Abstract
Catastrophic wildfire is increasingly common in forests of the western United States because climate change is increasing ambient temperatures and periods of drought. In 2011, the Las Conchas wildfire burned in the Santa Fe National Forest of New Mexico, including portions of ponderosa pine and mixed-conifer forests, and grasslands in the Valles Caldera National Preserve, a large, high-elevation volcanic caldera. Following the fire, Caldera staff began monitoring abiotic, plant, and animal responses. In this study, ground-dwelling arachnids were collected in pitfall traps in burned and unburned habitats from 2011–2015. Permutational multivariate analysis of variance (PERMANOVA) mostly at the genus level with some higher taxon levels showed significant fire, year, and interaction effects. Abundance was at or near unburned levels by 2014, but species composition changed in burned areas. Pardosa and Haplodrassus were dominant genera across habitats. Linyphiids were strong indicators of unburned sites. Harvestmen were among the dominant species in the forest habitats, and erythraeid mites were abundant in the burned ponderosa pine forest and the grassland. Years were not significantly autocorrelated, unsurprising given the interannual variation in precipitation in this generally arid region. Although fire is a common feature of these habitats, future fires may be outside of historical patterns, preventing spider communities from re-establishing fully.
1. Introduction
In 2011, a drought year, the largest wildfire (63,371 ha) in New Mexico, USA up to that time burned in the Santa Fe National Forest, from 26 June to 01 August [1]. The catastrophic Las Conchas fire was one more example of increasing wildfires in the western region of the U.S., as temperatures and drought periods have been increasing [2,3,4]. In the southern Rocky Mountains, several climate change factors are influencing the frequency and severity of fires in this region: trends toward larger fires, warmer maximum air temperatures between September and November, less precipitation between June and November, increased drought severity [2]. Results from a study of the years 1973–2012 in the western United States suggested trends of overall lengthening of the fire season from 37–117 days and mean burn time from 5 to 37 days [4]. Early snowmelt from the mountains also increased the likelihood of large summer wildfires [4]. In the Colorado Front Range of the Rocky Mountains, Rother and Veblen [3] looked at stands of ponderosa pine from past fires to estimate what future climate changes could mean for tree establishment. They found that the severity of the burn was less important than the extent of the fire, making the distance to seed sources for new trees longer. More vulnerable stands of ponderosa pine were at their lower elevation level or were found on south-facing slopes. The effects of fires in forests are complex; season, tree species, and local habitat features (such as slope or aspect) are all important in affecting the outcome of wildfire.
This study took place in the Jemez Mountains at the Valles Caldera National Preserve, which is within the National Forest and which experienced stand-replacing burns in the ponderosa pine forest, moderate to severe burns in the mixed-conifer forest, and less severe burns in mountain grassland in 2011. At the Caldera, monitoring of abiotic factors and biotic responses began as soon as the fire was contained, through the Collaborative Forest Landscape Restoration Project (CFLRP) for the southwest Jemez Mountains. The CFLRP had been established in 2010 to restore forest resilience to wildfire and other disturbances and improve conditions for wildlife, watersheds, and vegetation [5]. Ground-dwelling spiders, harvestmen, and selected mite groups were among the animal taxa chosen to be monitored from 2011 to 2015. They had not previously been surveyed at the Caldera, so this monitoring effort also provided the opportunity to add to the known diversity and distribution of these groups in a region that is relatively understudied (but see [6,7,8]).
These three arachnid taxa are widespread generalist predators, an important arthropod trophic group, with over 3800 species of spiders alone in North America, and are also a food source for larger animals, particularly birds and reptiles [9,10]. Because they are not herbivores, their response to post-fire changes in vegetation is based less on plant species and more on plant structure and litter amount [9,11]. They are frequent early colonizers to disturbed areas, whether walking in from nearby areas or through dispersal by ballooning [9,10].
There are numerous papers on spider responses to prescribed fires, with and without the combined treatment of timber thinning [12,13,14], an increasing number on wildfire [6,15,16,17], but understandably fewer with information on pre-fire conditions for the target species [18]. In different forests, spider abundance was either not affected by prescribed burns or returned to pre-fire levels within 3 years: Oregon, USA [12], in Swedish boreal forest [14], and in the juniper-poplar steppe in Hungary [17]. Wildfire sometimes more strongly affected spider communities. In the forest in Finland [15], spider assemblages were clearly different 3 years post-fire. In Canada spruce forest, a comparison of clear-cutting and wildfire [16] showed that spider responses to the two treatments were different and that wildfire (that is not catastrophic) could leave a more heterogeneous litter layer and thus had a less pronounced habitat effect than clear-cutting. In an Oregon, USA, grass/shrub steppe [18], study sites were in place before a wildfire occurred. There was no difference among sites before the fire, but afterward community composition changed, although, at the broader scale, richness and abundance did not. One study from Colorado, USA [6] looked at the effects of a 2002 catastrophic wildfire in pinyon-juniper woodland. Five to six years later, vegetation cover of grasses and annual plants had increased, litter decreased, and bare ground increased, compared with nearby old-growth stands. None of the 32 spider species was in the top 7 indicators of burned or unburned sites, but abundance and richness had not returned to levels found in old-growth controls. Four spider species were positively associated with the burned areas and 16 were negatively associated with the burned areas. Given the variability in extent and severity of wildfire, it is difficult to compare it to prescribed fire; therefore, additional studies from wildfire are needed. This is especially important for those areas that may be forested but situated in largely arid regions, as is the case for much of the southwestern United States.
For the arachnids in the two forest habitats and grassland that were burned in the Caldera, the main questions of interest for the CFLRP program were: (1) Did the Las Conchas fire have an effect on arachnid activity abundance and species composition from 2011 to 2015? If so, (2) By the end of 2015 were burned areas still different from the unburned areas in activity abundance, and species composition? These questions were important to land managers at the Caldera in helping them decide how long monitoring efforts should be. Wildfires in forests and grasslands in this region have been common historically [2,3,19], but currently are increasing in severity and frequency [20], so learning more about the ability of animal species resistance and resilience to them is necessary. Spiders in general are in the center of trophic food webs, affecting populations of soil mesofauna, insect larvae, and flying insects, and in turn being affected by other arthropod taxa and vertebrate animals.
2. Materials and Methods
The Valles Caldera National Preserve is located in the Jemez Mountains in north-central New Mexico, USA (35°55′12” N,106°31′15.6” W), part of the Rio Grande rift valley (Figure 1). A volcanic eruption about 1.25 Mya formed the caldera, which is now about 22 km in diameter [21] and ranges in elevation from about 2550 m to 3230 m at the highest point, Redondo Peak. The main vegetation types are forests of ponderosa pine, mixed-conifer, and spruce-fir, as well as grassy high-elevation meadows. For this study, vegetation types included ponderosa pine forest (PP), mixed-conifer forest (MC), and mountain valley grassland (MV), with elevations between 2590 m and 2750 m. Pinus ponderosa dominated the PP habitat, and Pseudotsuga menziesii (Douglas-fir) and Picea englemannii (Englemann spruce) were dominant at MC. At MV, C3 grasses, such as Festuca spp., Danthonia parryi, and Poa pratensis, along with the C4 grass Muhlenbergia montana, dominated [22].
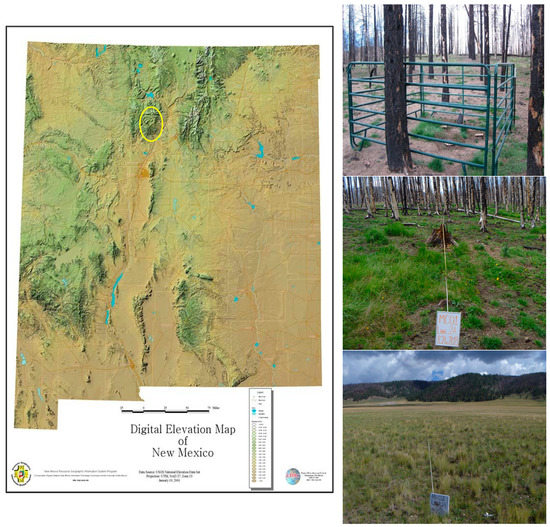
Figure 1.
Map of New Mexico. Yellow oval outlines the Valles Caldera National Preserve. The three habitats affected by the Las Conchas fire: Ponderosa pine forest, top; Mixed-conifer forest, middle; Mountain Valley Grassland, bottom. The ponderosa pine image shows the pitfall and fencing arrangement.
