Beta Diversity along an Elevational Gradient at the Pico da Neblina (Brazil): Is Spider (Arachnida-Araneae) Community Composition Congruent with the Guayana Region Elevational Zonation?
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Sampling Methods
2.3. Analyses
3. Results
3.1. Diversity and Dominance
3.2. Beta Diversity
3.3. Species Indicator Analysis
4. Discussion
4.1. Diversity and Dominance
4.2. Beta Diversity
4.3. Spider in Mountains
4.4. Montane Spider Conservation
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- McCain, C.M. Elevational gradients in diversity of small mammals. Ecology 2005, 86, 366–372. [Google Scholar] [CrossRef]
- McCain, C.M. Area and mammalian elevational diversity. Ecology 2007, 88, 76–86. [Google Scholar] [CrossRef]
- McCain, C.M. Could temperature and water availability drive elevational species richness patterns? A global case study for bats. Glob. Ecol. Biogeogr. 2007, 16, 1–13. [Google Scholar] [CrossRef]
- Janzen, D.H. Why mountain passes are higher in the tropics? Am. Nat. 1967, 101, 233–249. [Google Scholar] [CrossRef]
- Lomolino, M.K. Elevation gradients of species-density: Historical and prospective views. Glob. Ecol. Biogeogr. 2001, 10, 3–13. [Google Scholar] [CrossRef]
- Sanders, N.J.; Lessard, J.P.; Fitzpatrick, M.C.; Dunn, R.R. Temperature, but not productivity orgeometry, predicts elevational diversitygradients in ants across spatial grains. Glob. Ecol. Biogeogr. 2007, 16, 640649. [Google Scholar] [CrossRef]
- Orme, C.D.L.; Davies, R.G.; Burgess, M.; Eigenbrod, F.; Pickup, N.; Olson, V.A. Global hotspots of species richness are not congruent with endemism or threat. Nature 2005, 436, 1016–1019. [Google Scholar] [CrossRef]
- Rahbek, C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 2005, 8, 224–239. [Google Scholar] [CrossRef]
- Lieberman, D.; Lieberman, M.; Peralta, R.; Hartshorn, G.S. Tropical forest structure and composition on a largescale elevational gradient in Costa Rica. J. Ecol. 1996, 84, 137–152. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Givnish, T.J. Elevational gradients in tropical forest composition, structure, and diversity in the Sierra de Manantla´n. J. Ecol. 1998, 86, 999–1020. [Google Scholar]
- Brehm, G.; Homeier, J.; Fiedler, K. Beta diversity of geometrid moths (Lepidoptera: Geometridae) in an Andean montane rainforest. Div. Distrib. 2003, 9, 351–366. [Google Scholar] [CrossRef]
- Ghalambor, C.K.; Huey, R.B.; Martin, P.R.; Tewksbury, J.J.; Wang, G. Are mountain passes higher in the tropics? Janzens hypothesis revisited. Integr. Comp. Biol. 2006, 46, 5–17. [Google Scholar] [CrossRef] [Green Version]
- McCain, C.M. Vertebrate range sizes indicate that mountains may be higher in the tropics. Ecol. Lett. 2009, 12, 550–560. [Google Scholar] [CrossRef]
- Patterson, B.D.; Stotz, D.F.; Solari, S.; Fitzpatrick, J.W. Contrasting patterns of elevational zonation for birds and mammals in the Andes of southeastern Peru. J. Biogeogr. 1998, 25, 593–607. [Google Scholar] [CrossRef] [Green Version]
- Jankowski, J.E.; Ciecka, A.L.; Meyer, N.Y.; Rabenold, K.N. Beta diversity along environmental gradients: Implications of habitat specialization in tropical montane landscapes. J. Anim. Ecol. 2009, 78, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Willard, D.E.; Foster, M.S.; Barrowclough, G.F.; Dickerman, R.W.; Cannell, P.F.; Coats, S.L.; Cracraft, J.L.; O’Neill, J.P. The Birds of Cerro de la Neblina. Fieldiana 1991, 65, 27–51. [Google Scholar]
- Huber, O. Geographical and physical features. In Flora of the Venezuelan Guayana; Berry, P.E., Holst, B.K., Yatskievych, K., Eds.; Missouri Botanical Garden Press: St. Louis, MO, USA, 1995; Volume 1, pp. 161–191. [Google Scholar]
- Berry, P.E.; Riina, R. Insights into the diversity of the Pantepui flora and the biogeographic complexity of the Guayana Shield. Biol. Skr. 2005, 55, 145–167. [Google Scholar]
- Rull, V.; Nogué, S. Potential migration routes and barriers for vascular plants of the Neotropical Guyana Highlands during the Quaternary. J. Biogeogr. 2007, 4, 1327–1341. [Google Scholar] [CrossRef]
- Scharff, N.; Coddington, J.A.; Griswold, C.E.; Hormiga, G.; Bjorn, P.D.P. When to quit? Estimating spider species richness in a northern European deciduos forest. J. Arachnol. 2003, 31, 246–273. [Google Scholar] [CrossRef]
- Cardoso, P.; Arnedo, M.A.; Triantis, K.A.; Borges, P.A.V. Drivers of diversity in Macaronesian spiders and the role of species extinctions. J. Biogeogr. 2010, 37, 1034–1046. [Google Scholar] [CrossRef] [Green Version]
- World Spider Catalog. World Spider Catalog. Version 22.5; Natural History Museum Bern: Bern, Switzerland, 2021; Available online: http://wsc.nmbe.ch (accessed on 12 October 2021). [CrossRef]
