Abstract
The alpine belt hosts the treeless vegetation above the high elevation climatic treeline. The way alpine plants manage to thrive in a climate that prevents tree growth is through small stature, apt seasonal development, and ‘managing’ the microclimate near the ground surface. Nested in a mosaic of micro-environmental conditions, these plants are in a unique position by a close-by neighborhood of strongly diverging microhabitats. The range of adjacent thermal niches that the alpine environment provides is exceeding the worst climate warming scenarios. The provided mountains are high and large enough, these are conditions that cause alpine plant species diversity to be robust against climatic change. However, the areal extent of certain habitat types will shrink as isotherms move upslope, with the potential areal loss by the advance of the treeline by far outranging the gain in new land by glacier retreat globally.
Keywords:
biodiversity; high-elevation; mountains; phenology; snow; species distribution; treeline; topography; vegetation; warming 1. Introduction
The alpine world covers a small land fraction, simply since mountains get narrower with elevation [1]. Globally, 2.6% of the terrestrial area outside Antarctica meets the criteria for ‘alpine’ [2,3], a terrain still including a lot of barren or glaciated areas, with the actual plant covered area closer to 2% (an example for the Eastern Alps in [4]). Surprisingly, about 4% of all known angiosperm plant species live in that bioclimatic belt, thus, twice the number of one would be expected from the area alone, despite the often demanding life conditions [5]. The alpine world clearly facilitates high biodiversity. In part, this also holds the answer to why alpine terrain is a relatively safe place when it comes to coping with climatic change [6]. Due to the geographical distribution of mountains, the highest alpine plant species richness occurs at mid latitude, that is the temperate zone [7].
2. Geodiversity Drives Biodiversity
In order to explain this seemingly paradoxical conclusion, a definition of ‘alpine’ and of the life conditions in the alpine belt are required. By definition, the alpine belt’s downslope delineation is the climatic treeline. Therefore, the alpine belt is treeless. The term tundra should not be applied to the alpine belt, since it rather constitutes a certain land cover type in the arctic world. The treeline is defined as a life form limit, meaning no tree species can surpass the treeline, and most tree species do not reach this line, but a few do. Globally, the treeline tracks a common isotherm of seasonal mean temperature of about 6 °C, irrespective of latitude and thus, season length (a minimum of 3 months is required though, [8]). Using an algorithm based on freely available climatic data (e.g., from WorldClim), the climatic treeline, and with it, the lower edge of the alpine world, can be predicted with a precision of 78 m of elevation, with the remaining uncertainty partly related to the limited predictive power of data from scattered weather stations for air temperature at treelines [9]. Additionally, the treeline position always lags behind the regional climate changes. It may take more than a century for treeline to arrive at an equilibrium state with the climate.
It is evident that the transition from the montane forest to alpine vegetation is driven by size-dependent plant aerodynamics and not by tree-specific physiological shortcomings. Any tall structure such as a tree is aerodynamically coupled to the atmosphere, with convective heat transfer rapidly removing any warming of branches by the sun. This is also the reason why treelines reflect the thermal layering of the atmosphere. In stark contrast, smaller stature plants are nesting their shoots in a dense, short canopy in which heat convection is reduced, thus causing solar heat to become trapped. By their small stature and high foliage density, alpine plants engineer a microclimate that differs greatly from free air and what trees at treeline experience [6]. Counter expectation, alpine plants operate at much warmer temperatures than trees at treeline as long as the sun is out. This microclimate effect had been quantified for the entire growing seasons in temperate and arctic alpine environments [10,11]. Including all bad weather, the mean temperature for the growing season at the level of plant meristems may vary between 6 and 14 °C on a single alpine slope, depending on topography and canopy structure. Therefore, different plant species assemblages are living next to each other at seasonal mean temperatures that differ by 8 K (following applied physics, temperature differences are best addressed by the Kelvin), which is around twice the worst climate warming scenario for the same region. The striking role of exposure and its thermal effects was also evidenced for a broad spectrum of summits across Europe [12].
In order to understand alpine plant life, one has to account for the mosaic of life conditions produced by the interaction of topography (part of geodiversity), plant stature, solar radiation, and wind (Figure 1). The warmer living conditions during the day for alpine plants compared to treeline trees are well reflected in the thermal adjustment of basic metabolism. The temperature optimum of leaf net photosynthesis in alpine plants at 500–600 m above treeline is around 22 °C, that is, the temperature these plants actually experience during sunny weather [6,13]. An important message is that climatic data obtained from weather stations are unsuitable to define the life conditions of small stature plants in general and alpine plants in particular [10,11,14,15].
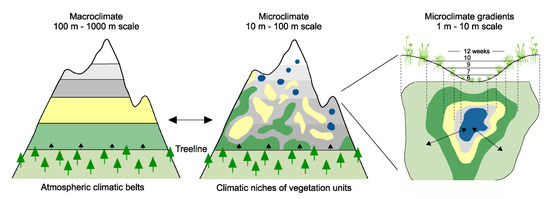
Figure 1.
Above the treeline, the alpine vegetation follows the patterns of life conditions created by topography (microclimate) not captured by air temperatures obtained from weather stations (macroclimate). The mosaics of thermal habitats provides steep climatic gradients at very small scales (zoomed-in example to the right). Snow accumulation in snowbeds can cause the length of the growing season to vary by 6 weeks across a distance of 10 m, with even larger differences across exposure gradients (e.g., N-S aspect, wind edges).
There is another facet to the central role of topography for alpine plant life at extratropical latitudes: The snow distribution during the dormant season. A snowpack can cause a difference in season length between convex and concave land surfaces from 2 (snowbeds) to 6 months (ridges). Since snow is perfectly insulating, soils and plants under snow rarely freeze in winter (Figure 2). Snow distribution caused by the interaction of wind and topography creates a mosaic of habitats with either no winter time freezing or most severe freezing stress over a distance of a few meters (Figure 3).
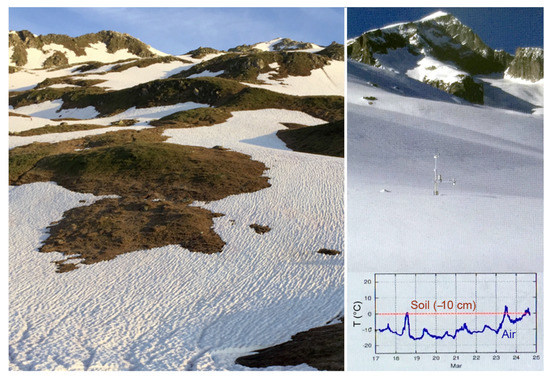
Figure 2.
Left: Snowpack determines the length of the growing season (photo taken on 15 June 2018 at 2440 m in the Swiss Alps). Right: Snow cover decouples life conditions from ambient atmospheric conditions and ensures often non-freezing conditions during winter (screen shot of the webcam at the same location, viewing a location slightly to the right of the same place, but on 24 March 2021).

Figure 3.