July maximum air temperatures ranged from 30 to 33 °C and January minimum air temperatures from −23 to −32 °C. Soil temperatures were buffered somewhat for summer temperatures (23–29 °C) and more strongly for winter minimums (−4 to −6 °C). Rainfall occurred mostly during the summer (“monsoon”) months [23]. Vegetation cover and exposed litter were regularly monitored by Caldera staff (Figure 2). In New Mexico, most rainfall occurs primarily during July–September (summer or monsoon pattern). At the Caldera from 2011–2015, rainfall ranged from 111 to 160 mm per month in the summer, while amounts ranged from 0 to 50 mm per month at other times of the year. Drought conditions were described by the Palmer Drought Severity Index, which measures departures from normal years in temperature and precipitation. Positive values are wetter years, negative values are dry or drought years. The year 2011 was in moderate to extreme drought (index values of −2.00 to −4.00), 2012 was in severe to extreme drought (−3.00 to −4.00), 2013 was in the mid-range (neither drought nor wet) to severe drought (−1.99 to −3.00), 2014 was in the mid-range to moderate drought (−1.99 to −2.00), and 2015 was in mid-range to very moist conditions (−1.99 to +3.99) [24].
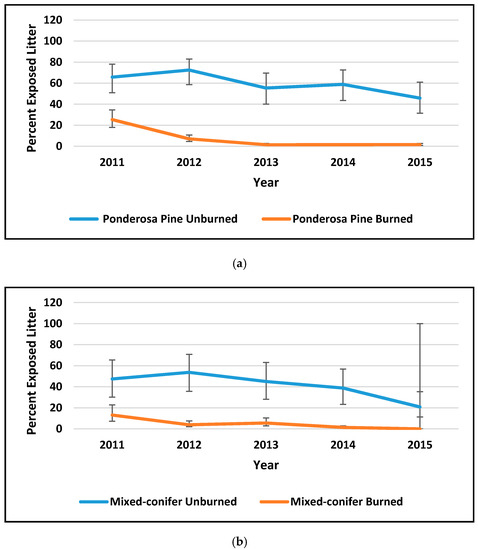
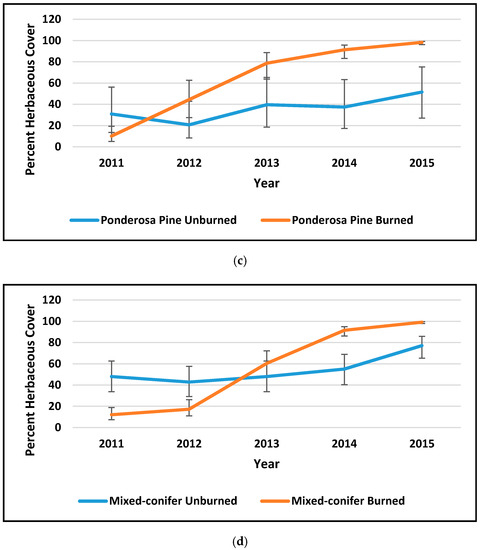
Figure 2.
Percent cover by year of exposed litter (a,b) and percent herbaceous cover (c,d) in ponderosa pine forest (PP) and mixed-conifer forest (MC) at the Valles Caldera (data courtesy Caldera staff). Error bars are 95% confidence intervals. Vegetation cover in Mountain Valley Grassland was always between 90% and 100%; see Suazo et al. (2018) for more detail.
Within each habitat, 12 sites were chosen, 6 in burned areas and 6 in unburned areas (Figure 3). Sites were chosen away from habitat edges and were at least 300–500 m apart, farther if possible, but burn patterns influenced site placement. Three pitfall traps with cups 9 cm in diameter × 12.5 cm in height were placed 1 m apart at each site, each trap about 2/3 filled with propylene glycol as the sample preservative and protected from elk disturbance by fencing (Figure 1). Traps were emptied every two to four weeks between May and early November, except in 2011, when trapping began after the fire, in July 2011. This produced 8–9 sampling periods per year in 2012–2015. In 2013, the Thompson Ridge fire burned 3 of the control sites each at PP and MC, which were removed from the analysis, resulting in an uneven sample set for 2013–2015; the MV sites were not affected by the Thompson Ridge fire.
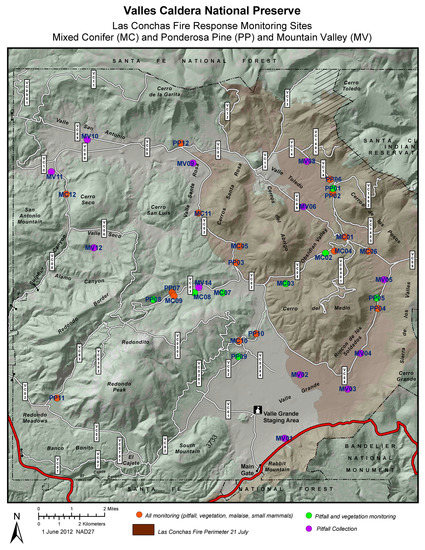
Figure 3.
Map of the extent of the Las Conchas wildfire study sites for arthropod pitfall traps at the Valles Caldera National Preserve, and the location of pitfall trap sites. Burned areas are to the right; unburned areas are to the left on the map.
Arachnids were separated from other arthropods, identified and counted in the lab at the University of New Mexico Museum of Southwestern Biology. More than 1700 representative specimens, in 70% ethanol, were deposited with the Museum; specimen collection information and catalog numbers were entered into the SCAN (Symbiota Collections of Arthropods Network) arthropod database.
Spiders, harvestmen, and selected mite groups and their abundances were included for analysis (Table A1, Table A2 and Table A3); however, if there were fewer than 10 individuals within a habitat and taxon over the course of the study, they were excluded. Activity abundance as used here is a combination of actual abundance in the habitat and a measure of activity since more active species are likely to be captured more often and so appear more abundant. Pitfall traps are designed to capture solitary, wandering arthropods [25]. Spiders are often rare, analysis at the species level results in matrices with many zeroes, therefore, I lumped species by genus for analysis. In cases where there was only one species for a given genus, the analysis is at the species level. This also allowed me to include juvenile stages that could be identified to genus, even if not to species. I excluded juveniles that could be identified only to family. If only one species was present, the juvenile stages were included in the count for the adults (e.g., the gnaphosid Drassodes neglectus). Whether or not to include juvenile stages is a long-standing issue with arachnids [26]. While they are not the same as adults, they are an important part of the spider biomass present at almost all collecting times, and they are the stages that readily disperse [9] and would recolonize burned areas. The two harvestman species (Togwoteeus biceps and Leiobunum sp.) and their immatures were included in the analyses. Mites that were included were divided into the following higher-taxonomic groups: Mesostigmata; Oribatida (the moss mites or beetle mites); and Trombidiformes, including Anystidae, Bdellidae, and a few undetermined groups. The Erythraeidae were most common. They are predators in adult and nymphal stages but as larvae are parasitic on other arthropods [27].
In PRIMER [28], species accumulation curves for the observed number of species, using Chao2 and Jackknife 2 estimators, showed sampling effort that was underestimating richness initially but approximated the estimated richness over the time course of the study (S1). Pairwise comparisons of sites within treatment and habitat were not significantly different in R’s adonis program [29].
Because sample collection dates varied, I grouped collection periods by season: spring (May–June), monsoon (July–September), and fall (October–November). Traps were open continuously from spring through fall; they were closed and often snow-covered in winter. Analyses were run at the site level.
I did not compare across habitats because the trap capture probability differed by habitat based on differences in amounts of open ground and litter [30] and the fire severity differed. I followed the same analyses for each habitat. Because the abundance levels varied widely, abundance numbers were log-transformed in PRIMER and R and grouped at the site level. In PRIMER, I used PERMANOVA to test for treatment (burned or unburned), year, and treatment x year effects. I used adonis in R for pairwise treatment and year comparisons of activity abundance for each habitat. I tested for autocorrelation (autocorr in R) over the years. Both PERMANOVA and autocorr took into account that the samples were collected from the same traps over time. I used non-metric multidimensional scaling (NMDS in R and in PRIMER, Bray-Curtis distances) to visualize the PERMANOVA results for treatment and year.
In PRIMER I used Similarity Percentages (SIMPER), which compared Bray–Curtis dissimilarities in the mean between-group differences in activity abundance among samples by treatment and among years. The method can be influenced by a large variation within the group. I also used this to show the percent contribution of the most abundant genera for treatment and year. Indicator genera (indicspp in R) showed taxa that were associated with a particular treatment or year.
