Abstract
Since the long-term application of synthetic chemicals as insecticides and the chemotherapy of protozoal diseases have had various negative effects (non-target effects, resistance), research on less harmful biological products is underway. This review is focused on lichens with potential insecticidal and antiprotozoal activity. Literature sources (27) were surveyed from five bibliographic databases and analyzed according to the taxonomic group of the insect, the protozoal disease and the lichen, the type of bioactive compounds (including method of application and mount applied), and the potential bioactivity based on mortalities caused after 24 h of exposure on insects and on parasitic protozoa. Six species of protozoa and five species of mosquitoes, three kinds of larval stages of insects and three protozoa stages were tested. Insecticidal and antiprotozoal effects of crude extracts and seven lichen secondary metabolites (mostly usnic acid) of 32 lichen species were determined. Physiological and morphological changes on parasitic protozoa were observed. Mortality rates caused by LSMs on insect vectors closer to (or somewhat above) the WHO threshold were considered to be insecticides. The results are based on laboratory experiments; however, the efficacy of metabolites should be confirmed in the field and on non-human primates to control the insect vectors and human protozoal diseases transmitted by insects.
1. Introduction
While insects have important roles in the ecosystem, several of them are problematic to human populations. Insects are known to be vectors of agents that cause serious illnesses to humans and domestic animals, as well as those which feed and/or damage crops thereby reducing yield [1]. The mosquito genera such as Aedes, Anopheles and Culex transmit dreadful pathogens that cause severe diseases in humans [2]. Mosquitoes are known to be vectors of diseases such as malaria, dengue fever, yellow fever, Japanese encephalitis, chikungunya and filariasis [2]. Hence, the control of insects is critical in the prevention of human diseases that are transmitted by blood-sucking vectors [3,4,5,6,7,8,9].
The management and control of insect vectors are commonly achieved using synthetic chemicals [10]. These chemical agents may act as ovicidal, larvicidal and adulticidal agents. However, they are faced with challenges such as high costs, residual effects in the environment leading to pollution problems, deleterious effects on non-target organisms, and ill effects in humans and animals through contamination of food and water [11].
The primary concern in vector control using synthetic insecticides is the emergence of resistance. This situation necessitated the search for safe alternatives that are not associated with the development of resistance.
Biopesticides can be used as alternative chemical agents when used in integrated pest management approaches, and biological control is one of the potential alternatives for insect control. Microorganisms (such as bacteria, fungi, and viruses) and natural products, including lichen secondary metabolites (LSMs), appear to be promising biopesticides [9,12,13,14,15,16,17,18,19,20,21].
Phytochemicals have a bioactive potential against insects, pests, human diseases and predators, and allelopathic effects of plant metabolites are known [22]; they also have a natural ability to protect plants against herbivores and help the plant adjust to abiotic stress [23].
The discovery of many phytochemicals may ensure a drop in the use of synthetic insecticides in insect control and give way for more eco-friendly and highly potent insecticidal activity. As an example, Calotropis procera (Aiton) Dryand. (Apocynaceae) found in West Africa, Asia, and tropical regions, shows defense strategies against insects, pests, fungi and viruses [24]. Its natural products possess insecticidal, fungicidal and pesticidal effects. Extracts of C. procera have ovicidal activities on almond moth, Cadra cautella (Walker) (Lepidoptera: Pyralidae). Leaf extracts of C. procera have shown larvicidal activities on Anopheles species. The latex of this plant also affects hatching in Aedes aegypti Linnaeus 1758 [25,26,27].
Vector-borne diseases (e.g., dengue fever, Lyme disease, malaria, West Nile virus) are human illnesses caused by microorganisms that are transmitted by arthropod and non-arthropod vectors (usually blood-feeding arthropods, such as mosquitoes, ticks, and fleas or other non-arthropod vectors such as snails) and account for more than 17% of all infectious diseases, causing more than 700,000 deaths every year. Those that are transmitted by an insect bite include malaria, dengue fever, schistosomiasis, human African trypanosomiasis, leishmaniasis, Chagas disease, yellow fever, Japanese encephalitis and onchocerciasis [2]. In Brazil, Chagas disease represents a serious health problem to humans; it is caused by a hemoflagellate protozoa Trypanosoma cruzi transmitted by means of a bite by triatomine bugs (Triatominae). Nifurtimox and benznidazole are used for treatment, but is toxic to humans [28]. Chemotherapy using these drugs has demonstrated cure rate of 60% among acute patients indicating that there is urgent need to develop alternative drugs with the potential to fill these limitations [29,30]. Leishmania species when inoculated by sandfly bites in humans cause two major forms of diseases, cutaneous leishmaniasis and mucocutaneous leishmaniasis, both of which are endemic in South America with high transmission rates in Paraguay [31]. Chemotherapy for leishmaniasis is achieved through pentavalent antimony as stibogluco-monate (Glucantime) and with pentamidine or amphotericin B with the limitations of the parasite developing resistance and toxicity to the host [32,33].
Based on a global report [2], it is estimated that 229 million malaria cases occurred in 2019 in 87 malaria endemic countries. Over 3.1 billion treatment courses of artemisinin-based combination therapy (ACT) were sold globally by manufacturers in 2010–2019 to reduce mortality and morbidity; at least 2.1 billion of these courses were delivered in the public sector and in malaria endemic countries. First-line treatments for Plasmodium falciparum include artemether-lumefantrine (AL), artesunate-amodiaquine (AS-AQ) and dihydroartemisinin-piperaquine (DHA-PPQ). Malaria chemotherapy using these combinations or as single drug for P. falciparum was 98.0% for AL, 98.4% for AS-AQ and 99.4% for DHA-PPQ and did not change over time. Partial resistance was seen to be independent with artemisinin-based chemotherapy in several foci in global malaria surveillance [2].
LSMs are attracting research on their application in other human diseases based on existing promising results on their bioactivity [17]. If such activity on insect vectors can be similarly applied on the pathogens they transmit, then just one single chemical substance from lichens would work on the vector as well as on the infectious agent with modification of the method of application, dosage and target host.
