Abstract
Despite coral community collapse, the mustard hill coral (Porites astreoides) is a species currently experiencing success throughout the Caribbean. The inshore reefs of Grenada were selected to study the influence of benthic factors on the abundance, size, and coverage of P. astreoides colonies. Surveys of reef communities along established 30 m transects were conducted at eight sites in 2014 and 2017 using a 0.5 m² quadrat. Coral Point Count was used to annotate the images, estimating the coverage of scleractinian corals, sponges, algae, and benthic substrates. Coverage, size, and abundance of P. astreoides colonies were quantified using the area measurement tool in ImageJ standardized against the quadrats. There were significant differences in benthic community assemblages between islands, selected sites, and between years. From 2014 to 2017 there was a significant decrease in the mean abundance of P. astreoides colonies and significant increases in mean colony size and coverage. The presence of P. astreoides colonies was significantly correlated with: rubble (−), sand (−); pavement (+); macroalgae (−); coralline algae (+); sponges (varying response); gorgonians (−); massive corals (+); and branching corals (−). P. astreoides follows similar recruitment patterns as other scleractinian corals. Observed changes in P. astreoides populations appear to indicate a recovery event following a disturbance, potentially tropical storm Chantal in 2013.
1. Introduction
Mirroring the state of global tropical reef ecosystems, coral reefs in the Caribbean have experienced dramatic declines in the last several decades, with live coral cover declining more than 80% since the 1970s [1,2]. The combined impacts of stressors such as rising sea temperatures, ocean acidification, overfishing, pollution, loss of herbivorous species, and disease outbreaks have been largely responsible for this decline [3,4,5,6]. Declines have not however impacted all scleractinian species in the Caribbean equally. They have been concentrated along select life-history or morphological lines, leading a shift towards non-framework building species [7]. These diverging responses among scleractinian species have led to changes in reef dynamics, with an as yet unclear impact on reef functionality [8]. Scleractinian species in the Caribbean can be broadly characterized as having one of four life history strategies based on colony morphology, growth rate, and reproductive method [9]. These life history strategies are: (1) weedy (high recruitment, brooding reproduction); (2) stress tolerant (large colonies, slow growing, broadcast spawning); (3) competitive (framework, fast-growing, broadcast spawning); and (4) generalist (mix of traits of all other categories) [9]. Competitive species such as Acropora palmata or A. cervicornis, historically responsible for much of the complex framework building on Caribbean reefs, have experienced the most dramatic declines since the 1970s [10,11].
Non-framework building scleractinian species with a weedy life history strategy have proven successful in the region, in an era of rapid environmental change and have been among the few ‘winners’ on Caribbean reefs [12]. Species exhibiting this life history strategy, such as the mustard hill coral (Porites astreoides), have increased in mean percent cover on Caribbean reefs and now account for 16–72% of all living coral in Caribbean reef communities [13,14,15].
P. astreoides is found throughout the Caribbean, along the coasts of Florida, Bermuda, Brazil, and along the coasts of West Africa. It is most abundant at depths of 0.5 m−15 m and tolerates a wide range of thermal, physical, and water quality conditions [16]. P. astreoides populations are dominated by medium and large-sized colonies and colony survival is typically high, making it highly unlikely for large colonies to reduce in size once they have reached certain growth stages [15]. P. astreoides has a brooding reproductive strategy and is capable of either sexual or asexual reproduction, facilitating a high rate of colony production [17].
For highly abundant species such as P. astreoides, it is presently unclear which factors influence population dynamics in the reef communities of the Caribbean, particularly at a local scale. It is also unclear what level of resilience a weedy life history strategy confers on a species to continued changes, necessitating regular updates on population status in the region [18]. To assess short-term temporal changes in P. astreoides populations and the extent of influence a gradient of interspecific and substrate factors may have on coverage, the present study quantified changes in P. astreoides coverage, abundance, and colony size, as well as coverage of other benthic features at eight near-shore reefs in the coastal waters of Grenada and Carriacou in 2014 and 2017.
2. Materials and Methods
2.1. Study Sites
Surveys were conducted at eight sites in the nearshore waters of Grenada and Carriacou in the Fall of 2014 and 2017. Four previously established study sites [19] were selected to complement a published dataset from Grenada [20,21] and four sites were also established on the nearby island of Carriacou (Figure 1, Table 1) [14,22].
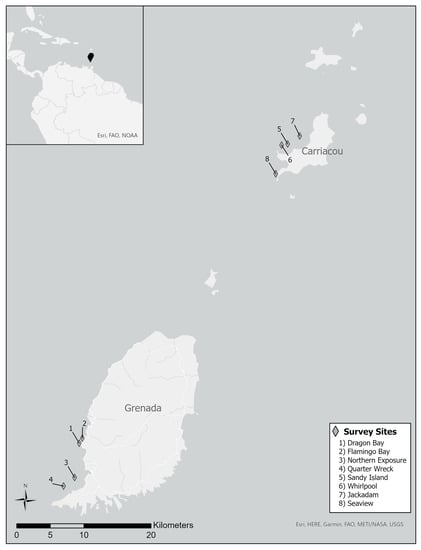
Figure 1.
Location of survey sites in Grenada and Carriacou, Eastern Caribbean (inset).

Table 1.
Benthic survey site names, site code for figures, locations, GPS coordinates, and similarity of species assemblages at study sites between 2014 and 2017 with p-values. Sites with values closer to 0 have similar species assemblages, those with values closer to 1 have very different species assemblages. All results are based on 200 permutations.
The sites on Carriacou were chosen to mirror those in Grenada in that they were approximately equivalent distances from shore, found at similar depths, had benthic community assemblages that appeared similar during initial visual assessments, and were all frequented by the recreational dive industry. All surveyed sites were comprised of shallow patch reefs on the leeward side of each island. Each site was marked with 1 m rebar stakes that were driven into coral rubble at the ends of 30 m long transects running across patch reefs in the nearshore waters of Carriacou. Each site in Grenada and Carriacou had four transects running parallel to each other spaced 5 m apart. All transects ranged in depth from 9.4 m to 12.2 m. Sites on Carriacou were established in the last week of August 2014 with the authorization of the Grenada Ministry of Forestry, Fisheries and Agriculture.
2.2. Benthic Community Assessment
Images were captured in Fall 2014 and 2017, every 0.5 m, using a standardized 0.5 m2 quadrat along each transect using a camera at a distance approximately 0.5 m above the benthos (including coral colonies that extend upwards). Images were imported into CPCe (Coral Point Count with Excel extensions) for image analysis [23]. A species accumulation curve was generated for the dataset from one full site [24]. The data were fit to a Shepard plot with a non-metric fit of 0.97 and a linear fit of 0.841. Eighteen points per image were distributed in a stratified random pattern (3 × 3 grid with 2 points distributed randomly per cell). This number of points per quadrat has been known to sufficiently capture the coverage of major benthic groups [24,25]. Individual points were identified to the species level for hard coral and to the genus level for gorgonians. Algae were classified as macroalgae (fleshy), turf, or coralline. Remaining benthic cover types were identified as sponges (encrusting and massive), zoanthids, or non-living (sand, pavement, rubble, and dead coral).
Percent cover of major benthic groups were arcsine square root transformed to satisfy the assumption of normality required for ANOVA. Nested ANOVA using sites nested within Island were used to test for differences in arcsine percent cover of coral, macroalgae, gorgonian, sponge, and non-living cover separately within each year. Two-way ANOVA were used to test for differences in the same benthic cover groups between years. Significant differences between groups were further investigated using a Welch’s t-test with a Bonferroni correction where appropriate.
Hard coral species abundance matrices were generated using the images processed in CPCe. Abundance data were square root transformed to remove potential bias due to very rare or very abundant species and Bray-Curtis dissimilarity matrices were generated for each year. Differences in Bray-Curtis dissimilarities between sites were assessed using ANOSIM within a year. A two-way PERMANOVA with site and year as factors was used to test for differences between the same sites over time. General linear mixed models were used to test for differences in species composition of the scleractinian coral community between islands and years. All statistical analyses were conducted in the base R environment Version 3.6.2 [26] or using the ‘vegan’ package [27].