Plant species growing on wind edges face severe winter conditions as exemplified for a 2440 m elevation ridge with Kalmia (=Loiseleuria) procumbens and tussock fragments of Carex curvula near Furka Pass in the Swiss Alps (4 February 2021), including low temperature extreme as well as heating to >20 °C under strong solar radiation in winter [16].
Furthermore, topography in the interaction with gravity and bedrock chemistry, shapes patterns of soil diversity. Soil properties have been successfully tested for adding explanatory power to predictive distribution models. By employing a suite of topography related ground phenomena, Buri et al. [17] arrived at concluding that soil pH and soil moisture are exerting the strongest additional effect in shaping alpine plant species distribution. These properties are not directly linked to the climate, but severe drought could have an influence.
In summary, topography (geodiversity) creates biodiversity in alpine landscapes, with the action of gravity adding gradients of soil moisture and nutrients, enhancing the diversification of life conditions. In order to understand the alpine plant life, the weather station derived climatic data are inept, making it very difficult to delineate the cold edge of the fundamental niche of alpine species.
3. Alpine Plant Life Strategies
Most alpine plants are ‘designed’ to live long and to persist. Some are older than the oldest living trees on Earth. Longevity is a result of clonal life. When age is counted from zygote formation, some alpine plants may date back to the early pioneer state after the retreat of the glaciers some 10–12 thousand years ago. For Carex curvula and several other important clonal species, ages of several thousand years have been calculated from estimated DNA-based clone size data and the known rate of clonal expansion [18,19]. This means, on the very same spot in the landscape the same genet had been present during the medieval warm period, when Vikings discovered Greenland and America, but also during the little ice age when glaciers came down to 1800 m elevation in some places. These clonal plants most likely also inhabited the same location during the warmest periods of the Holocene (see below).
An important trait ensuring longevity and thus surviving both seasonal climatic extremes as well as disturbances is the below-ground position of apical meristems as well as cohorts of preformed flowering buds (e.g., [6,20]). Below ground stems and buds, rather slow clonal spreading and massive belowground storage organs [21] together ensure that alpine plants are hardly affected by the year-to-year variation of weather conditions.
The situation is different though, for pioneer communities on newly released substrate such as glacier forefields or summit regions that became inhabitable due to climatic warming after the little ice age and ongoing warming (see below), tracking the global trends at a regionally enhanced speed. In the Alps warming since the late 19th century arrived at 2 K by the combination of the climatic ‘swing back’ after the little ice age and current acceleration of climatic warming (Figure 17.1 in [6]).
Not often considered a ‘strategy’, it is one of the most fundamental ones, namely how plants utilize the season and control their development (phenology). Outside tropical latitudes, the seasonality of the climate poses a selective force towards preventing plants from tracking the ever unpredictable weather, but rather sticks to an evolutionary pattern of initiating and ceasing seasonal activity based on a risk mitigation strategy. Such a phenology minimizes losses due to late freezing in spring and secures nutrient recovery, timely senescence, fruit maturation, and reserve formation before the freezing season starts, irrespective of the particular weather conditions in a given year. These species-specific phenology patterns may not be obvious in ‘normal years’ but become visible under extreme situations, with very early snowmelt or very late onset of winter. Photoperiod and, in part, an internal clock in winter-active species (e.g., [22]) ensure survival and fitness, but also constrain benefits of a warmer climate for which the genetic setting had not been selected for ([23], see below).
4. The Critical Role of Snow
Snow duration, snow pack, and topography related snow distribution are central towards an understanding of alpine plant life in extratropical regions. Snow entirely decouples alpine plant life from atmospheric conditions (Figure 2). While air temperatures may fall below −20 °C, less than half a meter of snow will suffice to maintain the soil surface as unfrozen. One of the reasons for a 0 °C interface between snow and soil is the warmth stored in soil over the growing season, and the high heat capacity of soil. As a consequence, plants on snow covered terrain experience rather mild winter conditions. It had been shown that plant growth can continue under several meters of snow [22] and unfrozen soil under snow permits microbial activity that causes much of the seasonal ecosystem carbon gain to become recycled over the snow-covered period [24]. A lack of snow can become fatal for plants adapted to life under a continuous snow cover in winter. Those adapted to survive without snow cover on ridges and wind edges (Figure 3) must tolerate temperatures that may be even colder than the concurrently measured air temperature, since the ground can cool several K below air temperature during clear winter nights due to radiative heat loss.
Since the topography-driven geometry of snowbeds is ‘carved into the landscape’ while the timing of snow release varies from year to year, the presence of so-called snowbed species is likely to become affected less by climatic warming than that of other groups of alpine species [25], since their snow cover is a result of the interaction of topography and wind rather than snowfall as such. Thus, the core of alpine snowbeds will exist no matter how warm the climate realistically might become, although snow release may occur earlier, depending on snow pack, and the size of these special habitats may shrink. Wind edge species will not become seriously affected either, since their phenology has been selected for coping with the absence of snow protection and solar warming of canopies at the wrong time. The classical wind edge species Kalmia procumbens in the Alps is not going to ‘wake up’ when the late winter sun heats its bare canopies to more than 20 °C ([16], Figure 4.8 in [6]). It is the large spectrum of alpine species that occupy habitats in between those two extremes (snowbeds versus wind edges) that are more likely to face new challenges.
In a recent alpine snow removal/snow addition experiment in combination with the natural year-to year variation of snowpack, Vorkauf et al. [23] showed that alpine grassland plants in the Swiss central Alps that do not belong to either of the above habitat types largely fall into two categories, those that track the date of release from snow and respond to temperature sums, and those that follow temperature only once the photoperiod indicates a safe period. The earlier, that is, the further away from that ecotypic photoperiod window they become released from snow, the more strongly the photoperiod restricts the onset of flowering. The authors arrived at concluding that the studied photoperiod-sensitive species are unlikely to profit from the earlier snow melt in a warmer climate in terms of additional growth by a longer growing season, nor will they suffer from accelerated freezing risk since they have their phenology under control. In a comparison of arctic and alpine plant species, Oberbauer et al. [26] also found that many species are not just tracking temperature but rather perform a conservative phenology that suggests a modulating role of photoperiod. Comparing snowmelt date and the occurrence and severity of freezing events soon after snowmelt over the past 46 years, Klein et al. [27] found no enhanced risk by frost in the Swiss Alps. This conclusion rests on the assumption that plants are tracking snowmelt data so that they arrive at similar developmental stages soon after snowmelt irrespective of when snowmelt occurs. However, species that do track snowmelt date in a current climate (rather than the tracking photoperiod) may not do so in a significantly warmer climate, further questioning the enhanced freezing damage hypothesis. Of the nival flora of the Alps, half of all >30 species tested by Keller and Körner [28] exhibited strong photoperiodism once exposed to an exceptionally early release from snow. Unpublished observations by P. Möhl and E. Hiltbrunner (Basel) suggested that species known as ‘snowmelt trackers’ (such as Carex curvula) also senesce earlier. Hence, they do not take any advantage of the resulting longer season in terms of biomass production, but rather complete their seasonal developmental cycle after c. 10 weeks despite the continuation of favorable conditions. In the long run, such conservative developmental responses may permit ‘native invasive’ species from lower elevation to outcompete these typical alpine species at their lower distributional edge or in habitats with incomplete plant cover.
