Abstract
Here we describe a new subgenus and three new species of parasitic water mites in the genus Unionicola (Acari: Hydrachnidia) from Myanmar: Myanmaratax subgen. nov., Unionicola (Myanmaratax) savadiensis subgen. and sp. nov. (hosts: Lamellidens savadiensis and L. generosus), U. (My.) generosa sp. nov. (the same hosts), and U. (My.) trapezidens sp. nov. (hosts: Trapezidens dolichorhynchus and T. angustior). These taxa were identified based on a two-gene phylogenetic analysis (COI + 28S), which also confirms the division of the genus Unionicola into numerous subgenera. The new species are cryptic species, which are morphologically indistinguishable but strongly resemble U. (Prasadatax) brandti Vidrine, 1985 described from Thailand (hosts: Lens spp. and Ensidens spp.). We also transfer the latter taxon from Prasadatax to Myanmaratax based on a set of morphological evidence and propose U. (My.) brandti comb. nov. The new subgenus contains a total of five species, one of which needs future sampling efforts and will be described elsewhere. Additionally, 56 valid subgenera, which were placed in the synonymy of the genus and in one case raised to the genus level, are restored here until robust phylogenetic evidence on their taxonomic status is available. Our results also confirm that Unionicola mites are narrow host specialists that are associated with either one or a few closely related freshwater mussel species belonging to one or two sister genera.
Keywords:
Unionicola; water mites; cryptic species; COI; 28S; Indochina; Myanmar; genetic differences; phylogeny; Unionicolidae 1. Introduction
The genus Unionicola Haldeman, 1842 is a diverse clade of water mites (Acari: Hydrachnidia: Unionicolidae), which contains both parasitic and free-living representatives. The parasitic members of the genus infest freshwater bivalve (Unionoida: Unionidae, Margaritiferidae, Mycetopodidae, Iridinidae, Etheriidae, and Hyriidae) and gastropod (Cyclophoracea: Viviparidae and Pilidae) mollusks and sponges (Demospongiae: Spongillidae) and use chironomid midges (order Diptera: Chironomidae) and caddisflies (order Trichoptera) for resettlement [1,2,3,4,5,6,7]. The Unionicola mites are known to inhabit all continents except Antarctica [7]. A few records of these mites from the freshwater razor clam Novaculina chinensis Liu and Zhang, 1979 (Veneroida: Pharidae) in China [8] need further confirmation, as the sampling locality is rather far from the general range of this mollusk species. This genus comprises 58 subgenera with more than 260 Unionicola species [7,9,10]. Despite this fact, new species are still being described every year from different geographical localities according to the subtle differences in morphology and genetic data [7,11,12,13,14]. Investigation of parasite species richness is a crucial step towards the evaluation of interactions between environment, chironomid and mussel hosts, and parasitic mites. It is clear that all of these factors must be considered when discussing the decline of freshwater bivalves observed in many regions [11]. The actual species richness of these parasitic water mites in the Oriental Region is still poorly known, although several studies have been conducted with morphological descriptions of mites in this genus from the Oriental tropics. Available published data reveal that the mussel-associated mite groups in Southeast and South Asia contain not less than 17 species and 8 subgenera [7]. It is expected that Southeast Asia represents a biodiversity hotspot for these water mites [7]. Moreover, each large river system of this region appears to be a separate evolutionary hotspot with a unique endemic fauna of freshwater mussels, housing a specific assemblage of Unionicola mites and other parasites [7,15,16,17]. Moreover, it was revealed that the unionid faunas of the Salween, Ayeyarwady, and Sittaung rivers are related to each other and should be considered to belong to a separate biogeographic domain, namely the Western Indochina Subregion [16,18,19]. This subregion is separated from the Sundaland Subregion by the Salween—Mekong drainage divide and houses largely endemic faunas of freshwater mussels [16,20,21], Unionicola mites [7], and mussel-associated leeches [17].
The results of recent studies [22,23] revealed a significantly positive relationship between host species richness and mite species richness, adding to a growing body of evidence that host diversity is an important determinant of parasite diversity. It is clear that species-rich freshwater mussel faunas of Southeast Asia house a number of undescribed mite species associated with mussels. For example, a new species of the genus Najadicola, Piersig, 1897, namely N. loeiensis Chapurina, Vikhrev, Kondakov, Tanmuangpak, 2019, was recently discovered from the Loei River, a tributary of the Mekong in Thailand [24]. Furthermore, the discovery of the new subgenus and species, Unionicola (Gibbosulicola) sella Chapurina, Bolotov, Vidrine, Kondakov and Vikhrev, 2021, from the margaritiferid species Gibbosula laosensis (Lea, 1863) (Margaritiferidae: Gibbosulinae) suggests that all six families in the order Unionida are infected with Unionicola mites [7], although the family-group placement of some genera such as Acostaea d’Orbigny, 1851 is questionable [25,26]. Two new free-living species of Unionicola from Thailand were recently described [10], and one parasitic species has been recently described from Myanmar [14]. Our earlier study [7] showed that members of seven subgenera of parasitic Unionicola mites were recorded in Southeast Asia: Dimockatax Vidrine, 1992, Fulleratax Vidrine, 1984, Imamuratax Vidrine, 1994, Pentatax Thor, 1922, Prasadatax Vidrine, 1992, Unionicola Haldeman, 1842 (hosts: Unionidae), and Gibbosulicula Chapurina, Bolotov, Vidrine, Kondakov and Vikhrev, 2021 (hosts: Margaritiferidae) [7]. The subgenus Majumderatax Vidrine, 1993, is a poorly known group of free-living mites but is known to occur in the mantle cavity of Lamellidens sp. from India [27,28]. Most species of Unionicola mites are narrow host specialists, which are known to associate with either one or only a few related species of molluscan host species belonging to one or two sister genera [7,22,29]. This feature could be used to detect cryptic species complexes in this group, as the association of a species with a range of hosts should indicate the presence of several morphologically indistinguishable species, of which all have a narrow host range, as it was shown in Asia [30], North America [31], and Europe [32].
This paper aims to (1) describe a new subgenus and three new species of parasitic mites discovered in Myanmar based on morphological and DNA-based analyses; (2) estimate the host range of these species; (3) discuss the subgeneric concept of the genus Unionicola on the basis of novel two-gene phylogenetic reconstruction, presented herein; (4) revise the taxonomic position of U. (Prasadatax) brandti Vidrine, 1985 based on newly obtained morphological data; and (5) develop identification keys for males and females for the new subgenus.
2. Materials and Methods
2.1. Collecting of Specimens, Map Creation, and Morphological Research
Samples of Unionicola mites were collected during field surveys carried out at 11 localities in Myanmar situated in the Ayeyarwady, Haungthayaw, Salween, Bago, and Sittaung river basins (Figure 1). The topographic base of the maps was created with Natural Earth Free Vector and Raster Map Data 4.0.0 (https://www.naturalearthdata.com, accessed on 1 September 2022); Global Self-consistent Hierarchical High-resolution Geography, GSHHG v2.3.7 (https://www.soest.hawaii.edu/wessel/gshhg, accessed on 1 September 2022); HydroBASINS dataset Version 1.0 (https://www.hydrosheds.org, accessed on 1 September 2022) [33,34]; The General Bathymetric Chart of the Oceans, GEBCO (https://www.gebco.net, accessed on 1 September 2022).
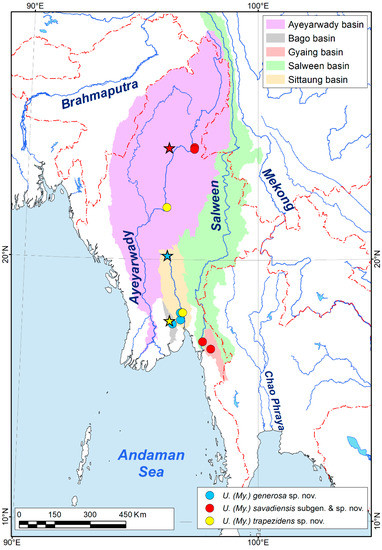
Figure 1.
Type localities (stars) and additional occurrences (circles) of the new species belonging to the subgenus Myanmaratax subgen. nov. from Myanmar. (Map: Mikhail Y. Gofarov).
Mites were collected from the mantle cavity and gills of unionid freshwater mussels by forceps. The samples were preserved in 96% ethanol immediately after collecting. For morphological research, permanent slides of mite specimens were prepared. For this, mites were incubated at 60 °C in 8% KOH for 30 min or more, depending on their size and the density and stiffness of the integument. The internal viscera were removed carefully with a dissecting needle; slides were mounted in Faure–Berlese fluid for observations with light microscopy, up to the magnification objective 40× with a research microscope (AXIO Lab.A1, Carl Zeiss, Oberkochen, Germany) equipped with a digital camera. Morphological details were measured with ZEN lite 2012 (blue edition) software (Carl Zeiss Microscopy Gmbh, 2011, Oberkochen, Germany). New taxonomic descriptions partly followed Vidrine [35,36,37] and Ding et al. [13]. The type specimens of the new species are deposited in the Russian Museum of Biodiversity Hotspots (RMBH), N. Laverov Federal Center for Integrated Arctic Research of the Ural Branch of the Russian Academy of Sciences (Arkhangelsk, Russia).
The multivariate analysis of variance (MANOVA) in PAST v. 3.06 [38] was used to test the differences between three studied Myanmaratax species for both sexes and separately for males and females based on 22 morphological measurements (all parameters are in the abbreviations section of the ‘Taxonomic account’). The measurements are presented in the Supplementary Table S2. Principal component analysis (PCA) was carried out for the species ordination in length space by 22 morphometric parameters separately for males and females using PAST v. 3.06 [38]. The differences in statistically significant morphological criteria between the three studied mite species for each sex were estimated by the One-Way-ANOVA with post-hoc pairwise comparisons based on Tukey’s HSD test using PAST v. 3.06 [38].
2.2. DNA Analyses
Total DNA was extracted from whole mite specimens using a standard phenol/chloroform procedure [39]. The barcode region of the mitochondrial cytochrome c oxidase subunit I (COI) gene was amplified and sequenced using the LoboF and LoboR as forward and reverse primers, respectively [40]. Partial sequences of the nuclear 28S ribosomal RNA gene were amplified and sequenced using the 23F or MiF/D2 primers. The PCR mix contained approximately 100 ng of total cellular DNA, 10 pmol of each primer, 200 μmol of each dNTP, 2.5 μL of PCR buffer (with 20 mmol MgCl2), and 0.8 units Taq DNA polymerase (SibEnzyme Ltd., Novosibirsk, Russia). Water was added for a final volume of 25 μL. The temperature cycling was as follows: 95 °C (5 min), 29 cycles of 95 °C (50 s), 46 °C (50 s), 72 °C (50 s), and a final extension at 72 °C (5 min) for the COI gene fragment; and 34–36 cycles of 95 °C (50 s), 56 °C (50 s), 72 °C (50 s), and a final extension at 72 °C (5 min) for the 28S gene fragment. Forward and reverse sequencing was performed on an automatic sequencer (ABI PRISM® 3730, Thermo Fisher Scientific, Waltham, MA, USA) using an ABI PRISM® BigDye™ Terminator v. 3.1 reagent kit, Waltham, MA, USA. The resulting sequences were analyzed with BioEdit v. 7.0.9 [41].
