Abstract
In the south of the Iberian Peninsula, many rivers are intermittent, a state most likely to be exacerbated by climate change, strongly affecting river biota. An additional challenge for native biota in this area is the arrival of new species, frequently aided by humans, and bivalves are particularly at risk. Here we assessed whether the native (Unio delphinus) and invasive (Corbicula fluminea) bivalves differed in habitat use. To address this question, we sampled populations of both species in six isolated permanent pools in the same river during summer in three consecutive years. U. delphinus occurred in all pools, while C. fluminea occurred only in the two most downstream pools. U. delphinus, but not C. fluminea, was found preferentially in patches under riparian vegetation cover. Both species were found in similar sediment types (coarse and fine gravel respectively). Although U. delphinus was present in all pools, recruitment was detected only in 2016, in one pool. We concluded that both species have the potential to compete for space, but a well-developed riparian vegetation cover may provide U. delphinus some advantage against C. fluminea.
1. Introduction
Bivalves have a pivotal importance in freshwater, filtering phytoplankton, bacteria and fine particulate organic matter from the water column and sediment []. With the exception of Invasive or exotic freshwater bivalves, bivalve biodiversity is declining rapidly at a global level [], and most native bivalves are highly endangered [,,]. This decline is caused mainly by habitat degradation and biological invasions [].
Bivalve introductions were fostered by the globalization of economic trade routes, increased watershed connectivity and recreational transport [] and most likely will continue to occur at a greater pace. In many systems the decline of native bivalves occurs concomitantly with the spread of the invasive Asian clam (Corbicula fluminea) or Zebra and Quagga mussels (Dreissena sp.) []. Invasive bivalves may often become dominant (by attaining high density and biomass rapidly [,]), and therefore alter the community structure and function of invaded systems [,,,].
The Oeiras (Guadiana basin, South Portugal; Mediterranean climate) is an intermittent river with superficial flow after winter rains but reduced to persistent isolated pools in the summer. These pools are harsh environments for many species since they may attain high temperature and conductivity and low dissolved oxygen in the summer. According to the IPCC [] global change predictions, an increase in the frequency and intensity of extreme events, such as droughts, is expected for this area. Under these conditions, biota in intermittent Mediterranean rivers may become extremely vulnerable since pools will be at increased risk of drying. Therefore, special conservation efforts may be needed for Mediterranean streams and rivers.
The Oeiras river has important populations of the unionids Unio delphinus, Unio tumidiformis and Anodonta anatina [,,]. However, there are also large populations of the invasive Corbicula fluminea. Corbicula fluminea is a potential threat to U. delphinus in other Iberian rivers [,,,] since C. fluminea was negatively related to native mussel abundance at small spatial scales []. Moreover, another exotic bivalve, Dreissena polymorpha has been recorded in nearby rivers [], and it is likely to reach South Portugal, further affecting native species and increasing the need to adopt urgent conservation measures for native bivalves.
To plan efficient conservation measures to protect native bivalves it is fundamental to obtain data on their current distribution, population structure and the appropriate conditions for these populations to thrive. Therefore, our objectives were: (i) to get insights on distribution of native (Unio delphinus) and invasive (Corbicula fluminea) in a 6 km stretch of the Oeiras river; (ii) to understand the microhabitat features influencing the distribution of these two species, and (iii) to estimate population parameters (densities, population structure). To fulfill these objectives, we sampled specimens and measured environmental conditions in six summer pools on three consecutive years from 2015 to 2017.
2. Materials and Methods
2.1. Environmental Conditions
We sampled six permanent pools in the Oeiras river: Ossada Montante (A), Monte Ossada (B), Monte Bentes (C), Pego do Inferno (D), Pego do Linho (E) and Pego dos Cágados (F) (Figure 1) in three consecutive summers (September) between 2015 and 2017. Each pool was mapped using satellite imagery and in loco measurements, and the extent of submerged area, the total length and width, the maximum depth, the micro habitat’s composition including sediment type (see below), were determined each year.
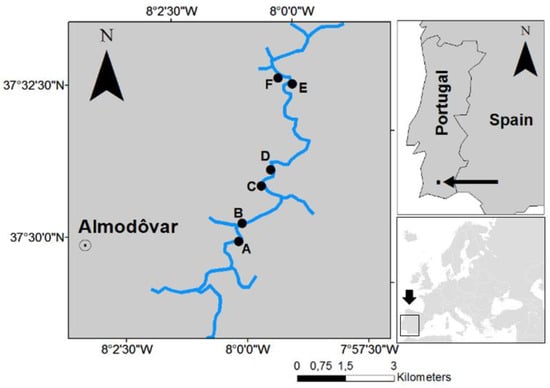
Figure 1.
