Abstract
Rock outcrops have promoted a high level of species diversity and provided a stable microclimate for long time periods. The present study is devoted to plant diversity of natural Quaternary outcrops of basaltic rocks. Chorological and ecological investigations were carried out at 35 such outcrops, located within five physiogeographic units of the Sudetes Mountains. The focus was on 120 xerothermic taxa of vascular plants: 62 strictly xerothermic (steppe) taxa of the Festuco valesiacae-Brometea erecti class, and 58 thermophilous taxa representing classes Trifolio medii-Geranietea sanguinei and Quercetea pubescentis. Limited geographical ranges of these plants are manifested by variable frequency of their occurrence. Species distribution is determined by natural factors, like surface area of the outcrop, the type of basaltic rock and the type of plant communities developed. Basaltic outcrops in the Sudetes meet the criterion of habitat islands (inselbergs), serve as regional centers of vascular flora, and are refugia for marginal populations of relict species.
1. Introduction
An important feature of different areas on Earth, in all of the geographical regions, is the spatial diversity of habitats. Places with nearly uniform abiotic parameters of environment usually form mosaics of more or less isolated habitats, which often become “islands” for various groups of organisms. Habitat fragmentation is a process of gradual division into smaller, more isolated parts. The fragmentation may result in different spatial configurations of habitat patches in the landscape and different patterns of species richness, at local to continental scales [1,2,3,4,5,6,7]. Habitat islands may have both natural and anthropogenic origin. Examples of natural habitat islands on land [8,9,10,11,12,13,14] are: Lakes, oxbow lakes and peatlands of different sizes—habitat islands for aquatic and marsh organisms, summit parts of high mountains isolated by deep valleys—for high mountain species, small forest complexes in the agricultural landscape—for shrub and forest species and rock outcrops (inselbergs) of a structure different from their surroundings—for rock species. The latter are often examples of small natural objects sensu small natural features (SNF) or small island effect (SIE) [13,15,16,17,18,19,20,21,22], which are characterized by high heterogeneity of abiotic factors, the presence of contrasting plant communities and species diversity and separateness, in comparison to the surrounding landscape diversity, as well as the risk of extinction events. Due to their geographic nature, rock outcrops (monadnocks) are regarded as specific environmental islands [23,24,25,26,27,28,29,30,31,32,33,34] because most of them show a clear ecological isolation from the surrounding landscape. Regardless of their surface area, habitat islands of the rock outcrops type are often the only places of occurrence or refugia for many groups of plants, including xerothermic and thermophilous species.
The development of steppe vegetation in Central Europe took place in the Preboreal period when xerothermic plants from the Pontic and Mediterranean regions began to enter forestless parts of Central Europe [8,35,36]. Next, geographical ranges of xerothermic plant communities shrank as the climate continued to warm up, which resulted in an increase of forest cover. This process was followed by their re-development that was in turn stimulated by human activity from the beginning of Neolithic settlement. Therefore, some researchers [37,38,39] believe that the majority of Central European xerothermic grasslands are relatively young communities and that their character is to a large extent anthropogenic. Formation of the current isolated localities of xerothermic plants and shaping of their geographical ranges is explained by phylogeographic studies using molecular markers [36]. However, due to the heterogeneous history of postglacial migration, it is difficult to find a common scenario for the evolution of extrazonal xerothermic flora in this part of Europe. The xerothermic species entered the Sudetes Mountains and spread from two regions: (i) on the south, from the Pannonian Plain (Hungarian Plain) through the Moravian Gate along the Odra valley and the Sudetes foreland; (ii) on the west, from Czech Republic and Germany refugia (České Sředohoři Mountains and Thuringian Highlands) along the Elbe valley and the northern depression of the Sudetes [40,41,42]. At the beginning of the last century, there were still relatively numerous traces of the former distribution of xerothermic vegetation clusters in the foreland of the Sudetes [43,44]. Currently, many of these species occur in the Sudetes only on rock hills. They were reported from limestones, serpentinites, as well as basaltic hills [45,46,47].
In Central Europe, xerothermic vegetation occurs only in places with special orographic, soil and microclimate conditions, i.e., the most dry places, with high ground temperatures and low rainfall (<600 mm per year), often related to rock habitats with reaction close to alkaline. Fragments of this vegetation that developed extrazonally are located far away from the Eurasian zone of steppes. Due to the history of their development in Postglacial and the simultaneous influence of the sub-oceanic and sub-continental climate, these fragments are characterized by a mixed floristic composition. Such transitory floristic nature makes the xerothermic grasslands of Central Europe unique natural objects [38,39,48]. Places of their occurrence, usually limited to small areas, are very often located within hills composed of various types of rocks. They include, among others, the hills of the Cainozoic Central European Volcanic Formation [49,50,51]. Their remains in the Sudetes have been preserved in the landscape till present and are referred to as volcanism relics or “palaeovolcanoes”.
Palaeovolcanoes, i.e., basaltic outcrops, differ in terms of age range, mineralogical composition, as well as petrographic and geochemical properties. They are necks, which are remnants of former volcanic cones, lava flows or intrusive forms. As a product of the alkali volcanism from Oligocene–Pliocene, they are built of various types of fine-grained rocks of the basaltic formation [52,53,54], including basanites, plagioclase basalts, nephelenites, phonolites, trachites and trachybasalts. These rocks are distinguished by an increased content of magnesium and a low content of silicon, sodium and potassium. Basalt is a characteristic type of bedrock, on which strongly skeletal soils are formed, mainly from the group of lithosols or rankers. The chemical composition of the basic minerals that build basaltic rocks (alkaline plagioclases, pyroxenes, amphiboles, feldspars, olivines and biotytes), the availability of magnesium compounds and high temperature of rock heating, altogether determine the richness and separateness of flora and promote the settlement of plants that have higher thermal requirements and are adapted to water scarcity.
Among basaltic outcrops in the Sudetes there are objects of various sizes—from small intrusions, which occur within hills built of other types of rocks, to vast basaltic hills with a characteristic conical shape (Figure 1). They provide habitats of semi-natural dry grasslands that require conservation according to the Directive 92/43 EEC (EUR 27). So far, however, only a few of these objects have been the subject of floristic and phytosociological studies [55,56,57,58,59,60]. Therefore, chorological and ecological investigations were carried out at basaltic outcrops, located within five physiogeographic units of the Sudetes Mountains. Possible relationships between regional distribution and floristic richness of xerothermic plant species, abiotic environmental factors and locally developed classes of vegetation, were examined.

Figure 1.
Basaltic outcrops of the Sudetes Mountains. (A) isolated tephrite cone of Knorrberg; (B) isolated nephelenite cone of Landeskrone; (C) peak nephelenite outcrop of Stożek Perkuna; (D) isolated basanite cone of Ostrzyca; (E,F) xerothermic grasslands of tephrite outcrop Krzyżowa Góra and (G,H) complex of xerothermic and thermophilous vegetation of the nephelenite outcrop Czartowska Skała.
2. Materials and Methods
2.1. Basaltic Outcrops
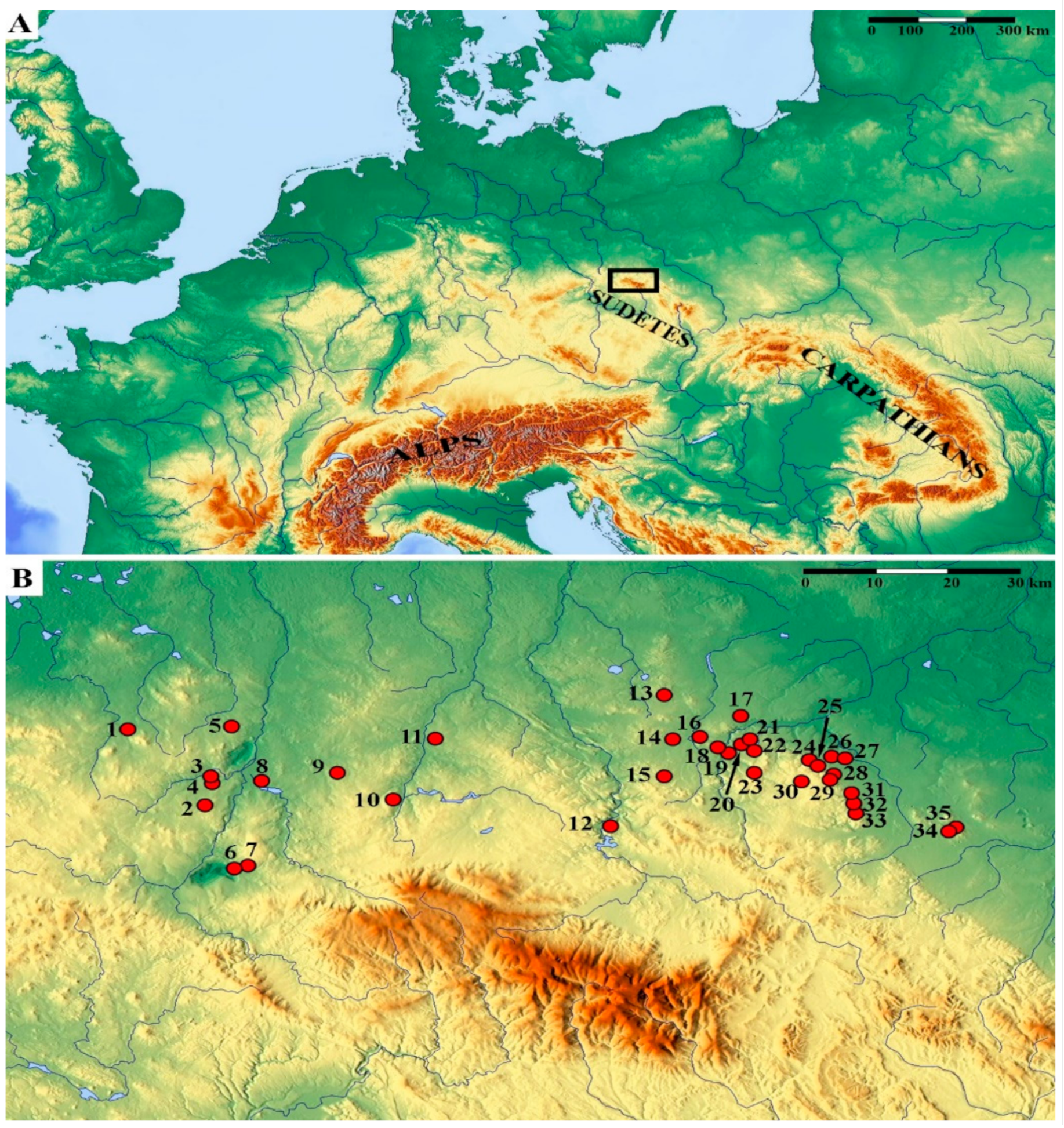
The Sudetes Mountains are part of the Czech Massif [61,62], which together with the Vosges, Black Forest, Massif Central and Harz belong to the Hercynian (Variscan) mountains of Central and Western Europe. They are distinguished by a complex geological structure [63] and numerous exposures of volcanic rocks of various age. The investigations were carried out at 35 basaltic outcrops located in the Sudetes Mountains, in the ranges of Lusatian Hills (Lausitzer Hügelland), Izera Plateau (Pogórze Izerskie), Western Kaczawa Plateau (Pogórze Zachodniokaczawskie), Eastern Kaczawa Plateau (Pogórze Wschodniokaczawskie) and Strzegom Hills (Wzgórza Strzegomskie) (Figure 2, Table 1), during vegetation seasons 2014–2020.
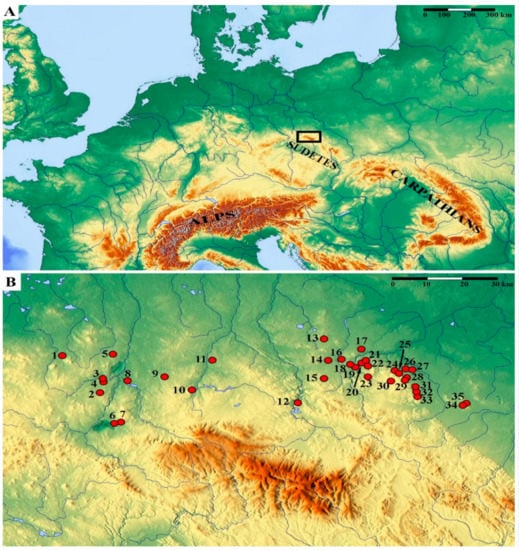
Figure 2.
Location of the studied area, marked by a black rectangle (A), and distribution of the investigated basaltic outcrops (B). The locality labels (numbers) are explained in Table 1. Relief maps were obtained from www.maps-for-free.com.

Table 1.
General information about location, geological structure and xerothermic vascular flora at the investigated outcrops. Upper indices after the outcrop names refer to physiographic units: a—Lusatian Hills; b—Izera Plateau; c—Western Kaczawa Plateau; d—Eastern Kaczawa Plateau and e—Strzegom Hills.