2.3. Phylogenetic Analyses
For phylogenetic analyses, we used 110 in-group COI + 28S haplotype sequences. Two sequences of Neumania spp. (Unionicolidae; GenBank acc. no. MN359295 for Neumania sp. and MK889600 for Neumania verrucosa) were used as an outgroup. The sequence dataset was aligned by the MUSCLE algorithm of MEGA7 [42]. The maximum likelihood phylogeny was reconstructed with IQ-TREE v. 1.6.11 [43] through an online resource of Los Alamos National Laboratory, National Nuclear Security Administration, USA. The node support values were estimated using an ultrafast bootstrap algorithm [44]. The Bayesian phylogeny was calculated with MrBayes v. 3.2.7a [45] through CIPRES Science Gateway in San Diego Supercomputer Center, USA [46]. The MrBayes analyses were carried out in two separate runs of 25,000,000 cycles long, each with four Markov chains, one cold and three heated (temperature set at 0.1). Trees were sampled every 5000th generation. The first 15% of trees were discarded as burn-in, and the majority rule consensus tree was calculated from the remaining trees. The GTR + G substitution model was applied in both the maximum likelihood and the Bayesian analyses.
The molecular diagnoses were carried out as follows: all sequences at the request of «Unionicola» in NCBI GenBank were selected, then those of them with sequenced gene regions matching our dataset were aligned using the MUSCLE algorithm implemented in MEGA7 [42]. This was necessary to search a large sample for unique or rare nucleotide substitutions in the analyzed sequences to select a unique set of nucleotide substitutions for each new species.
2.4. Nomenclatural Acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence, the new names contained herein are available under that code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The LSID for this publication is: https://zoobank.org/urn:lsid:zoobank.org:pub:F27A2E9A-2187-44AA-BCDC-0AF33839D9F8 (accessed on 27 September 2022). The electronic edition of this work was published in a journal with an ISSN and has been archived and is available from the following digital repositories: PubMed Central and LOCKSS.
3. Results
3.1. New Subgenus of Mussel-Associated Mites from Southeast Asia
Our two-locus phylogeny of Unionicola members is based on the mitochondrial cytochrome c oxidase subunit I (COI) and nuclear 28S ribosomal RNA (28S) gene fragments. The subgenus Myanmaratax subgen. nov. was recovered as a fully supported clade (BS/BPP = 100/1.00). We identified four species-level Myanmaratax lineages corresponding to Unionicola (My.) savadiensis sp. nov., U. (My.) generosa sp. nov., U. (My.) trapezidens sp. nov. (Table 1), and U. (My.) sp. ‘Yaukthwa’. All the new species described here represent well-supported subclades with diagnostic nucleotide substitutions. The molecular diagnoses were based on 281 COI and 61 28S Unionicola sequences. The level of genetic divergence (uncorrected COI p-distance) of each new species from its nearest neighbor varies from 14.5 to 16.1% (Table 2). Additionally, we transfer Unionicola (Prasadatax) brandti Vidrine, 1985 from Prasadatax to Myanmaratax subgen. nov. based on morphological features alone and propose U. (My.) brandti comb. nov. (Table 1). The fifth member of the new subgenus, U. (My.) sp. ‘Yaukthwa’ (Figure 2) was discovered in a few specimens and needs future collecting efforts. Morphologically, this species largely resembles U. (My.) brandti comb. nov. It will be described elsewhere.

Table 1.
Checklist of mussel-associated Unionicola mites (Unionicolidae) from Southeast Asia and India (without species that have been identified phylogenetically but have not been described yet, and free-living taxa).

Table 2.
Molecular diagnoses of new Unionicola (Myanmaratax) species.
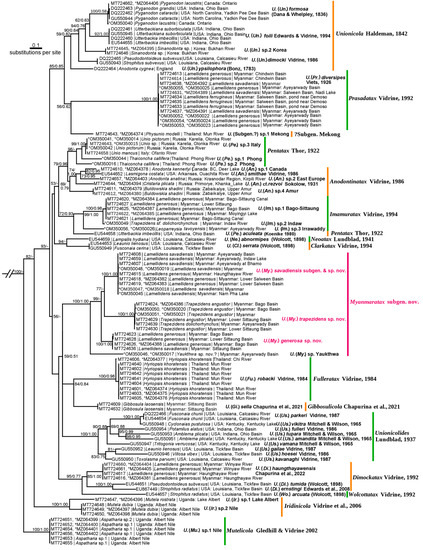
Figure 2.
Two-locus maximum likelihood phylogeny of the genus Unionicola (COI + 28S). Each sequence name contains the following information: GenBank accession number | mussel host name | collecting locality. The numbers near nodes are BS/BPP values. Subgenus-level clades are highlighted by different line colors. The new taxonomic names are in red. New sequences generated in this study are marked with an asterisk. Outgroup is not shown.
Available data on host ranges of Unionicola mites from South and Southeast Asia are compiled in Table 1. In Myanmar, members of the new subgenus represent a possible example of a host-driven radiation associated with two genera of freshwater mussels belonging to the tribe Lamellidentini, namely Lamellidens and Trapezidens. In particular, Unionicola (My.) savadiensis and U. (My.) generosa are parasites of Lamellidens savadiensis and L. generosus, while U. (My.) trapezidens use Trapezidens angustior and T. dolichorhynchus as hosts. The fourth member of the new subgenus, U. (My.) brandti comb. nov., was reported to use freshwater mussels of the tribes Contradentini and Rectidentini as hosts, while its record in a Pseudodontini member is doubtful, as species of this subgenus are cryptic and tend to be highly specialized to the host (Table 1). Finally, a few available specimens of U. (My.) sp. ‘Yaukthwa’ were also collected from freshwater mussels belonging to the tribe Contradentini (genus Yaukthwa Konopleva et al., 2019).
3.2. Taxonomic Account
Abbreviations are as follows: walking legs and pedipalps: I-(II-, III-, IV-)L = 1st (2nd, 3rd, 4th) walking leg; I-L-1(-2, -3, and so on) = 1st (2nd, 3rd, etc.) segment of the 1st walking leg; P-1(-2, -3, etc.) = 1st (2nd, 3rd, and so on)segment of the pedipalp [49,50]. LI = length of idiosoma; wGF = width of the genital field; PCG = length of posterior coxal group. All measurements are given in micrometers (μm).
Collection depositories: RMBH = Russian Museum of Biodiversity Hotspots, N. Laverov Federal Center for Integrated Arctic Research of the Ural Branch of the Russian Academy of Sciences (Arkhangelsk, Russia).
- Family Unionicolidae Oudemans, 1909
- Genus Unionicola Haldeman, 1842
- Subgenus Myanmaratax Chapurina, Vidrine, Kondakov, Vikhrev and Bolotov subgen. nov.
- LSID: urn:lsid:zoobank.org:act:C242AA35-C194-40E1-B3EA-4D0A924B6F09 (accessed on 27 September 2022)
- Type species. Unionicola (Myanmaratax) savadiensis subgen. and sp. nov.
Diagnosis (partly based on Vidrine [36]). Dorsum without dorsal platelets; coxal plates I and II with complete dividing suture. Anterior coxal group bears moderately long posterior apodeme; coxal plates III and IV fused together and divided by incomplete sutur. Posterior coxal group without an apparent posterior apodeme, square or rectangular shape with rounded inner corners (females have more rounding along the inner caudal margin). Both male and female genital fields consist of a single pair of acetabular plates; genital acetabula with many pairs (13–20) concentrated in anterior and posterior groups and may be without a distinct visible border between these groups; leg I with large setae; leg IV rather long, especially IV-L-5 noticeably longer than other segments, tarsus of every leg with bifid claws. Chaetotaxy of walking legs in females and males is different, P-3 with one seta for females and without setae for males; the idiosoma size for females is slightly larger than for males.
Etymology. The subgenus is named for the country of Myanmar, where these mites were discovered.
Host range. Mussel-associated gill mites who are members of this subgenus use freshwater mussel species as hosts. In Western Indochina, Lamellidens and Trapezidens (Bivalvia: Unionidae: Parreysiinae: Lamellidentini) house these mites, while Contradens and Ensidens (Bivalvia: Unionidae: Gonideinae: Contradentini and Rectidentini) are the primary hosts in the Sundaland Subregion (Table 1).
Comments. This group contains U. (My.) brandti Vidrine, 1985 comb. nov. and three species new to science, which are described here.
- Unionicola (Myanmaratax) savadiensis Chapurina, Vidrine, Kondakov, Vikhrev and Bolotov subgen. and sp. nov.
LSID: urn:lsid:zoobank.org:act:85D0F96D-A9DF-46D7-9439-CC792688B7B5 (accessed on 27 September 2022)
Holotype. MYANMAR: Indaw Lake, Thett Kel Chin village, 24°15′59″ N 96°7′22″ E, Ayeyarwady River basin (Figure 3G), from the gills of Lamellidens savadiensis (host voucher RMBH biv603), 13.xi.2018, 1♂ (slide RMBH Hyd 603), Vikhrev, Bolotov, and Nyein Chan leg. The type series of the new species and mussel host vouchers are deposited in RMBH (Arkhangelsk, Russia).
Paratypes. MYANMAR: the type locality, the same host, date, and collectors, 1♀, 2♂ (slide RMBH Hyd 603) and one sequenced specimen (sample ID RMBH Hyd_603_2; COI sequence accession number MT724659); ox-bow lake south of Hpa-An airport, 16°52′55″ N 97°39′46″ E, Salween River basin, from the gills of Lamellidens generosus (host voucher RMBH Biv365), 10.ii.2018, 3♀ (slide RMBH Hyd 365) and two sequenced specimens (sample ID RMBH Hyd_365_1; COI sequence accession number MT724618, 28S sequence accession number MZ064382; sample ID RMBH Hyd_365_2; COI sequence accession number MT724619, 28S sequence accession number MZ064383); Vikhrev, Bolotov, and Nyein Chan leg.; a tributary of the Haungthayaw River, 16°36′37″ N 98°0′39″ E, Salween River basin, from the gills of Lamellidens generosus (host voucher RMBH Biv359), 09.ii.2018, 2♂, 1♀ (slide RMBH Hyd 359) and one sequenced specimen (sample ID RMBH Hyd_359_2; COI sequence accession number MT724615), Vikhrev, Bolotov, and Nyein Chan leg.; Shwe Kyi Lake, 24°17′33″ N, 97°13′47″ E, Ayeyarwady River basin, from the gills of Lamellidens savadiensis (host voucher RMBH Biv261), 29.xi.2016, 1♂ (slide RMBH Hyd 261) and one sequenced specimen (sample ID RMBH Hyd_261; COI sequence accession number MT724607), Vikhrev and Nyein Chan leg.; Nam Sa Yi River, 24°13′10″ N, 97°13′20″ E, Ayeyarwady River basin, from the gills of Lamellidens savadiensis (host voucher RMBH Biv262), 30.xi.2016, 1♀ (slide RMBH Hyd 262) and one sequenced specimen (sample ID RMBH Hyd_262; COI sequence accession number MT724608), Vikhrev and Nyein Chan leg.; Nam Pha Lake near Bhamo, 24°17′49″ N, 97°15′39″ E, Ayeyarwady River basin, from the gills of Lamellidens savadiensis (host voucher RMBH Biv257), 29.xi.2016, one sequenced specimen (sample ID RMBH Hyd_257_1; COI sequence accession number OM350045), Vikhrev and Nyein Chan leg.; pond in Mezali village, 23°58′11″ N, 96°26′9″ E, Ayeyarwady River basin, from the gills of Lamellidens savadiensis (host voucher RMBH Biv914), 10.iii.2020, 2 sequenced specimens (sample ID RMBH Hyd_914_1; COI sequence accession number OM350047; 28S sequence accession number OM350018; sample ID RMBH Hyd_914_2; COI sequence accession number OM350048 28S sequence accession number OM350019), Bolotov, Vikhrev, Gofarov, and Kondakov leg.