Location of the six permanent pools in the Oeiras river, flowing north in this section. Ossada Montante (A), Monte Ossada (B), Monte Bentes (C), Pego do Inferno (D), Pego do Linho (E) and Pego dos Cágados (F).
Concomitantly we characterized the pools in terms of riparian vegetation and possible human-impacted riparian features (e.g., presence of artificial structures and livestock access). We also measured turbidity and conductivity.
2.2. Qualitative Sampling
We firstly searched for bivalves with a batiscope (adapted from []) until the total pool area was explored or to a maximum of one hour of searching. Specimens were identified, measured, weighed and placed back at the same sampling location. For each pool, a capture- per unit of effort value (C.P.U.E.) was calculated, and results were expressed as the number of bivalves captured per person, per hour [].
2.3. Quantitative Sampling
After the qualitative sampling, we established perpendicular transects starting at the tip of the submerged area, with a minimum distance of five meters, making sure that all micro-habitats were included. As pools length vary yearly with different submerged areas this procedure was repeated each year. In each transect, we established 0.25 m2 quadrats with one quadrat randomly taken within the first meter of each margin (left and right) and two random quadrats in the middle of each transect to a maximum depth of 1.5 m [,,]. The minimum distance between quadrats was three meters. Bivalves were identified, measured and weighed. We also recorded the water depth, distance to the margins, type of substrate and micro-habitat (see below) within the quadrat. Sediment grain size in each quadrat was classified according to American Society for Testing and Materials (ASTM) [] as boulders (>300 mm), cobblers (75–300 mm), coarse gravel (19–75 mm), fine gravel (4.75–19 mm), sand (0.075–4.75 mm) and silt/clay (<0.075 mm).
Micro-habitats were defined by taking into consideration the sediment type, presence, type and extension of vegetation as well as whether quadrats were sampled under riparian vegetation shade. All native bivalves were left in their location while C. fluminea specimens were removed from the pools. Yearly population estimates were obtained multiplying population density by pool size.
2.4. Data Analysis
When appropriate, environmental data was tested for normality (Kolmogorov–Smirnov normality test) and homogeneity of variances (Levene’s test) []. If normality and homogeneity of variances were not achieved, non-parametric tests were used.
Sediment data was expressed as percentages of cover at the sampling quadrats. A Multiple Correspondence Analysis (MCA) from FactoMineR package in R (Version 3.5.0, R. Core Team, 2018), was used to relate the presence/absence of bivalves and the environmental variables: sediment type, riparian gallery cover (presence or absence), distance to the nearest margin (m) and water depth (m).
We evaluated the correlation between the estimated densities and C.P.U.E. values and environmental variables using Spearman’s rank correlation (SPSS, version 22.0, IBM, Armonk, New York, NY, USA). For C. fluminea the data used corresponded to the two most downstream pools. To assess habitat preferences in terms of sediment types we applied the Ivlev’s electivity index, Ei (Ivlev, 1961) adapted for habitat preferences (e.g., [,]), given by:
where ri is the proportion of individuals in a habitat with a specific sediment type (i), and Pi is the relative abundance of that habitat in the study area. Values of Ei = −1 indicate avoidance, Ei = 0 represents non-selective use of habitat type, and Ei = 1 indicates exclusive use of habitat type.
Population structure (sum of all pools) were compared using a G-log likelihood ratio (SPSS, version 22.0) to test for differences among years (using the post hoc z test). Six size classes were considered for U. delphinus: <30 mm, [30–40[ mm, [40–50[ mm, [50–60[ mm, [60–70[ mm and ≥70 mm. Juveniles were grouped in the first size class according to Smith []. Corbicula fluminea were also grouped in five size classes: <10 mm, [10–20[ mm, [20–30[ mm, [30–40[ mm and ≥50 mm.
3. Results
3.1. Sites Description
The six pools differed in their physical conditions. Some locations had a well sustained riparian vegetation cover, mainly with ash and willow trees (Fraxinus angustifolia and Salix atrocinerea) and stable margins (e.g., pool C; Table 1), while others had discontinuous riparian corridors, unstable banks, some non-native tree species, modified river channels or cattle presence. Water conductivity (>600 µs cm−1), and turbidity (5.69–57.9 NTU’s) were high. Conductivity was high in all pools as sampling occurred in late summer with low water availability and varied between years due to shifts in water volume and depth. Turbidity varied because intrinsic pool characteristics were also different. Some pools are shallower, some pools have more fish fauna, some pools might experience increased anthropogenic pressure, all are variables that might influence turbidity differently.