3. Results
Spider taxa used in analyses of the monitoring effort were: 64 species in 29 genera and 10 families for PP; 69 species in 34 genera in 10 families for MC; and 58 species in 37 genera in 10 families for MV. The common co-dominant spider families in temperate areas, Lycosidae and Gnaphosidae, along with harvestmen and the erythraeid mites, made up about 60% of the abundance (Table A1, Table A2 and Table A3).
Even with uneven sampling, PERMANOVA effects of wildfire and year were significant in all three habitats, even for the lightly burned MV grassland. The interaction between fire and year was significant for PP and MC, but not for MV (Table 1). These effects were visualized with NMDS by treatment and year (Table 2, Figure 4, Figure 5 and Figure 6). Besides losing tree canopy cover, on the ground, the forest habitats lost litter cover, much of which was replaced with cover by grasses and herbaceous plants (Figure 2). The cover that had previously been there included pine needles, dead aspen leaves, and downed tree branches; a quite different microhabitat for small ground-dwelling arachnids. The directions of change within the habitats were similar in that the NMDS ellipses from 2012–2015 overlap (Figure 4, Figure 5 and Figure 6), but autocorrelation analysis showed that year was important only as a factor and not as a numerical time sequence. Arachnid abundance varied over three orders of magnitude, although most samples contained 10–40 individuals. The wolf spider Pardosa was the dominant genus in all habitats, frequently the highest contributor to abundance and never falling below the top 5 ranked taxa (Table 3). Pardosa remained the dominant genus; 5 of the 7 species were found in all 3 habitats (Table A1, Table A2 and Table A3). At PP, the wolf spider P. distincta was most abundant; all species except P. yavapa were collected mostly from burned sites. At MC, P. uncata, common in conifer forests [31], was most abundant in both burned and unburned sites. At MV, P. distincta and P. concinna were most abundant in burned and unburned sites; P. yavapa and P. uncata were the two species not found there. The species generally overlapped in time: P. coloradensis and P. concinna were adults in the spring and continued into the summer, while the other species were more abundant in the summer. Pardosa xerophila and P. montgomeryi were collected in rather low numbers in the forest habitats, mostly in burned sites. At MV, P. montgomeryi was collected more often than P. xerophila. Few Pardosa adults were present in fall collections.

Table 1.
PERMANOVA results for arachnid genera (and some higher taxa) for effects of fire, year, and their interaction for ponderosa pine forest, mixed-conifer forest, and mountain valley grassland at the Valles Caldera. ** 0.01> p >0.001, *** p = 0.001, n.s. = not significant.

Table 2.
Non-metric multidimensional scaling (NMDS) results for ponderosa pine forest (PP), mixed-conifer forest (MC), and mountain valley grassland (MV) at the Valles Caldera National Preserve by treatment (burned/unburned) and years. Significance values: * 0.05 > p >0.01, ** 0.01 > p > 0.001, *** p = 0.001, n.s. not significant.
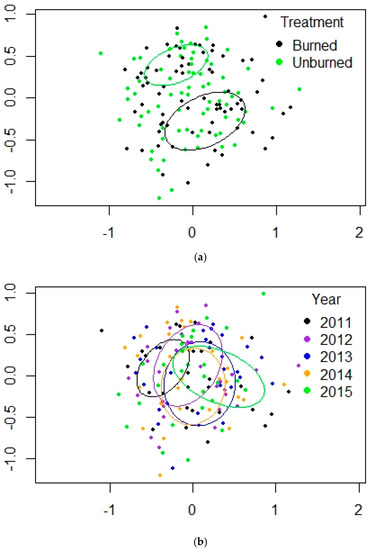
Figure 4.
(a,b). NMDS plots for PERMANOVA results for ponderosa pine forest at Valles Caldera. (a) treatment, (b) year. Ellipses are the 95% confidence intervals for the locations of the centroids.
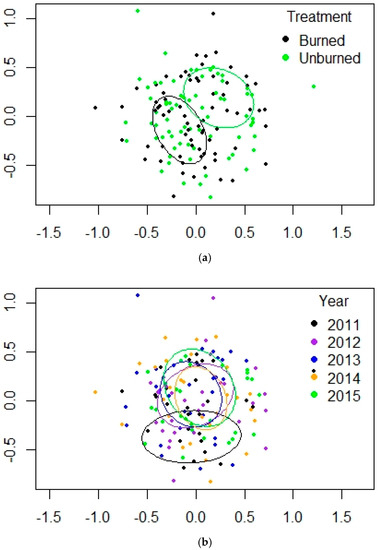
Figure 5.
(a,b). NMDS plots for PERMANOVA results for mixed-conifer forest at Valles Caldera. (a) treatment, (b) year. Ellipses are the 95% confidence intervals for the locations of the centroids.
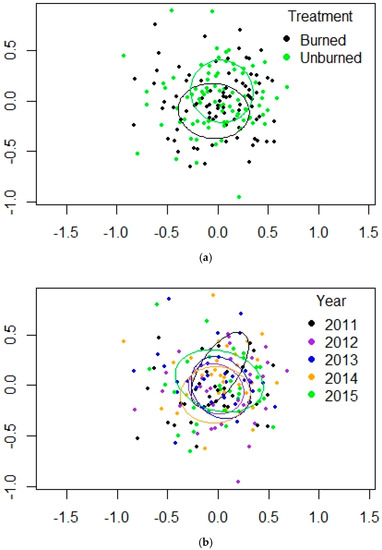
Figure 6.
(a,b). NMDS plots for PERMANOVA results for mountain valley grassland at Valles Caldera. (a) treatment, (b) year. Ellipses are the 95% confidence intervals for the locations of the centroids.

Table 3.
Arachnid community SIMPER results by treatment and year for Ponderosa Pine Forest, Mixed-conifer Forest, and Mountain Valley Grassland at the Valles Caldera National Preserve.
3.1. Ponderosa Pine Forest (PP)
In this habitat, the fire was severe enough to be stand-replacing; this area may become and remain a shrubfield for many years [20,32]. SIMPER results for the arachnids showed average similarity ranged from 28.77 to 39.33 across the five years, and the unburned sites were more similar to each other (42.14) than the burned ones (34.02). The top 5 taxa at PP accounted for 66.47% of abundance at burned sites and 70.78% at unburned sites and ranged from 63.17% to 69.68% across the 5 years of the study in percent contribution to activity abundance. Besides the high numbers of lycosids and gnaphosids, other families were also important: Dictynidae (Cicurina), Linyphiidae (Erigone, Agyneta), Sclerosomatidae (Togwoteeus), and Erythraeidae. Abundance was lowered from 2013–2015 because of the loss of 3 unburned sites to the Thompson Ridge Fire, as noted in the Sites and Methods section above. The number of individuals in samples varied greatly, but there was a general trend of increasing abundance in burned and unburned plots from 2011–2015. For the PP habitat, pairwise adonis tests of differences among pairs of years showed significant values for 2011 vs. 2012 (p = 0.0108), 2011 vs. 2013 (p = 0.0004), and 2013 vs. 2014 (p = 0.0133). All other paired comparisons for the year were not significant. Even though treatment differences were significant, there was overlap between the two over the 5 years (Figure S2a). PERMANOVA results and the NMDS visualization showed that the effect of the fire on arachnids was strong; the ellipses for the 95% confidence intervals for burned and unburned sites were widely separated, and the years remained relatively distinct, with increasing distance between the community of 2011 and 2015 (Table 1, Table 2, Figure 4a,b). Because of the amount of scatter in the 2-D plots, I also included the stress and fit values for the 3-D NMDS, which were an improvement in capturing the variability of the samples. However, the R2 values for the goodness of fit for treatment and year were not much changed.
In contrast, indicator analysis examined genera that contributed to the difference between burned and unburned areas. Few genera characterized the burned sites (Table 4). The linyphiid spider Erigone dentosa showed an unusual increase in the number of individuals in the burned sites from 0 in 2011, to 8 in 2012, to 148 in 2013, to 513 in 2014, and 253 in 2015 (Table A1 in Appendix A). Linyphiid spiders were speciose but often with few individuals in samples, as is common for this family [12]. Erythraeidae were also significant indicators of burned sites. The unburned sites had many more indicator genera across six spider families, one harvestman family, and several mite taxa. The dominant genera Pardosa wolf spider and Haplodrassus and Gnaphosa (both ground spiders) were generally not displaced (Table 3), but the unburned sites were clearly richer in genera, especially among the Linyphiidae, and among other lycosid and gnaphosid genera. The harvestman (Leiobunum), predatory mites (especially the Anystidae), and the beetle mites (Oribatida) were also important in distinguishing the unburned sites (Table 4).