2.3. P. astreoides Population Dynamics
The area measuring tool in ‘ImageJ’ standardized against the 0.5 m² quadrat in each image was used to assess the number of P. astreoides colonies, their respective size, and absolute coverage. Colonies found completely within the borders of the quadrat, or those qualitatively assessed as being 90% within the quadrat were counted. Images taken nearby were examined to ensure an accurate measurement of colonies with any overlap of the quadrat. Changes in mean percent cover, mean abundance, and mean colony size (cm2) of P. astreoides by year and island were assessed using a two-way ANOVA. A Kolmogorov-Smirnov two-sample t-test was used to assess differences in size-frequency distributions of mean colony size between years. Site-specific changes between 2014 and 2017 were assessed using Welch’s t-tests. A Grubbs’ test was used on each variable to identify and remove any potential outliers from the subsequent correlation analyses. Percentage values of coverage were arsine transformed. A scatterplot analysis of residuals vs. predicted values for all factors that were not significant in the Grubbs’ test was used to ensure homoscedasticity. Based on sample size calculations with predicted correlation coefficients of 0.2 [28], only predictors present in a minimum 123 quadrats, out of a total of 240 quadrats for each site, in each year (a potential maximum of four analyses), were included in the correlation. Pearson correlations were used to assess associations between the coverage of P. astreoides and habitat type (sand, rubble, and pavement), as well as biotic factors (scleractinian coral species, sponges, algae, and gorgonians; Table 2). Unless otherwise stated all statistical differences are at p < 0.05.

Table 2.
Interspecific and substrate factors tested for inclusion in the correlation analyses.
3. Results
3.1. Benthic Community Assessment
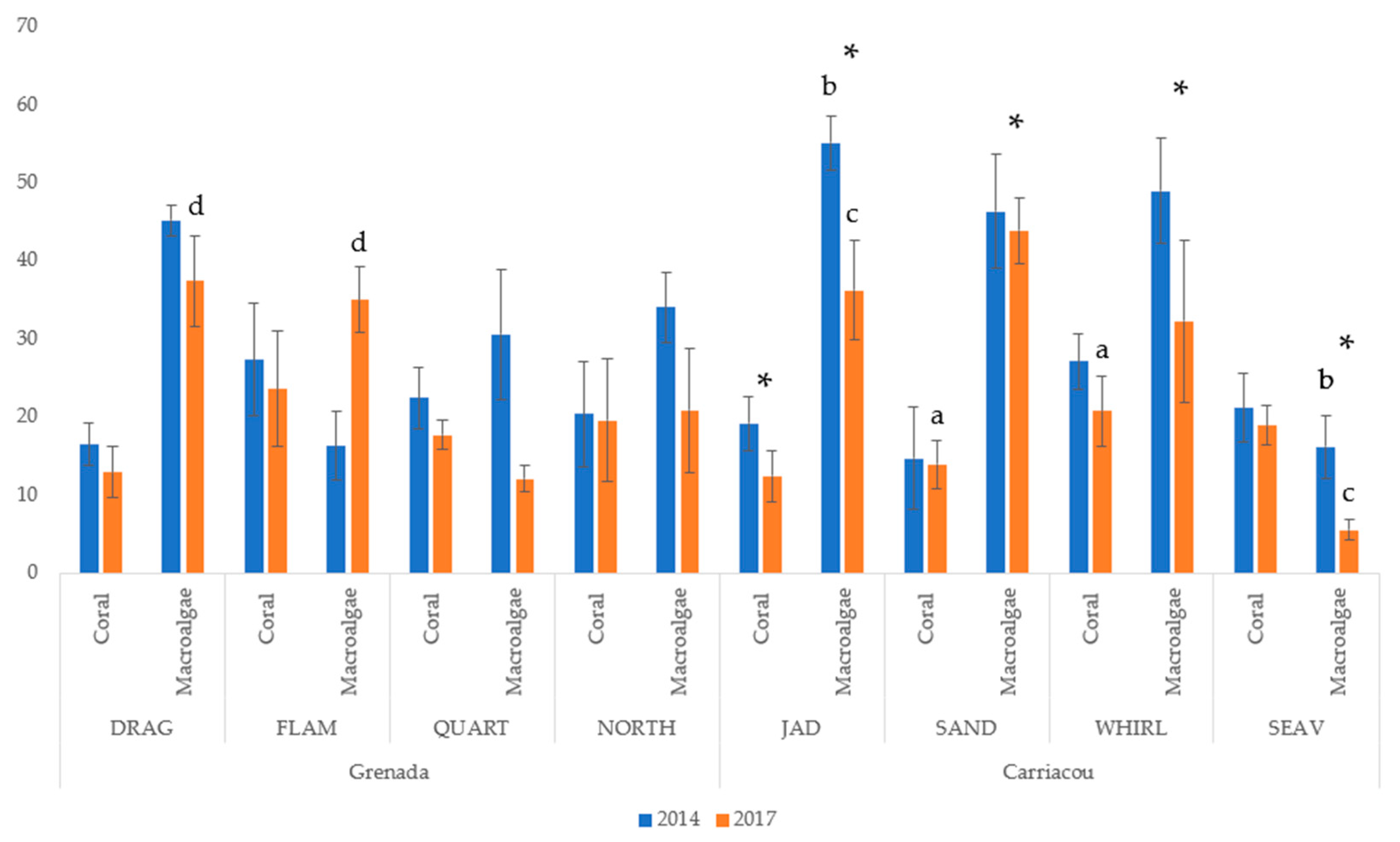
Hard coral species abundance matrices were generated using the images processed in CPCe. Most sites experienced a minor, non-significant decrease in mean coral cover between 2014 and 2017, except for a significant decrease at Jackadam (19.14% ± 3.51 to 12.38% ± 3.29), which drove the significant decrease in coral cover between years (Figure 2). Mean coral cover in 2014 was 21.08% ± 6.21 (average ± SE) for all study sites and decreased to a mean of 17.45% ± 5.67 in 2017. Within each year, mean percent coral cover was not significantly different between islands. The only sites that were significantly different within a year (post-hoc test with Bonferroni correction) were Seaview and Jackadam in 2017.
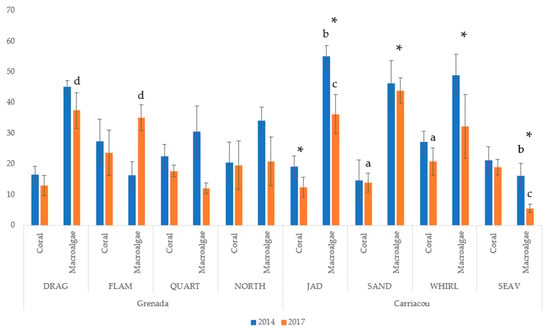
Figure 2.
Mean (±SD) of percent coral and macroalgal cover at study sites in Carriacou and Grenada in 2014 and 2017. (*) denotes a significant difference (p < 0.05) at a single site between years while letters denote a significant difference (p < 0.05) between sites in a given year.
There was a significant decrease in mean macroalgal cover in all sites from 2014 to 2017 (36.56% ± 14.90 to 27.88% ± 13.91). This change was driven by a significant decrease in Carriacou (41.62% ± 16.33 to 29.44% ± 16.00) at all four sites (Figure 2). Macroalgal cover was 28.91% ± 11.84 and did not change significantly in Grenada. In Carriacou, macroalgal cover was significantly higher at Jackadam than at Seaview for both 2014 and 2017 and there was a significant decrease at both sites between surveys. In Grenada, Dragon Bay had significantly higher macroalgal cover than Flamingo Bay in 2014, but not in 2017, as Flamingo Bay experienced a significant increase. Quarter Wreck and Northern exposure also experienced a significant decrease in macroalgal cover (Figure 2).