The weight of the different physiological drivers of phenology (internal clock, photoperiod, temperature, and their interactions) is mostly unknown, making predictions difficult: An exceptionally early snowmelt in 2020 revealed quite unexpected patterns of flowering phenology, causing species known for uniform and early date of peak flowering to exhibit a vast extension of the flowering period over almost a month (Geum montanum) [6]. Internal settings that do not come into action under ‘normal’ snowmelt regimes can be overrun by massive shifts of snowmelt (thus, buffering the effect of the date shift). Such a diversification of phenology may be the starting point for genotype selection for novel climatic settings. Rather than exploring mean responses, the existing, but commonly hidden response variation to early snow release within populations may reveal future success potential.
5. Species Range Limits
At this point, we like to emphasize that we are still unable to explain the low temperature range limits of alpine species on a mechanistic basis [6]. The reasons are manifold. Such questions had not been asked, the actual life conditions are unknown unless measured, the required experiments involve quite some laboratory equipment and experience (freezing tolerance), the developmental cues which protect plants from becoming active at the wrong time remain unknown, and the actual edges of distribution are hard to identify due to the fragmented microhabitas (Figure 1). Therefore, we are still left with mainly statistical space for time approaches, commonly tied to atmospheric conditions rather than the actual life conditions of plants, and such approaches yield scale (space) dependent effects [29,30,31,32,33,34]. The smaller the geographical scale, that is, the closer it comes to actual habitat mosaic size, the smaller is the predicted fraction of lost habitat types under different climate warming scenarios. In a study of soil and air temperatures, Löffler and Pape [35] observed pronounced spatial and seasonal variability of microhabitat temperature across an elevation gradient in the southern Scandes, related to species occurrence data. It seems imperative that the research community turns to the subject of range limit explanation and understanding the fundamental niche, but also for substantiating predictive attempts in a climate change context (see the epilogue in [6]).
6. Change in Alpine Land Area Due to Climatic Warming
Biodiversity needs space. It had been shown that the reduction of alpine species numbers with elevation across the globe can be explained by the reduction of available land area only (Figure 2.9 in [6]). Yet, the selective action of low temperatures is clearly limiting the number of species that can cope with increasingly high elevation, explaining global patterns of phylogenetic relatedness of alpine floras [36]. However, ongoing environmental changes at high elevation include more than just a rise in air temperature (Figure 4) [4]. When temperature comes into play, a distinction of gradual changes of means and the action of extremes needs to be distinguished [14]. Furthermore, a warmer atmosphere causes air masses to reach mountains with a higher water vapor content, potentially enhancing snowpack at high elevations, but warmer conditions will also increase evaporative forcing, and thus also incur more severe drought during heat wave episodes. While elevated CO2 concentrations have been shown to have no effect on alpine vegetation [37,38], atmospheric nitrogen deposition is a severe threat for these commonly rather oligotrophic systems that are adjusted to obtain their nutrients from nutrient cycling [39,40]. The individual and interacting influences of these changes act upon alpine plants through two primary pathways: The effects on development (phenology, internal state of preparedness to grow), and the effects on growth (the activity of meristems, the acquisition of resources; Figure 4).
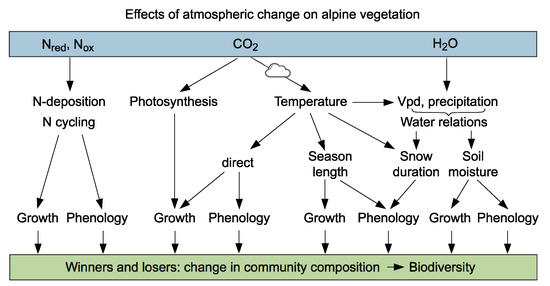
Figure 4.
Examples of environmental drivers of high elevation plant life that may change to a variable degree with time and geographic regions. As a consequence of these changes, some species may profit (gain abundance or space), while other species may lose terrain, potentially causing competitive shifts in community composition and plant species diversity.
A warmer climate alone will act upon the mosaics shown in Figure 1 in two ways: It may change patterns of life conditions at very small scales within patches (largely due to changes in snow duration at extratropical latitudes), and it can be assumed to move all thermal habitat types upslope, while the relative gradients within habitats are retained. The microclimatic benefits of dense, short stature plant communities will be retained, but the entire microhabitat will track atmospheric changes and thus, thermal habitat mosaics will shift in proportion to the climatic warming [30]. Since snow cover interacts with the potential action of temperature, the snow depth, and thus, duration of snowpack plays a role. Such a shift will thus have a time component (effective season length) and a temperature component within the growing season. A recent analysis in the Swiss Alps revealed that snowmelt strongly depended on spring air temperatures, more specifically when the first day of a 3-week running mean of daily air temperatures passed a 5 °C threshold (44% of the variance explained) and mean winter snow depth accounted for 30% of the variance [41].
At a larger scale, the alpine world faces two major influences on its areal extent: The opening of new land as glaciers retreat and the loss of land to an upslope advancing montane forest. The extent of the net change in alpine land area can be estimated on the basis of estimated future glacier wasting, and assuming that the treeline may arrive at a new steady state position that mirrors the global treeline isotherm of seasonal mean air temperature (weather service data will be a robust basis in this case).
Although this is a coarse estimation only, it provides the order of magnitude of the net changes that will result in the remaining terrain that is suitable for alpine plant species and their diversity in the future. It is important here, that mountainous land narrows with elevation, causing any change in bioclimate to exert greater absolute areal consequences at the lower edge of the alpine belt than in the colder, higher parts. However, any advance of lower belts to higher elevation exerts greater relative losses of an otherwise already small high alpine/nival terrain (Figure 5). A second issue is that soils are not going to shift, since their development takes several hundred years. This means, the late successional lower alpine vegetation on well-developed soils, in large, cannot shift into the often barren upper alpine/nival world. Thus, the following estimation neglects soil effects.
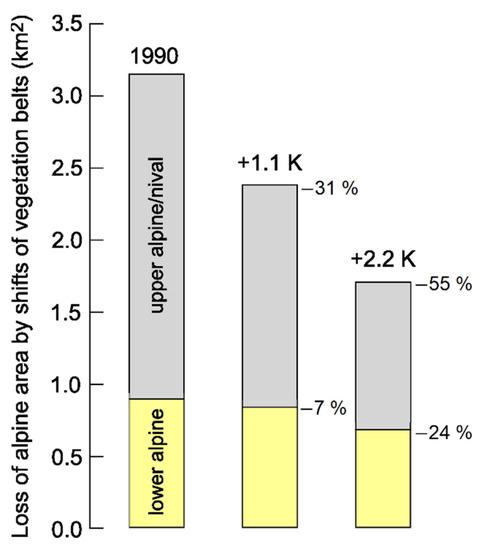
Figure 5.
Estimation of the losses of alpine terrain due to the upslope advance of treeline, assuming a new steady state at either +1.1 K (+200 m) or +2.2 K (+400 m of elevation). The lower alpine belt is assumed to shift irrespective of the state of soil development, inevitably diminishing space for the upper alpine/nival belt. The gain in global alpine space due to glacier wasting is too small (globally c. 0.126 Mio km2, see the text) to be visualized at that scale (data from [6]).