Table 4.
Indicator taxon analysis of arachnids for treatment and year for Ponderosa Pine Forest, Mixed-conifer Forest, and Mountain Valley Grassland at Valles Caldera National Preserve. Significance values: * 0.05 > p > 0.01, ** 0.01 > p > 0.001, *** p = 0.001. If a year is not listed, there was no indicator taxon for it.
3.2. Mixed-Conifer Forest (MC)
Fire severity ranged from moderate to severe in this forest. SIMPER showed arachnid similarity values of 37.84–40.07 across the five study years, and within treatments the similarities were close: 39.47 for burned and 40.44 for unburned (Table 3). The top 5 taxa at MC accounted for 73.70% of abundance at burned sites and 64.55% at unburned sites, and their contribution to activity abundance ranged from 63.69% to 81.97% across the 5 years of the study, a wider range than at PP. Besides the expected high numbers of lycosids and gnaphosids, 2 other spider families were also important: Dictynidae (Cicurina) and Linyphiidae (Erigone). The 2 harvestmen species T. biceps and Leiobunum sp. were dominants in unburned areas; the high abundance levels for 2012–2013 in unburned areas are largely due to these two species (Table A2 in Appendix A). The linyphiid E. dentosa also appeared in large numbers in burned sites (403 in 2014 and 324 in 2015), although lower than at PP (Table A1 and Table A2). Arachnid abundance was lower at burned sites through 2013 but increased steadily through 2015. For the MC habitat, pairwise adonis tests of differences among pairs of years showed significant values only for 2012 vs. 2015 (p = 0.0477). All other paired comparisons for the year were not significant. The scatter among the data points was less than at PP, which made comparisons by treatment among years less clear (Figure S2b). PERMANOVA results were significant for treatment, year, and the interaction of the two factors (Table 1). The NMDS plots illustrated clear separation between treatments, and 2011 was quite different from the remaining years (Table 2, Figure 5a,b); the 3-D NMDS improved the stress but did not much change the R2 for treatment or year. There were only three indicator genera for the burned sites at MC, including E. dentosa (Table 4). For the unburned sites, there were 20 indicator genera, including 9 linyphiids, both harvestmen genera, and four mite groups. The dominant genera Pardosa, Haplodrassus, and Gnaphosa were consistently in the top 5 taxa (Table 3). The unburned sites contained more genera, especially among the Linyphiidae. The harvestman (Leiobunum sp.), predatory mites (especially the Anystidae), and the beetle mites (Oribatida) were also important in distinguishing the unburned sites (Table 4).
3.3. Mountain Valley Grassland (MV)
The burn in this grassland habitat was of low severity. Within-group similarities for treatment and across the years were the highest of the three habitats, from 48.49 to 57.77 (Table 3). Pardosa ranked first for each year and both treatments, contributing 23–31% of the abundance. The gnaphosid Haplodrassus was also in the top 5 taxa for treatment and year, contributing 8–19% of abundance. The linyphiid E. dentosa appeared in large numbers only in 2015, in both burned and unburned sites (Table A3 in Appendix A). The harvestman T. biceps occurred in very low numbers here. Erythraeid mites were in the top 5 taxa for treatment and year, contributing 13–22% of abundance. Two genera were common at MV that were rare or not collected at PP or MC: the crab spider (Thomisidae) genus Xysticus (in low numbers in the forest sites) and the philodromid genus Thanatus (not collected at MC) (Table A1, Table A2 and Table A3). In every year, some samples contained well over 100 arachnids; samples at PP and MC only approached those numbers in 2014–2015, whether in burned or unburned sites. Recall that at PP and MC, 3 of the unburned sites were not included from 2013–2015 because they burned in the Thompson Ridge fire. For the MV habitat, pairwise adonis tests of differences among pairs of years showed significant values only for 2012 v. 2015 (p = 0.0477). All other paired comparisons for years were not significant. The NMDS showed greater overlap between the burned and unburned treatments (Figure 6) and the scatterplot of treatment and years showed several points from 2014 and 2015 farther from the rest (Figure S2).
PERMANOVA results were significant for treatment and year but not the interaction factor (Table 1). NMDS results showed the greatest amount of overlap of the three habitats, but 2011 was still somewhat different from the other years (Table 2, Figure 6a,b). There was less difference between burned and unburned sites, but the 3-D NMDS was able to reduce the stress and showed a significant effect of year. There were few indicator genera for treatment at MV. Seven taxa were indicators for the unburned sites, including the harvestman Leiobunum sp. and the philodromid genus Ebo, which was not collected at PP or MC.
4. Discussion
The arachnids within three habitats at the Caldera that were affected by the Las Conchas wildfire were similar in their responses (the ellipses largely overlapped) (Figure 4, Figure 5 and Figure 6), perhaps because many of the genera and species were shared. However, vegetation structure in each habitat was different, so that pitfall trap capture probabilities were not comparable across the three. Abundance returned to pre-fire levels in about 3 years (similar to results in Abbott et al. [33] in the Jarrah forest in Australia, Smith DiCarlo et al. [18] in Oregon grasslands, and Samu et al. in the Hungarian juniper-poplar steppe [17]). Nevertheless, at the level of species composition, there were a number of changes, even in unburned areas. Many spider species are rare in pitfall collections [16,30] (Table A1, Table A2 and Table A3), and so are not sampled each year, which can complicate what recovery means and makes long-term monitoring important for getting a more complete picture of species occurrence in an area. For example, over the five years of this study, the abundance of Pardosa, the dominant genus among ground-dwelling spiders, varied from 11% to 40% at PP, 5–24% at MC, but was more consistent in the lightly burned MV at 24–30% (Table 3). Interactions with other taxa are also likely to be important. The gnaphosid genus Micaria is well known for ant mimicry, both in associating with ants and preying on them [34]. Knudsen [35] reported increased ant abundance in PP burned sites after Spring 2012, which may have influenced Micaria activity abundance as well. For other less-abundant taxa, longer time scales may be needed to discern patterns in abundance and richness, particularly with influences from disturbance, such as fire or drought (for forests [6,33,36]; for grasslands [37]). It may turn out that there is no long-term pattern because the disturbances occur in a context of weather and vegetation structure that are themselves changing [4,32]. From 2011–2015, spring and fall precipitation at the Caldera was variable; every year showed different patterns and the summer monsoon period was delayed in 2011 and 2013. As a result, year was important as a factor but not as a time series influencing the arachnid community (Figure 4, Figure 5 and Figure 6), as was also the case in Oregon forest [12] and Australian grasslands [33]. At the Caldera there was considerable variation in numbers of individuals in samples across the years; often both burned and unburned sites increased or decreased together, suggesting annual weather was as big a factor as vegetation changes resulting from the fire.
Taxon dominance patterns at the Caldera were not greatly disrupted (Table 3). What affected more were the small litter-dwellers (such as Helophora, Sisicottus, and Wubana in the Linyphiidae, and Neoantistea in the Hahniidae), which made up a large part of the indicators for unburned sites (Table 4). Their numbers were generally low so that 5 years post-fire were not enough to say if they were moving back into the burned sites (Table A1, Table A2 and Table A3). Gillette et al. [13] found greater declines in spider abundance and richness in California forest stands that were structurally diverse, suggesting that the higher fuel loads there might require longer recovery times for the vegetation structure and the spider community. Lycosids and gnaphosids were common in burned areas in forests in Oregon [12] and Alberta, Canada [36] up to 15 years after a fire. Linyphiidae and Thomisidae were more common in unburned areas there, which matches the patterns found in the Caldera even after 5 years. It is unclear whether herbaceous and grass vegetation is acceptable for the species that had previously lived under the tree canopy and in the litter. Some of the philodromids, such as members of the genus Ebo [38], are common in grassy areas [10], yet did not increase in numbers in the forest sites, even though forb and grass cover increased there. Over the course of the study, there was no trend that the spider communities in the burned forest sites were becoming more like the grassland sites. In German forests of spruce and Douglas-fir, Ziesche and Roth [39] found tree canopy and litter were important to ground-dwelling spiders overall, but that microhabitats varied within forest stands, allowing the spiders to move around over the course of a year, as the seasons changed. The linyphiid spiders are a large family of small-bodied spiders that are generally rare at the species level and strongly associated with litter and low vegetation [10]. In Oregon forest [12], the linyphiids made up most of the species richness, but there was no dominant species because abundances were low. Linyphiids were dominants in Finnish forests 3 years post-fire [15], and Buddle et al. [36] showed linyphiids in Canadian forest were abundant in areas that had not burned for decades. At the Caldera, herbaceous cover increased and litter cover correspondingly decreased (Figure 2) at the burned sites, but the linyphiids that were indicator species of unburned areas did not move to burned areas over the course of the study, suggesting that litter and tree canopy cover provided important microhabitat in ways that herbaceous plants did not. Because of their small size (generally < 5 mm), they can disperse readily by ballooning [9], so that it seemed unlikely that the spiders were unable to colonize the burned areas. Hundreds of E. dentosa were collected in burned areas in PP (in 2013–2015), MC, and MV (in 2014–2015) (Table A1, Table A2 and Table A3). At PP and MC, numbers were lower in 2015, suggesting that the high numbers of E. dentosa were a short-lived occurrence, another reason for multi-year studies that can capture such transient changes. Members of this family are understudied in the southwestern US; therefore, the specimens and records from the Caldera are important additions to the knowledge of regional distribution and habitat association. While most of the linyphiids were indicators of unburned conditions, there were a few besides E. dentosa that were more abundant in burned areas, such as Islandiana flaveola at PP and Mermessus taibo at MC (Table A1 and Table A2).