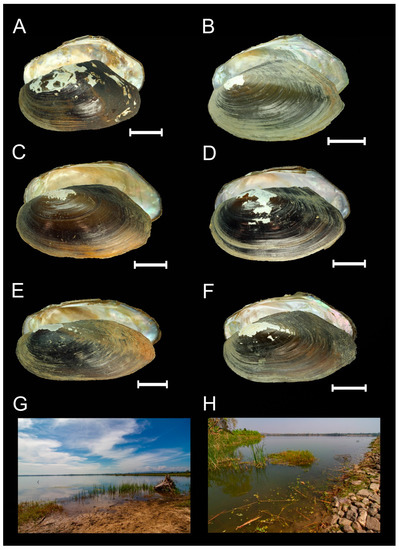
Figure 3.
Hosts and type localities of the new mussel-associated mite species from the subgenus Myanmaratax subgen. nov. from Myanmar. (A) Shell of Lamellidens savadiensis (Nevill, 1877) (specimen RMBH biv603_6), host of U. (My.) savadiensis subgen. and sp. nov., Indaw Lake, Ayeyarwady River basin. (B) Shell of L. generosus (Gould, 1847) (specimen RMBH biv359_3), host of U. (My.) savadiensis subgen. and sp. nov., a tributary of the Haungthayaw River. (C) Shell of L. savadiensis (specimen RMBH biv413_3), host of U. (My.) generosa sp. nov., Sin Thay Dam, Sittaug River basin. (D) Shell of L. generosus (specimen RMBH biv392_2), host of U. (My.) generosa sp. nov., Pagaing Stream, Sittaug River basin. (E) Shell of Trapezidens angustior (Hanley and Theobald, 1876) (specimen RMBH biv388_3), host of U. (My.) trapezidens sp. nov., Kyauk Phar Stream, Bago River basin. (F) Shell of T. dolichorhynchus (Tapparone Canefri, 1889) (specimen RMBH biv442_1), host of U. (My.) trapezidens sp. nov., Ayeyarwady River. (G) Type locality of U. (My.) savadiensis subgen. and sp. nov. (H) Type locality of U. (My.) generosa sp. nov. (Photos: Ekaterina S. Konopleva (A–F) and Ilya V. Vikhrev (G,H)).
Reference DNA data. Nine COI sequences and four 28S sequences were obtained from whole mites. These specimens were completely used for DNA extraction, but mites of the same sample were morphologically studied by us and are included here to the type series (see sequence accession numbers and locality data in the list of paratypes above).
Type host. The holotype was collected from the gills of Lamellidens savadiensis (Nevill, 1877) (Bivalvia: Unionidae: Parreysiinae: Lamellidentini) (Figure 3A). The paratypes were collected from the gills of the latter species and of Lamellidens generosus (Gould, 1847) (Figure 3A,B).
Etymology. This species is named after its host mussel, Lamellidens savadiensis.
Diagnosis. Features of the subgenus. P-2 with 4 lateral setae, male’s P-4 with one heavy and one thin ventral setae. Female’s P-3 with one slender lateral seta. Female’s genital field with 2 thin setae on the flap per side and one hair-like seta on every side of the genital field out of the flap. The tarsal claws of walking legs with 2 claws and spatulate seta over the tarsal claws of the walking legs is not prominent. The shape of female’s II-L-6 is weakly dumbbell, and the shape of female’s III-L-6 is dumbbell with a narrowing located distal part of the tarsus. Female’s IV-L-5 with 2 dorsal setae or absent dorsal setae. The chaetotaxy of male’s I-L-2, I-L-3 may be different, and the chaetotaxy of distal segments of IV-L varies rather strongly between individuals of the one species due to the large amount of these setae and ambiguity of the interpretation of setae position on the legs due to the preparation and distortion/foreshortening of fixation. The size of male’s idiosoma is 745–827 μm (average is 793 μm). The ratio of the whole length of the body to the length of the posterior coxal group for males is 2.7.
Description (Figure 5).
Male morphology. I-L: I-L-1 with 2 lateral and 2–4 dorsal blunt setae. I-L-2 with 2 dorsolateral and 2 dorso-distal blunt setae, one laterodistal and one lateral awl-shaped setae (the last may absent), one ventrodistal long swimming seta. I-L-3 with 5 blunt awl-shaped lateral setae, 2 lateral slender setae (may absent), 2 hair-like dorso-distal setae and 4 ventral swimming setae. I-L-4 with 4 blunt and 3 slender setae—laterally, 2 short dorsal setae, 2 hair-like dorso-distal setae and 3 ventral setae. I-L-5 with 4 awl-shaped, 3 thin latero-ventral setae, 2 short hair-like lateral setae. I-L-6 without setae. The claws of I legs are thick and each with 2 clawlets. IV-L: IV-L-1 with 3 latero-dorsal and one latero-distal setae. IV-L-2 with 4 lateral and 2 distal setae. IV-L-3 with one blunt dorso-distal seta, one distal heavy seta, a row of 6 dorsolateral heavy setae, 14 lateral heavy setae. IV-L-4 with 10 dorsal blunt, 10 blunt lateral setae, 3 ventral setae, one dorso-distal blunt seta, 3 distal elongated heavy blunt setae. IV-L-5 is almost twice long as IV-L-3 with 13 ventral heavy setae, 5 lateral setae, one prominent and 2 normal distal setae. IV-L-6 with one dorsal awl-shaped seta, 6 ventral setae. The claws of IV sickle-shaped and with a short dorsal and a long ventral clawlet. Genital field consists of 2 pairs of plates with 11–15 acetabula per side without flaps. Acetabula are concentrated in 2 groups on each side—anterior and posterior. One acetabulum of every group is larger than the rest.
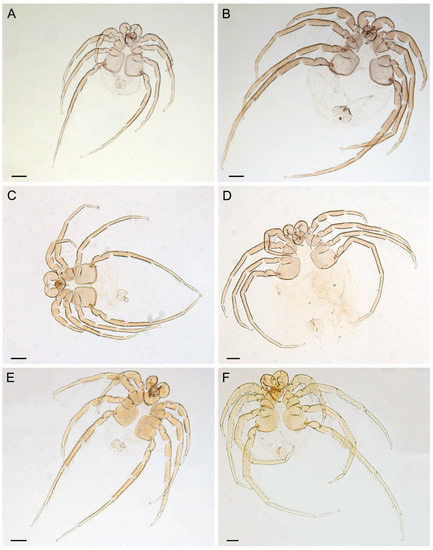
Figure 4.
Type series of new mussel-associated mites from Southeast Asia (photos of permanent slides). (A,B) U. (Myanmaratax) savadiensis subgen. and sp. nov.: male holotype RMBH Hyd 603 (E) and female paratype RMBH Hyd 603 (B). (C,D) U. (My.) generosa sp. nov.: male holotype RMBH Hyd 413 (C) and female paratype RMBH Hyd 413 (D). (E,F) U. (My.) trapezidens sp. nov.: male holotype RMBH Hyd 388 (E) and female paratype RMBH Hyd 388 (F). Scale bars = 200 µm. (Photos: Yulia E. Chapurina).
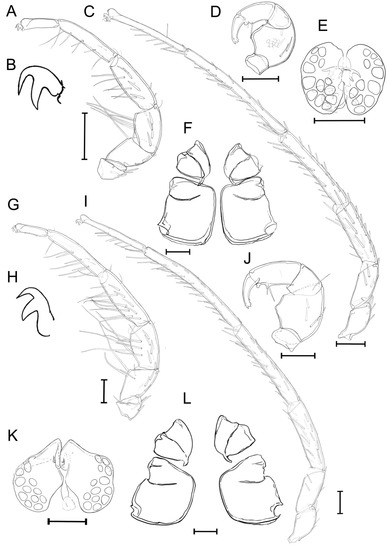
Figure 5.
Unionicola (Myanmaratax) savadiensis subgen. and sp. nov. The male, slide RMBH Hyd 603: (A) I-L, (B) claw of I-L, (C) IV-L, (D) P, (E) genital field, and (F) coxal plates. The female, slide RMBH Hyd 603: (G) I-L, (H) claw of I-L, (I) IV-L, (J) P, (K) genital field, and (L) coxal plate. Scale bars = 100 µm. (Graphics: Yulia E. Chapurina).
Female morphology. I-L: I-L-1 with one or 2 lateral blunt and one slender setae and 3 dorsal blunt setae (or 4 dorsal setae grouped in two pairs). I-L-2 with 3 lateral blunt setae and 2 lateral slender setae, not serrated, one distal short blunt seta and one distal heavy seta, and 2 ventro-distal long heavy setae. I-L-3 with one row of 4 or 5 lateral blunt setae, 4 lateral heavy elongated setae, 2 lateral slender setae, one laterodistal thin seta and 5 ventral long awl-shaped setae. I-L-4 with 5 blunt lateral setae, 4 lateral and 6 ventral heavy long setae (one seta is not long), one distal awl-shaped slender seta and 2 dorsal setae. I-L-5 with one dorsal seta, absent or 2 distal setae, one ventral and one lateral awl-shaped setae, 2 lateral thin setae and 7 ventrolateral heavy setae. I-L-6 with 3 ventral thin setae. The claws of I leg are thick and each with a shortened dorsal and a ventral clawlet. IV-L: IV-L-1with 5 dorsal awl-shaped blunt setae, IV-L-2 with 3 lateral setae, one distal peg-shaped seta and one dorsal seta. IV-L-3 with 22 lateral setae, one dorso-distal peg-shaped blunt seta and one distal heavy blunt seta. IV-L-4 with 20 lateral awl-shaped setae, one blunt lateral seta, 4 dorso-lateral setae, one ventral seta, one blunt seta dorso-distally and 2 heavy awl-shaped blunt setae distally. IV-L-5 with 6 short lateral setae, 14 awl-shaped blunt lateral setae (overall 20 lateral setae), 9 ventrolateral setae, absent or 2 dorsal setae, 2 short blunt and 2 heavy awl-like distal setae. IV-L-6 with 7 ventral and ventrolateral heavy setae (the number of these setae varies and may reach 9 or 10 setae in different specimens). The claws of IV leg are forked with a short dorsal clawlet and a long ventral clawlet.
Genital field consists of 2 pairs of plates with 12–20 acetabula per side, with a pair of flaps bearing 2 hair-like setae on top of the plates per side and one seta on the inner margin of the genital plate. Acetabula are concentrated in 2 groups on each side—anterior and posterior.
Male measurements (N = 5). Length of the idiosoma 793 (745–827); length of the posterior coxal group 298 (280–320); dorsal lengths of the P-1–5 segments: 33 (29–39), 148 (127–161), 66 (50–74), 109 (100–124), 75 (63–80); dorsal lengths of the I-L-1–6 segments: 77 (73–83), 106 (81–119), 145 (135–160), 194 (181–213), 204 (187–225), 116 (108–122); dorsal lengths of the IV-L-1–6 segments: 146 (130–171), 134 (124–149), 240 (221–267), 325 (305–365), 459 (425–510), 302 (286–319); length of the genital field 138 (114–164), width of the genital field 161 (144–177).
Female measurements. Length of the idiosoma (N = 6) 1402 (1216–1593); length of the posterior coxal group 342 (317–378). Dorsal lengths of the P-1–5 segments: 41 (40–43), 170 (163–181), 82 (75–89), 148 (142–159), 96 (88–106); dorsal lengths of the I-L-1–6 segments: 95 (83–107); 139 (134–144); 201 (199–203; 279 (272–292); 287 (280–301); 138 (134–148); dorsal lengths of the IV-L-1–6 segments: 181 (172–195), 188 (180–203), 366 (347–394), 479 (455–506), 608 (555–640), 360 (350–367); length of the genital field 199 (192–208), width of the genital field 256 (229–276).
Distribution. Ayeyarwady, Salween, and Haungthayaw basins in Myanmar.
Host range. This species is a narrow host specialist, which is known to occur on the gills of two Lamellidens species, namely L. savadiensis and L. generosus (Figure 3A,B).
Unionicola (Myanmaratax) generosa Chapurina, Vidrine, Kondakov, Vikhrev and Bolotov sp. nov.