For each outcrop the following parameters were considered. The summit coordinates, elevation and surface area were measured using a device with a global positioning system (Garmin GPS map; WGS 84 reference system). The type of basaltic rock was assigned to each outcrop based on the available geological materials [51,52,54,64]. In case of basaltic outcrops for which the literature data were available, habitat conditions were determined on the basis of the physico-chemical parameters of the bedrock (Table 2) [54,65,66,67,68]. Classification of the outcrops to specific types of volcanic rocks was done according to Le Maitre et al. [69]. Floristic richness of the outcrop was the total number of xerothermic species that were noted during field investigation.

Table 2.
Chemical composition of basaltic rocks from selected outcrops.
2.2. Plant Species
Classification of plants follows Jäger [70]. Among all the vascular plants encountered in the course of field investigations, two thermal groups were considered: Strictly xerothermic and thermophilous species. Plants were classified to the appropriate thermal group based on the phytosociological criterion [71,72,73,74], which distinguishes strictly xerothermic taxa of the Festuco valesiacae-Brometea erecti Br.-Bl. and Tüxen ex Br.-Bl. 1949 class and its lower syntaxa: Brometalia erecti Br.-Bl. and Tüxen ex Br.-Bl. 1949, Festucetalia valesiacae Br.-Bl. and Tüxen ex Br.-Bl. 1949; and thermophilous species included in communities of the Trifolio medii-Geranietea sanguinei Müller 1962 class (Antherico ramosi-Geranietalia sanguinei Julve ex Dengler in Dengler, Berg, Eisenberg, Isermann, Jansen, Koska, Löbel, Mathey, Bazolt, Spangenberg, Timmermann and Wollert 2003, Origanetalia vulgaris Müller 1962) and oak forests of the Quercetea pubescentis Doing Kraft ex Scamoni and Passarge 1959 class. Each of the examined taxa was affiliated to phytogeographical elements based on the general distribution ranges of vascular plant species [75,76,77,78]. Furthermore, Ellenberg indicator values [79], describing preferences to light, temperature and moisture, were assigned to each species.
2.3. Statistical Analysis
All the analyses were performed using the Statistics toolbox of Matlab (The Matworks, Natick, MA, USA). The hierarchical classification method included in the Matlab package was used in the ordination analysis. Statistical significance was tested using pairwise Student’s t-test.
3. Results
3.1. Phytogeographical Elements, Distribution of Xerothermic Species and Floristic Richness of Outcrops
Xerothermic flora of the investigated basaltic outcrops comprises 120 taxa of vascular plants, 62 of which are strictly xerothermic (steppe) taxa, which are typical of Euro-Asian steppe grasslands, and 58 are thermophilous species (see Appendix A—Table A1). The outcrops are located in the warmer part of Central Europe. Therefore, the xerothermic flora, next to the predominant Euro-Mediterranean, European and Euro-Siberian elements, includes also Pontic-Pannonian and Sub-Mediterranean species (e.g., Achillea pannonica, Bupleurum falcatum, Medicago minima, Ornithogalum angustifolium, Prunella grandiflora, Sorbus torminalis and Trifolium striatum). Moreover, some of the species attain the north-western limit of their geographical range in Europe, as also observed in other European countries [42,46,80,81,82]. These include Cotoneaster integerrimus, Festuca pallens, Melica transsilvanica and Staphyllea pinnata.
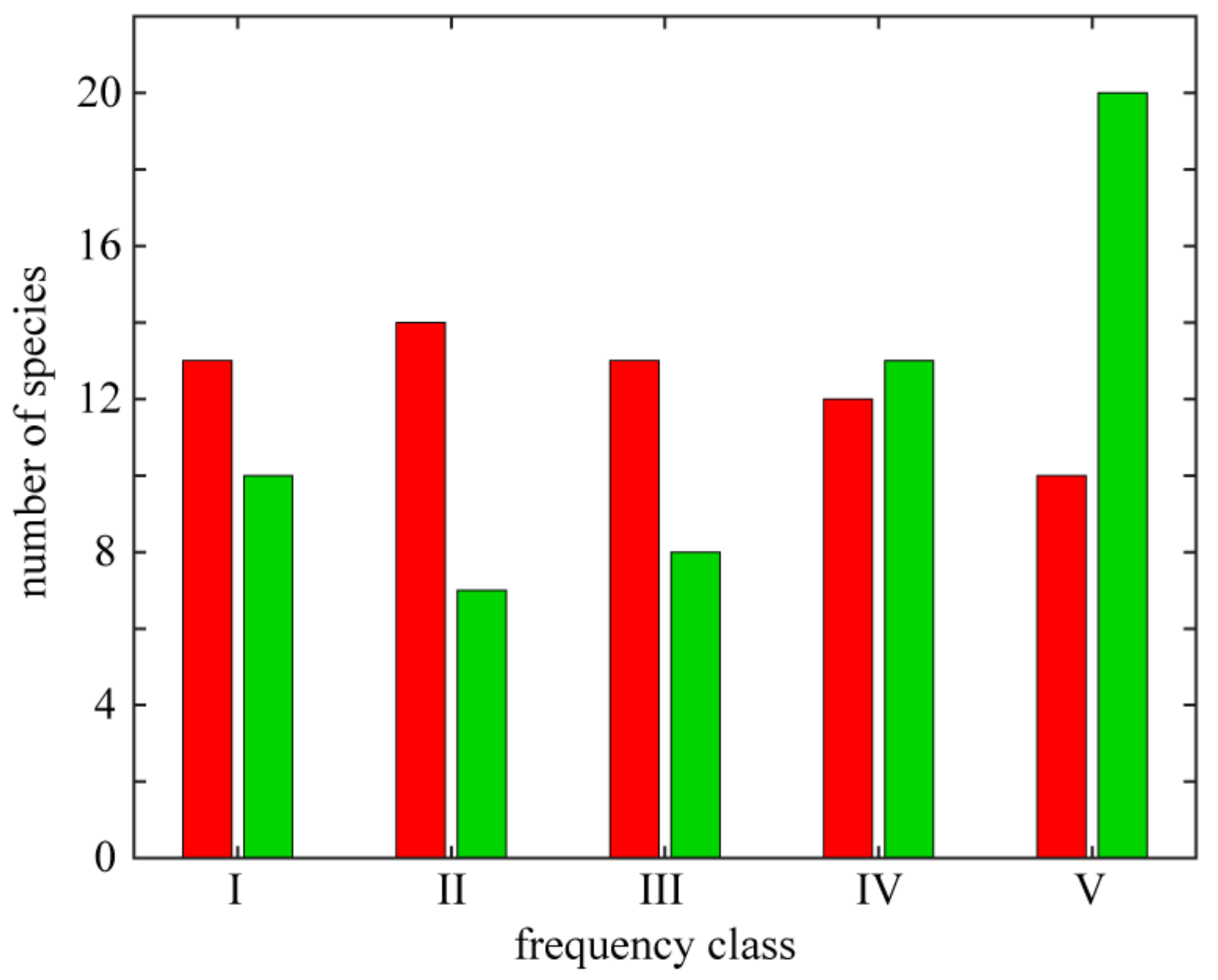
The distribution varies among individual species. It is manifested both by the total number of localities, from which the species was reported (frequency of species occurrence), and by species attachment to certain parts of the studied area (geographical range). On the basis of the occurrence frequency the species were divided into five classes (Figure 3). Among the thermophilous species, the majority of species are widespread and some were recorded from all of the basaltic outcrops (Astragalus glycyphyllos, Clinopodium vulgare, Lathyrus sylvestris and Securigera varia). The rarest species, i.e., those limited to one isolated locality, are either strictly xerothermic (e.g., Allium lusitanicum—Grodziec; Cirsium acaule—Landeskrone; Crepis praemorsa—Bazaltowa Góra; Medicago minima and Prunella grandiflora—Kopista and Trifolium striatum—Wilcza Góra) or thermophilous plants (e.g., Bupleurum falcatum—Borowa; Campanula cervicaria—Zamkowa; Hieracium schmidtii—Ostrzyca; Staphyllea pinnata—Grodziec and Thalictrum minus—Kopista).
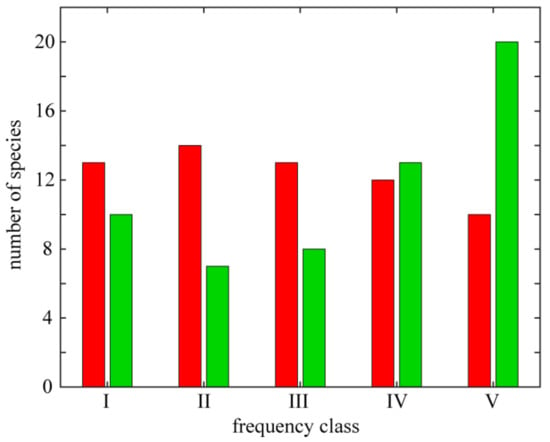
Figure 3.
Variation in frequency of strictly xerothermic (red) and thermophilous taxa (green). Frequency classes: I—species occurring on 1 locality, II—2-3 localities, III—4-8, IV—9-17 and V—18-35.
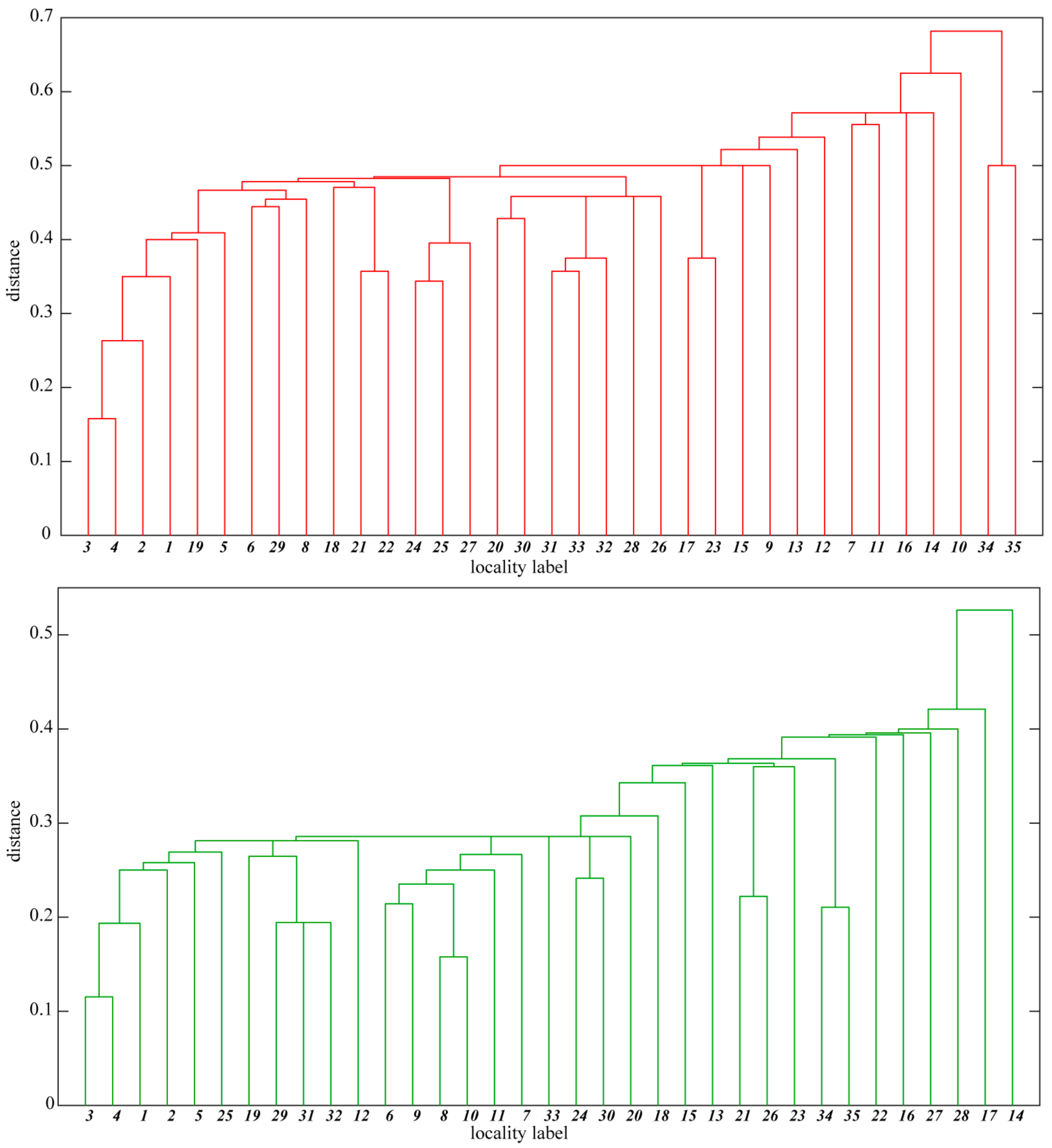
The distribution of some species is correlated with geographical regions of the Sudetes. Therefore, differences in the xerothermic flora of basaltic outcrops located in the different geographical regions are in the absence/presence of species with limited geographical range. This tendency is confirmed by the hierarchical cluster analysis of the basaltic outcrop flora. The floristic distance between the outcrop floras (measured by one minus Jaccard coefficient) is within the range 0.1–0.7 for strictly xerothermic species and 0.1–0.5 for thermophilous species (Figure 4). The dendrograms indicate the existence of outcrop clusters. The first cluster, apparent in both the dendrograms, comprises the outcrops situated in Lusatian Hills. They exhibit a similar level of floristic richness (Table 1) and are distinguished by the presence of several taxa: Anthemis tinctoria, Cerastium glutinosum, Cirsium acaule and Crataegus rhipidophylla. The second cluster, apparent in the dendrogram for strictly xerothermic species, includes some of the outcrops of the Eastern Kaczawa Plateau where fragments of Festuco valesiacae-Brometea erecti xerothermic grasslands have developed. Hence the presence of a number of species unknown from the remaining outcrops or species with the largest number of localities in this geographical region, like Alyssum alyssoides, Anthericum ramosum, Camelina microcarpa, Cerastium brachypetalum, Hypochaeris maculata, Medicago minima, Petrorhagia prolifera, Phleum phleoides, Prunella grandiflora, Salvia pratensis, Scabiosa ochroleuca and Thalictrum minus. The remaining basaltic outcrops, which are scattered within the investigated area, form only small clusters, including two or three outcrops. An example can be Góra Św. Jerzego-Krzyżowa Góra (Strzegom Hills—Figure 4, No. 34–35), for which Dianthus carthusianorum and Melica transsilvanica are the common locally distinguishing xerothermic species.