To promote these studies, our aim was to review the results so far achieved on the diversity of lichen species applied, their LSMs, the target organisms and details of application methods. This topic has been treated briefly in a review by Sachin et al. [34] who treated insecticide LSMs applied not only on insect vectors, but also against agricultural pests; however, studies on a possible antiprotozoal role of LSMS were not covered.
2. Materials and Methods
The public databases Google Scholar, PubMed, Recent Literature on Lichens, Scopus and Web of Science were used in the survey to search for literature in English language from the internet. The following common search words were used: “lichen secondary metabolites” AND “insect vectors” AND “human diseases” AND (“insecticidal” OR “bioassay” OR, “bioactive”) and (“insect vectors of parasitic” AND “human” AND “protozoa” AND “diseases”) at all times in order to get as many papers as possible according to basic guidelines from recommendations and proposed steps to follow when conducting systematic review [35].
Duplicates of published papers were grouped together and their contents were revised for the papers that addressed the topic on LSMs and their biological activities on insect vectors of human diseases and lichen substances on parasitic protozoa transmitted by insect vectors to humans were considered for this review. Those that contained studies on other insects and non-protozoa and not transmitted by insect bites were not included. Altogether 27 journal publications were filtered and used for the analysis below.
3. Diversity of Bioactivity of LSMs from Various Aspects
3.1. Lichen Species and Their LSMs Tested for Bioactivity on Insect Vectors of Human Diseases and for Antiprotozoal Activity
From the literature survey, 57 species of lichens were tested for biological activities on insect vectors of human diseases and a further 4 lichen species were tested on vector-borne diseases. Altogether 61 species were studied either for insecticide or antiprotozoal activity, however only those records of lichen species that exhibited high mortality effect of between 91% to 100% are included in Table 1. Nanayakkara et al. [18] applied 48 species on larvae of Aedes aegypti, however only those were selected that had a high mortality effect above 91% were selected.

Table 1.
Lichen species and LSMs having antivector or antiprotozoal bioactivity (N/A = not available).
These lichens contain the following 15 LSMs: (atranorin), 1′chloropannarin, (erythrin), evernic acid, (galbinic acid), gyrophoric acid, (lecanoric acid, norstictic acid, orsellinic acid), psoromic acid, pannarin, (salazinic acid, sekikaic acid), usnic acid, and vulpic acid. Only 7 of them were tested on insect vectors or on protozoa. Eight of the listed compounds (given in brackets) were identified by chromatography, but several of them were occasionally applied together and therefore it is not known which was the effective component. In other cases pure LSMs were used without mentioning the species from which the LSM was extracted from.
3.2. Groups of Insect Vectors and Parasitic Protozoa Tested on Effectiveness of LSMs
3.2.1. Mosquitoes
The following group of insects belonging to order Diptera and found to be vectors of human diseases were tested to determine the efficacy of LSMs in the cited literature sources. Nomenclature of the taxonomic groups follows the “Mosquito Taxonomic Inventory” [45].
Family: Culicidae
Subfamily: Anophelinae
Genus: Anopheles Meigen 1818
Anopheles stephensi Liston 1901 [38]
Subfamily: Culicinae
Genus: Aedes Meigen
Aedes aegypti Linnaeus 1758 [40]
Genus: Culex Linnaeus
Culex pipiens Linnaeus 1758 [13]
Culex quinquefasciatus Say 1823 [38]
Genus: Culiseta Felt 1904
Culiseta longiareolata Macquart 1838 [44]
The LSMs were mostly tested for insecticide efficacy on various larval stages of the above groups of mosquitoes. Second and third instar larvae [38,40,44] were usually tested, but the sensitivity of the fourth instar larvae was investigated in one study [13].
The first instar larvae were not used during the test period based on guidelines for laboratory and field testing of mosquito larvicides [46] and most studies show that the second instar larvae were the most preferred by the authors of the reviewed articles.
3.2.2. Parasitic Protozoa
The following parasitic protozoa (with the disease they cause indicated in brackets) and that are transmitted by insect bites to humans were found in the cited references.
Phylum: Euglenozoa
Class: Kinetoplastea
Order: Trypanosomatida Honigberg 1963 emend. Vickerman 1976
Family: Trypanosomatidae Doflein 1901 emend. Grobben 1905
Genus: Leishmania Borovsky 1898
Leishmania amazonensis Lainson & Shaw 1972 (cutaneous leishmaniasis) [37]
L. braziliensis Vianna 1911 (cutaneous leishmaniasis) [37]
L. donovani (Laveran & Mesnil 1902) Ross 1903 (cutaneous leishmaniasis) [37]
Genus: Trypanosoma Gruby 1843
Trypanosoma cruzi Chagas 1909 (Chagas disease) [36]
Phylum: Apicomplexa Levine 1980
Class: Aconoidasida
Order: Haemosporida
Family: Plasmodiidae
Genus: Plasmodium Marchiafava & Celli 1885
Plasmodium berghei Vincke & Lips 1948 (malaria) [43]
P. falciparum Welch 1897 (malaria) [43]
Furthermore, three developmental stages of T. cruzi (epimastigotes, trypomastigotes, amastigotes) were studied [36]. Generally, the liver stage of Plasmodium species and the stages amastigote and promastigote of Leishmania species were investigated in the publications analyzed [37,43]. Further details (concentration of LSMs showing potential bioactivity on parasites, enzyme inhibition as a biological target) are discussed in Section 4 and summarized in Table 2.

Table 2.
LSMs that have antiprotozoal activity on parasitic diseases transmitted by insect vectors, the target stage of the parasite and the effective concentrations (LS = liver stage; PfFabI, PfFabG, PfFabZ = enzymes of type II fatty acid biosynthesis (FAS-II) pathway in Plasmodium falciparum).
3.3. Methods of Extraction of LSMs for Bioassay on Insect Vectors and Antiprotozoal Activity
Field collected lichens were air dried [13,47] or dried under shade in room temperature [38,42]. A Soxhlet apparatus used for extraction and evaporation was done in a rotary evaporator. Methanol (e.g., [42]), acetone or chloroform (e.g., [47]) were the three solvents used to extract LSMs.