LSID: urn:lsid:zoobank.org:act:0B5256AD-6C1B-4D5E-AFC2-994A2E24CF1E (accessed on 27 September 2022)
Holotype. MYANMAR: Sin Thay Dam, 20°9′14″ N, 96°6′53″ E, Sittaung River basin (Figure 3H), from the gills of Lamellidens savadiensis (host voucher RMBH biv413), 01.iii.2018, 1♂ (slide RMBH Hyd 413), Vikhrev, Bolotov, and Nyein Chan leg. The type series of the new species and mussel host vouchers are deposited in RMBH (Arkhangelsk, Russia).
Paratypes. MYANMAR: the type locality, the same host, date, and collectors, 1♀, 2♂ (slide RMBH Hyd 413) and one sequenced specimen (sample ID RMBH Hyd_413_1; COI sequence accession number MT724636); pond near the Salu Dam, 17°32′57″ N 96°22′24″ E, Bago River basin, from the gills of Lamellidens generosus (host voucher RMBH Biv379), 18.ii.2018, 1♀ (slide RMBH Hyd 379) and one sequenced specimen (sample ID RMBH Hyd_379_2; COI sequence accession number MT724623), Vikhrev, Bolotov, and Nyein Chan leg.; Pagaing Stream, 17°42′28″ N, 96°42′55″ E, Bago River basin, from the gills of Lamellidens generosus (host voucher RMBH Biv392), 20.ii.2018, 3♂, 3♀(slide RMBH Hyd 392) and two sequenced specimen (sample ID RMBH Hyd_392_1; COI sequence accession number MT724625; 28S sequence accession number MZ064387; sample ID RMBH Hyd_392_2; COI sequence accession number MT724626; 28S sequence accession number MZ064388) Vikhrev, Bolotov, and Nyein Chan leg.; Pazunmyaung Stream, 17°58′36″ N, 96°45′57″ E, Sittaung River basin, from the gills of Lamellidens generosus (host voucher RMBH Biv394), 20.ii.2018, one sequenced specimen (sample ID Hyd_394_1; COI sequence accession number MT724628) Vikhrev, Bolotov, and Nyein Chan leg.
Diagnosis. Features of the subgenus. This species strongly resembles U. (Myanmaratax) savadiensis. P-2 with 4 lateral setae, male’s P-4 with one heavy and one thin ventral setae. Female’s P-3 with one slender lateral seta. Female’s genital field with two thin setae on the flap per side and one hair-like seta on every side of the genital field out of the flap. The tarsal claws of walking legs with 2 claws and spatulate seta over the tarsal claws of the walking legs is not prominent. The length of idiosoma in females may reach 1600–1700 μm and more, but the average parameter value is 1538 µm (N = 6). The shape of female’s II-L-6 and III-L-6 is a dumbbell with well-defined expansion closer to the basis of the tarsus and a narrowing located distally. Female’s IV-L-5 with 4 dorsal setae. Female’s I-L-2 with 2 or 3 well visible swimming ventrodistal setae (both variants of the trait may be observed within the same specimen).
The chaetotaxy of distal segments of I-L and IV-L varies rather strongly between individuals of the one species due to the large number of these setae and ambiguity of the interpretation setae position on the legs due to the preparation and distortion/foreshortening of fixation. The size range of male’s idiosoma is 860–1054 μm (average is 945 μm). The ratio of the whole length of the body to the length of posterior coxal group for males is 3.1.
Reference DNA data. Four COI and one 28S sequence were obtained from whole mites. These specimens were completely used for DNA extraction, but the mites of the same sample were studied by us morphologically and are included here as the type series (see sequence accession numbers and locality data in the list of paratypes above).
Type host. The holotype and several paratypes were collected from the gills of Lamellidens savadiensis (Nevill, 1877) (Bivalvia: Unionidae: Parreysiinae: Lamellidentini) (Figure 3C). Additional paratypes were obtained from the gills of Lamellidens generosus (Gould, 1847) (Bivalvia: Unionidae: Parreysiinae: Lamellidentini) (Figure 3D).
Etymology. This species is named after its host mussel, Lamellidens generosus.
Description (Figure 6). Male morphology. I-L: I-L-1 with 2 lateral and 3–4 dorsal awl-shaped setae. I-L-2 with 2 dorsolateral and 2 lateral awl-shaped setae, one dorsal and one dorso-distal peg-shaped setae, one lateral and one dorso-distal blunt setae, one ventrodistal long swimming seta. I-L-3 with 4 blunt awl-shaped lateral setae, 3 lateral slender setae, one distal seta, and 3 ventral swimming setae. I-L-4 with 4 or 5 blunt, one curved, 2 tiny, and 3 heavy setae laterally, and one slender distal, one blunt ventral and 2 ventral setae. I-L-5 with one thin, 3 spinous lateral setae, 2 slender and 2 short ventral setae. I-L-6 with one thin ventral seta. The claws of I legs are thick and each with 2 clawlets. IV-L: IV-L-1 with 3 lateral and 2 distal setae. IV-L-2 with 2 lateral and 2 distal setae. IV-L-3 with 2 blunt dorso-distal setae, one distal heavy seta, a row of 4 dorsolateral heavy setae, 3 lateral slender shortened setae, 9 lateral heavy setae and 2 ventrolateral heavy setae curved at the base. IV-L-4 with 4 dorsal blunt and one thin setae, 6 lateral, 9 ventral, 2 dorso-distal blunt setae, 2 distal elongated blunt setae. IV-L-5 is more than twice as long as IV-L-3 with 13 ventral heavy setae, 4 normal and one slender lateral setae, one normal dorsal seta, 3 dorsal and one dorso-lateral blunt setae, one dorso-distal thin seta and 2 distal slender setae. IV-L-6 with one curved, one elongated thin and 3 thin dorsal setae, 9 ventral setae. The claws of IV sickle-shaped and with a short dorsal and a long ventral clawlet. Genital field consists of 2 pairs of plates with 15–18 acetabula per side without flaps. Acetabula are concentrated in 2 groups on each side—anterior and posterior. One acetabulum of every group is larger than the rest.
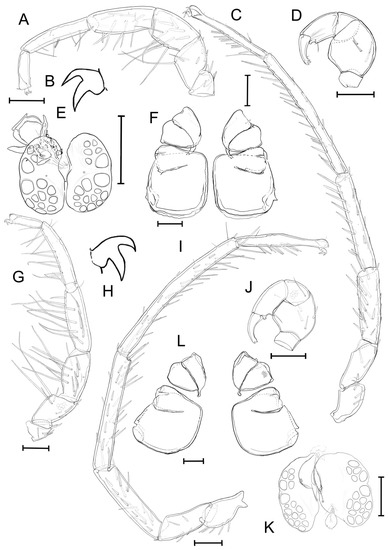
Figure 6.
Unionicola (Myanmaratax) generosa sp. nov. The male, slide RMBH Hyd 413: (A) I-L, (B) claw of I-L, (C) IV-L, (D) P, (E) genital field, and (F) coxal plate. The female, slide RMBH Hyd 413: (G) I-L, (H) claw of I-L, (I) IV-L, (J) P, (K) genital field, and (L) coxal plate. Scale bars = 100 µm. (Graphics: Yulia E. Chapurina).
Female morphology. I-L: I-L-1 with 2 lateral awl-shaped setae and 4 dorsal blunt setae grouped in 2 pairs. I-L-2 with 3 lateral blunt setae and 2 lateral slender setae, not serrated, one distal short blunt seta and one distal heavy seta, and 2 (maybe 3) ventro-distal long heavy setae. I-L-3 with 5 lateral blunt setae, 4 lateral heavy elongated setae, 2 lateral slender setae, one lateral short awl-shaped seta and 4 ventral long awl-shaped setae, one of them is blunt on the tip (or 4 ventral long awl-shaped setae). I-L-4 with 5 blunt lateral setae, 4 lateral and 5 heavy long ventral setae (one seta is not long), one distal awl-shaped slender seta and one dorso-distal seta. I-L-5 with 2 dorsal slender setae, one distal and one ventral awl-shaped slender seta and 9 ventrolateral heavy setae. I-L-6 with 3 ventral and 2 dorsal thin setae. The claws of I leg are thick and each with a shortened dorsal and a ventral clawlet. IV-L: IV-L-1 with 5 dorsal awl-shaped blunt setae, IV-L-2 with 4 lateral setae, one distal peg-shaped seta and one dorsal seta. IV-L-3 with a lateral row of 6 setae, and 12 lateral awl-shaped setae, 4 small setae (overall 22 lateral setae) and 2 distal heavy blunt setae. IV-L-4 with 18 lateral setae, 3 dorsal blunt setae, 4 heavy setae and one small ventral seta, 2 heavy and 2 shortened awl-shaped distal setae. IV-L-5 with 18 lateral setae, one of them is thin, 8 ventral setae, 4 dorsal setae, 2 of them are hair-like and 2 are blunt, 2 short blunt and 2 heavy awl-like distal setae. IV-L-6 with 6 ventral heavy setae, 3 heavy ventro-lateral setae, 2 hair-like dorsal setae. The claws of IV leg are forked with a short dorsal clawlet and a long ventral clawlet.
Genital field consists of 2 pairs of plates with 16–17 acetabula per side, with a pair of flaps bearing 2 hair-like setae on top of the plates per side and one seta on the inner margin of the genital plate. Acetabula are concentrated in 2 groups on each side—anterior and posterior.
Male measurements (N = 6). Length of the idiosoma 945 (860–1054); length of the posterior coxal group 303 (285–345); dorsal lengths of the P-1–5 segments: 32 (26–34), 144 (113–174), 70 (56–83), 119 (106–132), 79 (73–87); dorsal lengths of the I-L-1–6 segments: 74 (63–94), 113 (99–130), 158 (140–181), 217 (196–252), 224 (198–254), 120 (114–126); dorsal lengths of the IV-L-1–6 segments: 145 (130–157), 151 (134–174), 270 (240–329), 356 (312–433), 485 (400–579), 297 (285–326); length of the genital field (N = 5): 134 (121–150), width of the genital field (N = 6): 159 (142–176).
Female measurements. Length of the idiosoma (N = 4) 1538 (1358–1746); length of the posterior coxal group (N = 5): 324 (311–344); dorsal lengths of the P-1–5 segments: 40 (32–50), 163 (147–182), 82 (72–102), 144 (138–149), 93 (89–97); dorsal lengths of the I-L-1–6 segments: 91 (70–100); 137 (130–142); 195 (190–199); 272 (261–296); 277 (266–286); 134 (130–140); dorsal lengths of the IV-L-1–6 segments (N = 5): 168 (147–176), 184 (174–194), 349 (330–366), 455 (442–473), 587 (563–606), 342 (331–353); length of the genital field (N = 4): 207 (200–212), width of the genital field: 235 (227–242).
Distribution. Bago and Sittaung basins in Myanmar.
- Host range. This species is a narrow host specialist, which is known to occur on the gills of two Lamellidens species, namely L. savadiensis and L. generosus (Figure 3C,D).
- Unionicola (Myanmaratax) trapezidens Chapurina, Vidrine, Kondakov, Vikhrev and Bolotov sp. nov.
LSID: urn:lsid:zoobank.org:act:5EABC099-26DB-40B4-84D3-030358D416D8 (accessed on 27 September 2022)
Holotype. MYANMAR: Kyauk Phar Stream, 17°39′57″ N 96°14′47″ E, Bago River basin, from the gills of Trapezidens angustior [host voucher RMBH biv388], 19.ii.2018, 1♂ (slide RMBH Hyd 388), local collectors leg. The type series of the new species and mussel host vouchers are deposited in RMBH (Arkhangelsk, Russia).