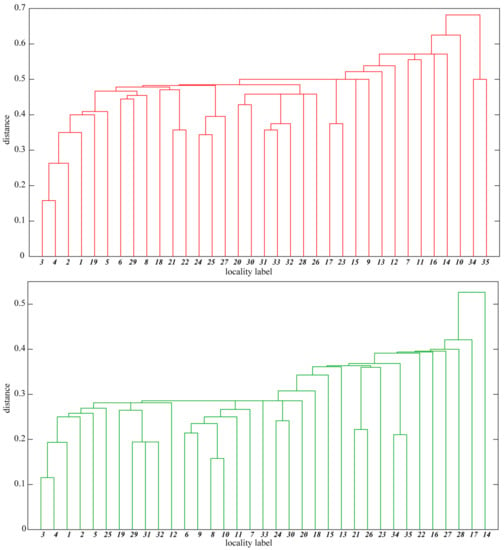
Figure 4.
Hierarchical cluster analysis showing floristic similarity of the investigated basaltic outcrops based on strictly xerothermic (red dendrogram) and thermophilous taxa (green). The locality labels (numbers) are explained in Table 1. The distance was computed as 1 minus Jaccard coefficient.
3.2. Relationships between Xerothermic Species Richness and Outcrop Parameters
The investigated basaltic outcrops are low hills (205–501 m above sea level) built of various basaltic rocks of the tertiary age (Table 1). They occur in open places as isolated hills surrounded by agricultural landscape or in forested areas (Figure 1). There are voids and quarries at most of the outcrops. Mining activities are currently carried out at only a few of them (Kozia Góra, Kopista, Łysanka and Wilcza Góra), the others are abandoned and unexploited workings with rock formations. Such places sometimes create secondary habitats for the development of rarer xerothermic species. However, no correlation was found between the presence of voids and quarries and the number of xerothermic species.
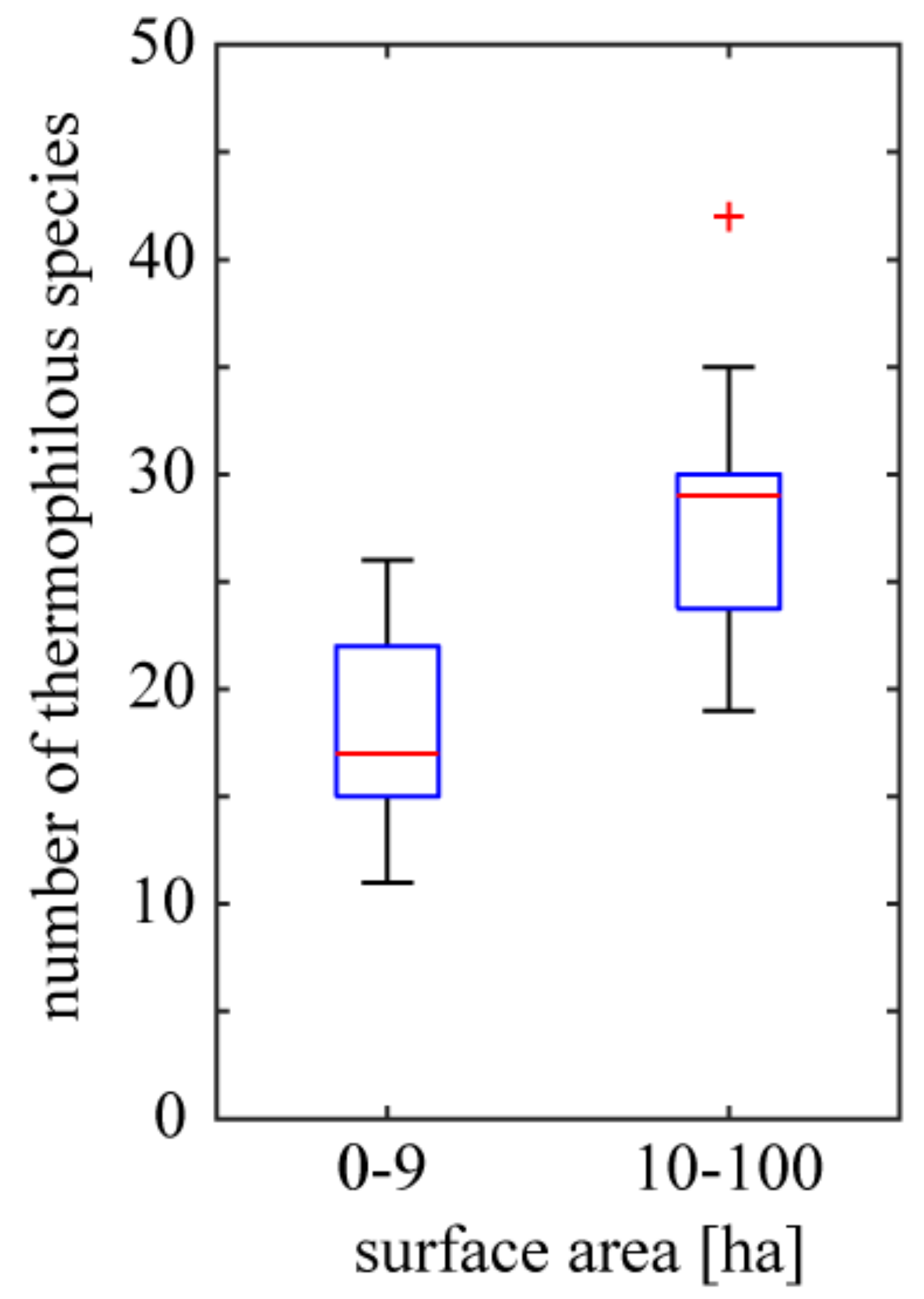
Individual basaltic outcrops differ considerably by the surface area, which ranges from 1 to 100 ha. This parameter of the outcrop affects the richness of thermophilous species but not that of strictly xerothermic species. In particular, heavily forested and quite extensive outcrops have relatively rich thermophilous flora while the richness of strictly xerothermic species is similar to that of small rock outcrops. Representatives of the former are: Rottstein (surface area of 100 ha /15 strictly xerothermic species /30 thermophilous species), Bazaltowa Góra (80/16/35) and Landeskrone (60/20/24); while of the latter: Krzyżowa Góra (3/29/26) and Winnik (2/24/22). The mean number of thermophilous species noted for outcrops of surface area smaller than 10 ha (mean = 17.78; standard error (SE) = 1.12; n = 18) is smaller than that noted for bigger outcrops (mean = 27.59; SE = 1.41; n = 17; statistically significant difference, Student’s t-test; p = 4.57 × 10−6) (Figure 5) while in the case of strictly xerothermic species the difference is not significant (p = 0.3067).
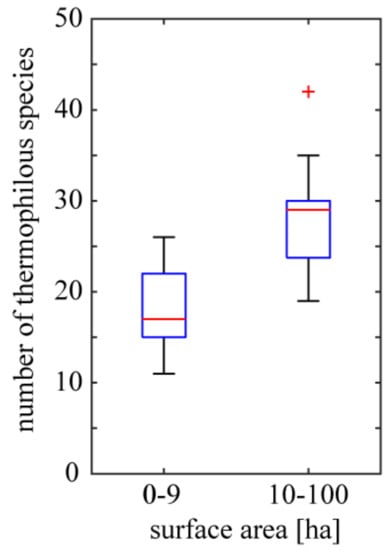
Figure 5.
Variation of thermophilous species richness of basaltic outcrops of different surface area. Boxes delimit the first and third quantiles; red lines within boxes are medians; whiskers extend from each end of the box to the adjacent values in the data on condition that the most extreme values are within 1.5 times the interquartile range from the ends of the box; red crosses represent outliers. Number of smaller outcrops = 18 and number of bigger outcrops = 17.
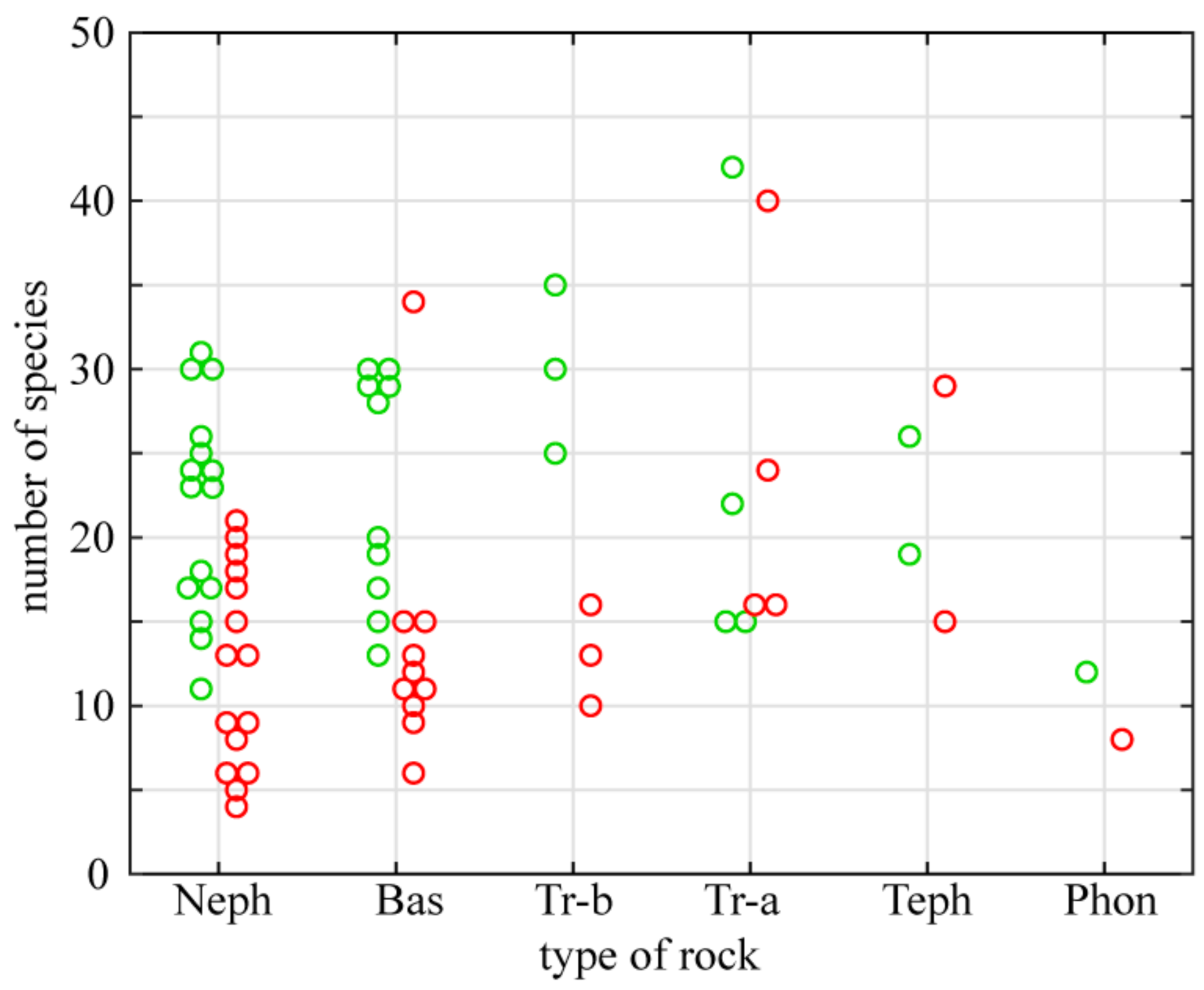
Chemical composition of basalts, of which the investigated outcrops are built, differs from other igneous rocks. Basalts are silicate rocks devoid of carbonates but the proportion of silicon compounds, occurring mainly in the form of silica (SiO2), is relatively low (up to 50%—Table 2). These rocks are also characterized by an increased content of magnesium oxides (MgO) and a low content of sodium and potassium oxides (Na2O and K2O). Moreover, they contain significant amounts of iron (Fe2O3), with a low content of phosphorus (P2O5). The black or dark green color of basaltic rocks causes relatively strong heating up of their surface. Six types of basalt build the investigated outcrops (Table 1). Nephelenites and basanites are much more common than the other types. The richness of strictly xerothermic and thermophilous flora on different types of basaltic rocks is presented in Figure 6. Outcrops of basanite (Wilcza Góra), tephrite (Krzyżowa Góra) and trachyandesite (Kopista) gather the largest number of strictly xerothermic taxa. A relatively low number of such species occurs on nephelenite, trachybasalt, and the majority of basanite outcrops. Similar relationships apply to thermophilous species with exception of trachybasalts, where the number of thermophilous species is relatively high, unlike that of strictly xerothermic species. The largest number of thermophilous species was noted for the Kopista hill (trachyandesite).