Table 1.
Environmental conditions and population parameters in six pools (A to F) in the Oeiras river in 2015–2017.
3.2. Bivalve Abundance and Distribution
Four bivalve species occurred in the Oeiras river. The native U. delphinus and the invasive C. fluminea were the most abundant, while Anodonta anatina and Unio tumidiformis were scarcer. Unio delphinus catch per unit effort (C.P.U.E.) ranged from zero (pools B and E in 2017) to 50.5 per researcher h−1 (pool A in 2017; Table 2). Unio delphinus densities attained up to 6.0 individuals per m2 (pool D in 2017) while maximum densities of C. fluminea were estimated in 12.7 individuals per m2 (pool E; Table 2, Figure 2). For C. fluminea C.P.U.E. and estimated densities were related (Spearman’s rank correlation: n = 6; rs = −0.829; p = 0.042) unlike for U. delphinus (n = 18; rs = −0.273; p = 0.274).

Table 2.
Densities (individuals per m2) and catch per unit of effort (C.P.U.E., number of bivalves collected per researcher per hour) of U. delphinus and C. fluminea in six pool sites in the Oeiras river in three years.
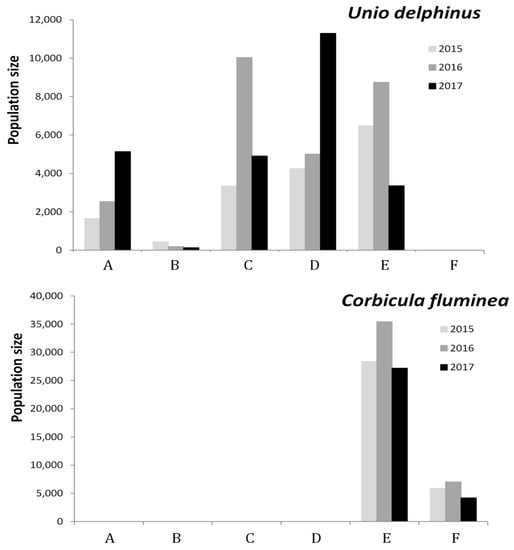
Figure 2.
Estimated population size (n) of U. delphinus (top) and C. fluminea (below) in six pool sites (A–F) along the Oeiras river.
C. fluminea and U. delphinus density was not correlated (n = 170; rs = 0.110; p = 0.154). In general U. delphinus density tended to increase from 2015 to 2017 in most pools (except B and E), while C. fluminea density increased only in pool E within the same period.
At patch scale, the presence of bivalves was explained by the distance to the margins and depth (more individuals of both species in the shallow margins) and fine gravel (C. fluminea) or coarse gravel substrates (U. delphinus) (Multiple correspondence analysis; Table 3 and Figure 3). These results were partially consistent with Spearman’s rank correlation: Densities of U. delphinus were correlated with the presence of fine gravel (rs = 0.126, p < 0.01) coarse gravel (rs = 0.160, p < 0.001) and riparian vegetation cover (rs = 0.153; p < 0.01). Densities of C. fluminea were positively correlated with fine gravel (n = 170; rs = 0.260; p < 0.001); sand (n = 170; rs = 0.170; p = 0.027) and silt/clay (n = 170; rs = 0.210; p < 0.01) and negatively correlated with depth (n = 170; rs = −0.207; p = 0.007) and rock presence (n = 170; rs = −0.302; p < 0.001). Although significant, all these correlations were low.

Table 3.
Statistical parameters from multiple correspondence analysis on factors explaining the distribution of U. delphinus and C. fluminea in six pools.
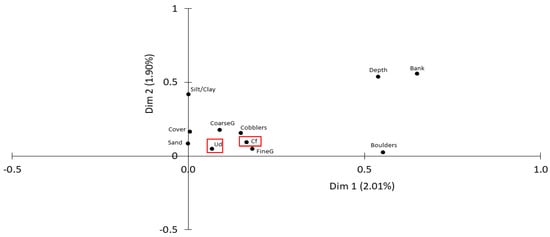
Figure 3.