3.4. Methods of Application of LSMs on Mosquitoes and the Strength of LSMs
The preferred stage of mosquitoes used in the reviewed studies was based on the fact that their larval stages live in water. Three different methods of application of the LSMs were found as follows:
- 1.0, 2.5 and 5.0 mg/mL LSMs in 10% DMSO in 100 mL water were used and mortality determined after 24 h, where 20 mosquito larvae were used in laboratory set up [40];
- 0.02 g of LSMs in 1 mL of acetone as stock solution was prepared and diluted to apply 1.0, 2.5 and 50 µg/mL solutions in 250 mL glass jars containing 100 mL of the solution, where 25 mosquito larvae were used in the laboratory set up [44];
- 0.02 g usnic acid (+) and (–) was dissolved in 1 mL acetone and six concentrations obtained, i.e., 0.1, 0.5, 1.0, 2.5, 5.0 and 10 µg/mL in distilled water in 250 mL glass jars containing 100–100 mL usnic acid solutions, where 25 mosquito larvae were used in thelaboratory set up.
For the above-mentioned applications the WHO protocol [46] developed and adopted in 1963 was used by the authors of reviewed articles with minor variation that did not significantly vary the outcomes from the mortality data obtained after 24 h.
Table 3 contains the names of lichens and the most important data of the tests (concentration of the known LSM or the crude extract of the lichen; mortality rate ranging from 50 to 100%) for comparison.

Table 3.
Applied concentration of the crude lichen extract or LSM extracted from the given lichen species and mortality of mosquitoes (Aedes and Culex) connected to tests carried out.
3.5. Dosage, Methods, Application of LSMs and Their Physiological and Morphological Effects on Protozoa
Effect of LSMs on parasitic protozoa in vivo or in vitro at various concentrations (dosage) and the methods applied (routes of administration) on test animal BALB c mice of the active compound are summarized in Table 4. The effect of LSMs on the organelles and various aspects of physiology of the given stages of parasitic protozoa species that could lead to their death are found in Table 5.

Table 4.
Concentration (dosage) and method (the route of administration) in applying LSMs on the given species of protozoa.

Table 5.
Physiological and morphological effects of LSMs on parasitic protozoa.
3.6. Effective LSMs Documented by Toxicity Tests
The ability of LSMs to control insects or human parasitic protozoa transmitted by insect vectors was evident in the reviewed articles, in this regard it is necessary to highlight in detail if such bioactive compounds can be used safely in the environment and on humans. Some of the documented results from such important toxicity tests done on bioactive LSMs were extracted from the reviewed papers and then compared. Table 6 contains more details on toxicological tests carried out in experiments with LSMs applied on parasitic protozoa.

Table 6.
The results of documented toxicological tests of LSMs applied to determine safety level.
4. Discussion
The five databases surveyed with the same search words (see Section 2) contained only 27 literature sources. It shows that in contrary to the wide application of LSMs [17], their research on bioactivity potential for antivector and antiprotozoal application is limited. The papers mentioned the application of 61 lichen species of which only 4 were used in the studies concentrating on protection against vector-borne protozoa. However, the lichen species contains only 15 bioactive components investigated, 7 of which were isolated and tested independently and 8 were present in lichens applied as crude extracts. The application of usnic acid is especially remarkable, since it is equally useful against insect vectors and parasitic protozoa transferred by them. Four additional LSMs, atranorin, diffractaic acid, gyrophoric acid and salazinic acid, were also tested against mosquitoes being one of the insect vectors of human protozoal diseases and results indicated that mortalities recorded are above the minimum 80% recommended by WHO [13,18,38,39,48], and a further 5, evernic acid, 1′chloropannarin, pannarin, psoromic acid and vulpic acid, are effective against protozoa [37,43].
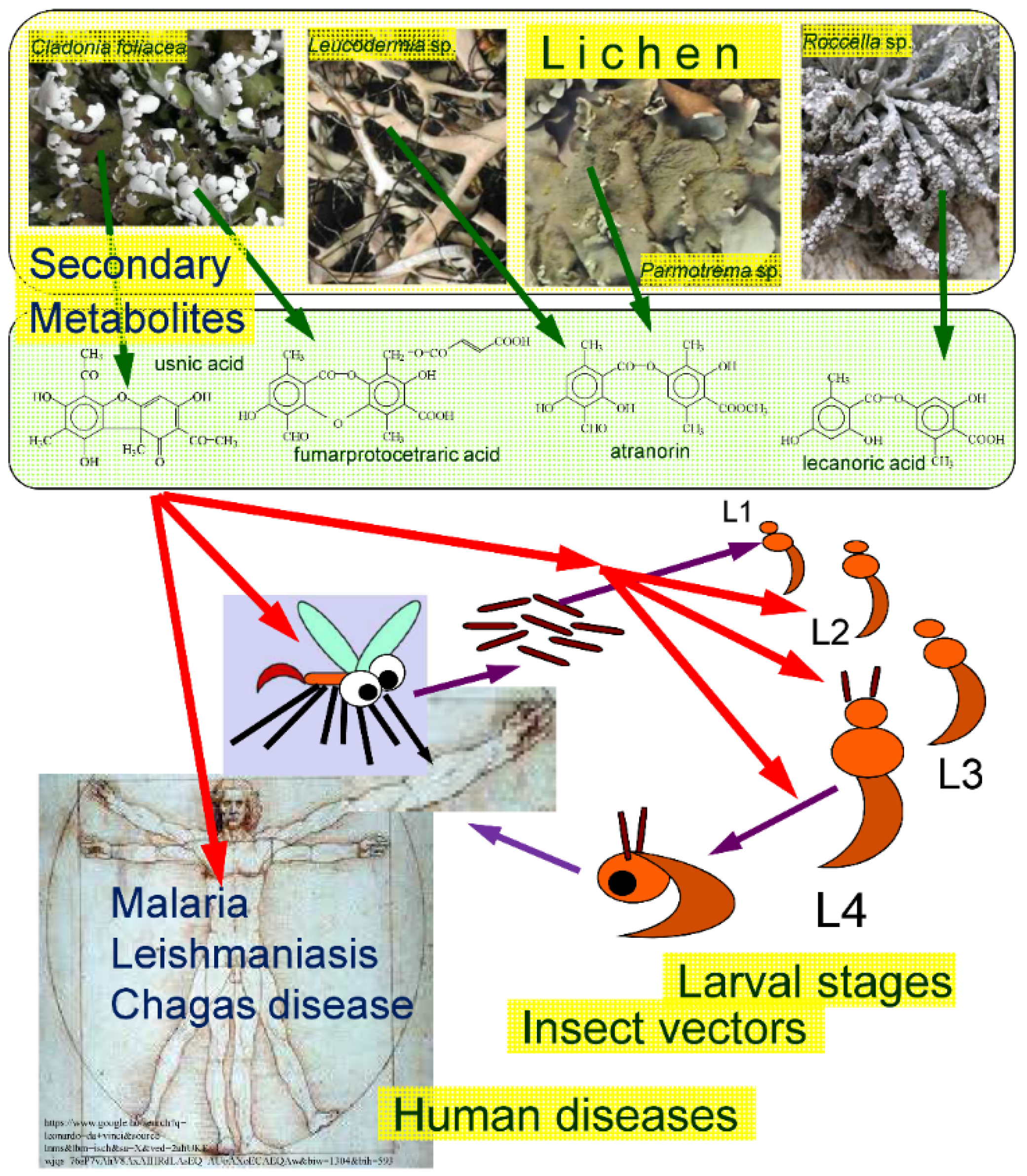
Main connections between lichens, LSMs, insect vectors and human diseases transmitted by parasitic protozoa are illustrated in Figure 1. The studied publications indicated that the larval stages had priority in application over the adult forms [38,40,44] and the various larval stages were not used with the same frequency [13]. The first instar larvae of the mosquito vectors were not used during the test period corresponding with the practice applied in the usual mosquito tests as proposed by the World Health Organization on larvicide activity [46]—and most studies show that the second instar larvae were the most preferred by the researchers [38,40,44].