The advance of the treeline in response to climatic warming is a dynamic process and may be faster or slower depending on stochastic events, including extreme weather conditions and all sorts of disturbances [8]. By the time the treeline will have reached a steady state position with a 1.1 K or 2.2 K warmer climate, it will be 200–400 m higher in elevation (assuming a lapse rate of seasonal air temperature by 0.55 K per 100 m). Using the treeline model by Paulsen and Körner [9] and the digital elevation model and climatic data from WorldClim, the future position of treeline will cause the global potentially vegetated land above the treeline to half (−55%) for a world 2.2 K warmer than the year 1990 (Figure 5). The relative effect rises with elevation since this estimation assumes that the late successional, lower alpine vegetation can shift upslope as well, and thus, will reduce the terrain currently inhabited by partly open vegetation on fragmented higher alpine/nival ground. As already mentioned, this estimation does not account for effects of soil development and altered moisture availability. It had been proposed that there is an alpine grassline in analogy to alpine treeline, meaning that connecting the position of patches of closed, very high elevation grassland communities also follows a common isotherm [42]. Since such grassland fragments are tied to stable substrate and certain topographic niches, they are unlikely to shift upslope, but are likely to retain their position, given they are currently representing the cold range limit of this type of alpine vegetation with a substantial leeway towards warmer conditions. They may become enriched with species from lower elevation, though.
There are also threshold phenomena that dampen the tracking of plants of a continuously warming climate. These thresholds may be related to a minimum temperature required for development and growth, as is the case in treeline formation [43] or they may exert singularities such as extreme freezing events that exceed the freezing tolerance of a species [44]. As long as such thresholds are not surpassed, there is a wide range of temperatures that may add to vigor, but do not exert a decisive role for species presence. This was shown for the recent history of growth responses of trees in the uppermost montane forest in the Alps that exhibit a wave-like growth enhancement as an isotherm passes upslope, with little change thereafter [45]. Similarly, the above mentioned grassline appears to represent a thermal threshold position, but once conditions facilitate the establishment of such communities, not much is going to change as the climate warms since these communities still operate within their thermal range [42]. Another example is the alpine rhizomatous tall herb Rumex alpinus. Rhizomes of these plants showed pronounced growth enhancement in response to recent climatic warming in the Tatra Mountains, but as warming induced more drought, the effect vanished [46]. Yet, whatever the climate, those species will remain confined to extremely nutrient rich patches (cattle resting places), with soil conditions delineating their presence.
Glaciers are currently covering 252,000 km2 globally (outside Greenland and Antarctica) [47]. At least half of that glaciated area is above an elevation where glaciers are likely to disappear in the near future and where alpine plant life could establish (the glaciers of Alaska alone cover 87,000 km2). If we assume the lower half of all these glaciers would be wasted globally, 126,000 km2 of raw substrate would become available for pioneer plants. This gain in ice-free land corresponds to 4% of the current alpine land area of 3.55 Mio km2 (an area that includes glaciers and arid parts, without which the reference terrain is 3.15 Mio km2 only). Obviously, the gain in glacier forelands by 0.126 Mio km2 provides no compensation for the 1.5 Mio km2 potential loss of alpine terrain to the advancing montane forests, once both processes reach an equilibrium state with a 2.2 K warmer climate. Therefore, the actual addition of novel, inhabitable land by glacier melting is negligibly small. Even the complete disappearance of mountain glaciers (0.25 Mio km2) would add little land to alpine/nival vegetation at a global scale. Since glaciation varied strongly in the younger geological past, current alpine vegetation needs to be viewed as the net outcome of these changes.
7. Viewing Alpine Plant Life over Geological Time Scales
The glacial cycles operated at roughly 100,000-year intervals and affected all high latitude mountains such as the Alps, the Rocky Mountains or the Hindukush Himalaya region. The ‘drama’ of glaciation and deglaciation of alpine terrain recurred 8–10 times during the last 1 million years, meaning that all but some rock flora on so-called nunatak outcrops [48] that protruded the ice shield became locally eradicated and pushed into refugia at the low elevation edges of the ice shield [49]. Since these refugia were often not connected, the fingerprints of genetic differentiation into clusters of greater similarity within species can still be found in the current alpine flora [4,50,51,52]. The different routes of re-colonisation added to such patterns [53]. The time frame of these massive shifts where much shorter than the mean speciation time [54], underpinning the great resilience but also ability to migrate up- and downslope of what we call alpine species today.
Over shorter time scales, climatic warming during the Holocene caused the treeline to shift upslope by 200–250 m compared to today’s position in the Alps during the so-called Atlanticum (6000–8000 years BP), the hitherto warmest period during the Holocene [55]. The warming persisted long enough, so that vegetation could track it. Therefore, if such warming would affect alpine biodiversity, the current alpine flora in the Alps is the left-over of that period.
Over longer time-scales, environmental changes associated with the uplifting of mountains added to speciation and diversity, with the climatic ups and downs even exerting a sort of ‘species-pump’ effect as some authors have suggested [56]. Such longer-term climatic cycles should be kept in mind, when judging the consequences of current climatic shifts on the alpine flora. The important point is that ‘change as such’ is not necessarily negative to alpine plant species diversity, provided there is sufficient space, elevation, and time. Species are capable of tracking such changes quite rapidly on geological time scales [57]. Smaller mountains with a narrow alpine belt are more likely at risk to lose species than mountain ranges with a wide range of elevations and large areal extent. It is important to make a clear distinction between the local disappearance and the extinction of a species. This distinction often gets lost in the public debate. In our view, the term extinction should be strictly and exclusively be applied to the loss of a species from plant Earth (no such evidence for alpine taxa due to the current warming). All other cases would best be addressed as a regional or local loss or reduced abundance of species, clearly avoiding misinterpretation.
8. Alpine Plant Diversity under Global Change
There is clear evidence that plant communities at the upper edge of plant life on summits have been changing in response to the little ice age cooling with a significant reduction in species, followed by a recovery as the climate warmed in the early part of the 20th century [58,59,60]. This trend became substantially accelerated as 20th century climatic warming occurred, which exceeded global mean trends by a factor of two for the Alps and even more in some arctic-alpine regions. For the Swiss Alps the climate warmed already by 2 K since 1874 (0.14 K per decade with the most rapid change since the early 1980s; MeteoSwiss, Figure 17.11 in [6]). The warming observed in the Hindukush Himalaya region is running at 0.104 K per decade, again with the fastest change in most recent decades [61]. The global GLORIA network evidences world-wide rises of species numbers on summits in response to these warming trends, and the data for Europe show a clear thermophilisation of pioneer communities at the upper edge of plant life [62,63,64], with the exception of Mediterranean mountains, which become affected by severe drought. There are examples for rising species numbers from many parts of the humid alpine world (for a synthesis see [6]). This rebuilding of communities at decadal scales also includes local disappearance of otherwise common species [65], which does not mean that these species got extinct. The more mobile species are, the more likely they are capable of tracking climatic changes by migrating, which includes animals, birds in particular [66,67]. Along the entire climatic gradients, the phenology of plants changes, and in part is tied to this, the seasonal development of animals and fungi that interact with them [68].