Multiple Correspondence Analysis (MCA) plot analysis for independent variables. Zoomed plot to 0.0 coordinates. Dim 1—Dimension 1 and Dim 2—Dimension 2. Dimensions represent categories with the highest contribution and the highest fraction of the total variance in the data. Codes for qualitative variables (presence/absence): Silt/Clay; Sand; FineG—fine gravel; CoarseG—coarse gravel; Cobblers; Boulders; Cover—Riparian gallery cover; Ud—U. delphinus species presence; Cf—C. fluminea species presence. Variable codes for quantitative variables (continuous): Bank—Distance to near margin (m); Depth—Water column depth (m).
The previous findings were also mostly consistent with the Ivlev’s electivity index results regarding the native Unionidae (Figure 4; Table A1 of Appendix). Unio delphinus preferred coarse (E = 0.47) and fine gravel (E = 0.34) and avoided sandy substrates (E = −0.28). Results are similar for C. fluminea, which was found preferentially in fine (E = 0.53) and coarse gravel (E = 0.49), avoiding boulders (E = −0.22) and sand (E = −0.16; an opposite result from Spearman rank correlations).
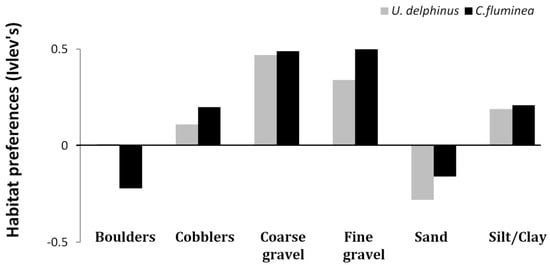
Figure 4.
Habitat preferences of U. delphinus and C. fluminea according to Ivlev’s electivity index. Ei is scaled so that Ei < 0 indicates avoidance and Ei > 0 indicates preference.
The dominant size classes of U. delphinus were [40–45[ mm in 2017, [60–65[ mm in 2016 and [55–60[ mm in 2015, and for C. fluminea [20–25[ mm both in 2015 and 2017 and [30–35[ mm in 2016. However, for both species, population structure was different among years (G-log likelihood ratio: G = 198, p < 0.01, d.f. = 10, n = 762 for U. delphinus and G = 201, p < 0.01, d.f. = 10, n = 750 for C. fluminea; Table 4, Figure 5). In 2016 and 2017 there was an increase of U. delphinus smaller size classes (especially in the classes >30 mm and [30–40[ mm (in 2017) (Figure 5). A similar pattern was observed for C. fluminea in 2017 (size classes >10 mm and [10–20[ mm).

Table 4.
Results from the post hoc z test after the G likelihood ratio was performed comparing size structure for both bivalve species. Same letters from a, b and c denote a subset of Year categories whose column proportions do not differ significantly from each other at the 0.05 level.
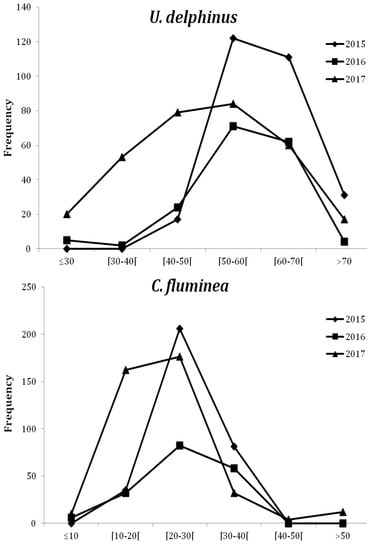
Figure 5.
Size frequency distributions (according to the different size classes) of U. delphinus and C. fluminea from 2015 to 2017 (pooled data from six pools).
4. Discussion
The native U. delphinus and the introduced C. fluminea, when co-occurring, they roughly coexist in the same substrate types, preferring coarse and fine gravel, respectively. Nevertheless, U. delphinus was more abundant in locations under riparian tree cover, unlike C fluminea. The importance of vegetation for U. delphinus is unclear, but it may be related to protection against high temperatures caused by direct sunlight. Other authors reported U. delphinus burrowing in banks between tree roots in hydraulically more stable locations []. Related species, such as U. tumidiformis, U. mancus, and U. ravoisieri seem to have the same preference for river locations shaded by riparian vegetation [,].