Paratypes. MYANMAR: the type locality, the same host, date, and collectors, 1♀, 1♂ (slide RMBH Hyd 388) and one sequenced specimen (sample ID RMBH Hyd_388_1; COI sequence accession number MT724624; 28S sequence accession number MZ064386); Ayeyarwady River, Kywe Zoon Seik Area, Mandalay, 21°59′27″ N, 96°3′39″ E, from the gills of Trapezidens dolichorhynchus (host voucher RMBH Biv442), 04.iii.2018, 2♀, 1♂ (slide RMBH Hyd 442) and one sequenced specimen (sample ID RMBH Hyd_442_1; COI sequence accession number MT724639), Vikhrev, Bolotov, and Nyein Chan leg.; Pazunmyaung Stream, 17°58′36″ N, 96°45′57″ E, Sittaung River basin, from the gills of Trapezidens angustior (host voucher RMBH Biv394), 20.ii.2018, two sequenced specimens (sample ID Hyd_394_2; COI sequence accession number MT724629; sample ID Hyd_394_3; COI sequence accession number MT724630), Vikhrev, Bolotov, and Nyein Chan leg.; Ka Lein Stream, 17°37′20″ N, 96°18′55″ E, Bago River basin, from the gills of Trapezidens angustior (host voucher RMBH Biv987), 21.iii.2020, one sequenced specimen (sample ID Hyd_987; COI sequence accession number OM350050; 28S sequence accession number OM350020), Bolotov, Vikhrev, Gofarov, and Kondakov leg.; Ba Mawe Khong Stream, 17°56′45″ N, 95°46’7″ E, Ayeyarwady River basin, from the gills of Trapezidens cf. dolichorchinhus (host voucher RMBH Biv1010), 25.iii.2020, one sequenced specimen (sample ID Hyd_1010_2; COI sequence accession number OM350051; 28S sequence accession number OM350021), Bolotov, Vikhrev, Gofarov, and Kondakov leg.
Reference DNA data. Six COI and three 28S sequences were obtained from whole mites. These specimens were completely used for DNA extraction, but the mites of the same sample were studied by us morphologically and are included here to the type series (see sequence accession numbers and locality data in the list of paratypes above).
Type host. The holotype and several Paratypes were collected from the gills of Trapezidens angustior (Hanley and Theobald, 1876) (Bivalvia: Unionidae: Parreysiinae: Lamellidentini) (Figure 3E). Additional paratypes were collected from the gills of Trapezidens dolichorhynchus (Tapparone Canefri, 1889) (Figure 3F).
Etymology. This species is named after its host mussel genus, Trapezidens Bolotov, Vikhrev and Konopleva, 2017.
Diagnosis. Features of the subgenus. Male’s P-2 with 2 lateral and 2 dorsal setae. Female’s P-2 with 4 lateral awl-shaped setae, P-3 with one thin, slender, lateral seta. Female’s P-3 with one hair-like lateral seta. Both for males and females P-IV with ventral tubercle bearing one spinous seta and having one small spatulate seta over the tarsal claws of the walking legs. The shape of female’s II-L-6 and III-L-6 is a dumbbell with a well-defined expansion closer to the basis of the tarsus and a narrowing located distally. Female’s I-L-2 with one or 2 well visible swimming ventrodistal setae (both variants of the trait may be observed within the same specimen).
The chaetotaxy of distal segments of I-L and IV-L varies rather strongly between individuals of the one species due to the big amount of these setae and ambiguity of the interpretation setae position on the legs due to the preparation and distortion/foreshortening of fixation. The size of male’s idiosoma is 878–919 μm (average is 899 μm; N = 3). The length of male’s I-L-6 and IV-L-6 is significantly longer than that for U. (My.) savadiensis and U. (My.) generosa: for I-L-6 length is 141–144 (average is 143); IV-L-6 is 319–338 (average is 330). I-L-4 for males with a row of lateral 3 awl-shaped prominent setae and 2 thin setae. The length of the following parameters for females is significantly longer than that for U. (My.) savadiensis and U. (My.) generosa: P-4 length 163–182 (average is 171); I-L-3 218–225 (average is 222); I-L-4 is 300–303 (average is 301); I-L-5 is 317–329 (average is 322); I-L-6 is 158–164 (average is 162); IV-L-4 is 373–395 (average is 382).
Description (Figure 7). Male morphology. I-L: I-L-1 with 2 lateral and 4 dorsal awl-shaped setae and with one lateral small seta. I-L-2 with 4 dorsolateral awl-shaped setae, one dorsal and one ventral tiny seta, 2 lateral setae and one distal long swimming seta. I-L-3 with 3 curved and 5 awl-shaped lateral setae, 2 lateral slender and one shortened seta, one ventral seta and 3 ventral swimming setae. I-L-4 with 3 blunt, 2 curved, one short and 3 normal setae laterally, one distal and 4 ventral setae. I-L-5 with 2 thin, 2 slender and one normal lateral seta and 8 short ventral slender and thin setae. I-L-6 with one thin ventral seta. The claws of I legs are thick and each with a shortened dorsal clawlet and a long ventral clawlet. IV-L: IV-L-1 with one lateral and one dorsal peg-shape setae, one awl-shaped dorsal, 4 lateral and one-two dorsal blunt setae. IV-L-2 with 5 lateral and 2 distal blunt setae of various length, one lateral and one distal awl-shaped setae. IV-L-3 with one slender dorso-lateral seta, a row of 6–7 dorsal heavy setae, some of them serrated and blunt on tips, 15 lateral, 4–5 ventral and 3 distal heavy setae, some of them are serrated and blunt on tips, and one distal short awl-shaped seta. IV-L-4 with 7 dorsal, 9 lateral, 8 ventral heavy setae, some of them serrated and blunt on tips, and 4 distal blunt heavy setae. IV-L-5 is twice as long as IV-L-3 with 15 ventral heavy setae, 3 normal and 3 heavy blunt lateral setae, 6 dorsal and one distal blunt seta, one ventral and one distal short slender seta, and one latero-distal slender seta. IV-L-6 with 2 dorsal slender setae, 2 dorsal, one lateral, one ventral thin seta, and 7 ventral setae. The claws of IV leg are sickle-shaped and with a short dorsal clawlet and a long ventral clawlet. Genital field consists of 2 pairs of plates with 13 acetabula per side without flaps. Acetabula are concentrated in 2 groups on each side—anterior and posterior. One acetabulum of the posterior group is larger than the rest. There 2 slender setae left and right of the genital field.
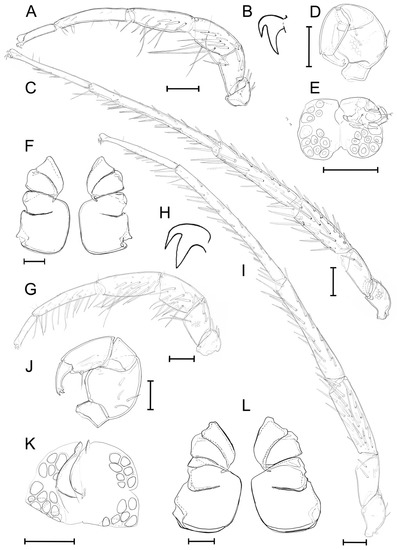
Figure 7.
Unionicola (Myanmaratax) trapezidens sp. nov. The male, slide RMBH Hyd 388: (A) I-L, (B) claw of I-L, (C) IV-L, (D) P, (E) genital field, and (F) coxal plate. The female, slide RMBH Hyd 388: (G) I-L, (H) claw of I-L, (I) IV-L, (J) P, (K) genital field, and (L) coxal plate. Scale bars = 100 µm. (Graphics: Yulia E. Chapurina).
Female morphology. I-L: I-L-1 with 3 lateral peg-shaped setae, one lateral and 3 dorsal setae. I-L-2 with 5 lateral blunt setae, some of them are serrated on tips, one lateral heavy seta, and one distal swimming seta (or one and 2 distal swimming setae within one specimen for each side), and one short dorsolateral peg-shaped seta. I-L-3 with 4 lateral blunt setae, 8 heavy lateral setae, 2 lateral and one distal slender seta, one lateral short awl-shaped seta, and one lateral and one dorsal thin setae. I-L-4 with 4 blunt lateral setae, 11 heavy lateral setae, 4 lateral and 2 dorsal thin setae, and 2 short lateral setae. I-L-5 with 5 lateral and 2 dorsal slender setae, 3 short blunt lateral setae, 3 distal, one ventral and 9 lateral heavy setae. I-L-6 with 2 ventral and 2 dorsal thin setae, 2 lateral and one ventral short seta. The claws of I-L are thick and each with a shortened dorsal and a ventral clawlets. IV-L: IV-L-1 with 4 dorsal awl-shaped setae, one dorso-distal heavy serrated seta, IV-L-2 with 4 lateral serrated setae and 2 distal serrated setae, one of them is peg-shaped. IV-L-3 with some rows of lateral heavy setae, some of them serrated and blunt on tips, a total of 27 lateral setae, one dorso-distal blunt seta, and 2 distal setae. IV-L-4 with 27 lateral setae, some of them are serrated, 4 ventral serrated setae, 2 dorso-distal blunt setae, and 2 distal spinous setae. IV-L-5 with 25 lateral setae, 8 ventral setae, 2 short lateral setae and 2 distal slender setae, and one distal blunt seta. IV-L-6 with 7 ventral setae, 2 heavy, one serrated peg-shaped and 2 tiny hair-like lateral setae, one dorsal seta. The claws of IV leg are forked with a short dorsal and a long ventral clawlet. Genital field consists of 2 pairs of plates with 14–16 acetabula per side, with a pair of flaps bearing 2 hair-like setae on top of the plates per side, and one seta on the inner margin of the genital plate. Acetabula are concentrated in 2 groups on each side—anterior and posterior.
Male measurements (N = 3). Length of the idiosoma: 900 (878–900), length of the posterior coxal group: 287 (262–300); dorsal lengths of the P-1–5 segments: 37 (32–42), 153 (115–160), 70 (57–86), 131 (119–144), 73 (70–77); dorsal lengths of the I-L-1–6 segments: 84 (71–93), 119 (104–127), 174 (153–184), 224 (204–237), 250 (224–265), 142 (138–146); dorsal lengths of the IV-L-1–6 segments: 145 (138–147), 139 (130–152), 260 (246–271), 364 (345–380), 520 (484–539), 330 (312–339); length of the genital field: 127 (118–141), width of the genital field: 171 (162–182). Female measurements. Length of the idiosoma (N = 2): 1265 (1074–1456), length of the posterior coxal group (N = 3): 287 (262–300); dorsal lengths of the P-1–5 segments: 47 (44–52), 189 (157–220), 96 (85–106), 169 (154–182), 91 (85–101); dorsal lengths of the I-L-1–6 segments: 96 (82–103); 153 (148–157); 223 (218–228); 303 (300–307); 321 (311–333); 162 (158–168); dorsal lengths of the IV-L-1–6 segments (N = 3): 183 (173–193), 179 (175–187), 377 (362–398), 524 (505–539), 696 (674–731), 385 (373–397); length of the genital field (N = 3): 198 (182–212), width of the genital field (N = 2): 229 (225–233).
Distribution. Ayeyarwady, Bago and Sittaung basins in Myanmar
Host range. This species is a narrow host specialist, which is known to occur on the gills of two Trapezidens species, namely Trapezidens angustior and T. dolichorhynchus (Figure 3G,H).
3.3. Morphometric Analysis
The statistical analysis was performed to confirm the interspecific differences of measured morphological structures. The species identification by the criteria of the lengths of various morphologic structures is different between males and females for the three new species Unionicola (Myanmaratax) (Table 3).
- For U. (My.) savadiensis, females are significantly larger than males by the idiosoma length (LI) and by other tested parameters (Wilk’s Lambda = 0.00033; F1,9 = 330, 14; p = 0.043).