Communities in the lower part of the alpine belt have not yet shown significant change [69] and the advance of lower elevation taxa into the montane belt was found to increase species richness [70]. Scherrer and Körner [11] illustrate, why a moderate rise in temperature should rise the overall plant species richness: Climate warming opens new habitats for species from lower elevations without losing the coldest types of habitats from a region due to topography related habitat diversity and cooler upslope escape routes. Such microtopography related patterns of species distribution (Figure 1) provide unbeaten monitoring possibilities in order to assess longer-term trends that cannot be spotted by the eye [4,71]. At such scales, other environmental drivers such as soil conditions, nutrient availability, accumulative influence of nitrogen deposition or changes in moisture regime strongly co-influence plant life (e.g., [72]). As mentioned above, accounting for topographic diversity, also modelling approaches arrive at smaller than often expected changes in species distribution at high elevation [30,31].
Legacy effects represent an important issue (often called ‘extinction debt’), meaning that plants hardly ever exhibit instantaneous range changes in response to external forces, but may need time to respond, with various attempts at estimating time to ‘extinction’ in alpine species [73,74,75,76,77]. In some clonal taxa such response times may exceed several thousands of years [6,18,19], since they do not depend on regular sexual reproduction. Predicting population shifts by sexual reproduction and/or propagule dispersal requires parameterization of life tables [78,79], that is, attributing validated transition probabilities from one life stage to the next: Seed to established seedling, seedling to reproductive state (with a clonal bypass), senescence and mortality (Figure 13.6 in Körner 2013 [80]). Such transition probabilities are also influenced by biotic interactions (herbivory, pathogens) and by physical disturbances. Attempts at a parameterization of such life tables help in identifying critical life stages (for an alpine species that switches between sexual and clonal reproduction see Weppler et al. 2006 and Scherrer et al. 2017 [78,79]). Yet, modelling time-to-extinction without such probability data comes close to a lottery, and accounting for clonal life and habitat mosaics is imperative.
9. Conclusions
Overall, the debate on alpine biodiversity under global change has suffered from various assumptions and confusions, and in particular from the restriction to climatic warming. First, in the case of climatic warming, a classical assumption was that alpine plant life prior to climatic warming was constrained by low temperature (‘stressed’ alpine plants). Now, a warming by 2 K is supposed to exert stress on the same species. These two views at alpine plant life obviously contradict each other and reflect an anthropocentric bias, ignoring the massive contrast between weather station based climatic trends and actual life conditions of plants. As Scherrer and Körner [10,11] showed, (1) the mean temperatures for an entire growing season on alpine slopes varies by a multiple of the worst IPCC climate warming scenarios. (2) The changes that had been observed so far, mostly concern pioneer species, and suitable habitats for such species became richer in species, as one would expect. (3) Clonal life strategies stabilize plant communities over very long periods. (4) It was noted with surprise, whenever tested, that isolated populations of alpine plant species are genetically very diverse [81,82]. (5) The advance of trees is much slower than the ongoing upslope shift of the isotherm, leaving trees and adjacent alpine terrain behind, and thus, exposed to higher than treeline-temperature [8,83]. As tree recruitment follows the isotherm shift, a new, fragmented, topography-related ecotone will be established at higher than the current elevation. The upslope shift of the lower alpine belt (to the extent soils permit) will reduce the areal extent of the upper alpine/nival belt (neglecting soil constraints) modulated by the height of mountains and the (small) compensatory effect of new land released from melting glaciers. Yet, due to persistent topographic diversity, the elevational narrowing of suitable land will unlikely eliminate rock and fellfield species from a region. It should be recalled that nunatak ‘islands’ had substantially contributed to the re-establishment of alpine belts in the early Holocene.
The populistic and alarmistic tone in many comments addressing alpine plant life in a changing environment rests on the assumption that plants ‘at the limit’ should be more vulnerable. However, the majority of these plants are not at all operating at their physiological limit. They are rather inhabiting habitats they were selected for, with robustness against variability and unpredictability of climatic conditions one of the major selective forces. It is part of the very nature of the alpine environment that life conditions can change rapidly over short periods of time and across very short geographical distances. The latter, in fact, secures the presence of suitable habitats in a region, and makes the alpine world one of the safest for plants when the climate changes [6]. The situation may differ for certain highly specialized animals such as the ptarmigan that has lost a third of its population in the central Alps in recent decades, although it remains open to which extent predation played a role (e.g., the return of predators) [84]. Because of their mobility, insect herbivores may move upslope faster than plants do [66], and impact taller (mainly) graminoids more than small herbs, thus promoting species coexistence [85].
To a large degree, it is the human population in mountainous terrains, who will experience multiple challenges (in the sense of vulnerability) as glaciers melt, permafrost-thawing is destabilizing infrastructure, hydrological extremes come into action, as winter tourism fades, and as some regions face problems with pastoralism or enhanced forest fire frequency. All this hardly affects the regional presence of alpine plant species, although there may be abundance changes and spatial shifts. We consider the general attribution of ‘high vulnerability to climatic change’ to alpine plant species unwarranted, given our current understanding of alpine plant life. The critical future research questions in a climatic change context are (1) the identification of plant species range limits on a mechanistic basis, (2) accounting for the actual microclimatic life conditions of alpine species, (3) their spatial distribution across habitat mosaics, and (4) obtaining field data on transition probabilities among life stages in order to apply life table analysis to the future range of alpine plants. The gateway to such information is hands-on fieldwork and ground-truth data.
Author Contributions
C.K. and E.H. jointly wrote the manuscript. Both authors have read and agreed to the published version of the manuscript.
Funding
The research presented here greatly profited from the Alpine Research and Education Station ALPFOR at the Furka pass in the Swiss central Alps (www.alpfor.ch).