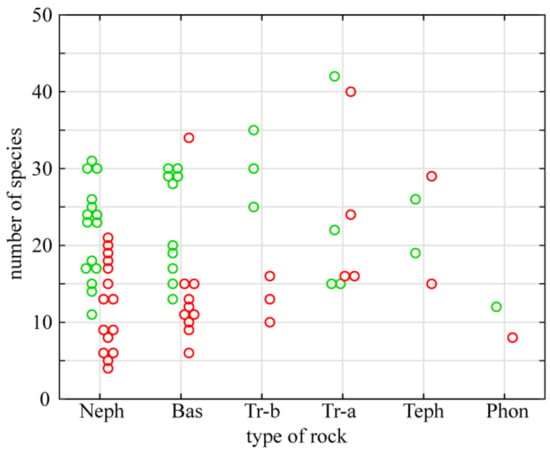
Figure 6.
Numbers of strictly xerothermic (red circles) and thermophilous taxa (green) reported from outcrops built of different basaltic rocks. Abbreviations: Neph—nephelenite, Bas—basanite, Tr-b—trachybasalt, Tr-a—trachyandesite, Teph—tephrite and Phon—phonolite.
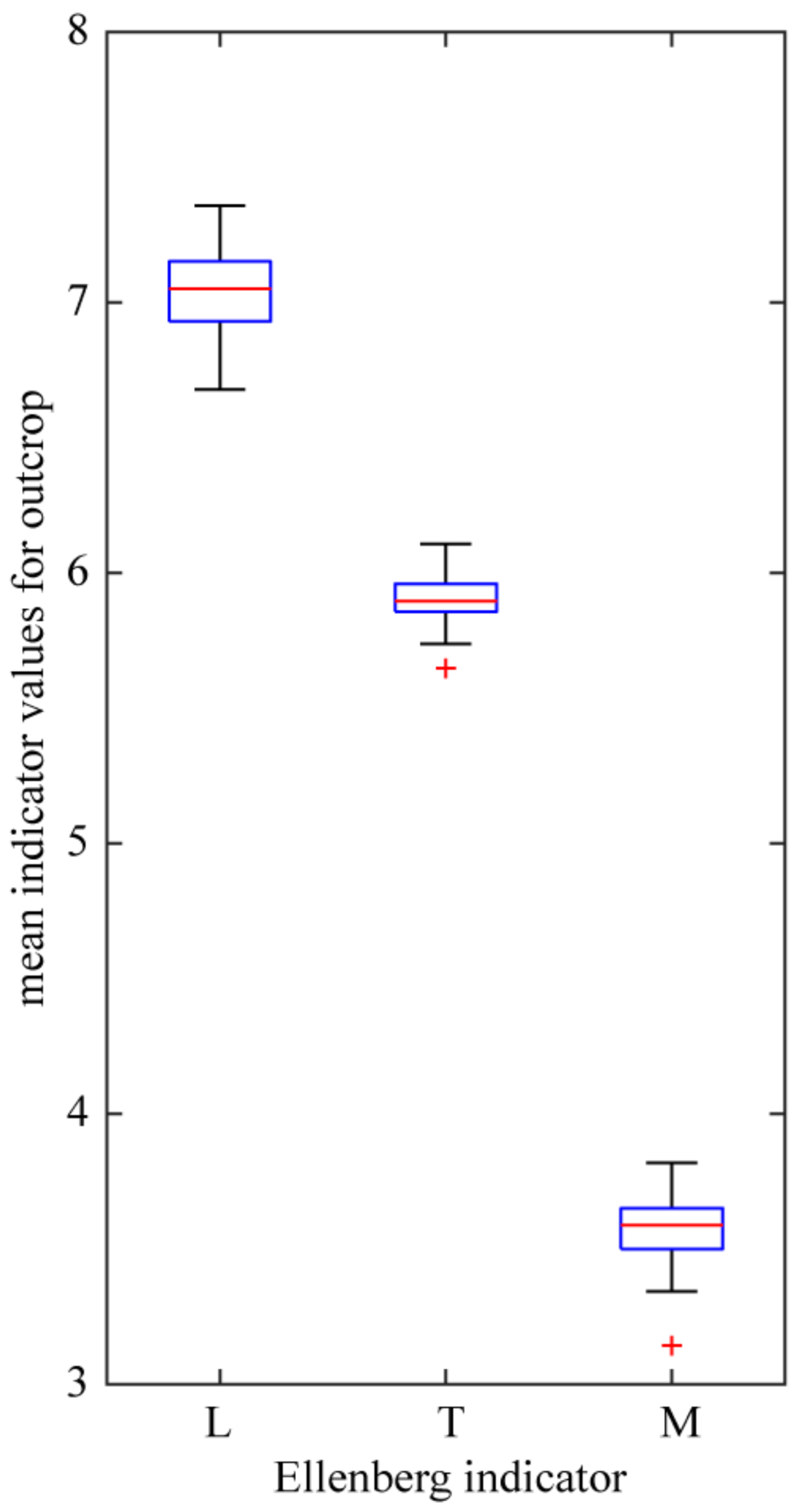
Habitats can be indirectly characterized using the system of Ellenberg indicators [79], the values of which represent relationships between plant species and environment, in particular the species preferences to light, temperature and moisture. Indicator values for species, the distribution of which was investigated, are in accordance with their xerothermic character (Table 1). Values of the indicator of light for all the xerothermic species (the strictly xerothermic and thermophilous species taken together) encountered at the investigated outcrops range from four (plants preferring shade) to nine (always full sun). Contribution of heliophilous plants, with indicator of light values eight to nine, is high (66%) especially in the group of species regarded as strictly xerothermic. Mean values of this indicator computed for all the species from a given locality fluctuate around seven, the value which represents species associated with the “open” habitat type (Figure 7). As already mentioned, basaltic habitats are distinguished by a strong degree of heating of the rock surface. Accordingly, mean values of the indicator of temperature for xerothermic species reported from the outcrops are high, close to six, which indicates high contribution of plants preferring high substrate temperature. On the other hand, low values of the indicator of moisture, between three and four, which represent mainly species preferring very dry and extremely dry habitats, apply to both strictly xerothermic and thermophilous species of basaltic outcrops (Figure 7).
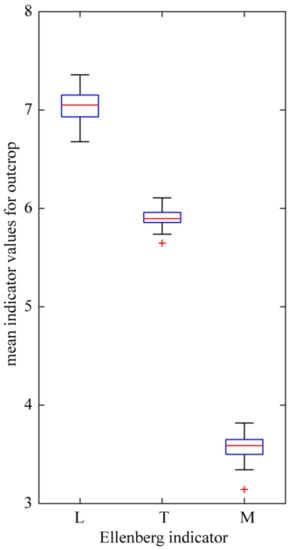
Figure 7.
Variation of mean Ellenberg indicators computed for species reported from given outcrop: Ellenberg indicators: L—light; T—temperature and M—moisture. See Figure 5 legend for box plot explanation.
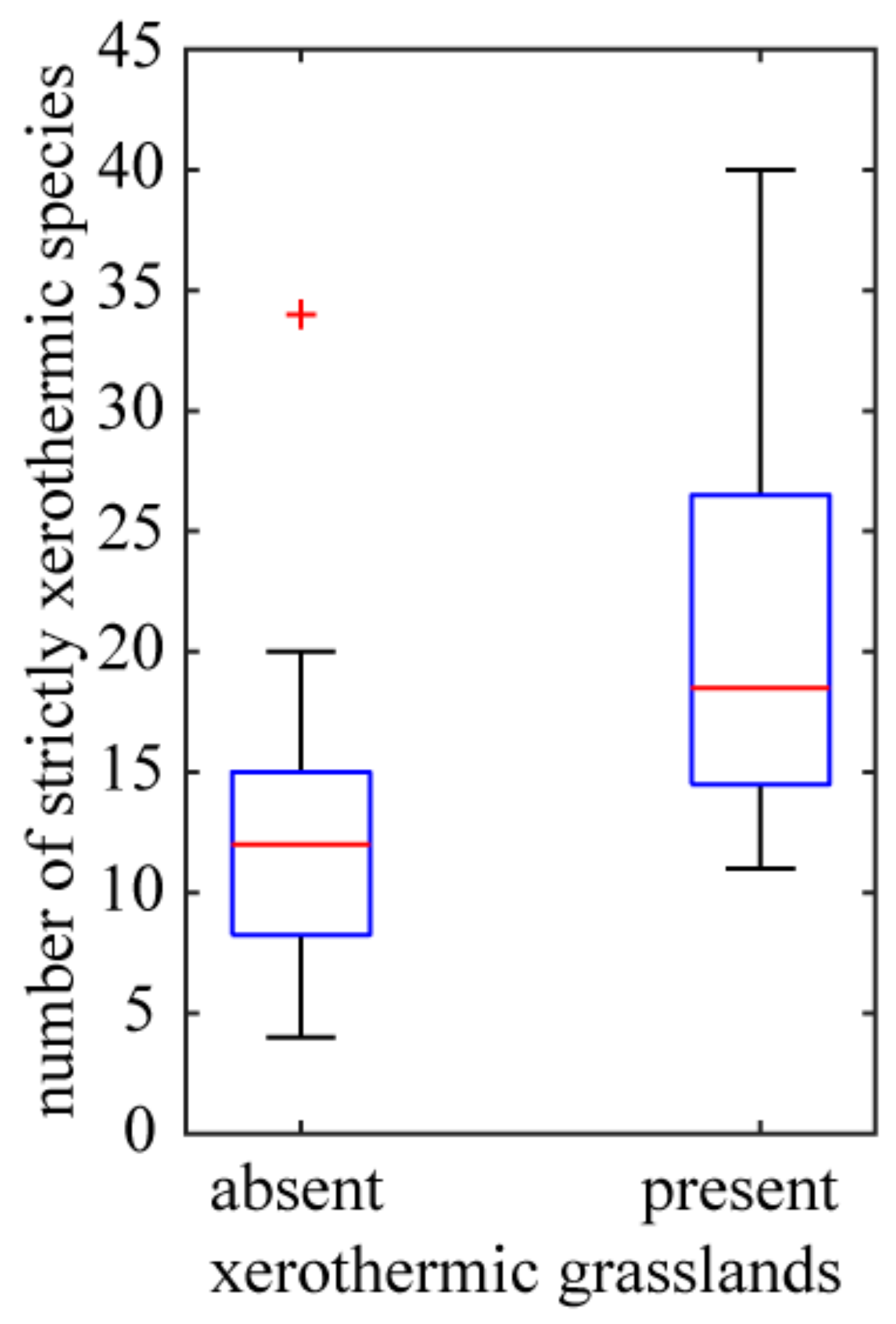
The investigated species that are associated with different biotopes belong to different syntaxonomic units. Some of species (51.66% of the investigated taxa) have narrow habitat requirements and are species of xerothermic grasslands of the Festuco valesiacae-Brometea erecti class. As a rule, they occupy exposed, unshaded fragments of the slopes of the basalt hills with southern exposure. The presence of such xerothermic grasslands is manifested in the increased floristic richness of strictly xerothermic species (Figure 8) (outcrops without grasslands: Mean number of strictly xerothermic species = 12.44; SE = 1.19; n = 27; outcrops with grasslands: Mean = 21.25; SE = 3.40; n = 8; the difference between means is statistically significant, Student’s t-test; p = 0.004). Distribution of species of xerothermic grasslands of the Festuco valesiacae-Brometea erecti class to a large extent coincides with one of the above-described clusters of basaltic outcrops. In particular, the largest number of these species were found in the Eastern Kaczawa Plateau. On the other hand, the lowest contribution of the xerothermic grassland species distinguishes the Izera Plateau. Smaller groups are species of thermophilous fringes of the Trifolio medii-Geranietea sanguinei class (32.23%), and species of thermophilous oak forests of the Quercetea pubescentis class (16.11%). The latter group plays a greater role in the outcrops of Bazaltowa Góra and Kopista, where fragments of submontane thermophilous oak forest with service-tree Sorbo torminalis-Quercetum petraeae Svoboda ex Blažková 1962 have developed. These localities mark the northern border of the geographical range of this association in Europe. The fact that the association is locally attached to the basaltic substrate resulted in the development of its endemic form Sorbo torminalis-Quercetum petraeae cephalantheretosum longifoliae Kwiatkowski 2003 [83].