Spiders made up the majority of the arachnid taxa, but harvestmen and mites also figured prominently in the top 5 of abundant taxa at PP and MC. New Mexico is in the southern part of the range of the harvestman T. biceps, where it occurs at high elevations, from dense forest to above treeline [40]. Leiobunum sp. occurred in comparable numbers to T. biceps at MC but was less abundant at PP (Table A1 and Table A2). Both species were rare at MV, but Leiobunum sp. was an indicator of unburned sites there. Pitfall traps do not sample mites fully but at least collect those active on the ground surface (see Reference [41] for results from Berlese funnel extractions). The erythraeids were numerous (Table A1, Table A2 and Table A3) and were indicators in burned sites at all three habitats. In ponderosa pine forest in California [41], one year following the prescribed fire, mites from litter and loose soil declined across all groups, except Prostigmata (now in Trombidiformes). Mites from pitfall samples in the same area collected in the following year had increased in abundance [13]. In Swedish boreal forest, Malmstrom et al. [14] found that the Trombidiformes needed only a year or so to return to pre-fire abundance levels, but that the Mesostigmata needed about 5 years. At the Caldera, results were similar, with Mesostigmata numbers low until 2015 in burned areas in all three habitats (Table A1, Table A2 and Table A3). Possible reasons for the longer recovery time include life history features, such as low reproductive or growth rates. For some soil-dwelling mesostigmatans, Krantz and David [27] reported that the females spend considerable time and energy finding plant rootlets to lay their eggs on, so the larvae hatch in a place where their symphylan prey occur.
Anystid (whirligig mites) and bdellid (snout mites) numbers were generally low, but they were indicators for unburned sites (Table 4). These taxa are rarely reported, but make up a part of the predator trophic level of surface-active arthropods.
There was a high overlap at the family and genus level among the habitats; most families occurred in each (but sometimes not in high enough numbers to be included in these analyses), except that Amaurobiidae were only at MC and Anyphaenidae were only at PP and MC. Amaurobiids occur in dense forests with tree canopy cover and downed trees [10]. Anyphaena marginalis is common in pine/oak forests of the southwestern US [42] and was collected mostly at PP, with few in MC. Samu et al. [17] and Smith DiCarlo et al. [18] found high overlap at their sites so that analysis at higher taxonomic levels masked differences at the species level. Within the spiders, many species are prey generalists but are strongly specialized in habitat needs, whether for web construction (the styles are extremely variable), or for temperature or humidity preferences, or for sites for depositing egg sacs. Nevertheless, suitable microhabitats are often available to them within broader habitat types [39], but these may come and go with disturbance, such as fire or drought. Studies assessing the extent of determinism in structuring spider assemblages have had mixed results. In grassland, Langlands et al. [37] suggested that species composition in burned areas over time did come to resemble the unburned species composition, but it took at least 10 years in that arid system to begin to see that pattern, and different sites could take different paths to reach the unburned condition. Ferrenberg et al. [43] worked with post-fire arthropod assemblages in the Jemez Mountains, including the Caldera. Their question was also about determinism in arthropod assemblages in the context of the number of fires and time since fire. Those factors were significant, but the habitat features that were measured did not strongly correspond to the indicator taxa. It is possible that they were not “seeing the habitat from the spiders’ point of view,” to know which habitat features were important or at what scale. Like Langlands et al. [37], they suggested that both stochastic and deterministic processes occur in the communities but are of variable importance. In the short term, the changes in abundance appeared to be stochastic, particularly in the context of variable weather conditions from one year to the next.
With fire returning more frequently and more severely to forests of the southwestern U.S. [2,4], arachnid communities are “reset” over and over, with little chance to develop their long-term patterns, which may require up to 15+ years [6,36,37,38]. Some areas of ponderosa pine forest that have undergone stand-replacing fire may persist as shrubfields of oak or other species for many decades [20,31]. For the arachnids of the Caldera, this could mean loss of rarer species locally for a time, even though a higher taxon analysis might show that richness and abundance were generally not changing. Nevertheless, for centuries, fire has been one of the producers of the Caldera landscape mosaic and the inhabitants are accustomed to the variation in past climate factors. The main concern is that future changes may well be outside the historic range of conditions [19], having an outsized impact on species that are poorly known to begin with, such as arthropods that are small, cryptic, or strongly seasonal [44].
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/12/10/396/s1, Figure S1: Species accumulation curves for samples in (a) ponderosa pine forest (PP), (b) mixed-conifer forest (MC), and (c) mountain valley grassland (MV) at the Valles Caldera National Preserve. Sobs is the observed number of taxa compared with the estimators Chao2 and Jackknife 2. Figure S2: NMDS scatterplots from PRIMER by treatment (B = Burned, U = Unburned) and year for (a) ponderosa pine forest (PP), (b) mixed-conifer forest (MC), and (c) mountain valley grassland (MV) at the Valles Caldera National Preserve, New Mexico, USA. Figure S3: R code for NMDS, autocorr, and indicspp.
Funding
This research was funded by the Collaborative Forest Landscape Restoration Project (CFLRP).
Acknowledgments
My sincere thanks go to Robert Parmenter, chief scientist at the Valles Caldera National Preserve for including post-fire arthropod monitoring and helpful comments on the manuscript; Mark Ward, field work and manager of sample sorting, and field crews; Martina Suazo for vegetation data; field and lab crews, especially Joaquin Garcia, Lozen Benson, and Marlo McCarter; and to J. Rudgers for her R class. I am grateful to the reviewers for their thorough reading and thoughtful comments on the manuscript. This paper is in memory of Dr. Norman I. Platnick (1951–2020), spider systematist and mentor, whose revisions were essential to my learning spider taxonomy.
Conflicts of Interest
The author declares no conflict of interest. The funders designed the collection of samples; my work was a part of a larger post-fire monitoring effort. The funders did not have a role in my data preparation or analysis; or in the preparation or writing of the manuscript; or the decision to publish the results.
Appendix A

Table A1.
Species and numbers collected for Ponderosa Pine Forest at Valles Caldera National Preserve 2011–2015. B = burned sites, U = unburned sites. Undet. = undetermined taxa.
Table A1.