Acknowledgments
We thank Susanna Riedl for preparing the artwork.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Körner, C.; Paulsen, J.; Spehn, E.M. A definition of mountains and their bioclimatic belts for global comparison of biodiversity data. Alp. Bot. 2011, 121, 73–78. [Google Scholar] [CrossRef]
- Körner, C.; Jetz, W.; Paulsen, J.; Payne, D.; Rudmann-Maurer, K.; Spehn, E.M. A global inventory of mountains for bio-geographical applications. Alp. Bot. 2017, 127, 1–15. [Google Scholar] [CrossRef]
- Körner, C. Mountain ecosystems in a changing environment. J. Prot. Mt. Areas Res. Manag. 2014, 6, 71–77. [Google Scholar] [CrossRef][Green Version]
- Körner, C. Mountain biodiversity, its causes and function. Ambio Spec. Rep. 2004, 13, 11–17. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life, 3rd ed.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Testolin, R.; Attorre, F.; Borchardt, P.; Brand, R.F.; Bruelheide, H.; Chytrý, M.; De Sanctis, M.; Dolezal, J.; Finckh, M.; Haider, S.; et al. Global patterns and drivers of alpine plant species richness. Glob. Ecol. Biogeogr. 2021, 30, 1218–1231. [Google Scholar] [CrossRef]
- Körner, C. The cold range limit of trees. Trends Ecol. Evol. 2021. [Google Scholar] [CrossRef]
- Paulsen, J.; Körner, C. A climate-based model to predict potential treeline position around the globe. Alp. Bot. 2014, 124, 1–12. [Google Scholar] [CrossRef]
- Scherrer, D.; Körner, C. Infra-red thermometry of alpine landscapes challenges climatic warming projections. Glob. Chang. Biol. 2009, 16, 2602–2613. [Google Scholar] [CrossRef]
- Scherrer, D.; Körner, C. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J. Biogeogr. 2011, 38, 406–416. [Google Scholar] [CrossRef]
- Winkler, M.; Lamprecht, A.; Steinbauer, K.; Hülber, K.; Theurillat, J.-P.; Breiner, F.; Choler, P.; Ertl, S.; Girón, A.G.; Rossi, G.; et al. The rich sides of mountain summits-a pan-European view on aspect preferences of alpine plants. J. Biogeogr. 2016, 43, 2261–2273. [Google Scholar] [CrossRef]
- Körner, C.; Diemer, M. In situ photosynthetic responses to light, temperature and carbon dioxide in herbaceous plants from low and high altitude. Funct. Ecol. 1987, 1, 179–194. [Google Scholar] [CrossRef]
- Körner, C.; Hiltbrunner, E. The 90 ways to describe plant temperature. Perspect. Plant Ecol. Evol. Syst. 2018, 30, 16–21. [Google Scholar] [CrossRef]
- Oldfather, M.F.; Ackerly, D.D. Microclimate and demography interact to shape stable population dynamics across the range of an alpine plant. New Phytol. 2019, 222, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Cernusca, A. Bestandesstruktur, Bioklima und Energiehaushalt von alpinen Zwergstrauchbeständen. Oecol. Plant 1976, 11, 71–102. [Google Scholar]
- Buri, A.; Grand, S.; Yashiro, E.; Adatte, T.; Spangenberg, J.E.; Pinto-Figeroa, E.; Varrecchia, E.; Guisan, A. What are the most crucial soil variables for predicting the distribution of mountain plant species? A comprehensive study in the Swiss. Alps. J. Biogeogr. 2019, 47, 1143–1153. [Google Scholar]
- Steinger, T.; Körner, C.; Schmid, B. Long-term persistence in a changing climate: DNA analysis suggests very old ages of clones of alpine Carex curvula. Oecologia 1996, 105, 94–99. [Google Scholar] [CrossRef]
- De Witte, L.C.; Armbruster, G.F.J.; Gielly, L.; Taberlet, P.; Stöcklin, J. AFLP markers reveal high clonal diversity and extreme longevity in four key arctic-alpine species. Mol. Ecol. 2012, 21, 1081–1097. [Google Scholar] [CrossRef]
- Mauracher, S.; Wagner, J. Flower preformation in the nival plant Ranunculus glacialis L.: Shoot architecture and impact of growing season length on floral morphogenesis and developmental dynamics. Alp. Bot. 2021, 131, 1–12. [Google Scholar] [CrossRef]
- Hiltbrunner, E.; Arnaiz, J.; Körner, C. Biomass allocation and seasonal non-structural carbohydrate dynamics do not explain the success of tall forbs in short alpine grassland. Oecologia 2021, 1–15. [Google Scholar] [CrossRef]
- Körner, C.; Riedl, S.; Keplinger, T.; Richter, A.; Wiesenbauer, J.; Schweingruber, F.; Hiltbrunner, E. Life at 0 °C: The biology of the alpine snowbed plant. Alp. Bot. 2019, 129, 63–80. [Google Scholar] [CrossRef]
- Vorkauf, M.; Kahmen, A.; Körner, C.; Hiltbrunner, E. Flowering phenology in alpine grassland strongly responds to shifts in snowmelt but weakly to summer drought. Alp. Bot. 2021, 131, 73–88. [Google Scholar] [CrossRef]
- Scholz, K.; Hammerle, A.; Hiltbrunner, E.; Wohlfahrt, G. Analyzing the effects of growing season length on the net ecosystem production of an alpine grassland using model-data fusion. Ecosystems 2018, 21, 982–999. [Google Scholar] [CrossRef]
- Sedlacek, J.; Wheeler, J.A.; Cortes, A.J.; Bossdorf, O.; Hoch, G.; Lexer, C.; Wipf, S.; Karrenberg, S.; Van Kleunen, M.; Rixen, C. The response of the alpine dwarf shrub Salix herbacea to altered snowmelt timing: Lessons from a multi-site transplant experiment. PLoS ONE 2015, 10, e0122395. [Google Scholar] [CrossRef]
- Oberbauer, S.F.; Elmendorf, S.C.; Troxler, T.G.; Hollister, R.D.; Rocha, A.V.; Bret-Harte, M.S.; Dawes, M.A.; Fosaa, A.M.; Henry, G.H.R.; Hoye, T.T.; et al. Phenological response of tundra plants to background climate variation tested using the International Tundra Experiment. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120481. [Google Scholar] [CrossRef]
- Klein, G.; Rebetez, M.; Rixen, C.; Vitasse, Y. Unchanged risk of frost exposure in subalpine and alpine plants after snowmelt in Switzerland despite climate warming. Int. J. Biomet. 2018, 62, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Keller, F.; Körner, C. The role of photoperiodism in alpine plant development. Arct. Antarct. Alp. Res. 2003, 35, 361–368. [Google Scholar] [CrossRef]
- Randin, C.F.; Engler, R.; Normand, S.; Zappa, M.; Zimmermann, N.E.; Pearman, P.B.; Vittoz, P.; Thuiller, W.; Guisan, A. Climate change and plant distribution: Local models predict high-elevation persistence. Glob. Chang. Biol. 2009, 15, 1557–1569. [Google Scholar] [CrossRef]
- Scherrer, D.; Schmid, S.; Körner, C. Elevational species shifts in a warmer climate are overestimated when based on weather station data. Int. J. Biometeorol. 2011, 55, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Feldmeier, S.; Schmidt, B.R.; Zimmermann, N.E.; Veith, M.; Ficetola, G.F.; Lötters, S. Shifting aspect or elevation? The climate change response of ectotherms in a complex mountain topography. Divers. Distrib. 2020, 26, 1483–1495. [Google Scholar] [CrossRef]