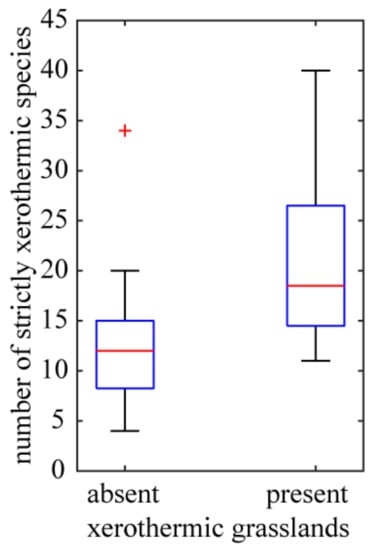
Figure 8.
Variation of richness of strictly xerothermic species for basaltic outcrops differing by the absence (27 outcrops) or presence (8 outcrops) of xerothermic grasslands. See Figure 5 legend for box plot explanation.
4. Discussion
The positive relation between species richness and the size of the investigated area is probably the most common pattern in nature—the larger the area, the more species one can expect. This relation is manifested by the so-called island effect on species richness [6,15,84,85,86,87]. Typically, when habitat islands are smaller and more isolated, the chances of the survival of species on the island are lower as well as the chances of the species colonization outside [88,89]. The hypothesis of habitat heterogeneity assumes that as the surface area increases, new habitats appear together with new species, which are associated with the new habitats, the effect of which is an increase of the total number of species [90,91,92]. The logarithmic relationship between species richness and the surface area is well known, but there are also many cases where small patches of habitats may have a more beneficial effect on the richness than a few larger ones [5,93,94]. Smaller islands, or their parts, are colonized mainly by narrowly specialized species. The lack of suitable habitats thus blocks the possibility of colonization of the islands by these “specialists”. For the basaltic outcrops, the investigation of which is presented in this paper, this phenomenon applies to a number of species that have a characteristic single locality. For example, Anthericum ramosum, Medicago minima, Prunella grandiflora and Pulmonaria angustifolia inhabit only one of the trachyandesite outcrops (Kopista). In this case, the floristic richness and species diversity are determined by the specific type of rock and the mosaic arrangement of microhabitats.
Habitat heterogeneity is therefore not a simple function of the size of the object—even within small areas, a number of ecological niches may appear, which are to a various extent preferable for specialized species. On the other hand, there is a hypothesis on richness-reducing disturbances that lead to a decline in species diversity in small areas [13,95,96]. However, the disturbance hypothesis does not apply to the results obtained from numerous basaltic outcrops investigated in this paper. The appearance of xerothermic taxa in secondary habitats, i.e., quarries of basalts, are against the disturbance hypothesis. It is indeed expected that the quarry exploitation in the initial phase resulted in the destruction of many species niches or even the complete disappearance of some species. On the other hand, the currently closed quarries, where no far-reaching succession phenomena took place, frequently became the main refugia of xerothermic species. This network of secondary habitats often gathers localities of specialized species that are regionally endangered taxa of the vascular flora of the Sudetes [97,98], including Campanula cervicaria (Zamkowa), Cerastium brachypetalum (Kopista), Ornithogalum angustifolium, Stachys germanica and Trifolium striatum (Wilcza Góra). Thus, “anthropogenic disturbances” in the structure of the rocky outcrops sometimes promote species diversity. Such tendencies have been described for the vascular flora of quarries composed not only of basalts but also of other types of rocks [99,100,101,102,103,104].
In the case of environmental islands that make up “archipelagos”, the distance between the islands is important. Regardless of the propagule carrier (wind, birds or people), quite high mutual floristic similarity of the islands located close to each other may result from easier colonization by species from the immediate vicinity [105,106,107]. In the investigated area, such relationships were found for several outcrops located in the Eastern Kaczawa Plateau (Krzyżowa Góra, Winnik, Srebrnik and Kopista), the distances between which oscillate around several kilometers. The result is a common group of several species (Camelina microcarpa, Hypochaeris maculata, Petrorhagia prolifera, Polygala comosa and Veronica prostrata), which here have the main center of occurrence in the investigated area. On the other hand, many species have a disjunctive pattern of distribution. Due to the fact that the basaltic outcrops where the species occur are tens of kilometers away, effective flow of genes and diaspores between them is not likely and the SIE may lead to extinction events. However, a transport of diaspores cannot be completely excluded. Examples of such local disjunctions are the geographical ranges of Bromus erectus (Ladenskrone/Krzyżna Góra—hills separated by 120 km), Festuca pallens (Ostrzyca/Góra Św. Jerzego—40 km), Potentilla inclinata (Wilcza Góra/Czartowska Skała—20 km), Trifolium rubens (Wilcza Góra/Bazaltowa Góra—20 km) and Verbascum lychnitis (Hutberg/Zamkowa—30 km).
From the ecological point of view, the most important chemical properties of basaltic rocks are slightly alkaline reaction, low content of phosphorus, potassium and calcium, and high magnesium content. The soils formed from such a substrate create specific, strongly heating, low-fertile habitats with a significant content of skeletal parts (rock fragments), which makes them permeable to water. Plants that occur in these extreme habitats are characterized by a scleromorphic structure and are adapted to water scarcity (xerism). Easily heating basaltic habitats with specific physical and chemical parameters can be treated as “edaphic islands”. Such features are typical also for serpentine habitats [47,108,109]. In general, low soil moisture and high insolation distinguish the areas occupied by xerothermic species of the Festuco valesiacae-Brometea erecti class, as confirmed in other studies regarding other European countries [110,111,112]. Their localities are usually related with steep, dry and often rocky slopes of the southern exposure. The habitats formed by such exposed rocks influence the flora also through the low albedo of their surface, which makes them warmer and drier. It is reported that slopes with such exposure receive on average up to 35% more direct sunlight per year than flat areas [27,113,114,115]. In turn, thermophilous species of the Trifolio medii-Geranietea sanguinei and Quercetea pubescentis classes, apart from slopes with southern or nearly southern exposure, occur also on the summits of basaltic outcrops and rocks with variable exposure and degree of slope.
The patterns of species diversity of the studied basaltic outcrops are therefore significantly influenced by the overall system of abiotic environmental factors generating a mosaic of developed habitat types. A similar relationship was shown for hills with a different geological structure [25,87,116,117,118].
Diagnosis of abiotic environment parameters can be complemented by the system of Ellenberg indicators [119,120,121,122,123]. In the present investigation, results of Ellenberg indicator analysis (the values of selected indicators representing species preferences for light, temperature and moisture) are to some extent obvious because only a specific group of plants, i.e., xerothermic species, was considered. Nevertheless, the results support the adequate selection of xerothermic species. In particular, within the analyzed flora, there is a significant predominance of species adapted to higher substrate temperature, high degree of insolation and very dry substrate.
The obtained results show a specific floristic composition of basaltic outcrops, that differs from the vascular flora of the surrounding areas (matrix vegetation). Thus, basaltic outcrops are specific habitats while the settlement of the outcrop plant species, which are often under stress conditions, into the surrounding matrix is often limited [27]. The basaltic outcrops, nevertheless, make a significant contribution to the local and regional species diversity.
5. Conclusions
Basaltic outcrops are not only specific environmental islands, which have a unique geological structure, topography, soils and microclimatic conditions, but also refugia for species with high thermal requirements. Due to the extremely northern location of the studied objects in the Sudetes and the influence of the humid oceanic climate, the investigated outcrops differ in terms of floristic richness from the more southern basalt hills of other parts of the Czech Massif (České Sředohoří Mountains), where numerous species representing sub-continental types of geographical range occur. Basalt outcrops also function as regional centers of xerothermic flora in the Sudetes.
A number of xerothermic species reach the northern limit of their geographical range in Europe in the investigated area. The localities of xerothermic taxa on the border of the geographical range seem to be very interesting both from a historical and evolutionary perspective. Marginal and relict populations are often subject to stronger local selection than those occupying the center of the species range, which can lead to the emergence of many genetically diverse populations, each adapted to its own habitat conditions. In times of global climate change, they can be the starting point for future migrations to other parts of Europe, which could be important for the long-term survival of populations. Therefore, one can conclude that due to their environmental vulnerability, geographical range and relict nature, it is appropriate to provide in situ conservation actions for those plant species that are at greater risk as well as for their habitats [124,125,126].
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are included within the article.
Acknowledgments
The author is grateful to the Academic Editor for launching this special issue and to the anonymous Reviewers for their valuable comments.
Conflicts of Interest
The author declares no conflict of interest.
Appendix A

Table A1.
Species list of the investigated basaltic outcrops and their Ellenberg indicators. Abbreviations: *—strictly xerothermic taxon, L—light, T—temperature and M—moisture.
Table A1.
Species list of the investigated basaltic outcrops and their Ellenberg indicators. Abbreviations: *—strictly xerothermic taxon, L—light, T—temperature and M—moisture.
