. Ecol. 2018, 55, 2263–2273. [Google Scholar] [CrossRef]
- Mainwaring, M.C. The use of man-made structures as nesting sites by birds: A review of the costs and benefits. J. Nat. Conserv. 2015, 25, 17–22. [Google Scholar] [CrossRef]
- Janiszewski, T.; Minias, P.; Wojciechowski, Z. Selective forces responsible for transition to nesting on electricity poles in the white stork Ciconia ciconia. Ardea 2015, 103, 39–50. [Google Scholar] [CrossRef]
- Steenhof, K.; Kochert, M.N.; Roppe, J.A. Nesting by raptors and common ravens on electricity transmission line towers. J. Wildl. Manag. 1993, 57, 271–281. [Google Scholar] [CrossRef]
- Jakubiec, Z. Causes of breeding losses and adult mortality in white stork Ciconia ciconia in Poland. Stud. Nat. 1991, 37, 107–124. [Google Scholar]
- Garrido, J.R.; Fernández-Cruz, M. Effects of power lines on a white stork Ciconia ciconia population in Central Spain. Ardeola 2003, 50, 191–200. [Google Scholar]
- Balmori, A. Possible Effects of Electromagnetic Fields from Phone Masts on a Population of White Stork (Ciconia ciconia). Electromagn. Biol. Med. 2005, 24, 109–119. [Google Scholar] [CrossRef]
- Vaitkuvienė, D.; Dagys, M. Possible effects of electromagnetic field on White Storks Ciconia ciconia breeding on low-voltage electricity line poles. Zool. Ecol. 2014, 24, 289–296. [Google Scholar] [CrossRef]
- Maricato, L.; Faria, R.; Madeira, V.; Carreira, P.; De Almeida, A.T. White stork risk mitigation in high voltage electric distribution networks. Ecol. Eng. 2016, 91, 212–220. [Google Scholar] [CrossRef]
- Newman, J.; Newman, C.; Lindsay, J.; Merchant, B.; Avery, M.; Pruett-Jones, S. Monk parakeets: An expanding problem on power lines and other electrical utility structures. In Environment Concerns in Rights-of-Way Management 8th International Symposium; Goodrich-Mahoney, J.W., Abrahamson, L., Ballard, J., Tikalsky, S., Eds.; Elsevier: New York, NY, USA, 2004; pp. 355–364. [Google Scholar]
- McIvor, G.E.; Rowe, C.; Healy, S.D. Deterring hooded crows from re-nesting on power poles. Wildl. Soc. Bull. 2012, 36, 729–734. [Google Scholar] [CrossRef]
- Red Eléctrica de España. Red Eléctrica and Birdlife: 15 Years of Applied Research; REE, S.A.: Madrid, Spain, 2005. [Google Scholar]
- Harness, R.E. Raptor Nest Management on Power Lines. In Proceedings of the Fifth IASTED International Conference on Power and Energy Systems, Benalmádena, Spain, 15–17 June 2005; pp. 534–538. [Google Scholar]
- Polat, Ö. An overview of bird related issues in electrical power systems. IOP Conf. Ser. Mater. Sci. Eng. 2016, 161, 012091. [Google Scholar] [CrossRef]
- Burnham, J.; Carlton, R.; Cherney, E.; Couret, G.; Eldridge, K.; Farzaneh, M.; Frazier, S.; Gorur, R.; Harness, R.; Shaffner, D.; et al. Preventive Measures to Reduce Bird-Related Power Outages—Part I: Electrocution and Collision. IEEE Trans. Power Deliv. 2004, 19, 1843–1847. [Google Scholar] [CrossRef]
- Kaluga, I.; Sparks, T.H.; Tryjanowski, P. Reducing death by electrocution of the white stork Ciconia ciconia. Conserv. Lett. 2011, 4, 483–487. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Kosicki, J.Z.; Kuźniak, S.; Sparks, T.H. Long-term changes and breeding success in relation to nesting structures used by the White Stork, Ciconia ciconia. Ann. Zool. Fenn. 2009, 46, 34–38. [Google Scholar] [CrossRef]
- Muriel, R.; Ferrer, M.; Casado, E.; Calabuig, C. First successful breeding of reintroduced ospreys Pandion haliaetus in mainland Spain. Ardeola 2010, 57, 175–180. [Google Scholar]
- Gyalus, A.; Végvári, Z.; Csörgő, T. Changes in the nest sites of white stork (Ciconia ciconia) in Hungary. Ornis Hung. 2018, 26, 65–88. [Google Scholar] [CrossRef]
- Molina, B.; Del Moral, J.C. La Cigüeña Blanca en España. VI Censo Internacional; SEO/BirdLife: Madrid, Spain, 2005. [Google Scholar]