- Saetersdal, M.; Birks, H.J.B. A comparative ecological study of Norwegian mountain plants in relation to possible future climatic change. J. Biogeogr. 1997, 24, 127–152. [Google Scholar] [CrossRef]
- Guisan, A.; Theurillat, J.P.; Kienast, F. Predicting the potential distribution of plant species in an Alpine environment. J. Veg. Sci. 1998, 9, 65–74. [Google Scholar] [CrossRef]
- Randin, C.F.; Dirnbock, T.; Dullinger, S.; Zimmermann, N.E.; Zappa, M.; Guisan, A. Are niche-based species distribution models transferable in space? J. Biogeogr. 2006, 33, 1689–1703. [Google Scholar] [CrossRef]
- Löffler, J.; Pape, R. Thermal niche predictors of alpine plant species. Ecology 2020, 101, e02891. [Google Scholar] [CrossRef]
- Qian, H.; Ricklefs, R.E.; Thuiller, W. Evolutionary assembly of flowering plants into sky islands. Nat. Ecol. Evol. 2021, 5, 640–646. [Google Scholar] [CrossRef]
- Körner, C.; Diemer, M.; Schäppi, B.; Niklaus, P.; Arnone, J. The responses of alpine grassland to four seasons of CO2 enrichment: A synthesis. Acta Oecologica 1997, 18, 165–175. [Google Scholar] [CrossRef]
- Inauen, N.; Körner, C.; Hiltbrunner, E. No growth stimulation by CO2 enrichment in alpine glacier forefield plants. Glob. Chang. Biol. 2012, 18, 985–999. [Google Scholar] [CrossRef]
- Hiltbrunner, E.; Schwikowski, M.; Körner, C. Inorganic nitrogen storage in alpine snow pack in the Central Alps (Switzerland). Atmos. Environ. 2005, 39, 2249–2259. [Google Scholar] [CrossRef]
- Kosonen, Z.; Schnyder, E.; Hiltbrunner, E.; Thimonier, A.; Schmitt, M.; Seitler, E.; Thöni, L. Current atmospheric nitrogen deposition still exceeds critical loads for sensitive, semi-natural ecosystems in Switzerland. Atmos. Environ. 2019, 211, 214–225. [Google Scholar] [CrossRef]
- Vorkauf, M.; Marty, C.; Kahmen, A.; Hiltbrunner, E. Past and future snowmelt trends in the Swiss Alps: The role of temperature and snowpack. Clim. Chang. 2021, 165, 1–19. [Google Scholar] [CrossRef]
- Bürli, S.; Theurillat, J.-P.; Winkler, M.; Lamprecht, A.; Pauli, H.; Rixen, C.; Steinbauer, K.; Wipf, S.; Abdaladze, O.; Andrews, C.; et al. A common soil temperature threshold for the upper limit of alpine glasslands in European mountains. Alp. Bot. 2021, 131, 41–52. [Google Scholar] [CrossRef]
- Körner, C. Alpine Treelines; Springer: Basel, Switzerland, 2012. [Google Scholar]
- Larcher, W.; Kainmüller, C.; Wagner, J. Survival types of high mountain plants under extreme temperatures. Flora Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 3–18. [Google Scholar] [CrossRef]
- Paulsen, J.; Weber, U.M.; Körner, C. Tree growth near treeline: Abrupt or gradual reduction with altitude? Arct. Antarct. Alp. Res. 2000, 32, 14–20. [Google Scholar] [CrossRef]
- Dolezal, J.; Kurnotova, M.; Sastna, P.; Klimesova, J. Alpine plant growth and reproduction dynamics in a warmer world. New Phytol. 2020, 228, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Hock, R.; Rasul, G.; Adler, C.; Cáceres, B.; Gruber, S.; Hirabayashi, Y.; Jackson, M.; Kääb, A.; Kang, S.; Kutuzov, S.; et al. High mountain areas (Chap. 3). In Special Report on Ocean and Cryosphere in a Changing Climate; Pörtner, H.O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; IPCC: Nairobi, Kenya, 2020. [Google Scholar]
- Birks, H.J.B. Statistical approaches to interpreting diversity patterns in the Norwegian mountain flora. Ecography 1996, 19, 332–340. [Google Scholar] [CrossRef]
- Schönswetter, P.; Tribsch, A.; Stehlik, I.; Niklfeld, H. Glacial history of high alpine Ranunculus glacialis (Ranunculaceae) in the European Alps in a comparative phylogeographical context. Biol. J. Linn. Soc. 2004, 81, 183–195. [Google Scholar] [CrossRef]
- Kuss, P.; Armbruster, G.F.J.; Aegisdóttir, H.H.; Scheepens, J.F.; Stöcklin, J. Spatial genetic structure of Campanula thyrsoides across the European Alps: Indications for glaciation-driven allopatric subspeciation. Perspect. Plant Ecol. Evol. Syst. 2011, 13, 101–110. [Google Scholar] [CrossRef]
- Scheepens, J.F.; Stöcklin, J. Glacial history and local adaptation explain differentiation in phenotypic traits in the Alpine grassland herb Campanula barbata. Plant Ecol. Divers. 2011, 4, 403–413. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Abdulhak, S.; Attorre, F.; Bergamini, A.; Carranza, M.L.; Chiarucci, A.; Ćušterevska, R.; Dullinger, S.; Gavilán, R.G.; del Galdo, G.G.; et al. Post-glacial determinants of regional species pools in alpine grasslands. Glob. Ecol. Biogeogr. 2021, 30, 1101–1115. [Google Scholar] [CrossRef]
- Parisod, C. Postglacial recolonisation of plants in the western Alps of Switzerland. Bot. Helv. 2008, 118, 1–12. [Google Scholar] [CrossRef]
- De Vos, J.M.; Joppa, L.N.; Gittleman, J.L.; Stephens, P.R.; Pimm, S.L. Estimating the normal background rate of species extinction. Cons. Biol. 2015, 29, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Heiri, O.; Wick, L.; van Leeuwen, J.F.N.; van der Knaap, W.O.; Lotter, A.F. Holocene tree immigration and the chironomid fauna of a small Swiss subalpine lake (Hinterburgsee, 1515 m asl). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 189, 35–53. [Google Scholar] [CrossRef]
- Muellner-Riehl, A.N.; Schnitzler, J.; Kissling, W.D.; Mosbrugger, V.; Rijsdijk, K.F.; Seijmonsbergen, A.C.; Versteegh, H.; Favre, A. Origins of global mountain plant biodiversity: Testing the ‘mountain-geobiodiversity hypothesis’. J. Biogeogr. 2019, 46, 2826–2838. [Google Scholar] [CrossRef]
- Tinner, W.; Kaltenrieder, P. Rapid responses of high-mountain vegetation to early Holocene environmental changes in the Swiss Alps. J. Ecol. 2005, 93, 936–947. [Google Scholar] [CrossRef]
- Kammer, P.M.; Schob, C.; Choler, P. Increasing species richness on mountain summits: Upward migration due to anthropogenic climate change or re-colonisation? J. Veg. Sci. 2007, 18, 301–306. [Google Scholar] [CrossRef]
- Stöckli, V.; Wipf, S.; Nilsson, C.; Rixen, C. Using historical plant surveys to track biodiversity on mountain summits. Plant Ecol. Divers. 2012, 4, 415–425. [Google Scholar] [CrossRef]