- Coddington, J.A.; Griswold, C.E.; Silva, D.; Larcher, L. Designing and testing sampling protocols to estimate biodiversity in tropical ecosystems. In The Unity of Evolutionary Biology: Proceedings of the Fourth International Congress of Systematic and Evolutionary Biology, College Park, MD, USA, 30 June–7 July 1991; Dioscorides Press: Portland, OR, USA, 1991; pp. 44–60. [Google Scholar]
- Janzen, D.H.; Ataroff, M.; Farinas, M.; Reyes, S.; Rincon, N.; Soler, A.; Soriano, P.; Vera, M. Changes in the arthropod community along an elevational transect in the Venezuelan Andes. Biotropica 1976, 8, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.V. The effect of architectural variation in habitat on a spider community: An experimental field study. Ecology 1981, 62, 73–80. [Google Scholar] [CrossRef]
- Greenstone, M.H. Determinants of web spider species diversity: Vegetation structural diversity vs. prey availability. Oecologia 1984, 62, 299304. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Ojeda, L.; Florez-Daza, E. Arañas orbitelares (Araneae: Orbiculariae) em tres formaciones vegetales de la Sierra Nevada de Santa Marta (Magdalena, Colombia). Rev. Iber. Aracnol. 2007, 16, 3–16. [Google Scholar]
- Chatzaki, M.; Lymberakis, P.; Markakis, G.; Mylonas, M. The distribution of ground spiders (Araneae, Gnaphosidae) along the elevational gradient of Crete, Greece: Species richness, activity and elevational range. J. Biogeogr. 2005, 32, 813–831. [Google Scholar] [CrossRef]
- Foord, S.H.; Dippenaar-Schoeman, A.S. The effect of elevation and time on mountain spider diversity: A view of two aspects in the Cederberg mountains of South Africa. J. Biogeogr. 2016, 43, 2354–2365. [Google Scholar] [CrossRef]
- Malumbres-Olarte, J.; Crespo, L.; Cardoso, P.; Szűts, T.; Fannes, W.; Pape, T.; Scharff, N. The same but different: Equally megadiverse but taxonomically variant spider communities along an elevational gradient. Acta Oecolog. 2018, 88, 19–28. [Google Scholar] [CrossRef]
- Miller, J.A.; Griswold, C.E.; Yin, C.M. The symphytognathoid spiders of the Gaoligongshan, Yunnan, China (Araneae, Araneoidea): Systematics and diversity of micro-orbweavers. ZooKeys 2009, 11, 9–195. [Google Scholar] [CrossRef]
- Tanasevitch, A.V. A new Palliduphantes Saaristo & Tanasevitch, 1996 from the Elburz Mountains, Iran (Araneae: Linyphiidae). Zool. Mid. East 2017, 63, 172–175. [Google Scholar]
- Dimitrov, D. A review of the linyphiid spider genus Proislandiana Tanasevitch, 1985 with description of a new high mountainous species from Turkey and Armenia (Araneae: Linyphiidae). Zootaxa 2020, 4743, 247–256. [Google Scholar] [CrossRef] [PubMed]
- RADAM. Folha NA19. Pico da Neblina; Ministério das Minas e Energia: Rio de Janeiro, Brazil, 1978.
- Pires, J.M.; Prance, T.G. The vegetation types of the Brazilian Amazon. In Key Environments: Amazonia; Prance, G.T., Lovejoy, T.E., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 109–145. [Google Scholar]
- Berry, P.E.; Huber, O.; Holst, B.K. Introduction. Floristic analysis and phytogeography. In Flora of the Venezuelan Guayana; Berry, P.E., Holst, B.K., Yatskievych, K., Eds.; Missouri Botanical Garden Press: St. Louis, MO, USA, 1995; Volume 1, pp. 161–191. [Google Scholar]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, W.H.; Parker, F.L. Diversity of planktonic foraminifera in deep-sea sediments. Science 1970, 168, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Kruskal, J.B. Nonmetric multidimensional scaling: A numerical method. Psychometrika 1964, 29, 115–129. [Google Scholar] [CrossRef]
- Fasham, M.J.R. A comparison of nometric multidimentsional scaling, principal components and reciprocal averaging for the ordination of simulated coenoclines, and coenoplanes. Ecology 1977, 58, 551–561. [Google Scholar] [CrossRef]
- Kenkel, N.C.; Orlóci, L. Applying metric and nonmetric multidimensional scaling to ecological studies: Some new results. Ecology 1986, 67, 919–928. [Google Scholar] [CrossRef]
- Brehm, G.; Konrad Fiedler, K. Ordinating tropical moth ensembles from an elevational gradient: A comparison of common methods. J. Trop. Ecol. 2004, 20, 165–172. [Google Scholar] [CrossRef]
- Smouse, P.E.; Long, J.C.; Sokal, R.R. Multiple regression and correlation extensions of the Mantel test of matrix correspondence. System. Zool. 1986, 35, 627–632. [Google Scholar] [CrossRef]
- Legendre, P. Comparison of permutation methods for the partial correlation and partial Mantel tests. J. Statist. Comput. Simul. 2000, 67, 37–73. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austr. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a fl exible asymetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 8.2. User’s Guide and Application. 2009. Available online: http://purl.oclc.org/estimates (accessed on 1 November 2010).