- For U. (My.) generosa, the lengths of PCG, P-2, P-3, I-L-1 between males and females were similar and do not show statistically significant differences between sexes by the length of tested morphological structures (Wilk’s Lambda = 0.001; F1,9 = 216.89; p = 0.053).
- For U. (My.) trapezidens, the lengths of only two of the 22 tested structures (P-2 and P-3) were similar for males and females, while morphological differences between sexes were not significant (Wilk’s Lambda = 0.008; F1,5 = 29.97; p = 0.136).

Table 3.
The ANOVA and MANOVA (total line) results of morphological structures between three Myanmaratax subgen. nov. species (genus Unionicola) based on linear dimensions (statistically different values are in bold). Abbreviations are given in the “Taxonomic account” section of the article.
Table 3.
The ANOVA and MANOVA (total line) results of morphological structures between three Myanmaratax subgen. nov. species (genus Unionicola) based on linear dimensions (statistically different values are in bold). Abbreviations are given in the “Taxonomic account” section of the article.
| Dependent Variable | Mixed Sample (Both Sexes) | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Square Sum, SS | F2,22 | p | Square Sum, SS | F2,22 | p | Square Sum, SS | F2,22 | p | |
| LI | 169,512.94 | 6.53 | 0.006 | 64,837.75 | 11.53 | 0.002 | 154,223.66 | 3.33 | 0.074 |
| wGF | 712.24 | 2.41 | 0.113 | 344.36 | 1.21 | 0.335 | 1867.61 | 6.11 | 0.016 |
| PCG | 569.08 | 0.67 | 0.522 | 591.84 | 0.53 | 0.602 | 915.45 | 1.56 | 0.253 |
| P-1 | 158.44 | 8.71 | 0.002 | 117.40 | 7.41 | 0.009 | 54.73 | 2.67 | 0.114 |
| P-2 | 1625.47 | 4.23 | 0.028 | 1153.36 | 3.24 | 0.078 | 541.00 | 1.31 | 0.309 |
| P-3 | 345.03 | 1.23 | 0.313 | 92.21 | 0.24 | 0.795 | 370.77 | 2.18 | 0.159 |
| P-4 | 2577.12 | 18.34 | 0.000 | 1294.61 | 5.65 | 0.020 | 1443.49 | 27.77 | 0.000 |
| P-5 | 51.99 | 0.80 | 0.463 | 116.60 | 1.85 | 0.203 | 11.84 | 0.18 | 0.841 |
| I-L-1 | 203.78 | 1.96 | 0.165 | 90.95 | 0.87 | 0.448 | 134.48 | 1.30 | 0.311 |
| I-L-2 | 578.65 | 3.56 | 0.046 | 336.36 | 1.12 | 0.361 | 558.10 | 22.59 | 0.000 |
| I-L-3 | 2694.11 | 16.41 | 0.000 | 1718.73 | 5.46 | 0.023 | 1462.02 | 108.04 | 0.000 |
| I-L-4 | 2521.37 | 5.80 | 0.009 | 2209.81 | 2.81 | 0.103 | 2067.17 | 24.97 | 0.000 |
| I-L-5 | 6515.00 | 14.49 | 0.000 | 3742.71 | 4.58 | 0.036 | 3952.99 | 47.70 | 0.000 |
| I-L-6 | 3071.93 | 56.74 | 0.000 | 1515.34 | 18.99 | 0.000 | 1621.22 | 56.90 | 0.000 |
| IV-L-1 | 876.21 | 3.61 | 0.044 | 193.14 | 0.62 | 0.556 | 862.48 | 4.98 | 0.029 |
| IV-L-2 | 183.82 | 0.79 | 0.468 | 576.20 | 1.75 | 0.220 | 83.46 | 0.61 | 0.563 |
| IV-L-3 | 760.33 | 0.87 | 0.434 | 2058.31 | 1.40 | 0.287 | 1281.20 | 4.50 | 0.037 |
| IV-L-4 | 6942.79 | 3.93 | 0.035 | 3079.19 | 0.98 | 0.406 | 10,032.55 | 25.73 | 0.000 |
| IV-L-5 | 23,001.09 | 9.60 | 0.001 | 6875.27 | 1.77 | 0.215 | 23,021.99 | 25.17 | 0.000 |
| IV-L-6 | 5564.89 | 27.75 | 0.000 | 2231.11 | 7.47 | 0.009 | 3754.15 | 36.71 | 0.000 |
| III-L-5 | 1228.85 | 1.00 | 0.384 | 3241.41 | 1.55 | 0.256 | 5401.17 | 15.01 | 0.001 |
| III-L-6 | 1283.30 | 6.45 | 0.006 | 767.77 | 3.43 | 0.070 | 1388.56 | 7.97 | 0.007 |
| total | Wilk’s Lambda = 0.000003 F2,22 = 25.709; p = 0.038 | Wilk’s Lambda = 0.0001 F2,22 = 8994; p = 0.104 | Wilk’s Lambda = 0.0002 F2,22 = 6.796; p = 0.135 | ||||||
More morphological differences have been found among the lengths of measured morphological structures for females of the three Myanmaratax species (14 out of 22 structures) than among those for males (7 out of 22 structures).
- The PCA scatterplot by the length of 22 morphological structures on the first 2 canonical axes showed that the males of the three species overlap with each other (Figure 8).
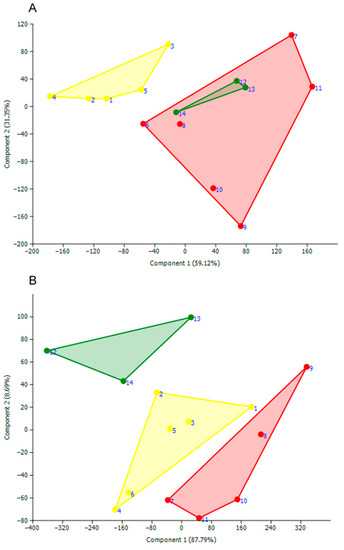 Figure 8. PCA ordination plot of the three new mite species according to the length values of 22 morphological parameters: U. (My.) savadiensis subgen. and sp. nov. (yellow), U. (My.) generosa sp. nov. (red), and U. (My.) trapezidens (green) (see Supplementary Table S2 for detail). (A) males; (B) females. Numbers near points are serial numbers of measured mites.
Figure 8. PCA ordination plot of the three new mite species according to the length values of 22 morphological parameters: U. (My.) savadiensis subgen. and sp. nov. (yellow), U. (My.) generosa sp. nov. (red), and U. (My.) trapezidens (green) (see Supplementary Table S2 for detail). (A) males; (B) females. Numbers near points are serial numbers of measured mites. - In particular, the samples of U. (My.) generosa and U. (My.) trapezidens males share similar ordination in the length space by both axes, and only the sample of U. (My.) savadiensis has a distinct position from the two other species. In contrast, the sample of each species’ females represents a separate cloud in the morphometric space (Figure 8).
It was found that six morphologic structures such as P-4, I-L-3, I-L-5, I-L-6, IV-L-6, III-L-6 are significantly different in length between the three new species for both sexes (Table 3).
- The pairwise comparisons indicated that the lengths of three morphological structures could be used for species identification (Table 4).
 Table 4. Pairwise comparisons of morphological structures between three Myanmaratax subgen. nov. species based on linear dimensions. Abbreviations are given in the “Taxonomic account″ section of the article.
Table 4. Pairwise comparisons of morphological structures between three Myanmaratax subgen. nov. species based on linear dimensions. Abbreviations are given in the “Taxonomic account″ section of the article.- The idiosoma length (LI) of U. (My.) savadiensis is significantly smaller compared with that of U. (My.) generosa and U. (My.) trapezidens (Table 4).
- The tarsus and tibia lengths of the first and forth walking legs (IL-6 and IV-L-5, respectively) of U. (My.) trapezidens are significantly larger than those for the two other species, namely U. (My.) generosa and U. (My.) savadiensis (Table 4).
- Only male examples of U. (My.) generosa do not share significant differences in the length of idiosoma and leg segments compared with those in the two other species, indicating the need for additional morphological features for species identification.
- As for females, the pairwise comparisons have shown that the linear dimensions of telofemur, genu, and tibia of the first and fourth walking legs (I-L-3, I-L-4, and IV-L-5, respectively) were significantly different between the three new species (Table 4). The longest leg segments were detected for U. (My.) trapezidens, while U. (My.) generosa revealed the shortest ones (Table 4).
- Thus, the ratios of linear dimensions of leg segments and idiosoma are useful for identification of the studied cryptic mite species in the subgenus Myanmaratax.
3.4. Taxonomic Key
Finally, taxonomic keys were designed for more accurate identification of the males and females of the species belonging to the subgenus Myanmaratax:
Key to the Unionicola (Myanmaratax) Species based on Morphological Features for Males
| 1 | P-2 with 3 lateral setae and P-3 with 1 seta. Distal seta dorsal to claws at the end of each walking leg is prominent……… | U. (My.) brandti Vidrine, 1985 |
| – | P-2 with 4 lateral setae and P-3 without setae. Distal seta dorsal to claws at the end of each walking leg is insignificantly noticeable……… | 2 |
| 2 | I-L-6 length is 141–144 µm (average is 143 µm); IV-L-6 is 319–338 (average is 330). I-L-4 with a row of lateral three awl-shaped prominent setae and two thin setae. The ratio of the width of the genital field to its length is 1.35 (varies from 1.28 to 1.44)……… | U. (My.) trapezidens sp. nov. |
| – | Average I-L-6 length is 115–120 µm (the size varies from 108 to 132 µm). I-L-4 with a row of lateral setae of the same shape (awl-shaped and blunt). The ratio of the width of the genital field to its length is 1.17–1.18 (varies from 0.95 to 1.34)……… | 3 |
| 3 | The idiosoma size is 745–827 (average is 793). The ratio of the whole length of the body to the length of posterior coxal group for males is 2.7……… | U. (My.) savadiensis sp. nov. |
| – | Length of the idiosoma 860–1054 (average is 945). The ratio of the whole length of the body to the length of posterior coxal group for males is 3.1……… | U. (My.) generosa sp. nov. |
Key to the Unionicola (Myanmaratax) Species based on Morphological Features for Females
| 1 | IV-L-3 is 200–300 µm, IV-L-4 is less than 430 µm, IV-L-5 varies from 310 to 525, IV-L-6 is 250–300 µm……… | U. (My.) brandti Vidrine, 1985 |
| – | IV-L-3 is more than 320 µm, IV-L-4 is more than 437 µm, IV-L-5 is more than 563 µm, IV-L-6 is more than 330 µm……… | 2 |
| 2 | The length of I-L-4 varies from 300 to 304 µm; I-L-5 is 317–329 µm, I-L-6 is 158–164 µm, I-L-2 with one/one or one/two ventrodistal swimming setae (both variants of the trait may be observed within the same specimen)……… | U. (My.) trapezidens sp. nov. |
| – | The length of I-L-4 less than 292 µm, length of I-L-5 less than 302 µm, I-L-6 less than 150 µm, I-L-2 with two or more ventrodistal swimming setae……… | 3 |
| 3 | The shape of II-L-6 and III-L-6 is a dumbbell with a well-defined expansion closer to the basis of the tarsus and a narrowing located distally……… | U. (My.) generosa sp. nov. |
| – | The shape of II-L-6 is weakly dumbbell, and the shape of III-L-6 is a dumbbell with a narrowing located at the distal part of the tarsus……… | U. (My.) savadiensis sp. nov. |
4. Discussion
4.1. Fauna of Mussel-Associated Unionicola Mites in Southeast and South Asia
Here, we describe a new subgenus and three new species of mussel-associated mites, increasing the fauna of this group in Southeast and South Asia to 8 subgenera and 15 species (Table 1). Our comprehensive two-locus phylogeny (COI + 28S) largely supports the subgenus- and species-level clades that were delineated earlier on the basis of the COI marker alone [7]. However, the positions of a few taxa have been changed. For example, a mite species from the freshwater mussel, Physunio modelli Brandt, 1974 (Unionidae: Gonideinae: Contradentini), in Thailand was recovered as a member of the subgenus Unionicola s. str., while the novel two-locus phylogeny indicates that it may belong to a separate subgenus.