Mean gorgonian cover for all sites was 3.82% ± 6.47 and did not change significantly between years. There were significant differences in gorgonian cover between islands (not shown). Flamingo Bay and Sandy Island had significantly higher cover than all other sites on their respective islands. Mean gorgonian cover was 18.12% ± 8.6 at Flamingo Bay versus 1.92% ± 1.09 on Grenada and 4.18% ± 4.5 at Sandy Island versus 0.85% ± 0.78 on Carriacou. Mean sponge cover for all sites was 2.67% ± 3.66 and did not change significantly between years or islands. Only Seaview underwent a significant reduction in sponge cover between years. Mean cover of non-living substrate for all sites did not change significantly between years or between islands within a year (not shown).
Site and island had a significant (ANOSIM) effect on predicting similarity of benthic species assemblages. The high dissimilarity (R) values obtained suggest that, for most sites, the majority of the variation in Bray-Curtis dissimilarity can be explained by site grouping. Island had a significant effect in predicting community dissimilarity in both 2014 and 2017. Between-years analysis using PERMANOVA showed a significant effect of time only. All sites were significantly different from the previous year when assessed using ANOSIM (Table 1).
A total of 25 unique hard coral species were identified in the photo quadrats using the stratified random method (Table 3). Of these, the most commonly observed species in Carriacou in both 2014 and 2017 were P. astreoides, P. divaricata and Orbicella annularis. In Grenada, the hard coral species assemblage was dominated by Madracis mirabilis, P. astreoides, and O. annularis in 2014 (Table 3) and M. mirabilis, P. astreoides, and P. divaricata in 2017. The proportions of O. annularis, P. porites, and P. divaricata in the hard coral species assemblage changed significantly between years and islands using a general linear mixed model (Table 3).

Table 3.
Mean (±SD) relative proportion of hard coral species colony counts in Carriacou (CAR) and Grenada (GND) in 2014 and 2017.
3.2. P. astreoides in Grenada
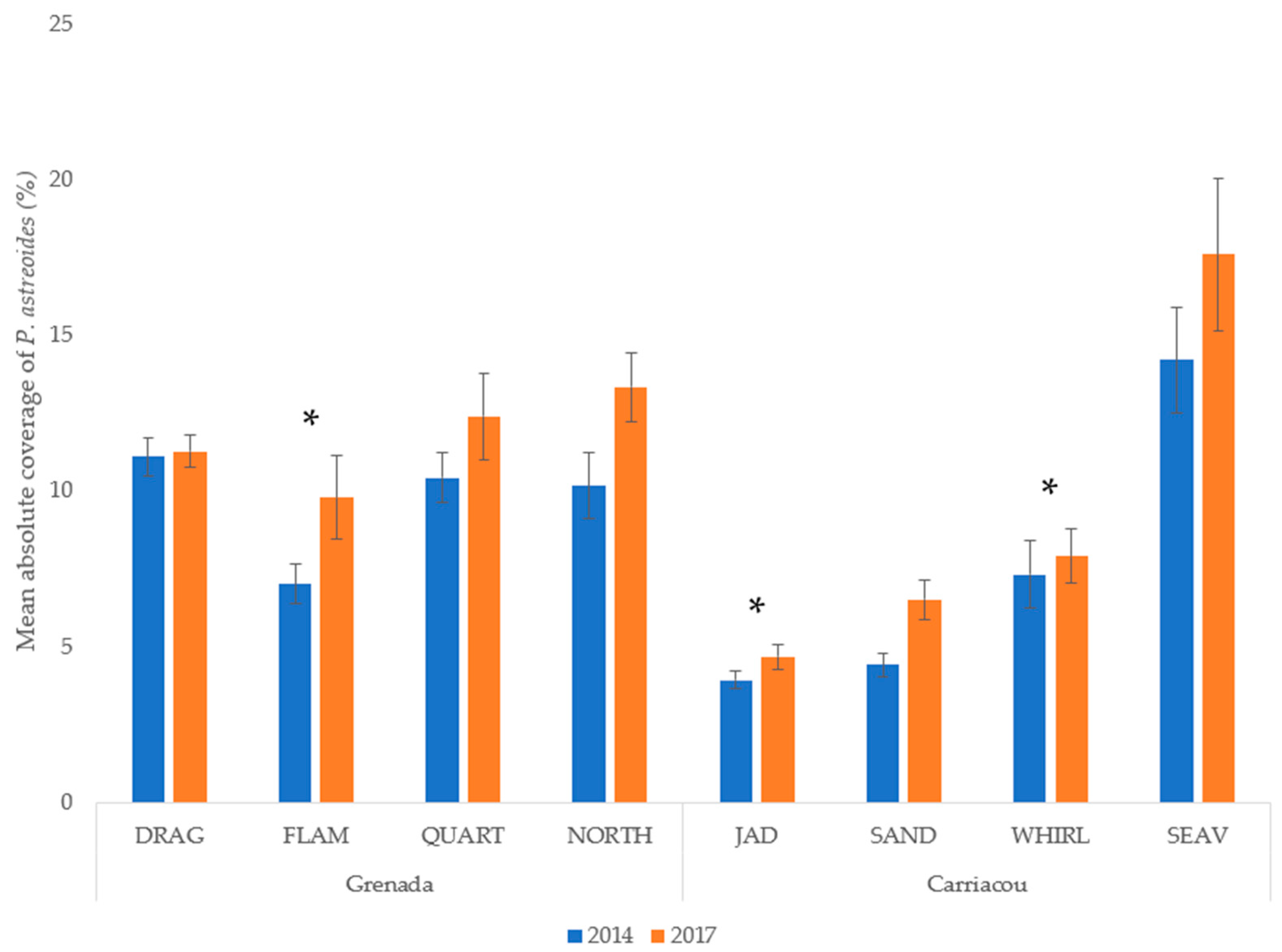
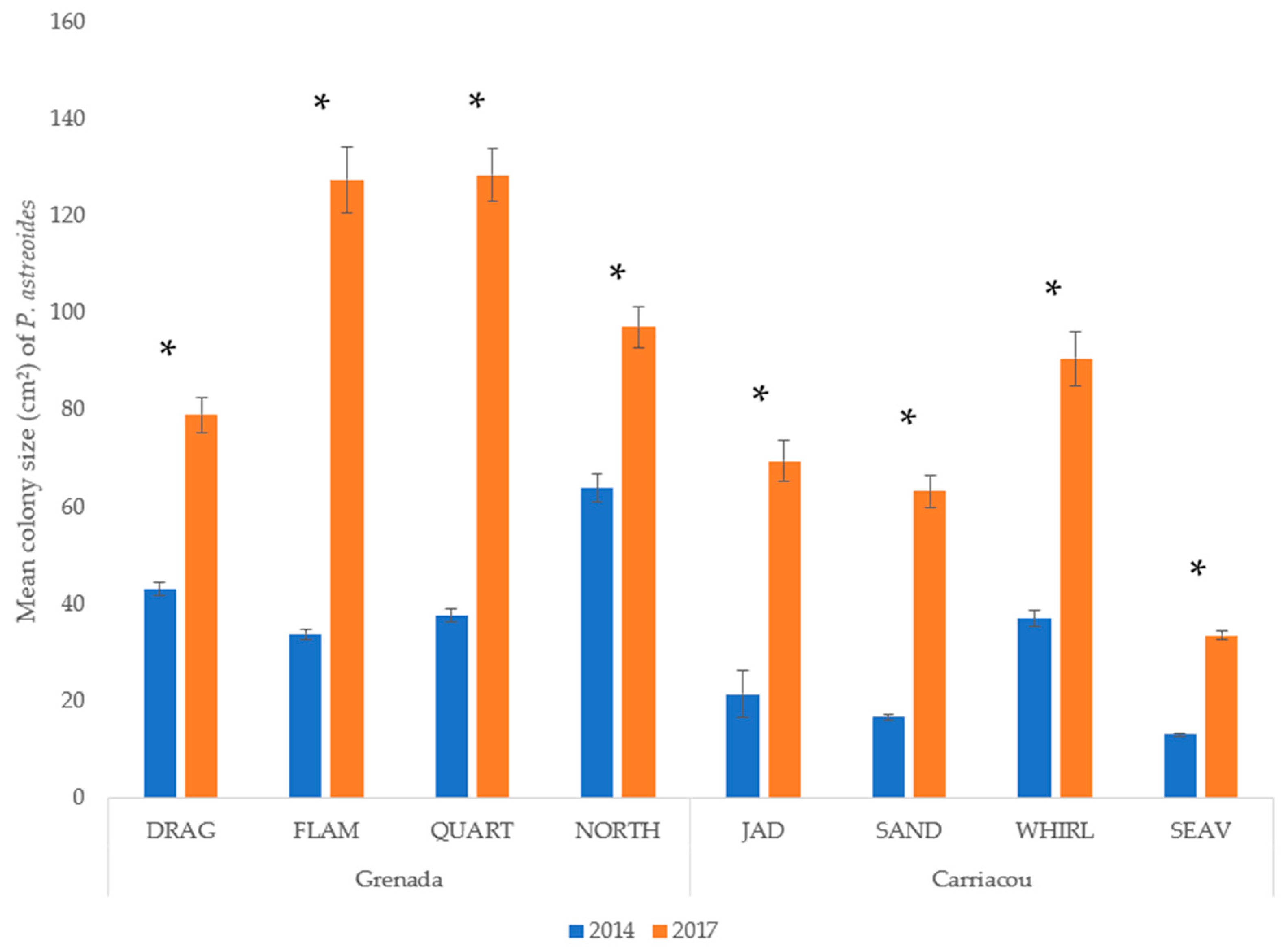