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 2 October 2011).
- Rosenberg, M.S. PASSAGE. Pattern Analysis, Spatial Statistics, and Geographic Exegesis. Version 1.0.; Department of Biology, Arizona State University: Tempe, AZ, USA, 2001. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD: Multivariate Analysis of Ecological Data. Version 5·12; MjM Software: Gleneden Beach, OR, USA, 1999. [Google Scholar]
- Nogueira, A.A.; Venticinque, E.M.; Brescovit, A.D.; Lo-Man-Hung, N.F.; Candiani, D.F. List of species of spiders (Arachnida, Araneae) from the Pico da Neblina, state of Amazonas, Brazil. Check List 2014, 10, 1044–1060. [Google Scholar] [CrossRef]
- Choi, S.W.; Jeong-Seop, N.A. Elevational distribution of moths (Lepidoptera) in Mt. Jirisan National Park, South Korea. European. J. Entom. 2010, 107, 229–245. [Google Scholar]
- McGill, B.J.; Etienne, R.S.; Gray, J.S.; Alonso, D.; Anderson, M.J.; Benecha, H.K.; White, E.P. Species abundance distributions: Moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 2007, 10, 995–1015. [Google Scholar] [CrossRef] [PubMed]
- Hurlbert, A.H. Species–energy relationships and habitat complexity in bird communities. Ecol. Lett. 2004, 7, 714–720. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Diamond, J.M.; Karr, J.R. Density compensation in island faunas. Ecology 1972, 53, 330–342. [Google Scholar] [CrossRef]
- Longino, J.T.; Colwell, R.K. Density compensation, species composition, and richness of ants on a neotropical elevational gradient. Ecosphere 2011, 2, 1–20. [Google Scholar] [CrossRef]
- Halaj, J.; Ross, D.W.; Moldenke, A.R. Habitat structure and prey availability as predictors of the abundance and community. J. Arachnol. 1998, 26, 203–220. [Google Scholar]
- Toti, D.S.; Coyle, F.A.; Miller, J.A. A structured inventory of Appalachian grass bald and heath bald spider assemblages and a test of species richness estimator performance. J. Arachnol. 2000, 28, 329–345. [Google Scholar] [CrossRef]
- Nogueira, A.A.; Pinto-da-Rocha, R.; Brescovit, A.D. Comunidade de aranhas orbitelas (Arachnida-Araneae) na região da Reserva Florestal do Morro Grande, Cotia, São Paulo, Brasil. Biota Neotrop. 2006, 6, 1–24. [Google Scholar] [CrossRef]