Furthermore, this updated phylogeny reveals several undescribed species from Southeast Asia. In particular, we choose not to describe U. (My.) sp. ‘Yaukthwa’ here because our series of specimens is too small for comparative morphological analyses. The Pentatax clade contains two new mite species discovered from members of the freshwater mussel genus Thaiconcha Bolotov et al., 2020 (Unionidae: Gonideinae: Pseudodontini) in Thailand and appears to be a polyphyletic taxon, as U. (Pentatax) aculeata from North America takes a separate position on the tree. It was suggested that U. (Pe.) aculeata should be placed in a new subgenus because these transient mantle mites are morphologically distinctive from the resident gill mites known as U. (Pe.) bonzi (Claparède, 1869) [9]. One new Imamuratax species was also discovered from freshwater mussels belonging to the genus Leoparreysia Vikhrev, Bolotov and Aksenova, 2017 (Unionidae: Parreysiinae: Leoparreysiini). All the new species need future research efforts and will be described elsewhere.
The Myanmaratax subgen. nov. contains four species from Myanmar (including one undescribed species) and U. (My.) brandti comb. nov. from Thailand and Laos. Morphologically, Unionicola (Wolcottatax) arcuata (Wolcott, 1898) and U. (Wo.) weni Vidrine et al., 2008 are similar to Myanmaratax by the shape of coxal plates, but the morphology of L-IV-6 differs between these taxa. In addition, Myanmaratax remotely resembles members of the subgenus Dimockatax, but these mites are larger (length >2 mm) [9] and the structure of the female genital field differs between the subgenera. Species in the new subgenus are characterized by very large COI p-distances from other taxa. Perhaps, this pattern is generally common among Unionicola mites because similar distance values were recorded in other groups [11].
The species, U. (My.) brandti comb. nov., could be distinguished from other Myanmaratax species by the morphology of male pedipalp, since its P-2 with 2 lateral setae and P-3 with 1 seta [36], while males of U. (My.) savadiensis, U. (My.) generosa, and U. (My.) trapezidens have four lateral setae on P-2 and do not have setae on P-3. The other prominent feature distinguishing U. (My.) brandti from others is a noticeable spatulate seta over the tarsal claws of the walking legs [36]. In contrast, this seta is practically not developed in the three new species of this subgenus (see key to Myanmaratax species for males). The chaetotaxy of U. (My.) brandti male’s leg I (I-L) also differs from other Myanmaratax species.
4.2. Subgeneric Taxonomic Concept of the Genus Unionicola
Recently, Smit [51] proposed an updated concept of the genus Unionicola. From his point of view, all the subgenera, except Vietsatax that he elevated to a genus, were placed as synonyms of the genus. Smit [51] noted that the existing subgeneric concept does not correspond to the Unionicola phylogeny and that only a few of the described subgenera could be considered natural groups. Based on the high levels of morphological variability of taxa within the genus, Smit [51] proposed to abandon the use of subgenera until more convincing evidence of their validity will be available.
However, this author [51] did not present any phylogenetic evidence to support the radical, above-mentioned taxonomic solution. Moreover, our novel two-gene phylogenetic reconstruction (COI + 28S) largely supports the morphology-based subgeneric classification of Unionicola that was proposed by earlier scholars [9,29]. Anyway, large and species-rich genera such as Unionicola need to be subdivided into intrageneric groups, at least for identification purposes. Smit [51] does this for other subgenera, e.g., Neumania Lebert, 1879 and Koenikea Wolcott, 1900, in the family Unionicolidae. There are additional examples of widely accepted and useful subgeneric classifications in other invertebrate groups, e.g., bumblebees (genus Bombus, Apidae, Hymenoptera, Hexapoda) [52]. We agree with the opinion of Smit [51] that the subgenera need to be revised, but we think that an updated synonymy of the genus Unionicola must be developed based on the application of modern DNA-based approaches to available subgeneric categories.
Guided by these considerations, we have recently restored the subgenus Dimockatax when describing the new species, Unionicola (Dimockatax) haungthayawensis [14]. Now, we here restored the valid subgenera in this genus as follows: Unionicola Haldeman, 1842 stat. rev. (the type subgenus as defined by Harvey [53] and Edwards and Vidrine [9] [= Parasitatax K. Viets, 1949], Pentatax Thor, 1922 stat. rev., Hexatax Thor, 1926 stat. rev., Polyatax K. Viets, 1933 stat. rev., Unionicolides Lundblad, 1937 stat. rev., Atacella Lundblad, 1937 stat. rev. [= Atacellides Lundblad, 1941], Neoatax Lundblad, 1941 stat. rev., Polyatacides Lundblad, 1941 stat. rev., Heteratax Lundblad, 1941 stat. rev., Unionicolella Lundblad, 1941 stat. rev., Bassatax Cook, 1966 stat. rev., Giselatax K.O. Viets, 1975 stat. rev., Unionicolopsis K.O. Viets, 1980 stat. rev., Armatax Bader, 1981 stat. rev., Fulleratax Vidrine, 1984 stat. rev., Ampullariatax Vidrine, 1985 stat. rev., Anodontinatax Vidrine, 1986 stat. rev., Berezatax Vidrine, 1986 stat. rev., Australatax Vidrine, 1986 stat. rev., Kovietsatax Vidrine, 1986 stat. rev., Cookatax Vidrine, 1986 stat. rev., Baderatax Vidrine, 1988 stat. rev., Heversatax Vidrine, 1988 stat. rev., Lundbladatax Vidrine, 1988 stat. rev., Ferradasatax Vidrine, 1988 stat. rev., Crameratax Vidrine, 1988 stat. rev., Wolcottatax Vidrine, 1992 stat. rev., Prasadatax Vidrine, 1992 stat. rev., Mitchellatax Vidrine, 1992 stat. rev., Everittatax Vidrine, 1992 stat. rev., Poundsatax Vidrine, 1992 stat. rev., Wilsonatax Vidrine, 1992 stat. rev., Bakeratax Vidrine, 1992 stat. rev., Gledhillatax Vidrine, 1992 stat. rev., Davidsatax Vidrine, 1992 stat. rev., Downesatax Vidrine, 1992 stat. rev. [= Smithatax Vidrine, 1992], Lasalleatax Vidrine, 1992 stat. rev., Conroyatax Vidrine, 1992 stat. rev., Curryatax Vidrine, 1992 stat. rev., Edwardsatax Vidrine, 1992 stat. rev., Crowellatax Vidrine, 1992 stat. rev., Australionicola Smit, 1992 stat. rev., Majumderatax Vidrine, 1993 stat. rev., Imamuratax Vidrine, 1994 stat. rev., Causeyatax Vidrine, 1994 stat. rev., Breaudatax Vidrine, 1994 stat. rev., Clarkatax Vidrine, 1994 stat. rev., Mutelicola Gledhill and Vidrine, 2002 stat. rev., Chambardicola Vidrine, Borsari and Bastian-Stanford, 2005 stat. rev., Iridinicola Vidrine, Lötter, Van As, Bastian-Stanford and Hazelton-Robichaux, 2006 stat. rev., Hyricola Vidrine, Bogan and Hazelton-Robichaux, 2007 stat. rev., Coelaturicola Vidrine, Lötter, Van As, Bogan and Bastian-Stanford, 2007 stat. rev., Vidrineatax Smit, 2008 stat. rev., Geikienicola Smit, 2008 stat. rev., and Vidrinatax Wen, 2009 stat. rev. To conclude, we reduce the genus Vietsatax Uchida and Imamura, 1938 stat rev. to subgeneric rank. Detailed descriptions of these taxa were discussed [9].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14100848/s1, Table S1: List of COI and 28S sequences of Unionicola mites used in this study. New sequences generated in this study are in bold. N/A—not available. Supplementary file Table S1 contains references to publications containing data about sequences used for phylogenetic analysis [54,55]. Table S2: Output data for statistical analysis. All lengths are given in µm. All parameters are clarified in the abbreviations section of the ‘Taxonomic account.’