Species and numbers collected for Ponderosa Pine Forest at Valles Caldera National Preserve 2011–2015. B = burned sites, U = unburned sites. Undet. = undetermined taxa.
| 2011 | 2012 | 2013 | 2014 | 2015 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Order Family | Genus Species | B | U | B | U | B | U | B | U | B | U |
| Araneae Anyphaenidae | Anyphaena marginalis (Banks) | 0 | 135 | 1 | 50 | 0 | 123 | 0 | 112 | 0 | 133 |
| Clubionidae | Clubiona oteroana Gertsch | 5 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 |
| Dictynidae | Cicurina sp. | 21 | 7 | 25 | 9 | 34 | 19 | 10 | 14 | 30 | 14 |
| Hackmania saphes (Chamberlin) | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 2 | 0 | 5 | |
| Gnaphosidae | Drassodes neglectus (Keyserling) | 7 | 5 | 8 | 31 | 2 | 15 | 7 | 5 | 1 | 1 |
| Gnaphosa immatures | 17 | 19 | 11 | 49 | 13 | 17 | 5 | 12 | 3 | 23 | |
| Gnaphosa muscorum (L. Koch) | 6 | 65 | 23 | 192 | 20 | 152 | 56 | 103 | 21 | 43 | |
| Gnaphosa parvula Banks | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Haplodrassus immatures | 105 | 26 | 47 | 17 | 10 | 16 | 10 | 5 | 5 | 7 | |
| Haplodrassus bicornis (Emerton) | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Haplodrassus eunis Chamberlin | 0 | 0 | 1 | 16 | 0 | 26 | 3 | 31 | 1 | 24 | |
| Haplodrassus signifier (C.L. Koch) | 11 | 16 | 21 | 23 | 26 | 6 | 12 | 8 | 14 | 7 | |
| Micaria immatures | 0 | 0 | 1 | 1 | 2 | 1 | 2 | 0 | 3 | 0 | |
| Micaria aenea Thorell | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | |
| Micaria foxi Gertsch | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Micaria gertschi Barrows & Ivie | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | |
| Micaria pulicaria (Sundevall) | 2 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 1 | 0 | |
| Micaria riggsi Gertsch | 0 | 0 | 5 | 0 | 5 | 0 | 1 | 0 | 2 | 0 | |
| Zelotes immatures | 16 | 7 | 2 | 13 | 1 | 4 | 0 | 5 | 1 | 4 | |
| Zelotes fratris Chamberlin | 17 | 9 | 3 | 10 | 3 | 17 | 0 | 15 | 4 | 9 | |
| Zelotes lasalanus Chamberlin | 0 | 0 | 1 | 2 | 2 | 0 | 2 | 0 | 0 | 0 | |
| Zelotes puritanus Chamberlin | 0 | 0 | 3 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | |
| Hahniidae | Neoantistea gosiuta Gertsch | 13 | 1 | 2 | 4 | 2 | 1 | 1 | 0 | 1 | 0 |
| Linyphiidae | Agyneta immatures | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Agyneta simplex (Emerton) | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Agyneta uta (Chamberlin) | 0 | 0 | 6 | 40 | 22 | 9 | 72 | 19 | 35 | 48 | |
| Erigone dentosa O. Pickard- Cambridge | 0 | 0 | 8 | 1 | 148 | 0 | 513 | 3 | 253 | 0 | |
| Grammonota gentilis Banks | 2 | 1 | 9 | 1 | 15 | 1 | 22 | 0 | 67 | 0 | |
| Helophora orinoma (Chamberlin) | 0 | 4 | 0 | 3 | 1 | 1 | 4 | 0 | 0 | 0 | |
| Incestophantes lamprus (Chamberlin) | 0 | 3 | 0 | 1 | 0 | 9 | 0 | 6 | 2 | 5 | |
| Islandiana immatures/undet. females | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| Islandiana coconino Ivie | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | |
| Islandiana flaveola (Banks) | 35 | 0 | 15 | 4 | 15 | 0 | 7 | 0 | 13 | 0 | |
| Islandiana lasalana (Chamberlin & Ivie) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | |
| Lepthyphantes immatures | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Lepthyphantes intricatus (Emerton) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | |
| Lepthyphantes turbatrix (O. P.-Cambridge) | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 3 | |
| Mermessus immatures | 6 | 0 | 7 | 1 | 4 | 3 | 5 | 2 | 2 | 1 | |
| Mermessus major Millidge | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Mermessus taibo (Chamberlin & Ivie) | 6 | 14 | 9 | 4 | 16 | 1 | 3 | 6 | 4 | 6 | |
| Mermessus trilobatus (Emerton) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | |
| Pocadicnemis occidentalis Millidge | 0 | 9 | 1 | 75 | 0 | 31 | 0 | 77 | 0 | 75 | |
| Spirembolus immatures | 1 | 4 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | |
| Spirembolus pallidus Chamberlin & Ivie | 0 | 4 | 0 | 7 | 0 | 9 | 4 | 31 | 2 | 18 | |
| Spirembolus spirotubus (Banks) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Tachygyna immatures | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | |
| Tachygyna haydeni Chamberlin & Ivie | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 0 | |
| Tachygyna tuoba Chamberlin & Ivie | 1 | 4 | 0 | 13 | 0 | 11 | 1 | 33 | 0 | 8 | |
| Walckenaeria immatures | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Walckenaeria communis (Emerton) | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 3 | |
| Walckenaeria maesta Millidge | 0 | 0 | 0 | 2 | 0 | 4 | 0 | 0 | 0 | 0 | |
| Walckenaeria spiralis (Emerton) | 0 | 5 | 0 | 4 | 0 | 41 | 0 | 12 | 0 | 28 | |
| Wubana drassoides (Emerton) | 0 | 9 | 0 | 13 | 0 | 8 | 1 | 8 | 0 | 10 | |
| Lycosidae | Alopecosa kochi (Keyserling) | 15 | 46 | 3 | 141 | 0 | 31 | 17 | 55 | 7 | 33 |
| Hogna sp. | 1 | 12 | 0 | 19 | 1 | 7 | 0 | 2 | 0 | 0 | |
| Pardosa immatures | 8 | 6 | 12 | 81 | 7 | 171 | 45 | 23 | 180 | 40 | |
| Pardosa coloradensis Banks | 0 | 0 | 0 | 0 | 10 | 0 | 11 | 0 | 52 | 0 | |
| Pardosa concinna (Thorell) | 0 | 0 | 11 | 1 | 97 | 1 | 137 | 2 | 142 | 0 | |
| Pardosa distincta (Blackwall) | 0 | 0 | 0 | 0 | 36 | 1 | 172 | 1 | 363 | 0 | |
| Pardosa montgomeryi Gertsch | 0 | 11 | 0 | 0 | 1 | 0 | 14 | 0 | 68 | 0 | |
| Pardosa uncata (Thorell) | 20 | 21 | 13 | 112 | 14 | 43 | 4 | 62 | 6 | 115 | |
| Pardosa xerophila Vogel | 0 | 0 | 0 | 0 | 9 | 0 | 31 | 0 | 23 | 1 | |
| Pardosa yavapa Chamberlin | 0 | 11 | 1 | 142 | 1 | 51 | 13 | 84 | 2 | 150 | |
| Philodromidae | Thanatus immatures | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Thanatus formicinus (Clerck) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Thanatus coloradensis Keyserling | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | |
| Thanatus vulgaris Simon | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Phrurolithidae | Scotinella pugnata (Emerton) | 8 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thomisidae | Xysticus immatures | 9 | 5 | 5 | 16 | 7 | 13 | 3 | 8 | 1 | 5 |
| Xysticus apachecus Gertsch | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Xysticus cunctator Thorell | 0 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 0 | 0 | |
| Xysticus emertoni Keyserling | 0 | 0 | 0 | 8 | 0 | 5 | 2 | 6 | 4 | 7 | |
| Xysticus ferox (Hentz) | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | |
| Xysticus gulosus Keyserling | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | |
| Xysticus locuples Keyserling | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Xysticus luctuosus (Blackwall) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Xysticus montanensis Keyserling | 1 | 0 | 1 | 3 | 0 | 8 | 3 | 1 | 0 | 2 | |
| Opiliones Sclerosomatidae | Leiobunum sp. | 2 | 0 | 1 | 15 | 6 | 92 | 2 | 0 | 6 | 38 |
| Togwoteeus biceps (Thorell) | 4 | 62 | 55 | 9 | 28 | 93 | 47 | 7 | 45 | 158 | |
| Acari Mesostigmata | 1 | 0 | 3 | 34 | 64 | 1 | 25 | 1 | 468 | 156 | |
| Oribatida | 2 | 1 | 1 | 15 | 1 | 6 | 0 | 15 | 0 | 9 | |
| Trombidiformes | Undetermined | 0 | 4 | 4 | 0 | 0 | 6 | 3 | 27 | 3 | 8 |
| Anystidae | 0 | 6 | 0 | 34 | 2 | 4 | 0 | 16 | 1 | 9 | |
| Erythraeidae | 27 | 28 | 182 | 20 | 183 | 43 | 96 | 8 | 326 | 2 |

Table A2.
Species and numbers collected for Mixed-conifer Forest at Valles Caldera National Preserve 2011–2015. B = burned sites, U = unburned sites. Undet. = undetermined taxa.
Table A2.