| Name of Species | Family | Ellenberg Indicator Value | ||
|---|---|---|---|---|
| L | T | M | ||
| * Achillea pannonica Scheele | Asteraceae | 7 | 7 | 3 |
| * Acinos arvensis (Lam.) Dandy | Lamiaceae | 9 | 6 | 2 |
| Agrimonia eupatoria L. | Rosaceae | 7 | 6 | 4 |
| * Ajuga genevensis L. | Lamiaceae | 8 | 6 | 3 |
| * Allium lusitanicum Lam. | Alliaceae | 9 | 6 | 2 |
| * Allium oleraceum L. | Alliaceae | 7 | 6 | 3 |
| * Allyssum alyssoides (L.) L. | Brassicaceae | 9 | 6 | 3 |
| * Anthemis tinctoria L. | Asteraceae | 8 | 6 | 3 |
| * Anthericum ramosum L. | Anthericaceae | 7 | 5 | 3 |
| * Anthyllis vulneraria L. subsp. vulneraria | Fabaceae | 8 | 6 | 3 |
| * Arabis hirsuta (L.) Scop. | Brassicaceae | 7 | 5 | 4 |
| * Artemisia campestris L. subsp. campestris | Asteraceae | 9 | 6 | 2 |
| Astragalus glycyphyllos L. | Fabaceae | 6 | 6 | 4 |
| * Brachypodium pinnatum (L.) P. Beauv. | Poaceae | 6 | 5 | 4 |
| * Bromus erectus Huds. | Poaceae | 8 | 5 | 3 |
| Bupleurum falcatum L. | Apiaceae | 6 | 6 | 3 |
| * Camelina microcarpa DC. | Brassicaceae | 7 | 6 | 4 |
| Campanula cervicaria L. | Campanulaceae | 6 | 6 | 5 |
| * Campanula glomerata L. subsp. glomerata | Campanulaceae | 7 | 6 | 4 |
| Campanula persicifolia L. | Campanulaceae | 5 | 5 | 4 |
| * Carex caryophyllea Latourr. | Cyperaceae | 8 | 5 | 4 |
| Carex montana L. | Cyperaceae | 5 | 6 | 4 |
| * Carlina vulgaris L. | Asteraceae | 7 | 5 | 4 |
| * Centaurea scabiosa L. subsp. scabiosa | Asteraceae | 7 | 6 | 3 |
| * Centaurea stoebe L. | Asteraceae | 8 | 7 | 3 |
| Cephalanthera longifolia (L.) Fritsch | Orchidaceae | 5 | 5 | 4 |
| * Cerastium brachypetalum Pers. subsp. brachypetalum | Caryophyllaceae | 9 | 7 | 3 |
| * Cerastium glutinosum Fr. | Caryophyllaceae | 9 | 7 | 3 |
| * Cerastium pumilum Curtis | Caryophyllaceae | 8 | 7 | 2 |
| Cervaria rivini Gaertn. | Apiaceae | 8 | 7 | 5 |
| * Cirsium acaule Scop. | Asteraceae | 9 | 5 | 3 |
| Clinopodium vulgare L. | Lamiaceae | 7 | 5 | 4 |
| Cotoneaster integerrimus Medik. | Rosaceae | 8 | 6 | 3 |
| * Crepis praemorsa (L.) Walther | Asteraceae | 6 | 7 | 3 |
| Crataegus rhipidophylla Gand. s.str. | Rosaceae | 7 | 6 | 5 |
| Dactylorhiza sambucina (L.) Soó | Orchidaceae | 7 | 5 | 4 |
| * Dianthus carthusianorum L. | Caryophyllaceae | 8 | 5 | 3 |
| Digitalis grandiflora Mill. | Plantaginaceae | 7 | 4 | 5 |
| Drymocallis rupestris (L.) Soják | Rosaceae | 7 | 7 | 4 |
| Festuca brevipila (L.) R. Tracey | Poaceae | 8 | 6 | 3 |
| * Festuca pallens Host | Poaceae | 9 | 7 | 2 |
| * Filipendula vulgaris Moench | Rosaceae | 7 | 6 | 3 |
| Fragaria moschata Weston | Rosaceae | 6 | 6 | 5 |
| Fragaria viridis Weston | Rosaceae | 7 | 5 | 3 |
| Gagea villosa (M. Bieb) Sweet | Liliaceae | 6 | 7 | 4 |
| Galium verum L. s.str. | Rubiaceae | 7 | 6 | 4 |
| Genista germanica L. | Fabaceae | 7 | 5 | 4 |
| Genista tinctoria L. subsp. tinctoria | Fabaceae | 8 | 6 | 6 |
| * Gentianopsis ciliata (L.) Ma | Gentianaceae | 7 | 6 | 3 |
| Geranium sanguineum L. | Geraniaceae | 7 | 6 | 3 |
| * Helianthemum nummularium (L.) Mill. subsp. obscurum (Wahlenb.) Holub | Cistaceae | 8 | 5 | 3 |
| Hieracium diapahanoides Lindeb. | Asteraceae | 5 | 5 | 4 |
| Hieracium schmidtii Tausch | Asteraceae | 8 | 6 | 4 |
| * Holosteum umbellatum L. | Caryophyllaceae | 8 | 6 | 3 |
| Hypericum montanum L. | Hypericaceae | 5 | 6 | 4 |
| * Hypochaeris maculata L. | Asteraceae | 7 | 6 | 4 |
| Inula conyzae (Griess.) DC. | Asteraceae | 6 | 6 | 4 |
| * Inula hirta L. | Asteraceae | 7 | 6 | 3 |
| * Jovibarba globifera (L.) J. Parn. subsp. globifera | Crassulaceae | 9 | 6 | 2 |
| * Koeleria macrantha (Ledeb.) Schult. | Poaceae | 7 | 6 | 3 |
| Lathyrus niger (L.) Bernh. | Fabaceae | 5 | 6 | 3 |
| Lathyrus sylvestris L. subsp. sylvestris | Fabaceae | 7 | 6 | 4 |
| Lychnis viscaria L. | Caryophyllaceae | 7 | 6 | 3 |
| Medicago falcata L. | Fabaceae | 8 | 6 | 3 |
| * Medicago minima (L.) L. | Fabaceae | 9 | 7 | 3 |
| * Melica transsilvanica Schur | Poaceae | 7 | 8 | 3 |
| Melittis melissophyllum L. | Lamiaceae | 5 | 7 | 4 |
| Orchis mascula (L.) L. subsp. speciosa (W. D. J. Koch) Hegi | Orchidaceae | 6 | 6 | 4 |
| Origanum vulgare L. subsp. vulgare | Lamiaceae | 7 | 6 | 3 |
| * Ornithogalum angustifolium Boreau | Hyacinthaceae | 9 | 8 | 2 |
| * Petrorhagia prolifera (L.) P. W. Ball et Heywood | Caryophyllaceae | 8 | 7 | 3 |
| Peucedanum oreoselinum (L.) Moench | Apiaceae | 6 | 6 | 3 |
| * Phleum phleoides (L.) H. Karst. | Poaceae | 8 | 6 | 3 |
| * Pilosella bauhinii (Schult.) Arv.-Touv. subsp. bauhinii | Asteraceae | 9 | 7 | 3 |
| * Poa angustifolia L. | Poaceae | 7 | 6 | 5 |
| * Poa bulbosa L. | Poaceae | 8 | 7 | 3 |
| * Polygala comosa Schkuhr | Polygalaceae | 8 | 6 | 3 |
| Polygonatum odoratum (Mill.) Druce | Ruscaceae | 7 | 5 | 3 |
| Potentilla alba L. | Rosaceae | 6 | 6 | 4 |
| * Potentilla inclinata Vill. | Rosaceae | 9 | 7 | 2 |
| * Potentilla leucopolitana P. J. Müll | Rosaceae | 9 | 6 | 2 |
| * Potentilla neumanniana Rchb. | Rosaceae | 8 | 6 | 3 |
| Potentilla recta L. | Rosaceae | 9 | 7 | 3 |
| * Prunella grandiflora (L.) Scholler | Lamiaceae | 9 | 7 | 3 |
| Pulmonaria angustifolia L. | Boraginaceae | 5 | 7 | 5 |
| Ranunculus polyanthemos L. subsp. polyanthemos | Ranunculaceae | 6 | 6 | 4 |
| * Salvia pratensis L. | Lamiaceae | 8 | 6 | 3 |
| * Sanguisorba minor Scop. subsp. minor | Rosaceae | 7 | 6 | 3 |
| * Saxifraga tridactylites L. | Saxifragaceae | 8 | 6 | 2 |
| * Scabiosa columbaria L. | Dipsacaceae | 8 | 5 | 3 |
| * Scabiosa ochroleuca L. | Dipsacaceae | 8 | 7 | 3 |
| Securigera varia (L.) Lassen | Fabaceae | 7 | 6 | 4 |
| * Seseli annuum L. | Apiaceae | 8 | 7 | 3 |
| Silene nutans L. | Caryophyllaceae | 7 | 6 | 3 |
| Sorbus torminalis (L.) Crantz | Rosaceae | 4 | 7 | 4 |
| * Stachys germanica L. | Lamiaceae | 7 | 7 | 3 |
| Staphyllea pinnata L. | Staphyleaceae | 7 | 7 | 5 |
| Tanacetum corymbosum (L.) Sch. Bip. | Asteraceae | 6 | 7 | 4 |
| * Taraxacum sect. Erythrosperma (H. Lindb.) Dahlst. | Asteraceae | 8 | 6 | 3 |
| * Teucrium botrys L. | Lamiaceae | 9 | 6 | 2 |
| Thalictrum minus L. subsp. minus | Ranunculaceae | 6 | 6 | 3 |
| Trifolium alpestre L. | Fabaceae | 7 | 6 | 3 |
| Trifolium medium L. | Fabaceae | 7 | 6 | 4 |
| * Trifolium montanum L. | Fabaceae | 8 | 6 | 3 |
| Trifolium rubens L. | Fabaceae | 7 | 6 | 3 |
| * Trifolium striatum L. | Fabaceae | 8 | 7 | 3 |
| * Turritis glabra L. | Brassicaceae | 6 | 6 | 3 |
| Valeriana pratensis Dierb. subsp. angustifolia (Soó) Kirschner et al. | Valerianaceae | 7 | 6 | 4 |
| Verbascum lychnitis L. | Scrophulariaceae | 7 | 6 | 3 |
| * Veronica prostrata L. s.str. | Plantaginaceae | 9 | 7 | 2 |
| Veronica teucrium L. | Plantaginaceae | 7 | 6 | 3 |
| Vicia dumetorum L. | Fabaceae | 6 | 6 | 5 |
| Vicia pisiformis L. | Fabaceae | 6 | 7 | 4 |
| Vicia sylvatica L. | Fabaceae | 7 | 6 | 4 |
| Vicia tenuifolia Roth s.str. | Fabaceae | 7 | 8 | 4 |
| Vincetoxicum hirundinaria Medik. | Apocynaceae | 6 | 5 | 3 |
| * Viola collina Besser | Violaceae | 6 | 5 | 3 |
| Viola hirta L. | Violaceae | 6 | 5 | 3 |
| * Viola rupestris F. W. Schmidt | Violaceae | 6 | 5 | 3 |
| Viola scabra F. Braun | Violaceae | 6 | 5 | 3 |
References
- Honnay, O.; Jacquemyn, H.; Bossuyt, B.; Hermy, M. Forest fragmentation effects on patch occupancy and population variability of herbaceous plant species (Tansley review). New Phytol. 2005, 166, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Lundholm, J.T. Plant species diversity and environmental heterogeneity: Spatial scale and competing hypotheses. J. Veg. Sci. 2009, 20, 377–391. [Google Scholar] [CrossRef]
- Tamme, R.; Hiiesalu, I.; Laanisto, L.; Szava-Kovats, R.; Pärtel, M. Environmental heterogeneity, species diversity and co-existence at different spatial scales. J. Veg. Sci. 2010, 21, 796–801. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- Fahrig, L. Ecological responses to habitat fragmentation per se. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Fahrig, L.; Arroyo-Rodriguez, V.; Bennett, J.R.; Boucher-Lalonde, V.; Cazetta, E.; Currie, D.J.; Eigenbrod, F.; Ford, A.T.; Harrison, S.P.; Jaeger, J.A.G.; et al. Is habitat fragmentation bad for biodiversity? Biol. Conserv. 2019, 230, 179–186. [Google Scholar] [CrossRef]
- Deák, B.; Kovács, B.; Rádai, Z.; Apostolova, I.; Kelemen, A.; Kiss, R.; Lukács, K.; Palpurina, S.; Sopotlieva, D.; Báthori, F.; et al. Linking environmental heterogeneity and plant diversity: The ecological role of small natural features in homogeneous landscapes. Sci. Total Environ. 2021, 763, 144199. [Google Scholar] [CrossRef]
- Szarzyński, J. Xeric Islands: Environmental Conditions on Inselbergs. In Inselbergs Biotic Diversity of Isolated Rock Outcrops in Tropical and Temperate Regions; Porembski, S., Barthlott, W., Eds.; Springer: Berlin/Heildelberg, Germany; New York, NY, USA, 2000; pp. 37–48. [Google Scholar]
- Oertli, B.; Joye, D.A.; Castella, E.; Juge, R.; Cambin, D.; Lachavanne, J.-B. Does size matter? The relationship between pond area and biodiversity. Biol. Conserv. 2002, 104, 59–70. [Google Scholar] [CrossRef]
- Rejmánková, R.; Rejmánek, M. Biogeography of artificial islands: Effects of age, area elevation, and isolation of plant species richness. Preslia 2002, 74, 307–314. [Google Scholar]
- Edvardsen, A.; Økland, R.H. Variation in plant species richness in and adjacent to 64 ponds in SE Norwegian agricultural landscapes. Aquat. Bot. 2006, 85, 79–91. [Google Scholar] [CrossRef]
- Vondrák, J.; Prach, K. Occurrence of heliophilous species on isolated rocky outcrops in a forested landscape: Relict species or recent arrivals ? Preslia 2006, 78, 115–121. [Google Scholar]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography. Ecology, Evolution, and Conservation; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Richardson, S.J.; Clayton, R.; Rance, B.D.; Broadbent, H.; McGlone, M.S.; Wilmshurst, J.M. Small wetlands are critical for safeguarding rare and threatened plant species. Appl. Veg. Sci. 2015, 18, 230–241. [Google Scholar] [CrossRef]
- Burns, K.C.; McHardy, R.P.; Pledger, S. The small-island effect: Fact or artefact? Ecography 2009, 32, 269–276. [Google Scholar] [CrossRef]
- Dengler, J. Robust methods for detecting a small island effect. Div. Distrib. 2010, 16, 256–266. [Google Scholar] [CrossRef]
- Triantis, K.A.; Sfenthourakis, S. Island biogeography is not a single-variable discipline: The small island effect debate. Div. Distrib. 2012, 18, 92–96. [Google Scholar] [CrossRef]
- Speziale, K.L.; Ezcurra, C. Rock outcrops as potential biodiversity refugia under climate change in North Patagonia. Plant Ecol. Divers. 2014, 8, 353–361. [Google Scholar] [CrossRef]