- Lo-Man-Hung, N.F.; Gardner, T.A.; Ribeiro-Júnior, M.A.; Barlow, J.; Bonaldo, A.L. The value of primary, secondary, and plantation forests for Neotropical epigeic arachnids. J. Arachnol. 2008, 36, 394–401. [Google Scholar] [CrossRef]
- Bosmans, R.; Maelfait, J.P.; De Kimpe, A. Analysis of the spider communities in an elevational gradient in the French and Spanish Pyrenees. Bull. Brit. Arachnol. Soc. 1986, 7, 69–76. [Google Scholar]
- Davis, A.L.V.; Scholtz, C.H.; Chown, S.L. Species turnover, community boundaries and biogeographical composition of dung beetle assemblages across an elevational gradient in South Africa. J. Biogeogr. 1999, 26, 1039–1055. [Google Scholar] [CrossRef]
- Bach, K.; Kessler, M.; Gradstein, S.R. A simulation approach to determine statistical significance of species turnover peaks in a species-rich tropical cloud forest. Div. Distrib. 2007, 13, 863–870. [Google Scholar] [CrossRef]
- Wu, F.; Yang, X.J.; Yang, J.X. Additive diversity partitioning as a guide to regional montane reserve design in Asia: An example from Yunnan Province, China. Div. Distrib. 2010, 16, 1022–1033. [Google Scholar] [CrossRef]
- Höfer, H.; Brescovit, A.D. Species and guild structure of a Neotropical spider assemblage (Araneae) from Reserva Ducke, Amazonas, Brazil. Andrias 2001, 15, 99–119. [Google Scholar]
- Nogueira, A.A.; Pena-Barbosa, J.P.P.; Venticinque, E.M.; Brescovit, A.D. The spider genus Chrysometa (Araneae, Tetragnathidae) from the Pico da Neblina and Serra do Tapirapecó mountains (Amazonas, Brazil): New species, new records, diversity and distribution along two elevational gradients. Zootaxa 2011, 2772, 33–51. [Google Scholar] [CrossRef]
- Turnbull, A.L. Ecology of the true spiders (Araneomorphae). Annual. Rev. Entomol. 1973, 18, 305–348. [Google Scholar] [CrossRef]
- Jetz, W.; Rahbek, C.; Colwell, R.C. The coincidence of rarity and richness and the potential signature of history in centres of endemism. Ecol. Lett. 2004, 7, 1180–1191. [Google Scholar] [CrossRef]