Densities of U. delphinus were higher than the reported for the northern Portuguese rivers Tua and Sabor (0.015–0.121 ind/m2; []), although sampling techniques differed, and those rivers were permanent. In the Oeiras river, while U. delphinus occurred in all the sampled pools, C. fluminea was only present in the two most downstream pools. It is possible that C. fluminea had fewer capabilities to expand upstream due to the river intermittency despite studies suggesting C. fluminea can move upstream up to 2.4 km per year []. In our study site the closest pool is approximately 4 km upstream (with the section in between pools being dry most of the year). Native bivalves are very adapted to summer conditions, unlike C. fluminea, which is known to be sensitive to summer environmental stress, suffering mass mortality events [] making upstream dispersal more difficult. Some studies also suggest that the success of C. fluminea invasion decreases with increasing abundance of adult native mussels, probably due to lack of space for the invaders, physical displacement by actively burrowing mussels and locally reduced food and oxygen resources []. C. fluminea is a hermaphroditic species. Larvae are incubated until being released as juveniles into the water column, settle and bury []. Before settling they can be dragged through currents or move attached to other organisms []. Unlike native mussels this invasive species does not need a fish host to successfully reproduce, which is an enormous competitive advantage. On the contrary, glochidium larvae of freshwater Unionidae need to find suitable fish hosts to attach themselves to and metamorphose into free-living juveniles [].
Our results were consistent with previous studies reporting C. fluminea preference for sediments with large organic matter content (lower grain sized sediments) such as sand mixed with silt and clay []. Organic material in sediments may be important for C. fluminea as this species is known for high filtration and growth/turnover rates, exacerbated during summer conditions [,]. Nonetheless in our pools sand was very uncommon, only detected in 2016. Therefore, the Ivlev’s index avoidance and the association observed in Spearman’s rank correlation have to be interpreted very carefully. Karatayev and co-workers [], found a correlation between C. fluminea occurrence and of other three unionids. The same study reported that both were mostly found in coarse detritus (as U. delphinus in our study) and clay substrates (C. fluminea), similar to this study, due to the higher organic matter content and at the same depth.
Corbicula fluminea presence can exacerbate the pressure on U. delphinus by competing for food [,] and reducing available habitats for juvenile unionids. Suspension and deposit feeding negatively impact unionid recruitment, and the ingestion of unionids sperm, glochidia and juveniles may contribute to population declines [,]. Also, the introduction of new parasites and diseases [], and increased ammonia toxicity as a result of massive C. fluminea die-offs, especially in the summer, may also increase native bivalves’ mortality [].
Changes in environmental conditions due to global warming may enable the colonization by new fish, which may not be suitable hosts for some native bivalve’s glochidia []. Additionally, invasive invertebrate species may predate or compete with U. delphinus [,,,,,]. Several invasive predatory species are already abundant in this river, such as the Pumpkinseed sunfish (Lepomis gibbosus), the Chameleon cichlid (Australoheros facetus) and the red swamp crayfish (Procambarus clarkii), which may partially explain the low number of juveniles detected during this three-year survey.
Finally, as a consequence of global warming, and water deviation for irrigation and livestock, a reduction in water availability is expected in pools in the coming years [] further increasing the pressure on U. delphinus.
5. Conclusions
In conclusion, the knowledge about U. delphinus preference for locations under riparian vegetation, with coarse and fine gravel, may aid conservation efforts. In this context, improvement of riparian vegetation should lead to better-quality habitat, potentially decreasing suitability for invasive species such as C. fluminea. Mitigation or protection measures should start by protecting or increasing riparian areas, enabling protection from high summer temperatures, and maintaining areas with coarse and fine gravel. Since our results suggest that native and invasive species prefer similar sediment types and may compete for space, extreme caution should be taken not to allow C. fluminea expansion upstream.
Author Contributions
Conceptualization, M.G. (Mafalda Gama), F.B. and P.A.; data curation, M.G. (Mafalda Gama) and F.B.; formal analysis, M.G. (Mafalda Gama), F.B. and P.A.; funding acquisition, H.G. and P.A.; investigation, P.A.; methodology, P.A.; resources, C.M., H.G. and P.A.; supervision, H.G., P.A. and M.G. (Manuel Graça); validation, C.M., H.G., P.A. and M.G. (Manuel Graça); writing—original draft, M.G. (Mafalda Gama) and F.B.; writing—review and editing, H.G., P.A. and M.G. (Manuel Graça). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SOMINCOR—Sociedade Mineira de Neves Corvo, project MUSSELFLOW (PTDC/BIA-EVL/29199/2017) and MARE (UID/MAR/04292/2019).