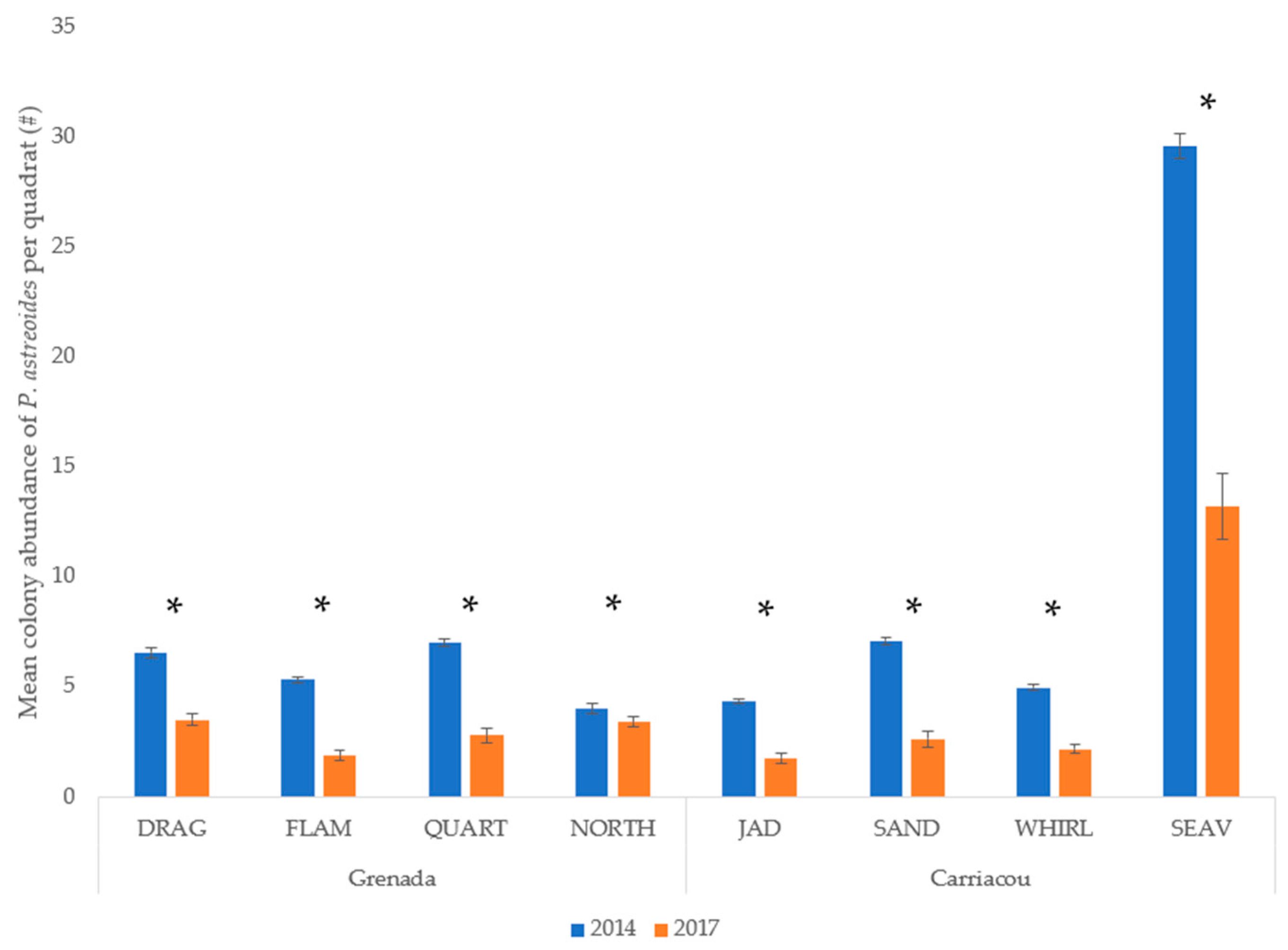
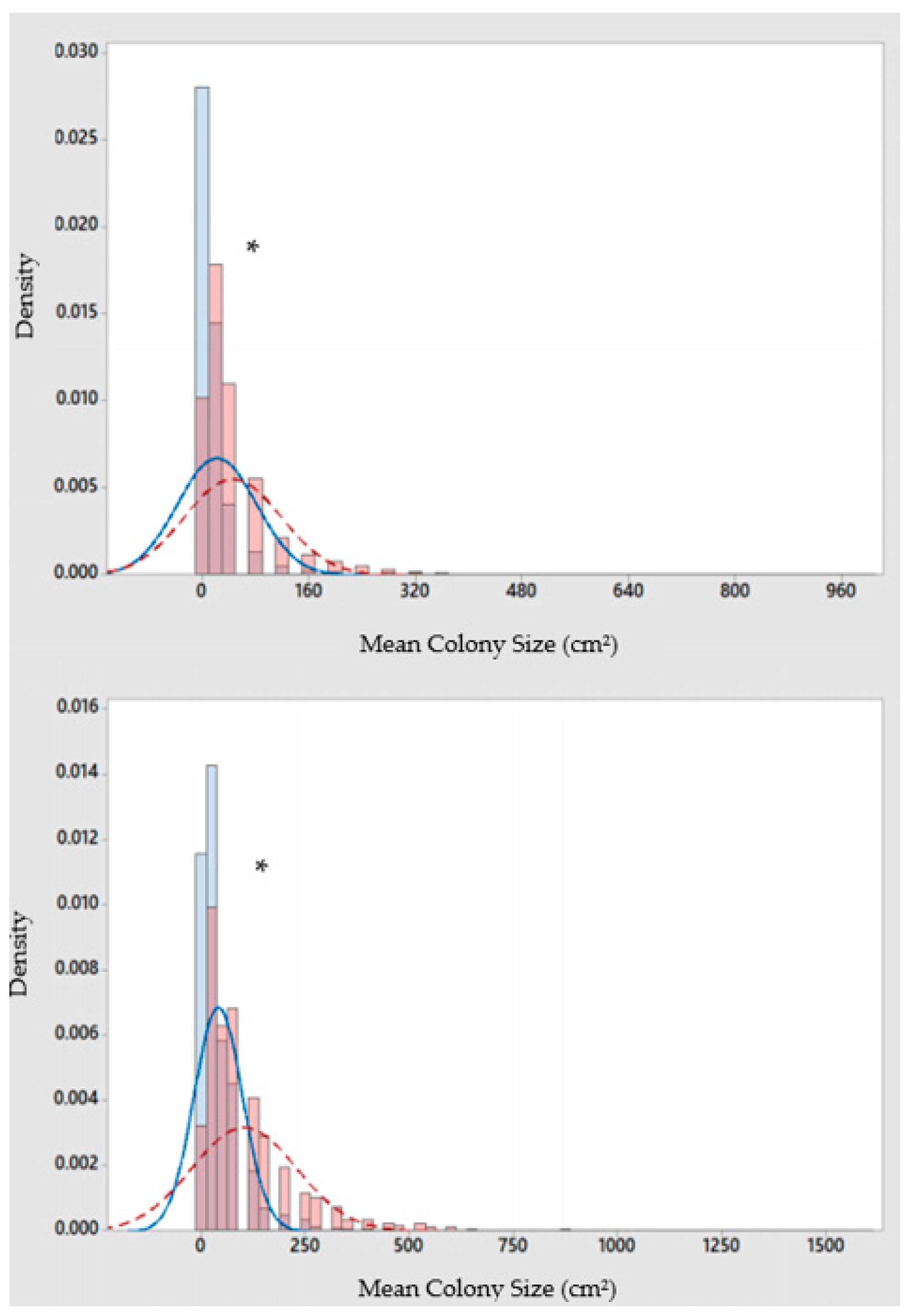
Mean percent coverage of the mustard hill coral increased at all eight sites, but significant increases were only observed at Flamingo Bay in Grenada, and at Whirlpool and Jackadam in Carriacou (Figure 3). Absolute colony coverage ranged from 0% to 38.9% in surveyed quadrats. Mean colony size of P. astreoides increased significantly at all sites in Carriacou and Grenada (Figure 4). Colony sizes from all surveyed quadrats ranged from 0.2 cm² to 1600 cm². Rates of colony size increases over the three years range from +87% to +329%. Mean colony abundance of P. astreoides significantly decreased at all sites in Carriacou and Grenada (Figure 5). P. astreoides colony abundance within a quadrat ranged from 0 to 134. There was a significant shift in the size frequency distribution (Kolmogorov-Smirnov 2-sample tests) of P. astreoides towards fewer, larger colonies at both islands from 2014 to 2017 (Figure 6).
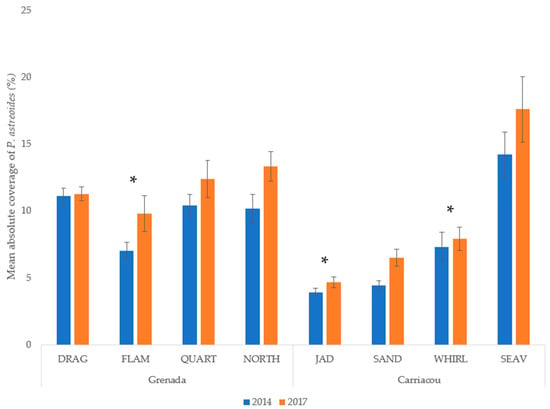
Figure 3.
Mean percent cover of P. astreoides in 2014 and 2017 in Carriacou and Grenada. (*) denotes significance (p < 0.05) between years.
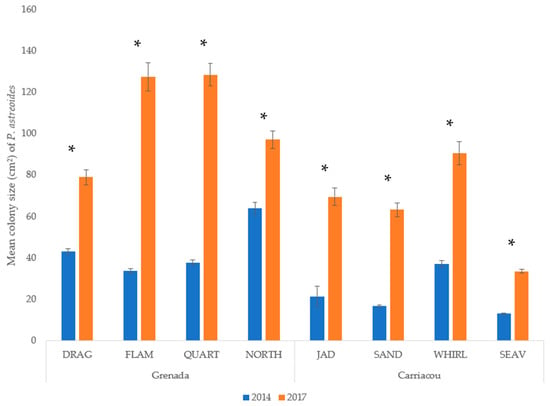
Figure 4.
Mean colony size (cm2) of P. astreoides in 2014 and 2017 in Carriacou and Grenada. (*) denotes significance (p < 0.05) between years.
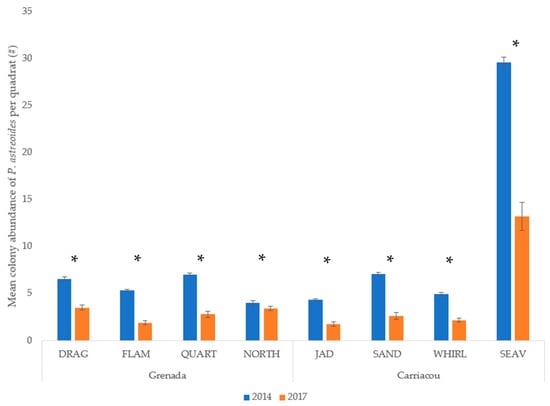
Figure 5.
Mean colony abundance of P. astreoides per quadrat in 2014 and 2017 in Carriacou and Grenada. (*) denotes significance (p < 0.05) between years.
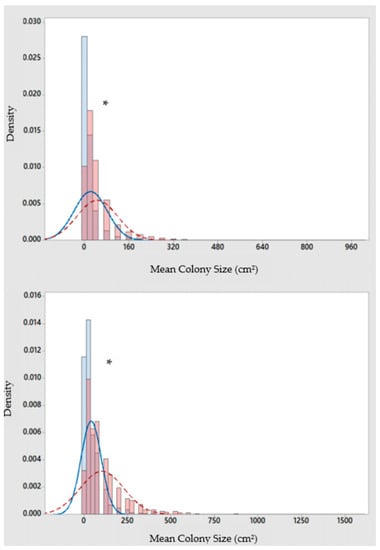
Figure 6.
Size-frequency distribution of P. astreoides colonies in Carriacou (top) and Grenada (bottom) in 2014 (dark grey bar; solid line) and 2017 (light grey bar; dashed line). * Indicates significance at p < 0.05.
Significant correlations between P. astreoides coverage and other biotic factors were identified in Carriacou and Grenada for both 2014 and 2017 (Table 4). Coverage of P. astreoides was significantly associated with sand (−), rubble (−), and coralline algae (+) in all analyses, (Table 4). Coverage was positively associated with sponges in Carriacou, and negatively associated in Grenada, although