- Wipf, S.; Stöckli, V.; Herz, K.; Rixen, C. The oldest monitoring site of the Alps revisited: Accelerated increase in plant species richness on Piz Linard summit since 1835. Plant Ecol. Divers. 2013, 6, 447–455. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Ren, G.Y.; Sun, X.B.; Shresta, A.B.; You, Q.L.; Zhan, Y.L.; Rajbhandari, R.; Zhang, P.-F.; Wen, K.-M. Observed changes in surface air temperature and precipitation in the Hindukush Himalaya region during 1901–2014. Adv. Clim. Chang. Res. 2017, 8, 148–156. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Akhalkatsi, M.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Fernández Calzado, R.; et al. Recent plant diversity changes on Europe’s mountain summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef]
- Lamprecht, A.; Semenchuk, P.R.; Steinbauer, K.; Winkler, M.; Pauli, H. Climate change leads to accelerated transformation of high-elevation vegetation in the central Alps. New Phytol. 2018, 220, 447–459. [Google Scholar] [CrossRef]
- Steinbauer, M.J.; Grytnes, J.-A.; Jurasinski, G.; Kulonen, A.; Lenoir, J.; Pauli, H.; Rixen, C.; Winkler, M.; Bardy-Durchhalter, M.; Barni, E.; et al. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 2018, 556, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Steinbauer, K.; Lamprecht, A.; Semenchuk, P.R.; Winkler, M.; Pauli, H. Dieback and expansions: Species-specific responses during 20 years of amplified warming in the high Alps. Alp. Bot. 2019, 130, 1–11. [Google Scholar] [CrossRef]
- Roth, T.; Plattner, M.; Amrhein, V. Plants, Birds and Butterflies: Short-Term Responses of Species Communities to Climate Warming Vary by Taxon and with Altitude. PLoS ONE 2014, 9, e82490. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; Illmer, P.; Querner, P.; Fischer, B.M.; Hofmann, K.; Lamprecht, A.; Praeg, N.; Schied, J.; Steinbauer, K.; Pauli, H. Side by side? Vascular plant, invertebrate, and microorganism distribution patterns along an alpine to nival elevation gradient. Arctic, Antarct. Alp. Res. 2018, 50, e1475951. [Google Scholar]
- Vitasse, Y.; Ursenbacher, S.; Klein, G.; Bohnenstengel, T.; Chittaro, Y.; Delestrade, A.; Monnerat, C.; Rebetez, M.; Rixen, C.; Strebel, N.; et al. Phenological and elevational shifts of plants, animals and fungi under climate change in the European Alps. Nat. Sci. 2021. [Google Scholar] [CrossRef]
- Vittoz, P.; Randin, C.; Dutoit, A.; Bonnet, F.; Hegg, O. Low impact of climate change on subalpine grasslands in the Swiss Northern Alps. Glob. Chang. Biol. 2009, 15, 209–220. [Google Scholar] [CrossRef]
- Rumpf, S.B.; Hülber, K.; Klonner, G.; Moser, D.; Schütz, M.; Wessely, J.; Willner, W.; Zimmermann, N.E.; Dullinger, S. Range dynamics of mountain plants decrease with elevation. PNAS 2018, 115, 18481853. [Google Scholar] [CrossRef]
- Körner, C. Comparative, long-term ecosystem monitoring across the Alps: Austrian Hohe Tauern National Park, South-Tyrol and Swiss central Alps. In 6th Symposium for Research in Protected Areas; Bauch, K., Ed.; Salzburger Nationalparkfonds, Mittersill, and Austrian Acad Sci: Vienna, Austria, 2018; pp. 331–337. [Google Scholar]
- Little, C.J.; Wheeler, J.A.; Sedlacek, J.; Cortes, A.J.; Rixen, C. Small-scale drivers: The importance of nutrient availability and snowmelt timing on performance of the alpine shrub Salix herbacea. Oecologia 2016, 180, 1015–1024. [Google Scholar] [CrossRef]
- Dullinger, S.; Gattringer, A.; Thuiller, W.; Moser, D.; Zimmermann, N.; Guisan, A.; Willner, W.; Plutzar, C.; Leitner, M.; Mang, T.; et al. Extinction debt of high-mountain plants under twenty-first-century climate change. Nat. Clim. Chang. 2012, 2, 619–622. [Google Scholar] [CrossRef]
- Svenning, J.C.; Sandel, B. Disequilibrium vegetation dynamics under future climate change. Am. J. Bot. 2013, 100, 1266–1286. [Google Scholar] [CrossRef]
- Rumpf, S.B.; Hülber, K.; Wessely, J.; Willner, W.; Moser, D.; Gattringer, A.; Klonner, G.; Zimmermann, N.E.; Dullinger, S. Extinction debts and colonization credits of non-forest plants in the European Alps. Nat. Commun. 2019, 10, 4293. [Google Scholar] [CrossRef]
- Usinowicz, J.; Levine, J.M. Climate-driven range shifts reduce persistence of competitors in a perennial plant community. Glob. Chang. Biol. 2021, 27, 1890–1903. [Google Scholar] [CrossRef]
- Nomoto, H.A.; Alexander, J.M. Drivers of local extinction risk in alpine plants under warming climate. Ecol. Lett. 2021, 24, 1157–1166. [Google Scholar] [CrossRef]
- Weppler, T.; Stoll, P.; Stöcklin, J. The relative importance of sexual and clonal reproduction for population growth in the long-lived alpine plant Geum reptans. J. Ecol. 2006, 94, 869–879. [Google Scholar] [CrossRef]
- Scherrer, D.; Stoll, P.; Stöcklin, J. Colonization dynamics of a clonal pioneer plant on a glacier foreland inferred from spatially explicit and size structured matrix models. Folia Geobot. Phytotaxon. 2017, 52, 353–366. [Google Scholar] [CrossRef]
- Körner, C. Ecology of populations and vegetation. In Strasburger’s Plant Sciences; Bresinsky, A., Kadereit, J.W., Körner, C., Neuhaus, G., Sonnewald, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1167–1215. [Google Scholar]
- Aegisdottir, H.H.; Kuss, P.; Stocklin, J. Isolated populations of a rare alpine plant show high genetic diversity and considerable population differentiation. Ann. Bot. 2009, 104, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Stöcklin, J.; Kuss, P.; Pluess, A.R. Genetic diversity, phenotypic variation and local adaption in the alpine landscape: Case studies with alpine plant species. Bot. Helv. 2009, 119, 125–133. [Google Scholar] [CrossRef]
- Körner, C. Fading of the temperature-growth coupling in treeline trees reflects a conceptual bias. Glob. Chang. Biol. 2021, 27, 3951–3952. [Google Scholar] [CrossRef]
- Knaus, P.; Antoniazza, S.; Wechsler, S.; Guélat, J.; Kéry, M.; Strebel, N.; Sattler, T. Schweizer Brutvogelatlas 2013–2016. Verbreitung und Bestandsentwicklung der Vögel in der Schweiz und im Fürstentum Liechtenstein; Schweizer Vogelwarte: Sempach, Switzerland, 2018. [Google Scholar]
- Descombes, P.; Pitteloud, C.; Glausen, G.; Defossez, E.; Kergunteuil, A.; Allard, P.M.; Rasmann, S.; Pellisier, L. Novel trophic interactions under climate change promote alpine plant coexistance. Science 2020, 370, 1469–1473. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).