Species and numbers collected for Mixed-conifer Forest at Valles Caldera National Preserve 2011–2015. B = burned sites, U = unburned sites. Undet. = undetermined taxa.
| 2011 | 2012 | 2013 | 2014 | 2015 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Order Family | Genus Species | B | U | B | U | B | U | B | U | B | U |
| Araneae Amaurobiidae | Callobius arizonicus (Chamberlin & Ivie) | 0 | 5 | 1 | 12 | 0 | 5 | 0 | 3 | 0 | 1 |
| Dictynidae | Cicurina sp. | 22 | 58 | 53 | 55 | 65 | 39 | 211 | 36 | 99 | 57 |
| Gnaphosidae | Drassodes neglectus (Keyserling) | 9 | 5 | 19 | 14 | 18 | 7 | 4 | 7 | 8 | 1 |
| Gnaphosa immatures | 17 | 7 | 21 | 13 | 66 | 24 | 9 | 8 | 72 | 10 | |
| Gnaphosa muscorum (L. Koch) | 3 | 8 | 71 | 73 | 40 | 56 | 137 | 71 | 57 | 45 | |
| Gnaphosa parvula Banks | 0 | 1 | 2 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | |
| Haplodrassus immatures | 64 | 8 | 13 | 22 | 11 | 21 | 9 | 25 | 11 | 13 | |
| Haplodrasus bicornis (Emerton) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Haplodrassus eunis Chamberlin | 0 | 0 | 9 | 15 | 8 | 6 | 27 | 12 | 8 | 14 | |
| Haplodrassus signifier (C.L. Koch) | 29 | 7 | 25 | 20 | 11 | 9 | 41 | 21 | 23 | 12 | |
| Micaria immatures | 0 | 0 | 2 | 2 | 6 | 2 | 2 | 1 | 1 | 0 | |
| Micaria aenea Thorell | 0 | 0 | 0 | 10 | 0 | 5 | 0 | 0 | 0 | 0 | |
| Micaria gertschi Barrows & Ivie | 0 | 0 | 0 | 3 | 6 | 7 | 1 | 0 | 1 | 1 | |
| Micaria pulicaria (Sundevall) | 0 | 1 | 1 | 5 | 1 | 0 | 1 | 0 | 1 | 1 | |
| Micaria riggsi Gertsch | 0 | 0 | 0 | 0 | 4 | 0 | 3 | 0 | 4 | 0 | |
| Micaria rossica Thorell | 0 | 0 | 1 | 0 | 8 | 0 | 32 | 0 | 44 | 1 | |
| Orodrassus coloradensis (Emerton) | 0 | 0 | 1 | 4 | 0 | 4 | 0 | 1 | 0 | 0 | |
| Zelotes immatures | 8 | 5 | 5 | 7 | 0 | 12 | 3 | 5 | 1 | 3 | |
| Zelotes fratris Chamberlin | 7 | 4 | 1 | 10 | 0 | 4 | 7 | 3 | 5 | 3 | |
| Zelotes puritanus Chamberlin | 1 | 0 | 5 | 0 | 11 | 0 | 5 | 1 | 4 | 0 | |
| Hahniidae | Neoantistea gosiuta Gertsch | 4 | 1 | 0 | 2 | 1 | 4 | 1 | 0 | 2 | 1 |
| Linyphiidae | Agyneta immatures/undet. females | 0 | 0 | 2 | 4 | 8 | 6 | 4 | 1 | 0 | 2 |
| Agyneta danielbelangeri Duperre | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 13 | |
| Agyneta uta (Chamberlin) | 0 | 0 | 0 | 2 | 3 | 1 | 52 | 7 | 7 | 35 | |
| Ceratinella brunnea Emerton | 2 | 1 | 0 | 3 | 0 | 2 | 0 | 1 | 0 | 2 | |
| Ceratinella ornatula (Crosby & Bishop) | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 5 | 3 | 0 | |
| Erigone dentosa O. Pickard-Cambridge | 1 | 5 | 3 | 10 | 43 | 0 | 403 | 1 | 324 | 25 | |
| Grammonota gentilis Banks | 0 | 1 | 0 | 1 | 4 | 0 | 1 | 0 | 24 | 1 | |
| Helophora orinoma (Chamberlin) | 0 | 18 | 0 | 34 | 1 | 28 | 2 | 39 | 3 | 70 | |
| Incestophantes lamprus (Chamberlin) | 0 | 0 | 0 | 2 | 3 | 2 | 0 | 0 | 0 | 3 | |
| Islandiana coconino Ivie | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | |
| Islandiana flaveola (Banks) | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 6 | |
| Lepthyphantes immatures | 3 | 1 | 0 | 3 | 1 | 1 | 0 | 0 | 0 | 1 | |
| Lepthyphantes intricatus (Emerton) | 0 | 1 | 1 | 1 | 2 | 0 | 5 | 0 | 0 | 0 | |
| Lepthyphantes turbatrix (O. Pickard-Cambridge) | 0 | 0 | 0 | 1 | 3 | 0 | 4 | 2 | 5 | 1 | |
| Mermessus immatures/undet. females | 17 | 6 | 4 | 0 | 3 | 0 | 6 | 0 | 3 | 0 | |
| Mermessus taibo (Chamberlin & Ivie) | 1 | 0 | 11 | 4 | 18 | 1 | 10 | 0 | 14 | 0 | |
| Mermessus trilobatus (Emerton) | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | |
| Pocadicnemis occidentalis Millidge | 0 | 2 | 1 | 3 | 0 | 3 | 0 | 2 | 0 | 0 | |
| Scotinotylus undet. females | 0 | 1 | 1 | 3 | 0 | 0 | 3 | 0 | 0 | 2 | |
| Scotinotylus pallidus (Emerton) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | |
| Scotinotylus pollucis Millidge | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 0 | 0 | |
| Scotinotylus sanctus (Crosby) | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Sisicottus immatures/undet. females | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Sisicottus montanus (Emerton) | 0 | 5 | 0 | 29 | 0 | 12 | 0 | 8 | 0 | 0 | |
| Sisicottus orites (Chamberlin) | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 53 | 0 | 43 | |
| Spirembolus immatures/undet. females | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Spirembolus pallidus Chamberlin & Ivie | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 6 | 0 | 1 | |
| Spirembolus spirotubus (Banks) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tachygyna immatures/undet. females | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 5 | 0 | 4 | |
| Tachygyna haydeni Chamberlin & Ivie | 0 | 1 | 0 | 7 | 0 | 4 | 0 | 10 | 0 | 30 | |
| Tachygyna tuoba Chamberlin & Ivie | 0 | 1 | 0 | 2 | 0 | 20 | 0 | 30 | 0 | 6 | |
| Tapinocyba sp. | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 4 | 2 | 23 | |
| Tapinocyba cf. cameroni Duperre & Paquin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Tapinocyba minuta (Emerton) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Walckenaeria communis (Emerton) | 2 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 3 | |
| Wubana drassoides (Emerton) | 0 | 26 | 0 | 8 | 0 | 8 | 0 | 12 | 0 | 17 | |
| Lycosidae | Alopecosa kochi (Keyserling) | 4 | 0 | 2 | 27 | 9 | 7 | 12 | 8 | 12 | 10 |
| Hogna sp. | 0 | 4 | 0 | 0 | 0 | 3 | 2 | 0 | 3 | 4 | |
| Pardosa immatures | 10 | 15 | 4 | 55 | 15 | 113 | 18 | 23 | 94 | 24 | |
| Pardosa coloradensis Banks | 0 | 0 | 1 | 0 | 0 | 0 | 8 | 0 | 94 | 1 | |
| Pardosa concinna (Thorell) | 0 | 0 | 9 | 0 | 22 | 0 | 196 | 1 | 476 | 1 | |
| Pardosa distincta (Blackwall) | 0 | 1 | 0 | 3 | 5 | 1 | 36 | 1 | 163 | 6 | |
| Pardosa montgomeryi Gertsch | 0 | 0 | 0 | 3 | 5 | 0 | 13 | 0 | 49 | 0 | |
| Pardosa uncata (Thorell) | 26 | 43 | 42 | 280 | 69 | 111 | 199 | 120 | 123 | 97 | |
| Pardosa xerophila Vogel | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 1 | 0 | 2 | |
| Pardosa yavapa Chamberlin | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 5 | 1 | |
| Trochosa terricola Thorell | 0 | 0 | 0 | 5 | 0 | 1 | 0 | 1 | 0 | 3 | |
| Phrurolithidae | Phrurolithus camawhitae Gertsch | 1 | 0 | 17 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phrurolithus connectus Gertsch | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Phrurolithus schwarzi Gertsch | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Salticidae | Pelegrina flavipes (Peckham & Peckham) | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 1 | 0 | 0 |
| Theridiidae | Steatoda immatues | 0 | 0 | 0 | 4 | 0 | 1 | 2 | 4 | 0 | 0 |
| Steatoda albomaculata (DeGeer) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Steatoda hespera Chamberlin & Ivie | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 3 | |
| Thomisidae | Xysticus immatures | 4 | 4 | 5 | 3 | 11 | 8 | 6 | 3 | 6 | 0 |
| Xysticus cunctator Thorell | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 9 | 0 | |
| Xysticus emertoni Keyserling | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 8 | 0 | |
| Xysticus ferox (Hentz) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Xysticus locuples Keyserling | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Xysticus luctuosus Keyserling | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Xysticus montanensis Keyserling | 0 | 2 | 0 | 1 | 1 | 4 | 0 | 0 | 0 | 1 | |
| Xysticus triguttatus Keyserling | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Opiliones Paronychidae | Sclerobunus robustus (Packard) | 5 | 0 | 2 | 5 | 1 | 2 | 1 | 4 | 0 | 0 |
| Sclerosomatidae | Leiobunum sp. | 0 | 0 | 23 | 1716 | 46 | 1259 | 30 | 139 | 51 | 673 |
| Togwoteeus biceps (Thorell) | 2 | 0 | 83 | 978 | 113 | 721 | 237 | 430 | 296 | 1102 | |
| Acari Mesostigmata | 0 | 0 | 0 | 16 | 16 | 1 | 18 | 8 | 440 | 16 | |
| Oribatida | 0 | 0 | 0 | 81 | 0 | 195 | 0 | 302 | 4 | 76 | |
| Trombidiformes | Undetermined | 0 | 0 | 0 | 14 | 1 | 1 | 0 | 14 | 0 | 0 |
| Anystidae | 0 | 0 | 0 | 10 | 1 | 10 | 0 | 32 | 0 | 6 | |
| Bdellidae | 0 | 0 | 0 | 6 | 0 | 6 | 0 | 7 | 0 | 3 | |
| Erythraeidae | 0 | 0 | 77 | 120 | 181 | 75 | 108 | 57 | 2 | 42 |

Table A3.