this was not statistically significant in Grenada in 2017 (Table 4). Coverage of P. astreoides was significantly associated with macroalgae (−) in all four correlation analyses (Table 4) and was significantly associated with gorgonians (−) in all three analyses that were possible (Table 4). Siderastrea siderea and O. annularis were the only scleractinian species present in all four analyses and no significant correlations were found with P. astreoides (Table 4). Porites divaricata was present in three of four analyses and was negatively correlated with P. astreoides coverage, although this was only significant in one of the three analyses (Table 4). Other scleractinian species were only present in one or two of the analyses with varying correlation relationships and statistical significance (Table 4).

Table 4.
Magnitude of the relationship between P. astreoides, scleractinian coral species, biotic factors, and habitat type. Pearson correlation coefficients indicated by r. * Indicates significance at p = 0.05.
4. Discussion
In this study, we were able to provide insight into factors relating to the success and population dynamics of P. astreoides. From 2014 to 2017, populations of this species were found to shift from being dominated by many smaller colonies, to fewer, larger and more mature colonies. Given the magnitude of changes in abundance and size of colonies (coupled with moderate coverage increases) over such a short timeframe, it is likely we observed the population dynamics of P. astreoides during and after a large-scale recruitment event, with a recent tropical storm in 2013 (Chantal) potentially acting as a trigger [29]. P. astreoides colonies were found to be positively associated with coralline algae, and pavement, while negatively associated with sand, rubble, and macroalgae. This mirrors the recruitment patterns found in other scleractinian species, with substrate type and algal status playing a critical role. In addition, recruitment patterns between P. astreoides and other scleractinian coral species were found to be potentially predicted by life-history strategy, being positively associated with massive corals, and negatively associated with branching corals.