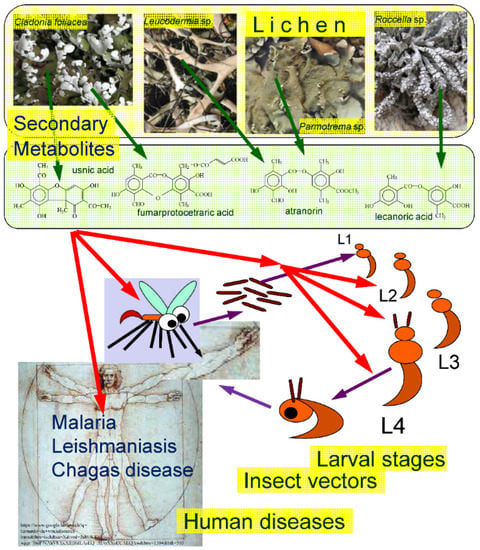
Figure 1.
The subject of the survey summarized. Green arrows symbolize the origin of LSMs from various lichens, red arrows represent the effects of LSMs on insect vectors, their various larval developmental stages (L1–L4) and human diseases caused by parasitic protozoa transmitted by insect vectors.
The various stages of protozoa were also distinguished during the analyses [36,37,43]. The stages amastigote, epimastigote and trypomastigote of the studied three developmental stages of Trypanosoma cruzi were affected by (+)-usnic acid extracted from Cladonia substellata [34]. In addition, (+)-usnic acid displayed the highest liver stage (LS) activity and stage specificity in Plasmodium species [43]. Furthermore the activity of (+)-usnic acid in the first place, and two other LSMs (pannarin and 1′-chloropannarin) were described on the amastigotes and promastigotes of Leishmania species [37].
The wide range of concentrations (c. 5–100 µg/mL of LSMs and c. 1000–5000(–20,000 µg/mL) for crude extracts) represent comparable dosage for insect vectors and protozoa [13,37,38,41]. The difference is explained by the high natural variation of the concentrations of LSMs in lichen thalli ranging from c. 0.1% to 10%(–30%) of their dry weight [17]. The larger necessary concentration of the crude extract is explained by the amount of the LSM present in the thallus [49].
The following results describe the complexity of these studies. Karthik et al. [39], for example, investigated insecticidal activities of Leucodermia leucomelos containing atranorin and salazinic acid on mosquito larvae (2nd and 3rd larval stages of Aedes aegypti with 20 larvae tested) using different concentrations at 1000, 1500, and 2000 µg/mL when mortality was recorded after 24 h. The mortality differed by concentrations: the highest mortality was 80% at 1000 µg/mL for the 2nd instar larvae and the lowest was 50% for the 3rd instar larvae at 1.5 mg/mL. The susceptibility was 80% for 3rd instar larvae and 100% for 2nd instar larvae, while at 2000 µg/mL survival rate was 0% indicating 100% mortality, hence 2000 µg/mL is the best concentration to kill 2nd instar larvae of A. aegypti if these results are compared to 10% dimethyl sulfoxide (DMSO) as a control in the investigation protocol [40].
LSMs, such as usnic acid (and its enantiomers), a widespread cortical dibenzofuran, have been known to show larvicidal activity based on bioassay studies against the 3rd and 4th instar larvae of the house mosquito (Culex pipiens); susceptibility to the bioactive compounds based on larval mortality was also dose-dependent [13]. LSMs from various taxonomic groups showed that bioactivity exhibited at LC50 was as follows: atranorin—0.52 µg/mL, 3-hydroxyphysodic acid—0.97 µg/mL, gyrophoric acid—0.41 µg/mL, (+)-usnic acid—0.48 µg/mL indicating that gyrophoric acid showed the highest toxicity [44].
When biological activities of LSMs are analyzed, their role in nature should also be considered through experimental studies as medicines in humans or animals. Their population size must be monitored while LSMs are applied to kill insects.
This review found that the insect transmitted human diseases, such as cutaneous leishmaniasis, malaria and Chagas disease, can be controlled using LSMs derived from Cladonia substellata, Erioderma leylandii, Protousnea malacea and Psoroma pallidum [36,37,43]. Based on laboratory results these contain (+)-usnic acid, pannarin, 1′chloropannarin, evernic acid, vulpic acid, and psoromic acid (Table 2) tested on parasitic protozoa. Usnic acid was applied the most widely by authors [36,37,43]. In vitro studies have also shown that (+)-usnic acid has a strong effect against Toxoplasma gondii Nicolle & Manceaux 1908 [50] and Trichomonas vaginalis Donné 1836 [51], which are also parasitic protozoa, although not transmitted through insect bites. (+)-usnic acid has also been shown to inhibit the viability of the tachyzoite of T. gondii. An in vivo experiment with (+)-usnic acid-liposome showed prolonged survival time of mice. The most promising result with (+)-usnic acid and (+)-usnic acid-liposome is that they have low toxicity on experimental mice and could still cause an inhibitory effect on the viability of toxoplasma tachyzoite by interfering with normal structures of organelles of T. gondii [50].