Author Contributions
Conceptualization, Y.E.C., M.F.V., and I.N.B.; Y.E.C., I.N.B., A.V.K., E.S.K., O.V.A., I.V.V., M.Y.G., T.W., N.C. and Z.L. collected data. A.V.K. and Y.E.C. processed DNA analyses of mite samples. Y.E.C. and E.S.K. performed phylogenetic reconstruction. N.A.Z. made statistical analyses. Y.E.C. prepared taxonomic descriptions and mite images. M.Y.G. created the map. Y.E.C. wrote the paper with input from M.F.V., I.N.B., A.V.K., O.V.A., Y.V.B., E.S.K., I.V.V., N.A.Z., M.Y.G., T.W., N.C. and Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by the Ministry of Science and Higher Education of the Russian Federation (projects No. FUUW-2022-0056 to Y.E.C., M.Y.G., I.N.B., and A.V.K.) and by the Russian Foundation for Basic Research (project No. 19-35-90085 to A.V.K. and Y.E.C.). Our research in Myanmar was performed under the survey permission No. 5/6000/LFR(210/2018) dated on 23 January 2018 issued by the Ministry of Agriculture, Livestock and Irrigation of Myanmar and the export permission No. NWCD/CITES/9/5666/2018 dated on 28 June 2018 issued by the Forest Department of the Ministry of Environmental Conservation and Forestry of Myanmar.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Frank Momberg (Director for Program Development and Asia-Pacific Program Director of Fauna and Flora International, UK), Mark Grindley (Country Director of Fauna and Flora International—Myanmar Program, Myanmar), and the staff of the Department of Fisheries of the Ministry of Agriculture, Livestock and Irrigation of Myanmar for their great help during this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mitchell, R. Host exploitation of two closely related water mites. Evolution 1967, 21, 59–75. [Google Scholar] [CrossRef]
- Böttger, K. Parasitologische Beziehungen zwischen Wassermilben und Trichopteren. Zool. Anz. 1972, 188, 154–156. [Google Scholar]
- Jones, R.K.H. Parasitism by Unionicola spp. larvae on chironomids. Hydrobiologia 1978, 60, 81–87. [Google Scholar] [CrossRef]
- Baker, R.A. Development and life strategies in mussel mites. In The Acari: Reproduction, Development and Life History Strategies; Schuster, R., Murphy, P.W., Eds.; Chapman and Hall: London, UK, 1991; pp. 65–73. [Google Scholar]
- Edwards, D.D.; Dimock Jr, R.V. Life history characteristics of larval Unionicola (Acari: Unionicolidae) parasitic on Chironomus tentans (Diptera: Chironomidae). J. Nat. Hist. 1995, 29, 1197–1208. [Google Scholar] [CrossRef]
- Proctor, H.C.; Smith, I.M.; Cook, D.R.; Smith, B.P. Subphylum Chelicerata, Class Arachnida. In Ecology and General Biology: Thorp and Covich’s Freshwater Invertebrates; Thorp, J., Rogers, D.C., Eds.; Academic Press: New York, NY, USA; London, UK, 2015; pp. 599–660. [Google Scholar]
- Chapurina, Y.E.; Bolotov, I.N.; Vidrine, M.F.; Vikhrev, I.V.; Lunn, Z.; Chan, N.; Win, T.; Bespalaya, Y.V.; Aksenova, O.V.; Gofarov, M.Y.; et al. Taxonomic richness and host range of tropical Asian mussel-associated mite assemblage (Acari: Unionicolidae) with description of a new subgenus and species of parasitic mite from freshwater pearl mussels (Unionida: Margaritiferidae). J. Zool. Syst. Evol. Res. 2021, 59, 613–634. [Google Scholar] [CrossRef]
- Wen, C.; Zhu, Z. Seven species of water mites in the genus Unionicola from Jiangxi (Acari: Unionicolidae). Acta Zootaxon Sin. 1999, 24, 30–37. [Google Scholar]
- Edwards, D.D.; Vidrine, M.F. Mites of Freshwater Mollusks; Malcolm, F., Ed.; Vidrine: Eunice, LA, USA, 2013; 336p. [Google Scholar]
- Savatenalinton, S.; Smit, H. New records of water mites from standing waters in Thailand, with the description of nine new species (Acari: Hydrachnidia). Zootaxa 2017, 4312, 69–91. [Google Scholar] [CrossRef]
- Lewisch, E.; Arnold, F.; Fuehrer, H.-P.; Harl, J.; Reichart, U.; Handschuh, S.; Thielen, F.; El-Matbouli, M. First description of freshwater mite Unionicola sauerensis sp. nov. infesting thick-shelled river mussel Unio crassus. Dis. Aquat. Organ. 2021, 145, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Smit, H. The water mites of Western Australia (Acari: Hydrachnidia), with the description of 13 new species. Acarologia 2021, 61, 928–966. [Google Scholar] [CrossRef]
- Ding, Z.; Jin, D.; Guo, J.; Yi, T. Three new species of the subgenus Unionicola Haldeman, 1842 (Acari, Hydrachnidia, Unionicolidae) from Guizhou, China. Zootaxa 2019, 4658, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Chapurina, Y.E.; Kondakov, A.V.; Chan, N.; Vikhrev, I.V.; Bolotov, I.N.; Konopleva, E.S.; Win, T.; Lunn, Z. A new species Unionicola (Dimockatax stat. rev.) haungthayawensis sp. nov.(Trombidiformes: Unionicolidae) from the freshwater mussel Lamellidens generosus (Gould, 1847) in Myanmar. Ecol. Monten. 2022, 56, 28–39. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Kondakov, A.V.; Vikhrev, I.V.; Aksenova, O.V.; Bespalaya, Y.V.; Gofarov, M.Y.; Kolosova, Y.S.; Konopleva, E.S.; Spitsyn, V.M.; Tanmuangpak, K.; et al. Ancient River Inference Explains Exceptional Oriental Freshwater Mussel Radiations. Sci. Rep. 2017, 7, 2135. [Google Scholar] [CrossRef] [PubMed]
- Bolotov, I.N.; Konopleva, E.S.; Vikhrev, I.V.; Gofarov, M.Y.; Lopes-Lima, M.; Bogan, A.E.; Lunn, Z.; Chan, N.; Win, T.; Aksenova, O.V.; et al. New freshwater mussel taxa discoveries clarify biogeographic division of Southeast Asia. Sci. Rep. 2020, 10, 6616. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Klass, A.L.; Kondakov, A.V.; Vikhrev, I.V.; Bespalaya, Y.V.; Gofarov, M.Y.; Filippov, B.Y.; Bogan, A.E.; Lopes-Lima, M.; Lunn, Z.; et al. Freshwater mussels house a diverse mussel-associated leech assemblage. Sci. Rep. 2019, 9, 16449. [Google Scholar] [CrossRef] [PubMed]
- Bolotov, I.N.; Vikhrev, I.V.; Kondakov, A.V.; Konopleva, E.S.; Gofarov, M.Y.; Aksenova, O.V.; Tumpeesuwan, S. New taxa of freshwater mussels (Unionidae) from a species-rich but overlooked evolutionary hotspot in Southeast Asia. Sci. Rep. 2017, 7, 11573. [Google Scholar] [CrossRef] [PubMed]
- Bolotov, I.N.; Pfeiffer, J.M.; Konopleva, E.S.; Vikhrev, I.V.; Kondakov, A.V.; Aksenova, O.V.; Gofarov, M.Y.; Tumpeesuwan, S.; Win, T. A new genus and tribe of freshwater mussel (Unionidae) from Southeast Asia. Sci. Rep. 2018, 8, 10030. [Google Scholar] [CrossRef] [PubMed]
- Bolotov, I.N.; Konopleva, E.S.; Vikhrev, I.V.; Lopes-Lima, M.; Bogan, A.E.; Lunn, Z.; Chan, N.; Win, T.; Aksenova, O.V.; Gofarov, M.Y.; et al. Eight new freshwater mussels (Unionidae) from tropical Asia. Sci. Rep. 2019, 9, 12053. [Google Scholar] [CrossRef]
- Pfeiffer, J.M.; Graf, D.L.; Cummings, K.S.; Page, L.M. Taxonomic revision of a radiation of South-East Asian freshwater mussels (Unionidae: Gonideinae: Contradentini+ Rectidentini). Invertebr. Syst. 2021, 35, 394–470. [Google Scholar] [CrossRef]
- Edwards, D.D.; Vidrine, M.F. Host specificity among Unionicola spp. (Acari: Unionicolidae) parasitizing freshwater mussels. J. Parasitol. 2006, 92, 977–983. [Google Scholar] [CrossRef]
- Edwards, D.D.; Vidrine, M.F. Host Diversity Affects Parasite Diversity: A Case Study Involving Unionicola spp. Inhabiting Freshwater Mussels. J. Parasitol. 2020, 106, 675–678. [Google Scholar] [CrossRef]
- Chapurina, Y.E.; Vikhrev, I.V.; Kondakov, A.V.; Tanmuangpak, K. A new Najadicola species (Acari: Hydrachnidia: Pionidae) from Asia. Ecol. Monten. 2019, 24, 32–37. [Google Scholar] [CrossRef]
- Bogan, A.E.; Hoeh, W.R. On becoming cemented: Evolutionary relationships among the genera in the freshwater bivalve family Etheriidae (Bivalvia: Unionoida). Geol. Soc. Spec. Publ. 2000, 177, 159–168. [Google Scholar] [CrossRef]
- Graf, D.L.; Cummings, K.S. A ‘big data’ approach to global freshwater mussel diversity (Bivalvia: Unionoida), with an updated checklist of genera and species. J. Molluscan. Stud. 2021, 87, eyaa034. [Google Scholar] [CrossRef]
- Majumder, M.Z.R.; Pal, S.G. Adaptations of Unionicola sp., a freshwater mite on Lamellidens marginalis from Bengal. Bicovas 1988, 1, 191–202. [Google Scholar]
- Vidrine, M.F. Majumderatax, new subgenus (Acari: Unionicolidae: Unionicolinae: Unionicola), in Europe and Asia. Int. J. Acarol. 1993, 19, 101–102. [Google Scholar] [CrossRef]
- Vidrine, M.F. North American Najadicola and Unionicola: Systematics and coevolution; Gail, Q., Ed.; Vidrine Collectibles: Eunice, LA, USA, 1996; p. 146. [Google Scholar]
- Wu, H.B.; Wen, C.G.; Guo, W. Sequence variation of the mitochondrial 12S rRNA gene among Unionicola (Wolcottatax) arcuata (Acari: Unionicolidae) from freshwater mussels in China. Int. J. Acarol. 2012, 38, 394–401. [Google Scholar] [CrossRef]
- Ernsting, B.R.; Edwards, D.D.; Timbrook, T.A.; Frerichs, M.M. Preliminary evidence of cryptic species among host-associated populations of Unionicola hoesei (Acari: Unionicolidae). Int. J. Acarol. 2014, 40, 358–365. [Google Scholar] [CrossRef]
- Stålstedt, J.; Bergsten, J.; Ronquist, F. “Forms” of water mites (Acari: Hydrachnidia): Intraspecific variation or valid species? Ecol. Evol. 2013, 3, 3415–3435. [Google Scholar] [CrossRef]
- Lehner, B.; Grill, G. Global river hydrography and network routing: Baseline data and new approaches to study the world’s large river systems. Hydrol. Process. 2013, 27, 2171–2186. [Google Scholar] [CrossRef]
- Lehner, B.; Verdin, K.; Jarvis, A. New global hydrography derived from spaceborne elevation data. Eos Trans. AGU 2008, 89, 93–94. [Google Scholar] [CrossRef]
- Vidrine, M.F. Fulleratax, new subgenus (Acari: Unionicolidae: Unionicolinae: Unionicola), in Southeast Asia. Int. J. Acarol. 1984, 10, 229–233. [Google Scholar] [CrossRef]
- Vidrine, M.F. Three new species of Unionicola (Acari Unionicolidae: Unionicolinae) inhabiting fresh water mussels (Unionacea) in Southeast Asia. Int. J. Acarol. 1985, 11, 125–131. [Google Scholar] [CrossRef]
- Vidrine, M.F. Five new species in the subgenus Parasitatax (Acari: Unionicolidae: Unionicola) from North America and Asia, with a re-evaluation of related species. Int. J. Acarol. 1986, 12, 141–153. [Google Scholar] [CrossRef]
- Hammer, Ø. PAST-Paleontological Statistics Reference Manual, v. 3.05.; Natural History Museum, University of Oslo: Oslo, Norway, 2015. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; p. 1659. [Google Scholar]
- Lobo, J.; Costa, P.M.; Teixeira, M.A.L.; Ferreira, M.S.G.; Costa, M.H.; Costa, F.O. Enhanced primers for amplification of DNA barcodes from a broad range of marine metazoans. BMC Ecol. 2013, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Huelsenbeck, J.P. MrBayes.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Miller, M.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES science gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Viets, K.H. Neue Wassermilben (Hydrachnellae, Acari) von Borneo, Indonesia. Abh. Hrsg. Vom Nat. Ver. Zu Brem. 1957, 35, 8–23. [Google Scholar]
- Viets, K.H. Indische Wassermilben. Zool. Jahrb. Abt. Syst. Geogr. Biol. Tiere 1926, 52, 369–394. [Google Scholar]
- Hevers, J. Unionicola species (Acari: Hydrachnidia: Unionicolidae) from Madagascar. Stuttg. Beiträge Zur Nat. A 2012, 5, 49–71. [Google Scholar]
- Pešić, V.; Zawal, A. A new species in the water mite subgenus Majumderatax Vidrine, 1993 from Sri Lanka (Acari: Hydrachnidia). Zootaxa 2018, 4457, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Smit, H. Water Mites of the World with Keys to the Families, Subfamilies, Genera and Subgenera (Acari: Hydrachnidia); Nederlandse Entomologische Vereniging: Leiden, The Netherlands, 2020; p. 774. [Google Scholar]
- Williams, P.H.; Cameron, S.A.; Hines, H.M.; Cederberg, B.; Rasmont, P. A simplified subgeneric classification of the bumblebees (genus Bombus). Apidologie 2008, 39, 46–74. [Google Scholar] [CrossRef]
- Harvey, M.S. The Australian Water Mites: A Guide to the Families and Genera. Monographs in Invertebrate Taxonomy; CSIRO Publishing: Collingwood, VIC, Australia, 1998; Volume 4, p. 150. [Google Scholar]
- Ernsting, B.R.; Edwards, D.D.; Vidrine, M.F.; Myers, K.S.; Harmon, C.M. Phylogenetic relationships among species of the subgenus Parasitatax (Acari: Unionicolidae: Unionicola) based on DNA sequence of the mitochondrial cytochrome oxidase I gene. Int. J. Acarol. 2006, 32, 195–202. [Google Scholar] [CrossRef]
- Edwards, D.D.; Vidrine, M.F.; Ernsting, B.R. Phylogenetic relationships among Unionicola (Acari: Unionicolidae) mussel-mites of North America based on mitochondrial cytochrome oxidase I sequences. Zootaxa 2010, 2537, 47–57. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).