The composition of Caribbean coral reefs has been relatively stable for much of the last 150,000 years, with species from all life history strategies persisting [29,30]. In the last several decades, increases in anthropogenic stressors and environmental impacts have resulted in up to 80% reductions in Caribbean live coral cover [1,2,31,32]. Communities once dominated by framework building corals are now primarily comprised of macroalgae and non-framework building species such as P. astreoides [7,33]. Prior species data for Carriacou and the nearby Tobago Cays show communities comprised of relatively similar species assemblages with the notable exception of acroporids [34,35]. In 1976, scleractinian coral cover in Carriacou was estimated at 30%, with A. palmata comprising approximately 10% of this value, with present densities being so low that the method used in the present study did not reflect their presence [34]. This lack of acroporids is attributed to their region-wide decline since the 1980s, with their near absence being noted in the nearby Tobago Cays in 1999 [35,36]. The percent coral cover values obtained in the present study for Grenada are slightly higher than those reported in recently published literature for the same study sites [20,21]. No significant differences in coral cover were detected between the islands. Unfortunately, there is a lack of extensive historical data for Carriacou and the only available published data is more than 40 years out of date and/or in the nearby Tobago Cays [34], hence the need for the present study. Statistically sound comparisons between the previous and present studies are not possible, however, broader comparisons can be made. The composition of the hard coral assemblages and the relatively low percent cover of hard corals combined with the high percent cover of macroalgae suggests that the current reefs of Carriacou and Grenada are stressed [2]. Non-reef building species such as Porites spp. now dominate these coral communities.
Size-frequency distributions (2014 vs. 2017) in Grenada and Carriacou revealed substantial increases in colony size of P. astreoides at all sites from 2014 to 2017, with a shift towards fewer, larger colonies in 2017. Although, large colonies (>400 cm2) observed in 2014 were still present in 2017. These increases ranged from +87% to +329% across the eight sites in only three years’ time. Moderate increases in mean percentage cover of P. astreoides was also observed at all sites, with statistical significance only found in Flamingo Bay, Whirlpool, and Jackadam. Although increasing in colony size and coverage, significant declines in P. astreoides abundance was observed at all sites. Considering the mean increase in colony size, coupled with a drop in colony abundance and low coverage increases, these results provide a further indication that this study observed the recruitment dynamics of P. astreoides [37]. First, local populations were dominated by juvenile colonies of P. astreoides (<40 cm2) in both 2014 and 2017 (Figure 6). Second, the large number of small colonies in 2014 could be reflective of a large-scale recovery event following a disturbance that took place prior to monitoring, such as tropical storm ‘Chantal’ that affected Grenada and Carriacou eight months prior to the first survey in 2014 [38]. Regional hurricane events in the windward islands of the Caribbean have been observed to negatively impact recruitment in scleractinian coral species, with it persisting at reduced levels [39]. These results corroborate previous research demonstrating that P. astreoides populations, much like other weedy coral species, are largely influenced by recruitment [18,40].
In addition to recruitment, which is thought to be the main explanation for the observed pattern, it is also possible that colony fusion likely played some role in the aforementioned trends observed in P. astreoides colonies. We maintain this possibility as increases in colony size do not correspond with the known growth rate of this species. The upper end of radial growth rates for P. astreoides has been estimated at ~5.78 mm year−1, comparable to other scleractinian corals, with colonies reaching maturity at eight to ten years of age [40,41]. Thus, for a 40 cm² colony to more than triple in size through growth alone over three years as observed (i.e., Flamingo Bay), radial extension of more than 8 mm year−1 would be required, exceeding known growth rates. Colony fusion has been observed in P. astreoides populations following hurricane Hugo in the Buck Island Reef National Monument [42]. Colony fusion rates year−1 as a share of the total population were observed to have increased from a mean of 0.4% to 4.1% following this disturbance [42]. Fusion is known to occur in the species and juvenile P. astreoides colonies are highly likely to be genetically related to one another since inbreeding through self-fertilization is common [41,43]. Asexual recruits are often found in close proximity and have irregular growth forms [37], an occurrence frequently noted observed in our image analysis (Figure 7). Yet, as we did not directly measure colony fusion in our study, this is an important aspect that future studies should consider when exploring populations of P. astreoides.

Figure 7.
Portion of a quadrat photo from the ‘Seaview’ site on Carriacou in 2014. P. astreoides is abundant, with many colonies growing in close proximity to one another.
In our study, P. astreoides colonies were significantly correlated with pavement (+), sand (−), rubble (−), macroalgae (−), and coralline algae (+) as observed with other scleractinian species [10,44,45,46]. Sandy and rubble substrates are habitat ‘sinks’ for coral recruits; the former results in poor attachment and smothering [46,47,48], while rubble can facilitate initial attachment and growth, but is easily shifted, destroying colonies [45,46,47,48,49]. Conversely, P. astreoides larvae can settle and thrive on pavement as this substrate is resistant to physical disturbance [45,46].
The negative relationship between P. astreoides coverage and macroalgae observed in the present study was expected. Scleractinian corals globally are unable to compete with macroalgae, resulting in shifts from coral-dominated communities to those dominated by macroalgae [44,50,51]. Herbivorous species are essential to clear macroalgae [44,52], however, not all herbivores impact P. astreoides success equally. Ocean surgeonfish grazing increased growth of P. astreoides, but parrotfish have no impact [52]. Herbivorous fish biomass in Grenada and Carriacou is ‘poor’ [53] and herbivorous invertebrates, such as Diadema antillarum, are also now largely absent from reef communities in Grenada [21]. Though not directly assessed in this study, high abundances of urchins were observed in transects with the low levels of macroalgae. Unlike macroalgae, coralline algae does not outcompete coral recruits, and attracts their settlement with chemical signatures in the water [46,54]. In the present study, a positive relationship was observed between P. astreoides and coralline algae coverage.
Varying responses of P. astreoides coverage to sponges were observed in the present study. Sponges were not classified by individual species or growth form, a potential source of this unclear relationship. The negative association between P. astreoides and gorgonians observed in the present study was expected as the elongate form of gorgonians can block essential sunlight for many scleractinian colonies [55]. Negative relationships have been observed between gorgonians and scleractinian corals, which has resulted in the classification of coral framework reefs being habitats distinct from gorgonian plains [53,55]. Care should be taken when considering this result as gorgonian cover was based on their respective presence in the image, not just the area where each colony was attached to the substrate.
Scleractinian species that were significantly positively correlated with P. astreoides had a massive colony growth pattern (both stress tolerant and generalist), while species that were significantly negatively correlated had a branching growth pattern (only weedy). Weedy corals with a shared life history strategy may compete with each other in disturbed environments [56]. Large colony size is typical of stress-tolerant species, with a greater reserve of polyps from which to draw energy, often increasing the resilience of these colonies to changes over time [57].
P. astreoides continues to be one of the more successful coral species in Grenada and the Caribbean at large and may continue to experience success in the future. P. astreoides faces similar settlement and competitive challenges as other coral species and its life-history strategy may be partially responsible for its success on a regional scale. Further work is required to assess the magnitude and range of environmental stressors this species can tolerate and the role colony fusion plays may play in the recovery dynamics of its’ populations, especially in the context of rapid climate change.
Author Contributions
Conceptualization, R.G.E., R.A.H. and J.S.L.; methodology, R.G.E., R.A.H., L.Á.-F. and J.S.L.; validation, R.G.E., R.A.H. and L.Á.-F.; formal analysis, R.G.E., R.A.H. and C.M.H.; resources, J.S.L.; data curation, R.G.E., R.A.H.; writing—original draft preparation, R.G.E., R.A.H.; writing—review and editing, R.G.E., R.A.H., L.Á.-F. and J.S.L.; supervision, C.M.H., L.Á.-F. and J.S.L.; project administration, J.S.L.; funding acquisition, J.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a NSERC Discovery Grant (Lumsden), the Ontario Veterinary College, University of Guelph, and St. George’s University.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is available from the corresponding author upon request.