However, based on the mode of application, the efficacy of usnic acid revealed notable variation by having no effect on target Leishmania parasites through oral and subcutaneous administration. Promising results were seen with usnic acid on Leishmania species through intralesional use including a 43.34% and 72.28% reduction of lesion and parasite load respectively (Table 3) [37]. The most susceptible stage of parasitic protozoa was trypomastigote where usnic acid had severe physiological and morphological effects on its membrane causing lysis and flagellar pocket. Effects of (+)-usnic acid on epimastigote forms were seen to have a target on mitochondria and kinetoplasts (Table 4). (+)-usnic acid had the highest inhibitory effects on the liver stage of Plasmodium berghei at 2.3 µΜ [43]. Evidence of reduced activity on the blood stage of Plasmodium falciparum exists for (+)-usnic acid and vulpic acid (Table 4). Psoromic acid was more effective on the blood stage and moderate on the liver stage, while evernic acid showed the lowest efficacy. When compared to their effect on FAS-II enzymes, evernic acid had high affinity for PfFabI and PfFabZ, but did not show effects on morphology on liver stage of P. falciparum [43].
Bioactivities of the LSMs on parasitic protozoa were conducted in vitro and in vivo using BALB c mice, hence the response from these studies can be compared to those expected in the case of human hosts. To confirm this claim, LSMs were subjected to toxicological assessments. Zebrafish were used for in vivo studies and human hepatocytes, murine peritoneal macrophages and cancer cells for in vitro studies [43]. However, according to studies by Fournet et al. [37], there was no toxicological test available as they only reported safety from their own observations and personal judgement and therefore the exact safety level was undetermined (Table 5). The (+)-usnic acid exhibited definite safety levels, but lower than at other acids. De Carvalho and coworkers (“unpubl.” in [36] (p. 160)) reported no side effects in Trypanosoma cruzi-infected mice treated for 5 days with 25 mg/kg/day of usnic acid, but other authors [52,53] have reported toxic effects of usnic acid and this information needs further study to exploit the unique properties of usnic acid in the future. Evernic, vulpic and psoromic acids showed a reduction in size and enlargement of the liver of zebrafish larvae [43]. These are cautions that also need to be considered during further experiments and intended applications. Finally, the fine difference between the two optical enantiomers of usnic acid—existing naturally in various species [54]—may cause differences in efficacy during various applications, as it was justified in a series of bioactivity analyses [55]. This topic was treated only exceptionally in the field of insect vectors (e.g., [13]), and is thus worthy of further studies.
5. Conclusions
If the number of lichen species known worldwide (c. 18–20,000 [56]) is compared to the number of insecticides justified so far on insect vectors and parasitic protozoa carried by them, it is obvious that there are potentially many more efficacious species. Similarly, the number of applicable LSMs of the existing c. 1000 [57] should be higher than those tested so far. Furthermore, more attention should be paid to the diversity due to optical enantiomers in the future cf. [13,55] when testing their insecticide and antiprotozoal role.
This review strongly revealed that very little information is available on the application of LSMs to determine their efficacy in the field compared to laboratory tests as well as toxicological information when using LSMs on non-target organisms in the environment. Higher vertebrates should also be considered for widening the range of taxa where LSMs are applied and their effects are controlled.
There is therefore the need to use those lichens that have been identified as having the highest biological activity against insects and perform field survey for consideration as new tools in insect vectors control and in management of parasitic protozoal diseases for commercial production in the market. In general the publications reviewed contained more detailed information on antiprotozoal application. These were more thorough studies based on recent sophisticated instrumental, ultrastructural and metabolomic studies [36,43]. There is a possible advantage in analyzing the so far less investigated and less frequent volatile LSMs by methods established in the study of natural products of vascular plants [58,59]. It is necessary to also consider recent methods in analysis for application, too, for example the possibilities of microencapsulation (where the active substances are protected by encapsulation and their activity kept constant after this process) in order to facilitate environmental protection and sustainable development [60]. This will supplement the existing control and management tools of human diseases, vectors of human and animal diseases and non-communicable and communicable diseases when further studies are done on several lichen groups that have demonstrated bioactive potentials.
Author Contributions
Conceptualization, initiated by E.É.F. and modified via discussions by A.M.M.; methodology, A.M.M.; writing—original draft preparation, review and editing, A.M.M. and E.É.F.; visualization, E.É.F.; supervision, E.É.F.; project administration, A.M.M. and E.É.F.; funding acquisition, A.M.M. and E.É.F. All authors have read and agreed to the published version of the manuscript.
Funding
The first author is holding a Stipendium Hungaricum Scholarship (2020–2024). This research was funded by the National Research Development and Innovation Fund, grant number NKFI K 124341. The APC was funded by Stipendium Hungaricum Scholarship (2020–2024).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The following public databases were used: Google Scholar (https://scholar.google.com, accessed on 20 June 2021), PubMed (https://pubmed.ncbi.nlm.nih.gov, accessed on 20 June 2021), Recent literature on lichens (http://nhm2.uio.no/botanisk/lav/RLL/RLL.HTM, accessed on 20 June 2021), Scopus (https://www.scopus.com, accessed on 20 June 2021) and Web of Science (https://www.webofknowledge.com, accessed on 20 June 2021).
Acknowledgments
Special thanks goes to László Lőkös (Hungarian Natural History Museum, Budapest) for reading the manuscript and for his useful advice and to Mark Seaward (Bradford University, UK) for his advice and revision of the English text.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Yildirim, E.; Aslan, A.; Emsen, B.; Cakir, A.; Ercisli, S. Insecticidal effect of Usnea longissima (Parmeliaceae) extract against Sitophilus granarius (Coleoptera: Curculionidae). Int. J. Agric. Biol. 2012, 14, 303–306. [Google Scholar]
- WHO 2020, World Health Organization/News Room/ Fact Sheets/Detail/Malaria, 1 April 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 15 April 2021).