Acknowledgments
We thank SOMINCOR—Sociedade Mineira de Neves Corvo for financing this research. Data analysis was performed within the scope of the project MUSSELFLOW (PTDC/BIA-EVL/29199/2017). F. Banha and M. Gama were supported by a Post-doc grant from the University of Évora within the scope of MARE (UID/MAR/04292/2019). We thank Godinho and Alfredo for their valuable field support, suggestions and patience when field work lasted forever.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Percentage of occurrence of U. delphinus and C. fluminea in sediment types and percentage of occurrence of each sediment type (yearly).
Table A1.
Percentage of occurrence of U. delphinus and C. fluminea in sediment types and percentage of occurrence of each sediment type (yearly).
| Year | Species | Sediment Type | |||||
|---|---|---|---|---|---|---|---|
| Boulders | Cobblers | Coarse Gravel | Fine Gravel | Sand | Silt/Clay | ||
| 2015 | U. delphinus | 8 | 15 | 42 | 25 | 1 | 8 |
| C. fluminea | 4 | 16 | 41 | 8 | 0 | 31 | |
| 2016 | U. delphinus | 29 | 16 | 24 | 10 | 4 | 18 |
| C. fluminea | 13 | 22 | 30 | 22 | 8 | 5 | |
| 2017 | U. delphinus | 23 | 11 | 11 | 43 | 0 | 12 |
| C. fluminea | 19 | 13 | 10 | 44 | 0 | 13 | |
| % of occurrence | |||||||
| 2015 | 29 | 14 | 7 | 0 | 0 | 10 | |
| 2016 | 13 | 10 | 8 | 7 | 1 | 10 | |
| 2017 | 14 | 10 | 8 | 12 | 0 | 6 | |
References
- Vaughn, C.C.; Hakenkamp, C.C. The functional role of burrowing bivalves in freshwater ecosystems. Freshw. Biol. 2001, 46, 1431–1446. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Bogan, A.E. Global diversity of freshwater mussels (Mollusca, Bivalvia) in freshwater. Hydrobiol 2008, 595, 139–147. [Google Scholar] [CrossRef]
- Osterling, M.E.; Arvidsson, B.L.; Greenberg, L.A. Habitat degradation and the decline of the threatened mussel Margaritifera margaritifera: Influence of turbidity and sedimentation on the mussel and its host. J. Appl. Ecol. 2010, 47, 759–768. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Burlakova, L.E.; Karatayev, A.; Mehler, K.; Seddon, M.; Sousa, R. Conservation of freshwater bivalves at the global scale: Diversity, threats and research needs. Hydrobiologia 2018, 810, 1–14. [Google Scholar] [CrossRef]
- Lydeard, C.; Cowie, R.H.; Ponder, W.F.; Bogan, A.E.; Bouchet, P.; Clark, S.A.; Cummings, K.S.; Frest, T.J.; Gargominy, O.; Herbert, D.G.; et al. The global decline of nonmarine mollusks. BioScience 2004, 54, 321–330. [Google Scholar] [CrossRef]
- Aldridge, D.C.; Elliot, P.; Moggridge, G.D. The recent and rapid spread of the zebra mussel (Dreissena polymorpha) in Great Britain. Biol. Conserv. 2004, 119, 253–261. [Google Scholar] [CrossRef]
- Strayer, D.L.; Malcom, H.M. Effects of zebra mussels (Dreissena polymorpha) on native bivalves: The beginning of the end or the end of the beginning? J. N. Am. Benthol. Soc. 2007, 26, 111–122. [Google Scholar] [CrossRef]
- Sousa, R.; Antunes, C.; Guilhermino, L. Ecology of the invasive Asian clam Corbicula fluminea (Müller, 1774) in aquatic ecosystems: An overview. Int. J. Lim. 2008, 44, 85–94. [Google Scholar] [CrossRef]
- Sousa, R.; Nogueira, A.J.A.; Gaspar, M.B.; Antunes, C.; Guilhermino, L. Growth and extremely high production of the nonindigenous invasive species Corbicula fluminea (Müller, 1774): Possible implications for ecosystem functioning. Estuar. Coast. Shelf Sci. 2008, 80, 289–295. [Google Scholar] [CrossRef]
- Sousa, R.; Gutiérrez, J.L.; Aldridge, D.C. Non-indigenous invasive bivalves as ecosystem engineers. Biol. Invasions 2009, 11, 2367–2385. [Google Scholar] [CrossRef]
- Sousa, R.; Varandas, S.; Cortes, R.; Teixeira, A.; Lopes-Lima, M.; Machado, J.; Guilhermino, L. Massive die-offs of freshwater bivalves as resource pulses. Ann. Limnol.-Int. J. Lim. 2012, 48, 105–112. [Google Scholar] [CrossRef]