Acknowledgments
We would like to thank EcoDive Grenada and Deefer Diving Carriacou for their assistance with diving logistics in the field. Funding for this research was provided by a NSERC Discovery Grant (Lumsden), the Ontario Veterinary College, University of Guelph, and St. George’s University. Horricks was the recipient of a St. George’s University post-doctoral and an Ontario Veterinary College Scholarship. Eagleson was the recipient of an Ontario Veterinary College Scholarship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gardner, T.A.; Côté, I.M.; Gill, J.A.; Grant, A.; Watkinson, A.R. Long-Term Region-Wide Declines in Caribbean Corals. Science 2003, 301, 958–960. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Donovan, M.K.; Cramer, K.L.; Lam, W. Status and Trends of Caribbean Coral Reefs: 1970–2012; International Union for the Conservation of Nature: Gland, Switzerland, 2014. [Google Scholar]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate Change, Human Impacts, and the Resilience of Coral Reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef]
- Castillo, K.D.; Ries, J.B.; Weiss, J.M.; Lima, F.P. Decline of forereef corals in response to recent warming linked to history of thermal exposure. Nat. Clim. Chang. 2012, 2, 756–760. [Google Scholar] [CrossRef]
- Okazaki, R.R.; Towle, E.K.; Hooidonk, R.; Mor, C.; Winter, R.N.; Piggot, A.M.; Cunning, R.; Baker, A.C.; Klaus, J.S.; Swart, P.K.; et al. Species-specific responses to climate change and community composition determine future calcification rates of Florida Keys reefs. Glob. Chang. Biol. 2017, 23, 1023–1035. [Google Scholar] [CrossRef]
- Anthony, K.R.; Marshall, P.A.; Abdulla, A.; Beeden, R.; Bergh, C.; Black, R.; Eakin, C.M.; Game, E.; Gooch, M.; Graham, N.; et al. Operationalizing resilience for adaptive coral reef management under global environmental change. Glob. Chang. Biol. 2015, 21, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.T.; Steneck, R.S.; Murphy, G.N.; Kench, P.S.; Edinger, E.N.; Smithers, S.G.; Mumby, P. Regional-scale dominance of non-framework building corals on Caribbean reefs affects carbonate production and future reef growth. Glob. Chang. Biol. 2015, 21, 1153–1164. [Google Scholar] [CrossRef]
- Van Woesik, R.; Sakai, K.; Ganase, A.; Loya, Y. Revisiting the winners and the losers a decade after coral bleaching. Mar. Ecol. Prog. Ser. 2011, 434, 67–76. [Google Scholar] [CrossRef]
- Darling, E.S.; Alvarez-Filip, L.; Oliver, T.A.; McClanahan, T.R.; Côté, I.M. Evaluating life-history strategies of reef corals from species traits. Ecol. Lett. 2012, 15, 1378–1386. [Google Scholar] [CrossRef]
- Schutte, V.G.W.; Selig, E.R.; Bruno, J.F. Regional spatio-temporal trends in Caribbean coral reef benthic communities. Mar. Ecol. Prog. Ser. 2010, 402, 115–122. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.; Banaszak, A.T.; McField, M.D.; Beltrán-Torres, A.U.; Álvarez-Filip, L. Assessment of Acropora palmata in the Mesoamerican Reef System. PLoS ONE 2014, 9, e96140. [Google Scholar] [CrossRef]
- Green, D.; Edmunds, P.; Carpenter, R. Increasing relative abundance of Porites astreoides on Caribbean reefs mediated by an overall decline in coral cover. Mar. Ecol. Prog. Ser. 2008, 359, 1–10. [Google Scholar] [CrossRef]
- Medina-Valmaseda, A.E.; Rodríguez-Martínez, R.E.; Alvarez-Filip, L.; Jordan-Dahlgren, E.; Blanchon, P. The role of geomorphic zonation in long-term changes in coral-community structure on a Caribbean fringing reef. PeerJ 2020, 8, e10103. [Google Scholar] [CrossRef]
- Eagleson, R. Spatial Distribution of Benthic Habitats and Ecological Patterns of the Mustard Hill Coral (Porites Astreoides) in the Nearshore Waters of Grenada. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2019. [Google Scholar]
- Edmunds, P. Population biology of Porites astreoides and Diploria strigosa on a shallow Caribbean reef. Mar. Ecol. Prog. Ser. 2010, 418, 87–104. [Google Scholar] [CrossRef]
- Baumann, J.H.; Townsend, J.E.; Courtney, T.A.; Aichelman, H.E.; Davies, S.W.; Lima, F.P.; Castillo, K.D. Temperature Regimes Impact Coral Assemblages along Environmental Gradients on Lagoonal Reefs in Belize. PLoS ONE 2016, 11, e0162098. [Google Scholar] [CrossRef] [PubMed]
- Goodbody-Gringley, G.; de Putron, S.J. Brooding Corals: Planulation Patterns, Larval Behavior, and Recruitment Dynamics in the Face of Environmental Change. In The Cnidaria, Past, Present and Future; Springer International Publishing: Cham, Switzerland, 2016; pp. 279–289. [Google Scholar]
- Edmunds, P.J.; Didden, C.; Frank, K. Over three decades, a classic winner starts to lose in a Caribbean coral community. Ecosphere 2021, 12, e03517. [Google Scholar] [CrossRef]
- Horricks, R.A.; Herbinger, C.M.; Lillie, B.N.; Taylor, P.; Lumsden, J.S. Differential protein abundance during the first month of regeneration of the Caribbean star coral Montastraea cavernosa. Coral Reefs 2018, 38, 45–61. [Google Scholar] [CrossRef]
- Anderson, R.; Morrall, C.; Nimrod, S.; Balza, R.; Berg, C.; Jossart, J. Benthic and fish population monitoring associated with a marine protected area in the nearshore waters of Grenada, Eastern Caribbean. Rev. Biol. Trop. 2012, 60, 71–87. [Google Scholar] [CrossRef]
- Anderson, R.A.; Morrall, C.B.; Nimrod, S.B.; Balza, R.A.; Berg, C.; Jossart, J.A. Marine Protected Area Monitoring in the Nearshore Waters of Grenada, Eastern Caribbean: Benthic Cover and Fish Populations. Rev. Biol. Trop. 2014, 62, 273–286. [Google Scholar] [CrossRef]
- Horricks, R.A. Tissue Regeneration of Artificially Induced Lesions in the Caribbean Great Star Coral (Montastraea Cavernosa) in the Nearshore Waters of Grenada. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2017. [Google Scholar]
- Kohler, K.E.; Gill, S.M. Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 2006, 32, 1259–1269. [Google Scholar] [CrossRef]
- Dumas, P.; Bertaud, A.; Peignon, C.; Léopold, M.; Pelletier, D. A “quick and clean” photographic method for the description of coral reef habitats. J. Exp. Mar. Biol. Ecol. 2009, 368, 161–168. [Google Scholar] [CrossRef]
- Alquezar, R.; Boyd, W. Development of rapid, cost effective coral survey techniques: Tools for management and conservation planning. J. Coast. Conserv. 2007, 11, 105–119. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; ISSN 17513243. ISBN 9780080554501. [Google Scholar]
- Oksanen, J. Vegan: Ecological Diversity. 2016, p. 12. Available online: https://www.semanticscholar.org/paper/Vegan%3A-ecological-diversity-Oksanen/6d1f21af6a90af203ab680657c215008e292cea6 (accessed on 27 November 2020).