- Fitzsimons, J.A.; Michael, D.R. Rocky outcrops: A hard road in the conservation of critical habitats. Biol. Conserv. 2017, 211, 36–44. [Google Scholar] [CrossRef]
- Hunter, M.L., Jr.; Acuña, V.; Bauer, D.M.; Bell, K.P.; Calhoun, A.J.K.; Felipe-Lucia, M.R.; Fitzsimons, J.A.; González, E.; Kinnison, M.; Lindenmayer, D.; et al. Conserving small natural features with large ecological roles: A synthetic overview. Biol. Conserv. 2017, 211, 88–95. [Google Scholar] [CrossRef]
- Poschlod, P.; Braun-Reichert, R. Small natural features with large ecological roles in ancient agricultural landscapes of Central Europe—History, value, status, and conservation. Biol. Conserv. 2017, 211, 60–68. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Millien, V. A global synthesis of the small-island effect in habitat islands. Proc. R. Soc. B 2018, 285, 20181868. [Google Scholar] [CrossRef]
- Danin, A. Sandstone outcrops—A major refugium of Mediterranean flora in the xeric part of Jordan. Isr. J. Plant Sci. 1999, 47, 179–187. [Google Scholar] [CrossRef]
- Porembski, S.; Barthlott, W. Granitic and gneissic outcrop (inselbergs) as centers of diversity for desiccation-tolerant vascular plants. Plant Ecol. 2000, 151, 19–28. [Google Scholar] [CrossRef]
- Harrison, S.; Inouye, B.D. High β diversity in the flora of Californian serpentine ‘islands’. Biodiv. Conserv. 2002, 11, 1869–1876. [Google Scholar] [CrossRef]
- Burke, A. Inselbergs in a changing world—Global trends. Div. Distrib. 2003, 9, 375–383. [Google Scholar] [CrossRef]
- Larson, D.W.; Matthes, U.; Kelly, P.E. Cliff Ecology: Pattern and Process in Cliff Ecosystems; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Jacobi, C.M.; do Carmo, F.F.; Vincent, R.C.; Stehmann, J.R. Plant communities on ironstone outcrops: A diverse and endangered Brazilian ecosystem. Biodiv. Conserv. 2007, 16, 2185–2200. [Google Scholar] [CrossRef]
- Partzsch, M. Flora, Vegetation und historische Entwicklung der Porphyrkuppenlandschaft zwischen Halle und Wettin (Sachsen-Anhalt). Schlechtendalia 2007, 15, 1–91. [Google Scholar]
- Pope, N.; Harris, T.B.; Rajakaruna, N. Vascular Plants of Adjacent Serpentine and Granite Outcrops on the Deer Isles, Maine, U.S.A. Rhodora 2010, 112, 105–141. [Google Scholar] [CrossRef]
- Speziale, K.L.; Ezcurra, C. The role of outcrops in the diversity of Patagonian vegetation: Relicts of glacial palaeofloras? Flora Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 141–149. [Google Scholar] [CrossRef]
- Djordjević, V.; Tsiftsis, S. Patterns of orchid species richness and composition in relation to geological substrates. Wulfenia 2019, 26, 1–21. [Google Scholar]
- Yates, C.J.; Robinson, T.; Wardell-Johnson, G.W.; Keppel, G.; Hopper, S.D.; Schut, A.G.T.; Byrne, M. High species diversity and turnover in granite inselberg floras highlight the need for a conservation strategy protecting many outcrops. Ecol. Evol. 2019, 9, 7660–7675. [Google Scholar] [CrossRef]
- Andrino, C.O.; Barbosa-Silva, R.G.; Lovo, J.; Viana, P.L.; Moro, M.F.; Zappi, D.C. Iron islands in the Amazon: Investigating plant beta diversity of canga outcrops. PhytoKeys 2020, 165, 1–25. [Google Scholar] [CrossRef]
- Taberlet, P.; Fumagalli, L.; Wust-Saucy, A.G.; Cossons, J.-F. Comparative phylogeography and post-glacial colonization routes in Europe. Molec. Ecol. 1998, 7, 453–464. [Google Scholar] [CrossRef]
- Kirschner, P.; Záveská, E.; Gamisch, A.; Hilpold, A.; Trucchi, E.; Paun, O.; Sanmartín, I.; Schlick-Steiner, B.C.; Frajman, B.; Arthofer, W.; et al. Long-term isolation of European steppe outposts boosts the biome’s conservation value. Nat. Commun. 2020, 11, 1968. [Google Scholar] [CrossRef]
- Pärtel, M. Local plant diversity patterns and evolutionary history at the regional scale. Ecology 2002, 83, 2361–2366. [Google Scholar] [CrossRef]
- Hejcman, M.; Hejcmanová, P.; Pavlů, V.; Beneš, J. Origin and history of grasslands in Central Europe—A review. Grass Forage Sci. 2013, 68, 345–363. [Google Scholar] [CrossRef]
- Leuschner, C.; Ellenberg, H. Ecology of Central European Non-Forest Vegetation: Coastal to Alpine, Natural to Man-Made Habitats. Vegetation Ecology of Central Europe Volume II; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Slavík, B. Přispěvek k fytogeografické charakteristice středočeského kraje. Stud. ČSAV 1980, 1, 45–107. [Google Scholar]
- Pott, R. Biototypen Schützenswerte Lebensräume Deutschlands und angrezender Regionen; Ulmer: Stuttgart, Germany, 1996. [Google Scholar]
- Hempel, W. Offenlandrelikte im Oberlausitzer Bergland und im angrezenden Nordböhmen. Ber. Naturforsch. Ges. Oberlausitz 2010, 18, 43–48. [Google Scholar]
- Ludwig, O. Das Pontische und Aquilonare Element in der Flora Schlesiens. Beibl. Bot. Jahrb. 1923, 130, 11–38. [Google Scholar]
- Schalow, E. Was lehrt die heutige Pflanzenverbreitung über die schlesische Urlandschaft? Mitt. Beuthen. Ges. Musver. 1931, 13/14, 250–259. [Google Scholar]
- Szeląg, Z. Rośliny naczyniowe Masywu Śnieżnika i Gór Bialskich; Instytut Botaniki im. W. Szafera, Polska Akademia Nauk: Kraków, Poland, 2000. [Google Scholar]
- Kwiatkowski, P. Current State, Separateness and Dynamics of Vascular Flora of the Góry Kaczawskie (Kaczawa Mountains) and Pogórze Kaczawskie (Kaczawa Plateau). II. Phytogeographical Analysis; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2007. [Google Scholar]
- Żołnierz, L. Zbiorowiska trawiaste występujące na Dolnośląskich serpentynitach—Wybrane aspekty ekologii. Zesz. Nauk. Uniw. Przyr. Wroc. Rozpr. 2007, 247, 1–231. [Google Scholar]
- Ewald, J. The calcareous riddle: Why are there so many calciphilous species in the Central European flora? Folia Geobot. 2003, 38, 357–366. [Google Scholar] [CrossRef]
- Lustrino, M.; Wilson, M. The circum-Mediterranean anorogenic Cenozoic igneous province. Earth Sci. Rev. 2007, 81, 1–65. [Google Scholar] [CrossRef]
- Ulrych, J.; Dostal, J.; Adamovič, J.; Jelínek, E.; Špaček, P.; Hegner, E.; Balogh, K. Recurrent Cenozoic volcanic activity in the Bohemian Massif (Czech Republic). Lithos 2011, 123, 133–144. [Google Scholar] [CrossRef]
- Janoška, M. Sopky a Sopečné Vrchy České Republiky; Academia: Praha, Czech Republic, 2013. [Google Scholar]
- Badura, J.; Pécskay, Z.; Koszowska, E.; Wolska, A.; Zuchiewicz, W.; Przybylski, B. New age and petrological constraints on Lower Silesian basaltoids, SW Poland. Acta Geodyn. Geomater. 2005, 2, 7–15. [Google Scholar]
- Tietz, O.; Büchner, J.; Suhr, P.; Abratis, M.; Goth, K. Die Geologie des Baruther Schafberges und der Dubrauker Horken—Aufbau und Entwicklung eines känozoischen Vulkankomplexes in Ostsachsen. Ber. Naturforsch. Ges. Oberlausitz Suppl. 2011, 18, 15–48. [Google Scholar]
- Buchner, J.; Tietz, O.; Viereck, L.; Suhr, P.; Abratis, M. Volcanology, geochemistry and age of the Lausitz Volcanic Field. J. Earth Sci. 2015, 104, 2057–2083. [Google Scholar]
- Anioł-Kwiatkowska, J.; Świerkosz, K. Flora i roślinność rezerwatu “Ostrzyca Proboszczowicka” oraz jego otoczenia. Acta Univ. Wrat. Prace Bot. 1992, 48, 45–115. [Google Scholar]
- Kwiatkowski, P. Zbiorowiska roślinne projektowanego rezerwatu “Rataj” koło Jawora. Parki Nar. Rez. Przyr. 1995, 14, 95–108. [Google Scholar]
- Kwiatkowski, P. Szata roślinna Bazaltowej Góry i jej otoczenia. Acta Univ. Wrat. Prace Bot. 1996, 70, 73–110. [Google Scholar]
- Kwiatkowski, P. Zmiany we florze roślin naczyniowych Masywu Grodźca (Pogórze Kaczawskie). Przyr. Sudet. 2013, 16, 45–66. [Google Scholar]
- Szczęśniak, E. Szata roślinna projektowanego rezerwatu “Krzyżowa Góra” koło Strzegomia (Dolny Śląsk). Ochr. Przyr. 1998, 55, 61–75. [Google Scholar]
- Ritz, C.; Wünsche, A. Basaltkuppen in der östlichen Oberlausitz. Tuexenia 2017, 10, 49–65. [Google Scholar]
- Jenik, J. Large-scale pattern of biodiversity in Hercynian massifs. In Spatial Processes in Plant Communities; Krahulec, F., Agnew, A.D.Q., Agnew, S., Willems, J.H., Eds.; SPB Academic Publishing and Academia: Praha, Czech Republic, 1990; pp. 251–259. [Google Scholar]
- Mazurski, K.R. Environmental problems in the Sudetes, Poland. GeoJournal 1999, 46, 271–277. [Google Scholar] [CrossRef]
- Sawicki, L. Mapa Geologiczna Regionu Dolnośląskiego z Przyległymi Obszarami Czech i Niemiec 1: 100,000. Podstawy Litostratygraficzne i Kodyfikacja Wydzieleń; Państwowy Instytut Geologiczny: Warszawa, Poland, 1997. [Google Scholar]
- Birkenmajer, K.; Jerzmański, J.; Nairn, A.E.M. Palaeomagnetic Studies of Polish Rocks. IV. Cenozoic Basalts of Lower Silesia. Ann. Soc. Geol. Pol. 1970, 40, 31–61. [Google Scholar]
- Birkenmajer, K.; Pécskay, Z.; Grabowski, J.; Lorenc, M.W.; Zagożdżon, P.P. Radiometric Dating of the Tertiary Volcanics in Lower Silesia, Poland. III. K-Ar and Palaeomagnetic Data from Early Miocene Basaltic Rocks near Jawor, Fore-Sudetic Block. Ann. Soc. Geol. Pol. 2002, 72, 241–253. [Google Scholar]
- Birkenmajer, K.; Pécskay, Z.; Grabowski, J.; Lorenc, M.W.; Zagożdżon, P.P. Radiometric Dating of the Tertiary Volcanics in Lower Silesia, Poland. IV. Further K-Ar and Paleomagnetic Data from Late Oligocene to Early Miocene Basaltic Rocks of the Fore-Sudetic Block. Ann. Soc. Geol. Pol. 2004, 74, 1–19. [Google Scholar]
- Birkenmajer, K.; Pécskay, Z.; Grabowski, J.; Lorenc, M.W.; Zagożdżon, P.P. Radiometric Dating of the Tertiary Volcanics in Lower Silesia, Poland. V. K-Ar and Paleomagnetic Data from Late Oligocene to Early Miocene Basaltic Rocks of the North-Sudetic Depression. Ann. Soc. Geol. Pol. 2007, 77, 1–16. [Google Scholar]
- Birkenmajer, K.; Pécskay, Z.; Grabowski, J.; Lorenc, M.W.; Zagożdżon, P.P. Radiometric Dating of the Tertiary Volcanics in Lower Silesia, Poland. VI. K-Ar and Paleomagnetic Data from Basaltic Rocks of the West Sudety Mountains and their Northern Foreland. Ann. Soc. Geol. Pol. 2011, 81, 115–131. [Google Scholar]
- Le Maitre, R.W.; Streckeisen, A.; Zanettin, B.; Le Bas, M.J.; Bonin, B.; Bateman, P.; Bellieni, G.; Dudek, A.; Efremova, S.; Keller, J. Igneous Rocks: A Classification and Glossary of Terms Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Igneous Rocks; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Jäger, E.J. (Ed.) Rothmaler-Exkursionsflora von Deutschland. Gefässpflanzen: Grundband, 21. durchgesehene Auflage; Spinger Spektrum: Berlin, Germany, 2017. [Google Scholar]
- Mucina, L. Conspectus of Classes of European Vegetation. Folia Geobot. Phytotax. 1997, 32, 117–172. [Google Scholar] [CrossRef]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2001. [Google Scholar]