- Chen, L.H.; Wilson, M.E. Non-vector transmission of dengue and other mosquito-borne Flaviviruses. Dengue Bull. 2005, 29, 18–31. Available online: https://www.researchgate.net/publication/228656801_Non-vector_transmission_of_dengue_and_other_mosquito-borne_flaviviruses (accessed on 20 June 2021).
- Dar, J.A.; Wani, K.A. Environmental changes and emerging vectorborne diseases: A review. Biol. Forum Int. J. 2010, 2, 78–83. Available online: https://www.researchtrend.net/bfij/bfij_volume-3.php (accessed on 20 June 2021).
- Ghaly, A.; Edwards, S. Termite damage to buildings: Nature of attacks and preventive construction methods. Am. J. Eng. Appl. Sci. 2011, 4, 187–200. [Google Scholar] [CrossRef]
- Oberemok, V.V.; Laikova, K.V.; Gninenko, Y.I.; Zaitsev, A.S.; Nyadar, P.M.; Adeyemi, T.A. A short history of insecticides. J. Plant Protect. Res. 2015, 55, 221–226. [Google Scholar] [CrossRef]
- Ricci, I.; Valzano, M.; Ulissi, U.; Epis, S.; Cappelli, A.; Favia, G. Symbiotic control of mosquito borne disease. Pathog. Glob. Health 2012, 106, 380–385. [Google Scholar] [CrossRef]
- Singh, N.; Shukla, S.; Gupta, V.; Tandia, N.; Singh, P. Mosquito borne zoonotic diseases: A review. J. Livest. Sci. 2015, 6, 65–72. Available online: http://livestockscience.in/volumes/ (accessed on 20 June 2021).
- Verma, M.; Sharma, S.; Prasad, R. Biological alternatives for termite control: A review. Int. Biodeterior. Biodegrad. 2009, 63, 959–972. [Google Scholar] [CrossRef]
- Frampton, G.K.; Jansch, S.; Scott-Fordsmand, J.J.; Rombke, J.; Van den Brink, P.J. Effects of pesticides on soil invertebrates in laboratory studies: A review and analysis using species sensitivity distributions. Environ. Toxicol. Chem. 2006, 25, 2480–2489. [Google Scholar] [CrossRef]
- Starks, S.E.; Hoppin, J.A.; Kamel, F.; Lynch, C.F.; Jones, M.P.; Alavanja, M.C.; Sandler, D.P.; Gerr, F. Peripheral nervous system function and organophosphate pesticide use among licensed pesticide applicators in the agricultural health study. Environ. Health Persp. 2012, 120, 515–520. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Santhanam, P.; Srinivasan, M. Larvicidal potency of marine actinobacteria isolated from mangrove environment against Aedes aegypti and Anopheles stephensi. J. Par. Dis. 2017, 41, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Cetin, H.; Tufan-Cetin, O.; Turk, A.O.; Tay, T.; Candan, M.; Yanikoglu, A.; Sumbul, H. Insecticidal activity of major lichen compounds, (-)- and (+)-usnic acid, against the larvae of house mosquito, Culex pipiens L. Parasitol Res. 2008, 102, 1277–1279. [Google Scholar] [CrossRef]
- Ghosh, A.; Chowdhury, N.; Chandra, G. Plant extracts as potential mosquito larvicides. Indian J. Med. Res. 2012, 135, 581–598. [Google Scholar] [PubMed]
- Gupta, S.; Dikshit, A.K. Biopesticides: An ecofriendly approach for pest control. J. Biopest. 2010, 3, 186–188. Available online: https://www.semanticscholar.org/paper/Biopesticides%3A-an-ecofriendly-approach-for-pest-Gupta-Dikshit/9dc875f76af9fd1ae59fac41311cc96ee3e7f875 (accessed on 20 June 2021).
- Kumar, S. Biopesticides: A need for food and environmental safety. J. Biofertil. Biopestic. 2012, 3, e107. [Google Scholar] [CrossRef]
- Molnár, K.; Farkas, E. Current results on biological activities of lichen secondary metabolites: A review. Z. Naturforsch. C. J. Biosci. 2010, 65, 157–173. [Google Scholar] [CrossRef]
- Nanayakkara, C.; Bombuwala, K.; Kathirgamanathar, S.; Adikaram, N.K.B.; Wijesundara, D.S.A.; Hariharan, G.N.; Wolseley, P.; Karunaratne, V. Effect of some lichen extracts from Sri Lanka on larvae of Aedes aegypti and the fungus Cladosporium cladosporioides. J. Natn. Sci. Found. Sri Lanka 2005, 33, 147–149. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Auad, A.M.; Mendes, S.M.; Frizzas, M.R. Crop losses and the economic impact of insect pests on Brazilian agriculture. Crop. Prot. 2014, 56, 50–54. [Google Scholar] [CrossRef]
- Garcia, A.R.M.; Rocha, A.d.P.; Moreira, C.C.; Rocha, S.L.; Guarneri, A.A.; Elliot, S.L. Screening of fungi for biological control of a triatomine vector of Chagas disease: Temperature and trypanosome infection as factors. PLoS Negl. Trop. Dis. 2016, 10, e0005128. [Google Scholar] [CrossRef]
- Ramanujam, B.; Rangeshwaran, R.; Sivakumar, G.; Mohan, M.; Yandigeri, M.S. Management of insect pests by microorganisms. Proc. Indian Natl Sci. Acad. 2014, 80, 455–471. [Google Scholar] [CrossRef]
- Bostan, C.; Butnariu, M.; Butu, M.; Ortan, A.; Butu, A.; Rodino, S.; Parvue, C. Allelopathic effect of Festuca rubra on perennial grasses. Rom. Biotechnol. Lett. 2013, 18, 8190–8196. Available online: https://www.researchgate.net/publication/273678898_Allelopathic_effect_of_Festuca_rubra_on_perennial_grasses (accessed on 20 June 2021).