- Melo, A.S.; Rangel, T.F.L.V.B.; Diniz-Filho, J.A.F. Environmental drivers of beta-diversity patterns in New-World birds and mammals. Ecography 2009, 32, 226–236. [Google Scholar] [CrossRef]
- Polotow, D.; Griswold, C. Chinja, a new genus of spider from the Eastern Arc Mountains of Tanzania (Araneae, Zoropsidae). Zootaxa 2018, 4472, 545–562. [Google Scholar] [CrossRef]
- Steinbauer, M.J.; Field, R.; Grytnes, J.A.; Trigas, P.; Ah-Peng, C.; Attorre, F.; Beierkuhnlein, C. Topography-driven isolation, speciation and a global increase of endemism with elevation. Glob. Ecol. Biogeogr. 2016, 25, 1097–1107. [Google Scholar] [CrossRef]
- Chaladze, G.; Otto, S.; Tramp, S. A spider diversity model for the Caucasus Ecoregion. J. Insect Conserv. 2014, 18, 407–416. [Google Scholar] [CrossRef]
- Deltshev, C.; Komnenov, M.; Blagoev, G.; Georgiev, T.; Lazarov, S.; Stojkoska, E.; Naumova, M. Faunistic Diversity of Spiders (Araneae) in Galichitsa Mountain (FYR Macedonia). Biodiv. Data J. 2013, 1, e977. [Google Scholar] [CrossRef] [PubMed]
- McDiarmid, R.W.; Donnelly, M.A. Herpetofauna of the Guyana Highlands: Amphibians and Reptiles of the Lost World. In Ecology and Evolution in the TropicsA Herpetological Perspective; Donnelly, M.A., Crother, B.I., Guyer, C., Wake, M.H., White, M.E., Eds.; The Univestity of Chicago Press: Chicago, IL, USA, 2005; 584p. [Google Scholar]
- Aisen, S.; Werenkraut, V.; Márquez, M.E.G.; Ramírez, M.J.; Ruggiero, A. Environmental heterogeneity, not distance, structures montane epigaeic spider assemblages in north-western Patagonia (Argentina). J. Insect Conserv. 2017, 21, 951–962. [Google Scholar] [CrossRef]
- Viterbi, R.; Cerrato, C.; Bionda, R.; Provenzale, A. Effects of temperature rise on multi-taxa distributions in mountain ecosystems. Diversity 2020, 12, 210. [Google Scholar] [CrossRef]
- Levi, H.W. The Neotropical orb-weaver genera Chrysometa and Homalometa (Araneae: Tetragnathidae). Bull. Mus. Comp. Zool. 1986, 151, 91–215. [Google Scholar]
- Henle, K.; Davies, K.F.; Kleyer, M.; Margules, C.; Settele, J. Predictors of Species Sensitivity to Fragmentation. Biodiv. Conserv. 2004, 13, 207–251. [Google Scholar] [CrossRef]