- Gilbert, N.I.; Correia, R.A.; Silva, J.P.; Pacheco, C.; Catry, I.; Atkinson, P.W.; Gill, J.A.; Franco, A.M.A. Are white storks addicted to junk food? Impacts of landfill use on the movement and behaviour of resident white storks (Ciconia ciconia) from a partially migratory population. Mov. Ecol. 2015, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zbyryt, A.; Dylewski, L.; Neubauer, G. Mass of white stork nests predicted from their size: Online calculator and implications for conservation. J. Nat. Conserv. 2021, 60, 125967. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS 3.14.0 Geographic Information System; Open Source Geospatial Foundation: Beaverton, OR, USA, 2020. [Google Scholar]
- Cramp, S.; Simmons, K.E. Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1977; Volume I. [Google Scholar]
- Alonso, J.C.; Alonso, J.A.; Carrascal, L.M. Habitat selection by foraging white storks, Ciconia ciconia, during the breeding season. Can. J. Zool. 1991, 69, 1957–1962. [Google Scholar] [CrossRef]
- Johst, K.; Brandl, R.; Pfeifer, R. Foraging in a patchy and dynamic landscape: Human land use and the White Stork. Ecol. Appl. 2001, 11, 60–69. [Google Scholar] [CrossRef]
- Lázaro, E.; Chozas, P.; Fernández-Cruz, M. Demografia de la Cigüeña Blanca (Ciconia ciconia) en España. Censo Nacional de 1984. Ardeola 1986, 33, 131–169. [Google Scholar]
- Carrascal, L.M.; Bautista, L.M.; Lázaro, E. Geographical variation in the density of the white stork Ciconia ciconia in Spain: Influence of habitat structure and climate. Biol. Conserv. 1993, 65, 83–87. [Google Scholar] [CrossRef]
- Bruederle, A.; Hodler, R. Nighttime lights as a proxy for human development at the local level. PLoS ONE 2018, 13, e0202231. [Google Scholar] [CrossRef]
- Shi, K.; Huang, C.; Chen, Y.; Li, L. Remotely sensed nighttime lights reveal increasing human activities in protected areas of China mainland. Remote Sens. Lett. 2018, 9, 467–476. [Google Scholar] [CrossRef]
- National Centers for Environmental Information Version 1 VIIRS Day/Night Band Nighttime Lights. Available online: https://ngdc.noaa.gov/eog/viirs/download_dnb_composites.html (accessed on 22 April 2020).
- Bivand, R.S.; Wong, D. Comparing implementations of global and local indicators of spatial association. TEST 2018, 27, 716–748. [Google Scholar] [CrossRef]
- Crase, B.; Liedloff, A.C.; Wintle, B.A. A new method for dealing with residual spatial autocorrelation in species distribution models. Ecography 2012, 35, 879–888. [Google Scholar] [CrossRef]
- Barbosa, M.A.; Real, R.; Muñoz, A.; Brown, J. New measures for assessing model equilibrium and prediction mismatch in species distribution models. Divers. Distrib. 2013, 19, 1333–1338. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Infante, O.; Peris, S. Bird nesting on electric power supports in northwestern Spain. Ecol. Eng. 2003, 20, 321–326. [Google Scholar] [CrossRef]
- Lazo, A.; Castelló, A.; Braña, S.; Conde, J.L. Análisis de los Resultados del Seguimiento de la Nidificación de Cigüeñas Blancas y Eficacia de los Dispositivos Disuasores Instalados en Torres de Transporte de Electricidad de Red Eléctrica de España Entre 1999 y 2014; Red Eléctrica de España: Madrid, Spain, 2016. [Google Scholar]
- Gadenne, H.; Cornulier, T.; Eraud, C.; Barbraud, J.C.; Barbraud, C. Evidence for density-dependent habitat occupancy at varying scales in an expanding bird population. Popul. Ecol. 2014, 56, 493–506. [Google Scholar] [CrossRef]
- Bachir, A.S.; Chenchouni, H.; Djeddou, N.; Barbraud, C.; Céréghino, R.; Santoul, F. Using self-organizing maps to investigate environmental factors regulating colony size and breeding success of the White Stork (Ciconia ciconia). J. Ornithol. 2013, 154, 481–489. [Google Scholar] [CrossRef]
- Vergara, P.; Aguirre, J.I. Age and breeding success related to nest position in a White stork Ciconia ciconia colony. Acta Oecol. 2006, 30, 414–418. [Google Scholar] [CrossRef]
- Djerdali, S.; Guerrero-Casado, J.; Tortosa, F.S. The effects of colony size interacting with extra food supply on the breeding success of the White Stork (Ciconia ciconia). J. Ornithol. 2016, 157, 941–947. [Google Scholar] [CrossRef]