- Dengler, J.; Eisenberg, M.; Schröder, J. Die grundwasserfernen Saumgesellschaften Nordostniedersachsens im europäischen Kontext—Teil I: Säume magerer Standorte (Trifolio-Geranietea sanguinei). Tuexenia 2006, 26, 51–91. [Google Scholar]
- Roleček, J. Teplomilné doubravy (Quercetea pubescentis). In Vegetace České Republiky. 4. Lesní a Křovinná Vegetace; Chytrý, M., Ed.; Academia: Praha, Czech Republic, 2013; pp. 296–346. [Google Scholar]
- Meusel, H.; Jäger, E.; Weinert, E. Vergleichende Chorologie der Zentraleuropäischen Flora. 1; Veb Gustav Fischer Verlag: Jena, Germany, 1965. [Google Scholar]
- Meusel, H.; Jäger, E.; Rauschert, S.; Weinert, E. Vergleichende Chorologie der Zentraleuropäischen Flora. 2; Veb Gustav Fischer Verlag: Jena, Germany, 1978. [Google Scholar]
- Meusel, H.; Jäger, E. Vergleichende Chorologie der Zentraleuropäischen Flora. 3; Veb Gustav Fischer Verlag: Jena, Germany, 1992. [Google Scholar]
- Finnie, T.J.R.; Preston, C.D.; Hill, M.O.; Uotila, P.; Crawley, M.J. Floristic elements in European plants: An analysis based on Atlas Florae Europaeae. J. Biogeogr. 2007, 34, 1848–1872. [Google Scholar] [CrossRef]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulissen, D. Zeigerwerte von Pflanzen in Mitteleuropa. 3. Durchgesehene Auflage. Scr. Geobot. 2001, 18, 1–262. [Google Scholar]
- Kwiatkowski, P. Chorological and phytogeographical diversity of tree and shrubs as a mean to regionalization: Kaczawa Mountains, Sudetes, Poland. Willdenowia 2014, 44, 363–376. [Google Scholar] [CrossRef]
- Wagensommer, R.P.; Perrino, E.V.; Silletti, G.N. Carex phyllostachys A. Mey. (Cyperaceae) new for Italy and phytogeographical considerations. Phyton 2014, 54, 215–222. [Google Scholar]
- Meindl, C.; Brune, V.; Listl, D.; Poschlod, P.; Reisch, C. Survival and postglacial immigration of the steppe plant Scorzonera purpurea to Central Europe. Plant Syst. Evol. 2016, 302, 971–984. [Google Scholar] [CrossRef]
- Kwiatkowski, P. Podgórska ciepłolubna dąbrowa brekiniowa Sorbo torminalis-Quercetum na Pogórzu Złotoryjskim. Fragm. Flor. Geobot. Polon. 2003, 10, 175–193. [Google Scholar]
- Krauss, J.; Klein, A.-M.; Steffan-Dewenter, I.; Tscharntke, T. Effects of habitat area, isolation, and landscape diversity on plant species richness of calcareous grasslands. Biodiv. Conserv. 2004, 13, 1427–1439. [Google Scholar] [CrossRef]
- Helm, A.; Hanski, I.; Partel, M. Slow response of plant species richness to habitat loss and fragmentation. Ecol. Lett. 2006, 9, 72–77. [Google Scholar] [CrossRef]
- Fletcher, R.J., Jr.; Didham, R.K.; Banks-Leite, C.; Barlow, J.; Ewers, R.M.; Rosindell, J.; Holt, R.D.; Gonzalez, A.; Pardini, R.; Damschen, E.I.; et al. Is habitat fragmentation good for biodiversity? Biol. Conserv. 2018, 226, 9–15. [Google Scholar] [CrossRef]
- Tsiftsis, S. The complex effect of heterogeneity and isolation in determining alpha and beta orchid diversity on islands in the Aegean archipelago. Syst. Biodivers. 2020, 18, 281–294. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography. With a New Preface by Edward O. Wilson; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Watling, J.I.; Arroyo-Rodríguez, V.; Pfeifer, M.; Baetenm, L.; Banks-Leite, C.; Cisneros, L.M.; Fang, R.; Hamel-Leigue, A.C.; Lachat, T.; Leal, I.R.; et al. Support for the habitat amount hypothesis from a global synthesis of species density studies. Ecol. Lett. 2020, 23, 674–681. [Google Scholar] [CrossRef]
- Triantis, K.A.; Mylonas, M.; Lika, K.; Vardinoyannis, K. A model for the species–area–habitat relationship. J. Biogeogr. 2003, 30, 19–27. [Google Scholar] [CrossRef]
- Cramer, M.J.; Willig, M.R. Habitat heterogeneity, species diversity and null models. Oikos 2005, 108, 209–218. [Google Scholar] [CrossRef]
- MacDonald, Z.G.; Anderson, I.D.; Acorn, J.H.; Nielsen, S.E. The theory of island biogeography, the sample-area effect, and the habitat diversity hypothesis: Complementarity in a naturally fragmented landscape of lake islands. J. Biogeogr. 2018, 45, 2730–2743. [Google Scholar] [CrossRef]
- Quinn, J.F.; Harrison, S.P. Effects of habitat fragmentation and isolation on species richness: Evidence from biogeographic patterns. Oecologia 1988, 75, 132–140. [Google Scholar] [CrossRef]
- Schrader, J.; Moeljono, S.; Keppel, G.; Krett, H. Plants on small islands revisited: The effects of spatial scale and habitat quality on the species-area relationship. Ecography 2019, 42, 1405–1414. [Google Scholar] [CrossRef]
- Huston, M. A general hypothesis of species diversity. Am. Nat. 1979, 113, 81–101. [Google Scholar] [CrossRef]
- Murphy, G.E.P.; Romanuk, T.N. A meta-analysis of declines in local species richness from human disturbances. Ecol. Evol. 2014, 4, 91–103. [Google Scholar] [CrossRef]
- Fabiszewski, J.; Kwiatkowski, P. Threatened vascular plants of the Sudeten Mountains. Acta Soc. Bot. Pol. 2002, 71, 339–350. [Google Scholar] [CrossRef][Green Version]
- Bräutigam, S.; Otto, H.-W. Rote Liste der Farn- und Samenpflanzen der Oberlauitz—Aktualisierte Fassung. Ber. Naturforsch. Ges. Oberlausitz 2012, 20, 99–116. [Google Scholar]
- Davis, B.N.K. Ecology of Quarries: The Importance of Natural Vegetation; Institute of Terrestrial Ecology: Cambridge, UK, 1982. [Google Scholar]
- Kwiatkowski, P. Kamieniołomy wapienia w Górach Kaczawskich ostoją rzadkich i ginących gatunków flory naczyniowej Sudetów. Gór. Odkryw. 1998, 40, 156–163. [Google Scholar]
- Mückschel, C. Floristische Beobachtungen in aufgelassenen Steinbrücken des Rheinischen Westerwaldes. Decheniana 1999, 153, 59–67. [Google Scholar]
- Novák, J.; Prach, K. Artificial sowing of endangered dry grassland species into disused basalt quarries. Flora Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 179–183. [Google Scholar] [CrossRef]
- Tropek, R.; Kadlec, T.; Karesova, P.; Spitzer, L.; Kocarek, P.; Malenovsky, I.; Banar, P.; Tuf, I.H.; Hejda, M.; Konvicka, M. Spontaneous succession in limestone quarries as an effective restoration tool for endangered arthropods and plants. J. Appl. Ecol. 2010, 47, 139–147. [Google Scholar] [CrossRef]
- Raška, P.; Riezner, J.; Pokorný, R.; Holec, M.; Raška, M. Relations between Biotic and Abiotic Diversity in Abandoned Basalt Quarry and Its Relevance for Ecologic Restoration (Radobyl Hill, Northern Czechia). Acta Univ. Agric. Silvc. Mendel. Brun. 2017, 65, 151–166. [Google Scholar] [CrossRef]
- Soons, M.B.; Heil, G.W. Reduced colonization capacity in fragmented populations of wind-dispersed grassland forbs. J. Ecol. 2002, 90, 1033–1043. [Google Scholar] [CrossRef]
- Murphy, H.T.; Lovett-Doust, J. Context and connectivity in plant metapopulations and landscape mosaics: Does the matrix matter? Oikos 2004, 105, 3–14. [Google Scholar] [CrossRef]
- Hannus, J.-J.; von Numers, M. Vascular plant species richness in relation to habitat diversity and island area in the Finnish Archipelago. J. Biogeogr. 2007, 35, 1077–1086. [Google Scholar] [CrossRef]
- Lombini, A.; Ferrari, C.; Carperiè, B. The ecology of ophiolitic scree vegetation: A survey on the northern Apennine outcrops (Italy). Bocconea 2001, 13, 561–571. [Google Scholar]
- Kruckerberg, A. Geology and Plant Life. The Effects of Landforms and Rock Types on Plants; Washington University Press: Seattle, WA, USA, 2004. [Google Scholar]
- Korneck, D. Xerothermvegetation von Rheinland-Pfalz und Nachbargebieten. Schriftenr. Vegetationskd. 1974, 7, 1–196. [Google Scholar]
- Royer, J.M. Synthèse eurosibérienne, phytosociologique et phytogéographique de la classe des Festuco-Brometea. Diss. Bot. 1991, 178, 1–296. [Google Scholar]
- Di Pietro, R.; Wagensommer, R.P. A new Sesleria juncifolia association from south-eastern Italy and its position in the amphi-Adriatic biogeographical context. Acta Bot. Croat. 2014, 73, 171–207. [Google Scholar]
- Michalik, S. Charakterystyka ekologiczna kserotermicznej i górskiej flory naczyniowej Ojcowskiego Parku narodowego. Stud. Nat. 1979, 19, 1–95. [Google Scholar]
- Slavíková, J.; Molíková, M.; Rejmánek, M.; Rydlo, J.; Studnič, M.; Studničková, I.; Suchara, I.; Štolcová-Březinová, J. Ecological and Vegetational Differentiation of a Solitary Conic Hill (Oblik in České Středohoři Mts.); Academia: Praha, Czech Republic, 1983. [Google Scholar]
- McCune, B.; Keon, D. Equations for potential annual direct incident radiation and heat load. J. Veg. Sci. 2002, 13, 603–606. [Google Scholar] [CrossRef]
- Cook, M.W.; Lane, K.T.; Foster, B.L.; Holt, R.D. Island theory, matrix effects and species richness patterns in habitat fragments. Ecol. Lett. 2002, 5, 619–623. [Google Scholar] [CrossRef]
- Löbel, S.; Dengler, J.; Hobohm, C. Species richness of vascular plants, bryophytes and lichens in dry grasslands: The effects of environment, landscape structure and competition. Folia Geobot. 2006, 41, 377–393. [Google Scholar] [CrossRef]
- Cox, C.B.; Moore, P.D.; Ladle, R.J. Biogeography. An Ecological Approach, 9th ed.; Wiley-Blackwell: Oxford, UK, 2016. [Google Scholar]
- Diekmann, M. Species indicator values as important tool in applied plant ecology—A review. Bas. Appl. Ecol. 2003, 4, 493–506. [Google Scholar] [CrossRef]
- Berg, C.; Welk, E.; Jäger, E.J. Revising Ellenberg’s indicator values for continentality based on global vascular plants species distribution. Appl. Veg. Sci. 2017, 20, 482–493. [Google Scholar] [CrossRef]
- Chytrý, M.; Tichý, L.; Dřevojan, P.; Sádlo, J.; Zelený, D. Ellenberg-type indicator values for the Czech Flora. Preslia 2018, 90, 83–103. [Google Scholar] [CrossRef]
- Hedwall, P.-O.; Brunet, J.; Diekmann, M. With Ellenberg indicator values towards the north: Does the indicate power-decrease with distance from Central Europe? J. Biogeogr. 2019, 46, 1041–1053. [Google Scholar] [CrossRef]
- Tyler, T.; Herbertsson, L.; Olofsson, J.; Olsson, P.A. Ecological indicator and traits values for Swedish vascular plants. Ecol. Ind. 2021, 120, 106923. [Google Scholar] [CrossRef]
- Heywood, V.H.; Dulloo, M.E. In Situ Conservation of Wild Plant Species: A Critical Global Review of Best Practices; International Plant Genetic Resources Institute (IPGRI): Technical Bulletin No 11; Bioversity International: Rome, Italy, 2005. [Google Scholar]
- Heywood, V.H. In situ conservation of plant species—An unattainable goal? Israel Plant Sci. 2015, 63, 211–231. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Crop Wild Relatives (CWR) Priority in Italy: Distribution, Ecology, In Situ and Ex Situ Conservation and Expected Actions. Sustainability 2021, 13, 1682. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).