- Dunn, R.R. Modern insect extinctions, the neglected majority. Conserv. Biol. 2005, 19, 130–136. [Google Scholar] [CrossRef]
- Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L.; Bruna, E.M.; Didham, R.K.; Stouffer, P.C.; Gascon, C.; Bierregard, R.O.; Laurance, S.G.; Sampaio, E. Ecosystem decay of amazonian forest fragments: A 22-year investigation. Conserv. Biol. 2002, 16, 605–618. [Google Scholar] [CrossRef] [Green Version]
- Dirnböck, T.; Essel, F.; Rabitsch, W. Disproportional risk for habitat loss of high-altitude endemic species under climate change. Glob. Chang. Biol. 2011, 17, 990–996. [Google Scholar] [CrossRef]
- Kuntner, M. Climate change and spider biodiversity: Redefining araneological research priorities. Crop Protect. 2014, 61, 109. [Google Scholar] [CrossRef]
- Rull, V.; Vegas-Vilarrúbia, T.; Nogué, S.; Huber, O. Conservation of the unique neotropical vascular flora of the Guayana Highlands in the face of global warming. Conserv. Biol. 2009, 23, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Nogué, S.; Rull, V.; Vegas-Vilarrúbia, T. Elevational gradients in the neotropical table mountains: Patterns of endemism and implications for conservation. Diver. Distrib. 2013, 19, 676–687. [Google Scholar] [CrossRef]
- Rull, V.; Vegas-Vilarr´ubia, T. Unexpected biodiversity loss under globalwarming in the Neotropical Guayana Highlands. Glob. Chang. Biol. 2006, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]

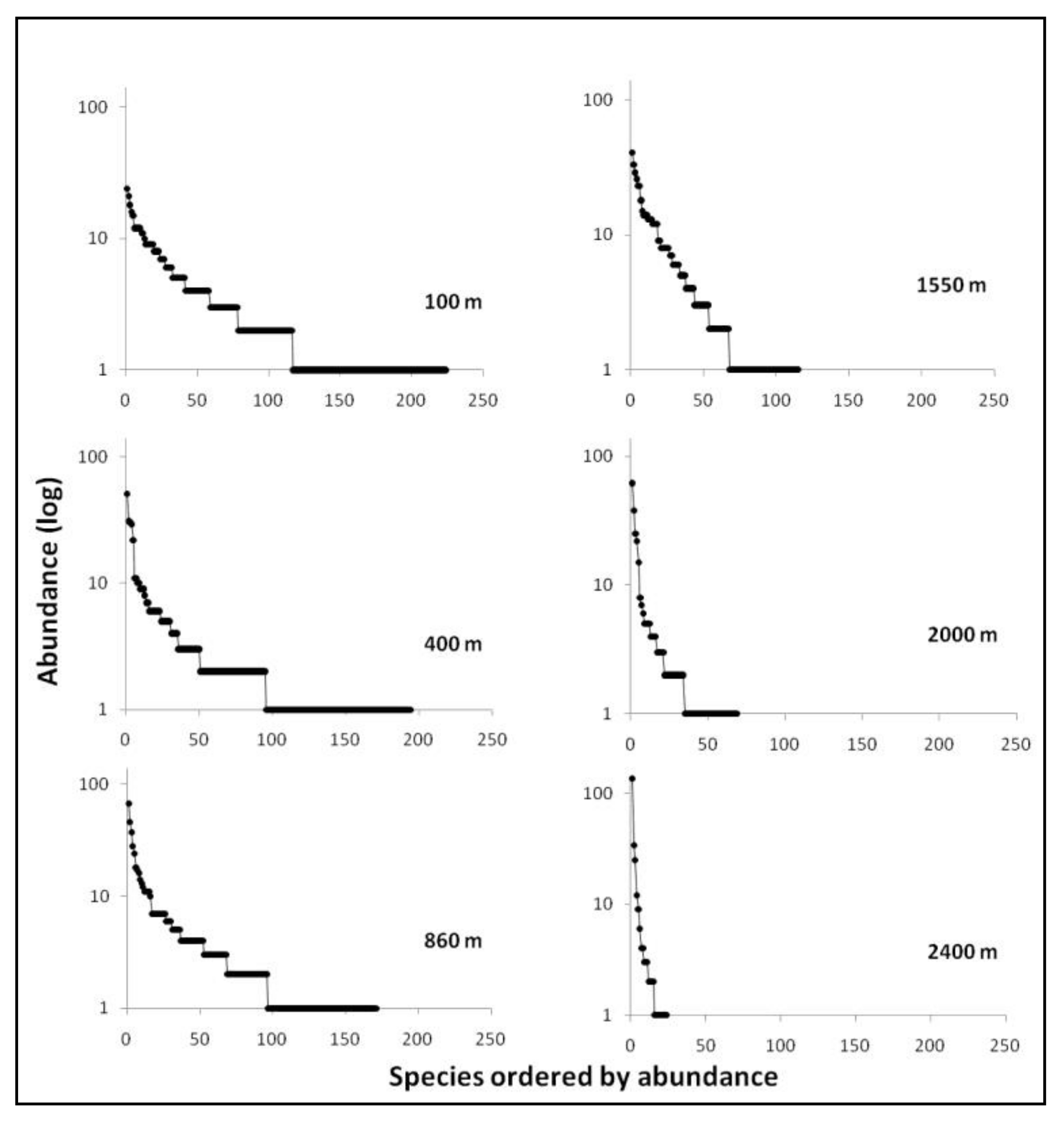
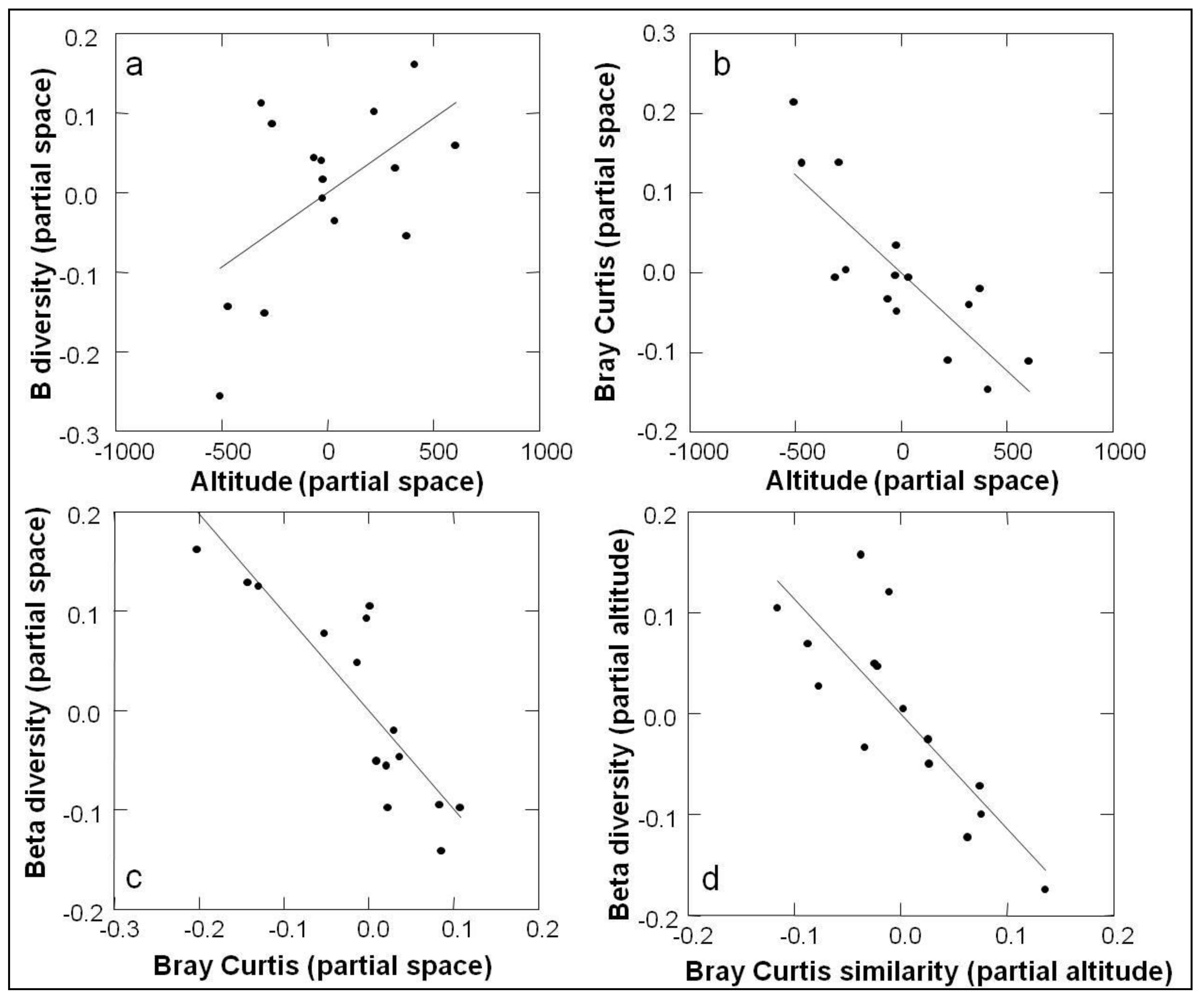
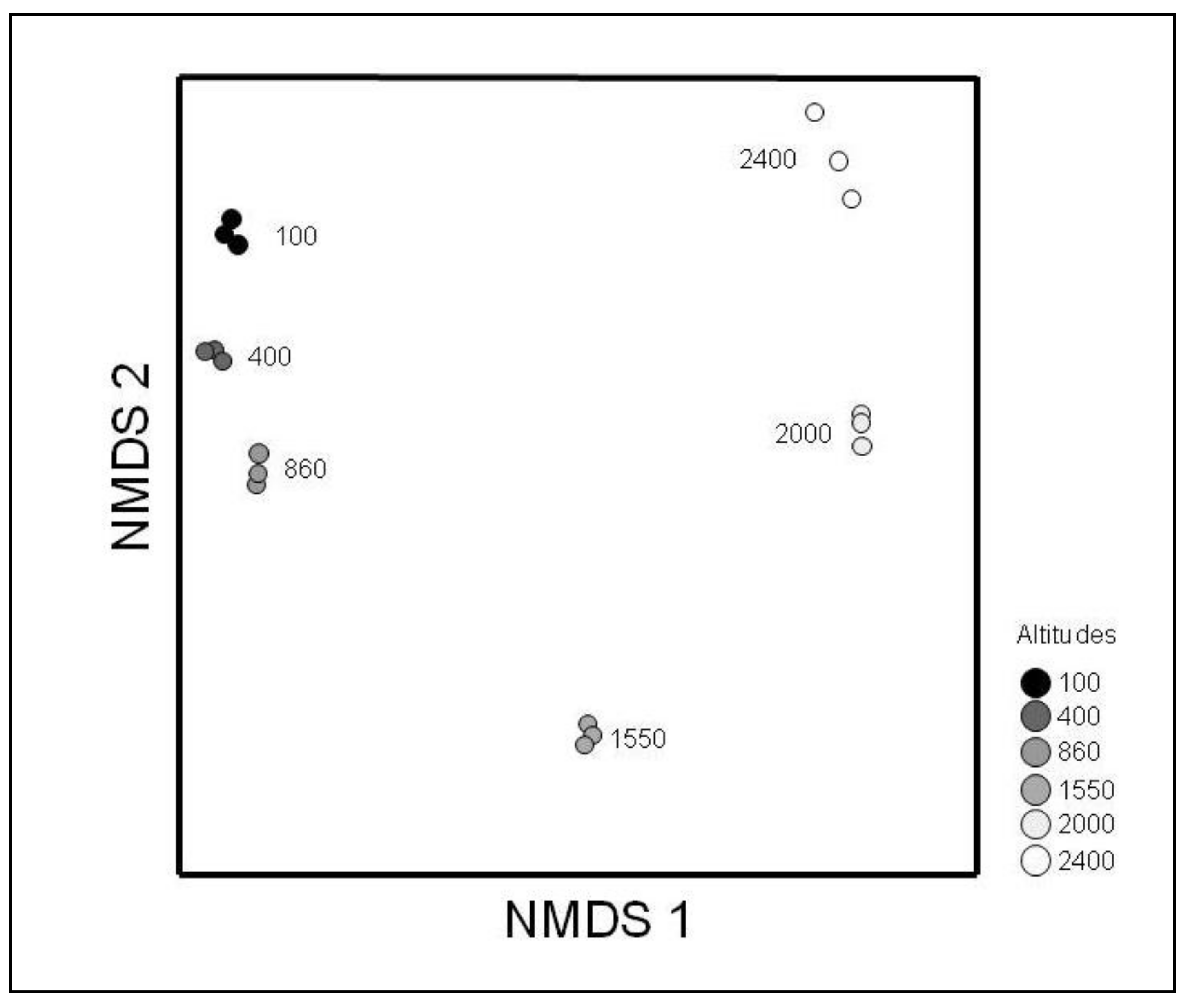
| Elevation (m.a.s.l.) | S(%S) | N(%N) | D | BP dom | % singl/S alt | % singl/S tot |
|---|---|---|---|---|---|---|
| 100 | 224 (42.4) | 688 (21.9) | 142.74 | 0.03 | 48.21 | 32.49 |
| 400 | 194 (36.7) | 590 (18.8) | 98.59 | 0.09 | 51.03 | 24.87 |
| 860 | 171 (32.4) | 713 (22.7) | 82.02 | 0.09 | 43.86 | 16.75 |
| 1550 | 115 (21.8) | 597 (19) | 61.68 | 0.07 | 41.74 | 14.21 |
| 2000 | 69 (13.1) | 295 (9.4) | 26.31 | 0.21 | 50.72 | 10.66 |
| 2400 | 24 (4.5) | 257 (8.2) | 6.10 | 0.53 | 37.5 | 1.02 |
| 100 | 400 | 860 | 1550 | 2000 | |
|---|---|---|---|---|---|