- Hadaček, F.; Müller, C.; Werner, A.; Greger, H.; Proksch, P. Analysis, isolation and insecticidal activity of linear furanocoumarins and other coumarin derivatives from Peucedanum (Apiaceae: Apioideae). J. Chem. Ecol. 1994, 20, 2035–2054. [Google Scholar] [CrossRef] [PubMed]
- Larhsini, M.; Bousaid, M.; Lazrek, H.B.; Jana, M.; Amarouch, H. Evaluation of antifungal and molluscicidal properties of extracts of Calotropis procera. Fitoterapia 1997, 68, 371–373. Available online: https://www.researchgate.net/publication/290792705_Evaluation_of_antifungal_and_molluscicidal_properties_of_extracts_of_Calotropis_procera (accessed on 20 June 2021).
- Alam, M.A.; Habib, M.R.; Nikkon, F.; Khalequzzaman, M.; Karim, M.R. Insecticidal activity of root bark of Calotropis gigantea L. against Tribolium castaneum (Herbst). World. J. Zool. 2009, 4, 90–95. [Google Scholar]
- Singh, R.K.; Mittal, P.; Dhiman, R.C. Laboratory study on larvicidal properties of leaf extract of Calotropis procera (Family-Asclepiadaceae) against mosquito larvae. J. Commun. Dis. 2005, 37, 109–113. Available online: https://www.researchgate.net/publication/7034248_Laboratory_study_on_larvicidal_properties_of_leaf_extract_of_Calotropis_procera_Family-Asclepiadaceae_against_mosquito_larvae (accessed on 20 June 2021). [PubMed]
- Singhi, M.; Joshi, V.; Sharma, R.C.; Sharma, K. Ovipositioning behaviour of Aedes aegypti in different concentrations of latex of Calotropis procera: Studies on refractory behaviour and its sustenance across gonotrophic cycles. Dengue Bull. 2004, 28, 184–188. Available online: https://apps.who.int/iris/handle/10665/164002 (accessed on 20 June 2021).
- Moncayo, A. Program Report of the UNPD/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR); World Health Organization: Geneva, Switzerland, 1993; pp. 67–75. [Google Scholar]
- Estani, S.S.; Porcel, B.M.; Segura, E.L.; Yampotis, C.; Ruiz, A.M.; Velazquez, E. Efficacy of chemotherapy with benznidazole in children in intermediate phase of Chagas’ disease. Amer. J. Trop. Med. Hyg. 1998, 59, 526–529. [Google Scholar] [CrossRef] [PubMed]
- De Castro, S.L. The challenge of Chagas’ disease chemotherapy: An update of drugs assayed against Trypanosoma cruzi. Acta Trop. 1993, 53, 83–98. [Google Scholar] [CrossRef]
- Curtin, J.M.; Aronson, N.E. Leishmaniasis in the United States: Emerging issues in a region of low endemicity. Microorganisms 2021, 9, 578. [Google Scholar] [CrossRef]
- Berman, J.D. Chemotherapy for leishmaniasis: Biochemical and mechanisms, clinical efficacy, and future strategies. Rev. Infect. Dis. 1988, 10, 560–586. [Google Scholar] [CrossRef]
- Croft, S.L. Recent developments in the chemotherapy of leishmaniasis. Trends Pharmacol. Sci. 1988, 9, 376–381. [Google Scholar] [CrossRef]
- Sachin, M.B.; Mahalakshmi, S.N.; Prashith Kekuda, T.R. Insecticidal efficacy of lichens and their metabolites—A mini review. J. Appl. Pharm. Sci. 2018, 8, 159–164. [Google Scholar] [CrossRef]
- Khan, K.S.; Kunz, R.M.; Kleinen, J.; Antes, G. Five steps to conducting a systematic review. J. R. Soc. Med. 2003, 96, 118–121. [Google Scholar] [CrossRef]
- De Carvalho, E.A.B.; Andrade, P.P.; Silva, N.H.; Pereira, E.C.; Figueiredo, R.C.B.Q. Effect of usnic acid from the lichen Cladonia substellata on Trypanosoma cruzi in vitro: An ultrastructural study. Micron 2005, 36, 155–161. [Google Scholar] [CrossRef]
- Fournet, A.; Ferreira, M.-E.; Rojas de Arias, A.; Torres de Ortiz, S.; Inchausti, A.; Yaluff, G.; Quilhot, W.; Fernandez, E.; Hidalgo, M.E. Activity of compounds isolated from Chilean lichens against experimental cutaneous leishmaniasis. Comp. Biochem. Physiol. C. 1997, 116, 51–54. [Google Scholar] [CrossRef]
- Syed Zameer Ahmed, K.; Sidhra, S.Z.A.; Kisore, P.V.; Kamaraj, C.; Nayaka, S. Larvicidal potential of selected indigenous lichens against three mosquito species—Culex quinquefasciatus, Aedes aegypti and Anopheles stephensi. Chin. Herb. Med. 2018, 10, 152–156. [Google Scholar] [CrossRef]
- Karthik, S.; Nandini, K.C.; Prashith Kekuda, T.R.; Vinayaka, K.S.; Mukunda, S. Total phenol content, insecticidal and amylase inhibitory efficacy of Heterodermia leucomela (L). Ann. Biol. Res. 2011, 2, 38–43. [Google Scholar]
- Vinayaka, K.S.; Krishnamurthy, Y.L.; Prashith Kekuda, T.R.; Praveen Kumar, S.V.; Sudharshan, S.J.; Chinmaya, A. Larvicidal and wormicidal efficacy of methanolic extracts of five macrolichens collected from Bhadra wildlife sanctuary. Biomedicine 2009, 29, 327–331. Available online: https://www.researchgate.net/publication/259174826_Larvicidal_and_Wormicidal_efficacy_of_methanolic_extracts_of_five_macrolichens_collected_from_Bhadra_wildlife_sanctuary (accessed on 20 June 2021).
- Kumar, P.S.V.; Kekuda, P.T.R.; Vinayaka, K.S.; Swathi, D.; Chinmaya, A. Insecticidal efficacy of Ramalina hossei H. Magn & G. Awasthi and Ramalina conduplicans Vain.—macrolichens from Bhadra wildlife sanctuary, Karnataka. Biomedicine 2010, 30, 100–102. Available online: https://www.researchgate.net/publication/259464166_Insecticidal_efficacy_of_Ramalina_hossei_H_Magn_G_Awasthi_and_Ramalina_conduplicans_Vain-Macrolichens_from_Bhadra_wildlife_sanctuary_Karnataka (accessed on 20 June 2021).