- Ilarri, M.; Sousa, R. Corbicula fluminea Müller (Asian clam). In A Handbook of Global Freshwater Invasive Species; Francis, R.A., Ed.; Routledge: London, UK, 2012; Chapter 15; p. 484. [Google Scholar]
- Anastácio, P.M.; Ribeiro, F.; Capinha, C.; Banha, F.; Gama, M.; Filipe, A.F.; Rebelo, R.; Sousa, R. Non-native freshwater fauna in Portugal: A review. Sci. Total Environ. 2019, 650, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for policymakers. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1–32. [Google Scholar]
- Araujo, R.; Toledo, C.; Machordom, A. Redescription of Unio gibbus Spengler, 1793, a west palaearctic freshwater mussel with hookless glochidia. Malacologia 2009, 51, 131–141. [Google Scholar] [CrossRef]
- Reis, J.; Machordom, A.; Araujo, R. Morphological and molecular diversity of Unionidae (Mollusca, Bivalvia) from Portugal. Graellsia 2013, 69, 17–36. [Google Scholar]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, J.; Bespalaya, Y.; Bódis, E.; Burlakova, L.; Van Damme, D.; et al. Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biol. Rev. 2017, 92, 572–607. [Google Scholar] [CrossRef] [PubMed]
- Novais, A.; Dias, E.; Sousa, R. Inter- and intraspecific variation of carbon and nitrogen stable isotope ratios in freshwater bivalves. Hydrobiologia 2016, 765, 149–158. [Google Scholar] [CrossRef]
- Modesto, V.; Castro, P.; Lopes-Lima, M.; Antunes, C.; Ilarri, M.; Sousa, R. Potential impacts of the invasive species Corbicula fluminea on the survival of glochidia. Sci. Total Environ. 2019, 673, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, C.C.; Spooner, D.E. Scale-dependent associations between native freshwater mussels and invasive Corbicula. Hydrobiologia 2006, 568, 331–339. [Google Scholar] [CrossRef]
- Rajagopal, S.; Pollux, B.J.A.; Peters, J.L.; Cremers, G.; Moon-van der Staay, S.Y.; van Alen, T.; Eygensteyn, J.; van Hoek, A.; Palau, A.; bij de Vaate, A.; et al. Origin of Spanish invasion by the zebra mussel, Dreissena polymorpha (Pallas, 1771) revealed by amplified fragment length polymorphism (AFLP) fingerprinting. Biol. Invasions 2009, 11, 2147–2159. [Google Scholar] [CrossRef]
- McRae, S.E.; Allan, J.D.; Burch, J.B. Reach and catchment scale determinants of the distribution of freshwater mussels (Bivalvia: Unionidae) in south-eastern Michigan, USA. Freshwat. Biol. 2004, 49, 127–142. [Google Scholar] [CrossRef]
- Obermeyer, B.K. Comparison of Quadrats Versus Timed Snorkel Searches for Assessing Freshwater Mussels. Am. Midl. Nat. 1998, 155, 307–320. [Google Scholar] [CrossRef]
- Schloesser, D.W.; Metcalfe-Smith, J.L.; Kovalak, W.P.; Longton, G.D.; Smithee, R.D. Extirpation of Freshwater Mussels (Bivalvia: Unionidae) Following the Invasion of Dreissenid Mussels in an Interconnecting River of the Laurentian Great Lakes. Am. Midl. Nat. 2006, 155, 307–320.42. [Google Scholar] [CrossRef]
- ASTM D2487—1966 Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System); Reapproved; ASTM International: West Conshohocken, PA, USA, 2006.
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1999; p. 663. [Google Scholar]
- Anastácio, P.M.; Banha, F.; Capinha, C.; Bernardo, J.M.; Costa, A.M.; Teixeira, A.; Bruxelas, S. Indicators of movement and space use for two co-occurring invasive crayfish species. Ecol. Indic 2015, 53, 171–181. [Google Scholar] [CrossRef]
- Loughman, Z.J.; Skalican, K.T.; Taylor, N.D. Habitat selection and movement of Cambarus chasmodactylus (Decapoda: Cambaridae) assessed via radio telemetry. Freshwat. Sci. 2013, 32, 1288–1297. [Google Scholar] [CrossRef]
- Smith, D.G. Sistematics and distribution of the recent Margaritiferidae. In Ecology and Evolution of the Freshwater Mussels Unionoida; Springer: Berlin/Heidelberg, Germany, 2001; pp. 33–49. [Google Scholar]
- Araujo, R.; Unio tumidiformis. The IUCN Red List of Threatened Species. 2011. Available online: http://dx.doi.org/10.2305/IUCN.UK.20112.RLTS.T171935A6810869.en (accessed on 10 November 2011).