| 400 | 1.50 | ||||
| 860 | 1.60 | 1.45 | |||
| 1550 | 1.90 | 1.89 | 1.71 | ||
| 2000 | 1.80 | 1.85 | 1.80 | 1.65 | |
| 2400 | 1.79 | 1.84 | 1.76 | 1.79 | 1.62 |
| Comparsion | Control | R | P |
|---|---|---|---|
| (Partial) | |||
| β diversity × BCS | space | −0.896 | 0.0051 |
| β diversity × BCS | elevation | −0.824 | 0.0018 |
| β diversity × BCS | −0.921 | 0.002 | |
| β diversity × Elevation | space | 0.554 | 0.0507 |
| β diversity × Elevation | 0.727 | 0.0102 | |
| β diversity × Space | 0.576 | 0.0532 | |
| BCS × Elevation | space | −0.829 | 0.0028 |
| BCS × Elevation | −0.797 | 0.0014 | |
| BCS × Space | −0.494 | 0.0964 |
| Partition | Category | IS | S | % IS | Aver IV | IV 100 | Total IV |
|---|---|---|---|---|---|---|---|
| Lower and | LH | 42 | 110 | 38.2 | 68 | 3 | |
| upper half | UH | 6 | 62 | 9.7 | 64.9 | 0 | |
| Total | 48 | 157 | 30.6 | 68.2 | 3 | 3272.6 | |
| Guayana region | Lowlands | 22 | 96 | 22.9 | 72.1 | 1 | |
| Uplands | 15 | 103 | 14.6 | 78.8 | 1 | ||
| Higlands | 3 | 24 | 12.5 | 92.9 | 1 | ||
| Total | 40 | 157 | 25.5 | 75.9 | 3 | 3034.5 | |
| Elevations | 100 | 21 | 72 | 29.2 | 89.2 | 12 | |
| 400 | 9 | 72 | 12.5 | 82.3 | 4 | ||
| 860 | 12 | 80 | 15.0 | 80.5 | 3 | ||
| 1550 | 19 | 52 | 36.5 | 90.9 | 9 | ||
| 2000 | 6 | 20 | 30.0 | 81.7 | 2 | ||
| 2400 | 4 | 12 | 33.3 | 96.6 | 3 | ||
| Total | 71 | 157 | 45.2 | 87.1 | 33 | 6183.1 |
| Partition 1 | Partition 2 | Partition 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Category | Category | Category | |||||||||||
| IS (S) | LH | UH | IS (S) | L | U | H | IS (S) | 100 | 400 | 860 | 1550 | 2000 | 2400 | |
| Anyphaenidae | 1 (4) | 1 | 1 (4) | 1 | 3 (4) | 1 | 2 | |||||||
| Araneidae | 13 (35) | 12 | 1 | 7 (35) | 5 | 1 | 1 | 14 (35) | 4 | 4 | 3 | 2 | 1 | |
| Corinnidae | 2 (4) | 2 | 1 (4) | 1 | 1 (4) | 1 | ||||||||
| Ctenidae | 4 (6) | 4 | 4 (6) | 2 | 2 | 4 (6) | 2 | 1 | 1 | |||||
| Deinopidae | 1 (1) | 1 | 1 (1) | 1 | ||||||||||
| Hahniidae | 1 (1) | 1 | ||||||||||||
| Linyphiidae | 1 (4) | 1 | 1 (4) | 1 | 2 (4) | 1 | 1 | |||||||
| Lycosidae | 1 (1) | 1 | 1 (1) | 1 | ||||||||||
| Mimetidae | 3 (6) | 2 | 1 | |||||||||||