- Bonett, D.G.; Wright, T.A. Sample size requirements for estimating pearson, kendall and spearman correlations. Psychometrika 2000, 65, 23–28. [Google Scholar] [CrossRef]
- Greenstein, B.J.; Curran, H.A.; Pandolfi, J.M. Shifting ecological baselines and the demise of Acropora cervicornis in the western North Atlantic and Caribbean Province: A Pleistocene perspective. Coral Reefs 1998, 17, 249–261. [Google Scholar] [CrossRef]
- Toth, L.T.; Stathakopoulos, A.; Kuffner, I.B.; Ruzicka, R.R.; Colella, M.A.; Shinn, E.A. The unprecedented loss of Florida’s reef-building corals and the emergence of a novel coral-reef assemblage. Ecology 2019, 100, e02781. [Google Scholar] [CrossRef]
- Burke, L.; Maidens, J. Reefs at Risk in the Caribbean; World Resources Institute: Washington, DC, USA, 2004; ISBN 1559632574. [Google Scholar]
- Neal, B.P.; Khen, A.; Treibitz, T.; Beijbom, O.; O’Connor, G.; Coffroth, M.A.; Knowlton, N.; Kriegman, D.; Mitchell, B.G.; Kline, D. Caribbean massive corals not recovering from repeated thermal stress events during 2005–2013. Ecol. Evol. 2017, 7, 1339–1353. [Google Scholar] [CrossRef]
- Aronson, R.B.; MacIntyre, I.G.; Wapnick, C.M.; O’Neill, M.W. Phase Shifts, Alternative States, and the Unprecedented Convergence of Two Reef Systems. Ecology 2004, 85, 1876–1891. [Google Scholar] [CrossRef]
- Goodwin, M.; Cole, M.; Stewart, W.; Zimmerman, B. Species density and associations in Caribbean reef corals. J. Exp. Mar. Biol. Ecol. 1976, 24, 19–31. [Google Scholar] [CrossRef]
- Deschamps, A.; DesRochers, A.; Klomp, K.D. St. Vincent. A Rapid Assessment of the Horseshoe Reef, Tobago Cays Marine Park, St. Vincent, West Indies (Stony Corals, Algae and Fishes). Atoll Res. Bull. 2003, 496, 438–459. [Google Scholar] [CrossRef][Green Version]
- Lewis, J.B. A preliminary description of the coral reefs of the Tobago Cays, Grenadines, West Indies. Atoll Res. Bull. 1975, 178, 1–9. [Google Scholar] [CrossRef][Green Version]
- Smith, S.R. Patterns of Coral Recruitment and Post-Settlement Mortality on Bermuda’s Reefs: Comparisons to Caribbean and Pacific Reefs. Am. Zool. 1992, 33, 663–673. [Google Scholar] [CrossRef]
- Votaw, G.S.; Rosa, L.; Snell, W.; Anselmi, C.; History, S. Tropical Storm Chantal; National Weather Service: Washington, DC, USA, 2013. [Google Scholar]
- Mallela, J.; Crabbe, M. Hurricanes and coral bleaching linked to changes in coral recruitment in Tobago. Mar. Environ. Res. 2009, 68, 158–162. [Google Scholar] [CrossRef]
- Madin, J.S.; Anderson, K.D.; Andreasen, M.H.; Bridge, T.C.; Cairns, S.D.; Connolly, S.R.; Darling, E.S.; Diaz, M.; Falster, D.S.; Franklin, E.C.; et al. The Coral Trait Database, a curated database of trait information for coral species from the global oceans. Sci. Data 2016, 3, 160017. [Google Scholar] [CrossRef]
- Chornesky, E.; Peters, E. Sexual Reproduction and Colony Growth in the Scleractinian Coral Porites Astreoides. Biol. Bull. 1987, 172, 161–177. [Google Scholar] [CrossRef]
- Bythell, J.C. Assessment of the Impacts of Hurricanes Marilyn and Luis and Post-Hurricane Community Dynamics at Buck Island Reef National Monument; Technical Report; US Department of Interior, National Park Service: Washington, DC, USA, 1997. [Google Scholar] [CrossRef]
- Brazeau, D.A.; Gleason, D.F.; Morgan, M.E. Self-Fertilization in Brooding Hermaphroditic Caribbean Corals: Evidence from Molecular Markers. J. Exp. Mar. Biol. Ecol. 1998, 231, 225–238. [Google Scholar] [CrossRef]
- Vega Thurber, R.; Burkepile, D.E.; Correa, A.M.S.; Thurber, A.R.; Shantz, A.A.; Welsh, R.; Pritchard, C.; Rosales, S. Macroalgae Decrease Growth and Alter Microbial Community Structure of the Reef-Building Coral, Porites Astreoides. PLoS ONE 2012, 7, e44246. [Google Scholar] [CrossRef]
- Cameron, C.M.; Pausch, R.E.; Miller, M.W. Coral Recruitment Dynamics and Substrate Mobility in a Rubble-Dominated Back Reef Habitat. Bull. Mar. Sci. 2016, 92, 123–136. [Google Scholar] [CrossRef]
- Ritson-Williams, R.; Arnold, S.; Paul, V. Patterns of larval settlement preferences and post-settlement survival for seven Caribbean corals. Mar. Ecol. Prog. Ser. 2016, 548, 127–138. [Google Scholar] [CrossRef]
- Ross, C.; Ritson-Williams, R.; Olsen, K.; Paul, V.J. Short-Term and Latent Post-Settlement Effects Associated with Elevated Temperature and Oxidative Stress on Larvae from the Coral Porites Astreoides. Coral Reefs 2013, 32, 71–79. [Google Scholar] [CrossRef]
- Olsen, K.; Sneed, J.; Paul, V.J. Differential larval settlement responses of Porites astreoides and Acropora palmata in the presence of the green alga Halimeda opuntia. Coral Reefs 2016, 35, 521–525. [Google Scholar] [CrossRef]
- Fava, F.; Ponti, M.; Scinto, A.; Calcinai, B.; Cerrano, C. Possible effects of human impacts on epibenthic communities and coral rubble features in the marine Park of Bunaken (Indonesia). Estuarine Coast. Shelf Sci. 2009, 85, 151–156. [Google Scholar] [CrossRef]
- Gilby, B.L.; Maxwell, P.S.; Tibbetts, I.; Stevens, T. Bottom-Up Factors for Algal Productivity Outweigh No-Fishing Marine Protected Area Effects in a Marginal Coral Reef System. Ecosystems 2015, 18, 1056–1069. [Google Scholar] [CrossRef]
- Olsen, K.; Paul, V.J.; Ross, C. Direct effects of elevated temperature, reduced pH, and the presence of macroalgae (Dictyota spp.) on larvae of the Caribbean coral Porites astreoides. Bull. Mar. Sci. 2015, 91, 255–270. [Google Scholar] [CrossRef]
- Burkepile, D.E.; Hay, M. Impact of Herbivore Identity on Algal Succession and Coral Growth on a Caribbean Reef. PLoS ONE 2010, 5, e8963. [Google Scholar] [CrossRef] [PubMed]
- Atlantic and Gulf Rapid Reef Assessment. Grenada’s Coral Reef Report Card; Ocean Research and Education Foundation: Key Largo, FL, USA, 2016; Available online: http://caribnode.org/documents/85 (accessed on 15 November 2017).
- Done, T.J. Coral Community Adaptability to Environmental Change at the Scales of Regions, Reefs and Reef Zones. Am. Zool. 1999, 39, 66–79. [Google Scholar] [CrossRef]
- Williams, S.; Mumby, P.; Chollett, I.; Cortés, J. Importance of differentiating Orbicella reefs from gorgonian plains for ecological assessments of Caribbean reefs. Mar. Ecol. Prog. Ser. 2015, 530, 93–101. [Google Scholar] [CrossRef]
- Riegl, B.; Purkis, S.J. Coral population dynamics across consecutive mass mortality events. Glob. Chang. Biol. 2015, 21, 3995–4005. [Google Scholar] [CrossRef]
- De Barros, M.M.L.; Pires, D.D.O. Colony size-frequency distributions among different populations of the scleractinan coral Siderastrea stellata in Southwestern Atlantic: Implications for life history patterns. Braz. J. Oceanogr. 2006, 54, 213–223. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).