- Rovira, Q.; Campos, M.; Feo-Quer, C.; Camós, I.; Martí, J.; Angelats, I.; Cros, C.; Casadevall, R.; Dalmau, G.; Cruset, E.; et al. Habitat preferences of Unio mancus and U. ravoisieri in northeast of Catalonia. In Proceedings of the 2nd International Seminar on the Rearing of Unionoid Mussels, Clervaux, Luxembourg, 24 November–27 November 2015. [Google Scholar]
- Patrício, C.I.M. Contribuição para o estudo da bioecologia dos mexilhões de água doce (Unionoida) do Nordeste de Portugal. Master Thesis, University of Oporto, Oporto, Portugal, 2013. [Google Scholar]
- Beran, L. Spreading expansion of Corbicula fluminea (Mollusca: Bivalvia) in the Czech Republic. Heldia 2006, 6, 187–192. [Google Scholar]
- Vohmann, A.; Borcherding, J.; Kureck, A.; bij de Vaate, A.; Arndt, A.; Weitere, M. Strong body mass decrease of the invasive clam Corbicula fluminea during summer. Biol. Invasions 2010, 12, 53–64. [Google Scholar] [CrossRef]
- Way, C.; Hornbach, D.; Miller-Way, C.; Payne, B.; Miller, A.C. Dynamics of filter feeding in Corbicula fluminea (Bivalvia: Corbiculidae). Can. J. Zool. 2011, 68, 115–120. [Google Scholar] [CrossRef]
- Karatayev, A.Y.; Burlakova, L.E.; Kesterson, T.; Padilla, D.K. Dominance of the Asiatic clam, Corbicula fluminea (Müller) in the benthic community of a reservoir. J. Shellfish Res. 2003, 22, 487–493. [Google Scholar]
- Ferreira-Rodriguez, N.; Pardo, I.; Sousa, R. Negative effects of Corbicula fluminea over native freshwater mussels. Hydrobiology 2018, 810, 85–95. [Google Scholar] [CrossRef]
- Graczyk, T.K.; Conn, D.B.; Marcogliese, D.J.; Graczyk, H.; de Lafontaine, Y. Accumulation of human waterborne parasites by zebra mussels (Dreissena polymorpha) and Asian freshwater clams (Corbicula fluminea). Parasitol. Res. 2003, 89, 107–112. [Google Scholar] [PubMed]
- Cherry, D.S.; Scheller, J.L.; Cooper, N.L.; Bidwell, J.R. Potential effects of Asian clam (Corbicula fluminea) die-offs on native freshwater mussels (Unionidae) I: Water-column ammonia levels and ammonia toxicity. J. N. Am. Benthol. Soc. 2005, 24, 369–380. [Google Scholar] [CrossRef]
- Taeubert, A.; Martinez, J.E.; Gum, M.P.B.; Geist, J. The relationship between endangered thick-shelled river mussel (Unio crassus) and its host fishes. Biol. Conserv. 2012, 155, 94–103. [Google Scholar] [CrossRef]
- Correia, A.M.; Bandeira, N.; Anastácio, P.M. Predator-prey interactions of Procambarus clarkii with aquatic macroinvertebrates in single and multiple prey systems. Acta Oecologica 2005, 28, 337–343. [Google Scholar] [CrossRef]
- Reis, J.; Araújo, R. Life history of the freshwater mussel Unio tumidiformis (Bivalvia: Unionidae) in a temporary Mediterranean-type stream. Invert. Biol. 2016, 135, 31–45. [Google Scholar] [CrossRef]
- Weiss, S.; Marić, S.; Snoj, A. Regional structure despite limited mtDNA sequence diversity found in the endangered Huchen, Hucho hucho (Linneaus, 1758). Hydrobiologia 2010, 658, 103–110. [Google Scholar] [CrossRef]
- Meira, A.; Lopes-Lima, M.; Varandas, S.; Teixeira, A.; Arenas, F.; Sousa, R. Invasive crayfishes as a threat to freshwater bivalves: Interspecific differences and conservation implications. Sci. Total Environ. 2018, 649, 938–948. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).