- Zbyryt, A.; Sparks, T.H.; Tryjanowski, P. Whitewashing improves relocated nest occupancy in the white stork: An experimental test of public information. J. Nat. Conserv. 2021, 59, 125929. [Google Scholar] [CrossRef]
- Cuadrado, M.; Sánchez, I.; Barcell, M.; Armario, M. Reproductive data and analysis of recoveries in a population of white stork Ciconia ciconia in southern Spain: A 24-year study. Anim. Biodivers. Conserv. 2016, 39, 37–44. [Google Scholar] [CrossRef]
- Bialas, J.T.; Dylewski, Ł.; Tobolka, M. Determination of nest occupation and breeding effect of the white stork by human-mediated landscape in Western Poland. Environ. Sci. Pollut. Res. 2020, 27, 4148–4158. [Google Scholar] [CrossRef] [PubMed]
- Bialas, J.T.; Dylewski, Ł.; Dylik, A.; Janiszewski, T.; Kaługa, I.; Królak, T.; Kruszyk, R.; Pawlukojć, K.; Pestka, Z.; Polakowski, M.; et al. Impact of land cover and landfills on the breeding effect and nest occupancy of the white stork in Poland. Sci. Rep. 2021, 11, 7279. [Google Scholar] [CrossRef] [PubMed]
- Benharzallah, N.; Bachir, A.S.; Barbraud, C. Nest characteristics and food supply affect reproductive output of white storks Ciconia ciconia in semi-arid areas. Biologia 2022, 77, 997–1006. [Google Scholar] [CrossRef]
- Tsachalidis, E.P.; Goutner, V. Diet of the White Stork in Greece in Relation to Habitat. Waterbirds 2002, 25, 417–423. [Google Scholar] [CrossRef]
- Latus, C.; Kujawa, K.; Glemnitz, M. The influence of landscape structure on White Stork’s Ciconia ciconia nest distribution. Acta Ornithol. 2000, 35, 97–102. [Google Scholar]
- Ferreira, E.; Grilo, F.; Mendes, R.; Lourenço, R.; Santos, S.; Petrucci-Fonseca, F. Diet of the White Stork (Ciconia ciconia) in a heterogeneous Mediterranean landscape: The importance of the invasive Red Swamp Crayfish (Procambarus clarkii). Airo 2019, 26, 33–47. [Google Scholar]
- Moritzi, M.; Maumary, L.; Schmid, D.; Steiner, I.; Vallotton, L.; Spaar, R.; Biber, O. Time budget, habitat use and breeding success of White Storks Ciconia ciconia under variable foraging conditions during the breeding season in Switzerland. Ardea 2001, 89, 457–470. [Google Scholar]
- Tryjanowski, P.; Kuzniak, S. Population size and productivity of the White Stork Ciconia ciconia in relation to common vole Microtus arvalis density. Ardea 2002, 90, 213–217. [Google Scholar]
- Kamiński, P.; Jerzak, L.; Kasprzak, M.; Kartanas, E.; Bocheński, M.; Hromada, M.; Baszyński, J.; Kozera, W.; Woźniak, A.; Ulrich, W. Do agricultural environments increase the reproductive success of White Stork Ciconia ciconia populations in South-Western Poland? Sci. Total Environ. 2020, 702, 134503. [Google Scholar] [CrossRef]
- Radović, A.; Tepić, N. Using Corine Land Cover habitat database for the analysis of breeding bird habitat: Case study of white storks (Ciconia ciconia) from northern Croatia. Biologia 2009, 64, 1212–1218. [Google Scholar] [CrossRef]
- Podlaszczuk, M.; Wojciechowski, Z.; Podlaszczuk, P.; Minias, P.; Janiszewski, T.; Wojciechowska, A. Shortening day length as a previously unrecognized selective pressure for early breeding in a bird with long parental care. J. Ornithol. 2014, 156, 389–396. [Google Scholar] [CrossRef]
- Jerzak, L.; Bocheński, M.; Czechowski, P. Unusual feeding behaviour of the White Stork Ciconia ciconia in the Kłopot colony (W Poland). In The White Stork in Poland: Studies in Biology, Ecology and Conservation; Tryjanowski, P., Sparks, T.H., Jerzak, L., Eds.; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2006; pp. 203–207. [Google Scholar]
- Zbyryt, A.; Sparks, T.H.; Tryjanowski, P. Foraging efficiency of white stork Ciconia ciconia significantly increases in pastures containing cows. Acta Oecol. 2020, 104, 103544. [Google Scholar] [CrossRef]
- Janss, G.F.E.; Ferrer, M. Avian electrocution mortality in relation to pole design and adjacent habitat in Spain. Bird Conserv. Int. 2001, 11, 3–12. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).