| Oonopidae | 1 (1) | 1 | ||||||||||||
| Pholcidae | 4 (8) | 4 | 4 (8) | 2 | 2 | 3 (8) | 1 | 2 | ||||||
| Pisauridae | 1 (2) | 1 | 1 (2) | 1 | 2 (2) | 2 | ||||||||
| Salticidae | 3 (15) | 3 | 2 (15) | 1 | 1 | 5 (15) | 2 | 1 | 1 | 1 | ||||
| Scytodidae | 1 (2) | 1 | 1 (2) | 1 | ||||||||||
| Senoculidae | 1 (1) | 1 | 1 (1) | 1 | ||||||||||
| Sparassidae | 3 (4) | 3 | 3 (4) | 3 | 2 (4) | 1 | 1 | |||||||
| Tetragnathidae | 3 (13) | 2 | 1 | 3 (13) | 2 | 1 | 6 (13) | 2 | 2 | 1 | 1 | |||
| Theridiidae | 3 (32) | 2 | 1 | 5 (32) | 2 | 3 | 15 (32) | 6 | 1 | 3 | 5 | |||
| Theridiosomatidae | 3 (5) | 2 | 1 | 1 (5) | 1 | 3 (5) | 2 | 1 | ||||||
| Thomisidae | 1 (2) | 1 | 1 (2) | 1 | ||||||||||
| Uloboridae | 4 (9) | 4 | 3 (9) | 3 | 3 (9) | 2 | 1 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, A.A.; Brescovit, A.D.; Perbiche-Neves, G.; Venticinque, E.M. Beta Diversity along an Elevational Gradient at the Pico da Neblina (Brazil): Is Spider (Arachnida-Araneae) Community Composition Congruent with the Guayana Region Elevational Zonation? Diversity 2021, 13, 620. https://doi.org/10.3390/d13120620
Nogueira AA, Brescovit AD, Perbiche-Neves G, Venticinque EM. Beta Diversity along an Elevational Gradient at the Pico da Neblina (Brazil): Is Spider (Arachnida-Araneae) Community Composition Congruent with the Guayana Region Elevational Zonation? Diversity. 2021; 13(12):620. https://doi.org/10.3390/d13120620
Chicago/Turabian StyleNogueira, André A., Antonio D. Brescovit, Gilmar Perbiche-Neves, and Eduardo M. Venticinque. 2021. "Beta Diversity along an Elevational Gradient at the Pico da Neblina (Brazil): Is Spider (Arachnida-Araneae) Community Composition Congruent with the Guayana Region Elevational Zonation?" Diversity 13, no. 12: 620. https://doi.org/10.3390/d13120620
APA StyleNogueira, A. A., Brescovit, A. D., Perbiche-Neves, G., & Venticinque, E. M. (2021). Beta Diversity along an Elevational Gradient at the Pico da Neblina (Brazil): Is Spider (Arachnida-Araneae) Community Composition Congruent with the Guayana Region Elevational Zonation? Diversity, 13(12), 620. https://doi.org/10.3390/d13120620