- Moreira, A.S.N.; Fernandes, R.O.S.; Lemos, F.J.A.; Braz-Filho, R.; Vieira, I.J.C. Larvicidal activity of Ramalina usnea lichen against Aedes aegypti. Rev. Bras. Farm. 2016, 26, 530–532. [Google Scholar] [CrossRef][Green Version]
- Lauinger, I.L.; Vivas, L.; Perozzo, R.; Stairiker, C.; Tarun, A.; Zloh, M.; Zhang, X.; Xu, H.; Tonge, P.J.; Franzblau, S.G.; et al. Potential of lichen secondary metabolites against Plasmodium liver stage parasites with FAS-II as the potential target. J. Nat. Prod. 2013, 76, 1064–1070. [Google Scholar] [CrossRef]
- Cetin, H.; Tufan-Cetin, O.; Turk, A.O.; Tay, T.; Candan, M.; Yanikoglu, A.; Sumbul, H. Larvicidal activity of some secondary lichen metabolites against the mosquito Culiseta longiareolata Macquart (Diptera: Culicidae). Nat. Prod. Res. 2012, 26, 350–355. [Google Scholar] [CrossRef]
- Mosquito Taxonomic Inventory. Available online: http://mosquito-taxonomic-inventory.info (accessed on 8 May 2021).
- WHO 2005, World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; World Health Organization: Geneva, Switzerland, 2005; pp. 1–39, No. WHO/CDS/WHOPES/GCDPP/2005.13; Available online: https://apps.who.int/iris/handle/10665/69101 (accessed on 20 June 2021).
- Bomfim, R.R.; Araújo, A.A.S.; Cuadros-Orellana, S.; Melo, M.G.D.; Quintans, L.J., Jr.; Cavalcanti, S.C.H. Larvicidal activity of Cladonia substellata extract and usnic acid against Aedes aegypti and Artemia salina. Lat. Am. J. Pharm. 2009, 28, 580–584. Available online: https://www.researchgate.net/publication/238114261_Larvicidal_Activity_of_Cladonia_substellata_Extract_and_Usnic_Acid_against_Aedes_aegypti_and_Artemia_salina (accessed on 20 June 2021).
- WHO 2006, World Health Organization. Guidelines for Testing Mosquito Adulticides for Indoor Residual Spraying and Treatment of Mosquito Nets; World Health Organization: Geneva, Switzerland, 2006; pp. 1–60, WHO/CDS/NTD/WHOPES/GCDPP/2006.3; Available online: https://apps.who.int/iris/handle/10665/69296 (accessed on 20 June 2021).
- Farkas, E.; Biró, B.; Szabó, K.; Veres, K.; Csintalan, Z.; Engel, R. The amount of lichen secondary metabolites in Cladonia foliacea (Cladoniaceae, lichenised Ascomycota). Acta Bot. Hung. 2020, 62, 33–48. [Google Scholar] [CrossRef]
- Si, K.; Wei, L.; Yu, X.; Wu, F.; Li, X.; Li, C.; Cheng, Y. Effects of (+)-usnic acid and (+)-usnic acid-liposome on Toxoplasma gondii. Expl. Parasit. 2016, 166, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, M.; Ding, D.; Tan, T.; Yan, B. Effect of Cladonia alpestris on Trichomonas vaginalis in vitro. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi [Chin. J. Parasitol. Parasit. Dis.] 1995, 13, 126–129. [Google Scholar] [PubMed]
- Han, D.; Matsumaru, K.; Rettori, D.; Kaplowitz, N. Usnic acid-induced necrosis of cultured mouse hepatocytes: Inhibition of mitochondrial function and oxidative stress. Biochem. Pharmacol. 2004, 67, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Pramyothin, P.; Janthasoot, W.; Pongnimitprasert, N.; Phrukudom, S.; Ruangrungsi, N. Hepatotoxic effect of (+)usnic acid from Usnea siamensis Wainio in rats, isolated rat hepatocytes and isolated rat liver mitochondria. J. Ethnopharm. 2004, 90, 381–387. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Yamamoto, Y.; Yoshimura, I.; Kurokawa, T.; Huneck, S. Distribution of optical isomers of usnic and isousnic acids analyzed by high performance liquid chromatography. J. Hattori Bot. Lab. 1997, 83, 173–178. [Google Scholar]
- Galanty, A.; Paśko, P.; Podolak, I. Enantioselective activity of usnic acid: A comprehensive review and future perspectives. Phytochem. Rev. 2019, 18, 527–548. [Google Scholar] [CrossRef]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Ainsworth & Bisby’s Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008; pp. 1–772. [Google Scholar]
- Stocker-Wörgötter, E. Metabolic diversity of lichen forming, ascomycetous fungi: Culturing, production of polyketides and shikimi acid derivatives, and PKS genes. Nat. Prod. Rep. 2008, 25, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Samfira, I.; Rodino, S.; Petrache, P.; Cristina, R.T.; Butu, M.; Butnariu, M. Characterization and identity confirmation of essential oils by mid infrared absorption spectrophotometry. Dig. J. Nanomat. Biostruct. 2015, 10, 557–566. Available online: https://www.researchgate.net/publication/224806602_Volatile_constituents_of_selected_Parmeliaceae_lichens (accessed on 20 June 2021).
- Stojanović, I.Ž.; Radulović, N.S.; Mitrović, T.L.J.; Stamenković, S.M.; Stojanović, G.S. Volatile constituents of selected Parmeliaceae lichens. J. Serb. Chem. Soc. 2011, 76, 987–994. [Google Scholar] [CrossRef]
- Barbat, C.; Rodino, S.; Petrache, P.; Butu, M.; Butnariu, M. Microencapsulation of the allelochemical compounds and study of their release from different products. Dig. J. Nanomat. Biostruct. 2013, 8, 945–953. Available online: https://www.researchgate.net/publication/249643665_Microencapsulation_of_the_allelochemical_compounds_and_study_of_their_release_from_different_products (accessed on 20 June 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).