Species and numbers collected for Mountain Valley Grassland at Valles Caldera National Preserve 2011–2015. B = burned sites, U = unburned sites. Undet. = undetermined taxa.
Table A3.
Species and numbers collected for Mountain Valley Grassland at Valles Caldera National Preserve 2011–2015. B = burned sites, U = unburned sites. Undet. = undetermined taxa.
| 2011 | 2012 | 2013 | 2014 | 2015 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Order Family | Genus Species | B | U | B | U | B | U | B | U | B | U |
| Araneae Clubionidae | Clubiona oteroana Gertsch | 32 | 13 | 12 | 14 | 2 | 6 | 7 | 9 | 19 | 26 |
| Dictynidae | Cicurina sp. | 1 | 6 | 0 | 2 | 2 | 6 | 0 | 3 | 0 | 4 |
| Gnaphosidae | Gnaphosa immatures | 0 | 13 | 2 | 16 | 1 | 34 | 0 | 78 | 2 | 34 |
| Gnaphosa borea Kulczynski | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Gnaphosa muscorum (L. Koch) | 2 | 0 | 5 | 3 | 5 | 0 | 5 | 1 | 5 | 0 | |
| Gnaphosa parvula Banks | 4 | 50 | 0 | 31 | 0 | 44 | 0 | 178 | 1 | 59 | |
| Haplodrassus signifier (C.L. Koch) | 327 | 116 | 239 | 209 | 177 | 170 | 170 | 210 | 103 | 88 | |
| Micaria immatures | 12 | 3 | 0 | 2 | 10 | 3 | 4 | 0 | 1 | 0 | |
| Micaria aenea Thorell | 0 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | 0 | 0 | |
| Micaria gertschi Barrows & Ivie | 10 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| Micaria riggsi Gertsch | 0 | 0 | 3 | 0 | 0 | 2 | 2 | 0 | 1 | 0 | |
| Micaria rossica Thorell | 1 | 0 | 26 | 3 | 93 | 11 | 79 | 4 | 41 | 0 | |
| Zelotes immatures | 6 | 2 | 11 | 0 | 3 | 2 | 2 | 6 | 0 | 2 | |
| Zelotes fratris Chamberlin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | |
| Zelotes lasalanus Chamberlin | 3 | 1 | 3 | 15 | 4 | 18 | 3 | 23 | 7 | 8 | |
| Hahniidae | Neoantistea gosiuta Gertsch | 16 | 19 | 6 | 15 | 7 | 18 | 4 | 48 | 7 | 19 |
| Linyphiidae | Agyneta immatures | 31 | 15 | 8 | 12 | 22 | 15 | 24 | 12 | 13 | 14 |
| Agyneta hedini Paquin & Duperre | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| Agyneta simplex (Emerson) | 15 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Agyneta uta (Chamberlin) | 0 | 0 | 9 | 18 | 11 | 14 | 59 | 36 | 34 | 16 | |
| Ceratinella brunnea Emerton | 6 | 2 | 1 | 2 | 10 | 12 | 4 | 6 | 3 | 1 | |
| Erigone dentosa O. Pickard-Cambridge | 6 | 2 | 1 | 15 | 4 | 20 | 88 | 23 | 212 | 110 | |
| Grammonota gentilis Banks | 40 | 130 | 95 | 189 | 26 | 116 | 18 | 169 | 63 | 273 | |
| Islandiana immatures | 0 | 0 | 0 | 0 | 6 | 7 | 8 | 20 | 9 | 30 | |
| Islandiana coconino Ivie | 18 | 1 | 8 | 0 | 27 | 4 | 17 | 1 | 33 | 3 | |
| Islandiana flaveola (Banks) | 14 | 14 | 18 | 18 | 28 | 32 | 37 | 43 | 15 | 12 | |
| Mermessus immatures/undet. females | 0 | 2 | 0 | 5 | 1 | 0 | 2 | 6 | 0 | 2 | |
| Mermessus major (Millidge) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Mermessus taibo (Chamberlin & Ivie) | 0 | 1 | 0 | 0 | 0 | 1 | 3 | 2 | 1 | 0 | |
| Mermessus trilobatus (Emerton) | 7 | 11 | 3 | 0 | 1 | 2 | 0 | 4 | 7 | 7 | |
| Spirembolus pallidus Chamberlin & Ivie | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 1 | |
| Spirembolus spirotubus (Banks) | 0 | 0 | 3 | 3 | 5 | 12 | 0 | 0 | 0 | 0 | |
| Tachygyna haydeni Chamberlin & Ivie | 0 | 0 | 8 | 0 | 34 | 5 | 0 | 0 | 0 | 0 | |
| Tachygyna tuoba Chamberlin & Ivie | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tapinocyba sp. | 0 | 0 | 0 | 0 | 0 | 0 | 30 | 0 | 0 | 0 | |
| Tapinocyba dietrichi Crosby & Bishop | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 6 | 0 | 1 | |
| Walckenaeria immatures | 0 | 2 | 0 | 2 | 0 | 5 | 0 | 15 | 1 | 4 | |
| Walckenaeria communis (Emerton) | 0 | 0 | 0 | 1 | 0 | 4 | 0 | 5 | 0 | 0 | |
| Walckenaeria dondalei Millidge | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | |
| Walckenaeria spiralis (Emerton) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Lycosidae | Alopecosa kochi (Keyserling) | 6 | 1 | 6 | 1 | 5 | 5 | 10 | 2 | 11 | 6 |
| Hogna sp. | 0 | 0 | 1 | 14 | 4 | 4 | 8 | 10 | 7 | 8 | |
| Pardosa immatures | 97 | 58 | 36 | 78 | 64 | 100 | 108 | 129 | 67 | 62 | |
| Pardosa coloradensis Banks | 0 | 0 | 0 | 6 | 9 | 37 | 10 | 9 | 4 | 14 | |
| Pardosa concinna (Thorell) | 100 | 190 | 392 | 595 | 218 | 214 | 449 | 368 | 416 | 367 | |
| Pardosa distincta (Blackwall) | 327 | 568 | 483 | 1002 | 439 | 798 | 893 | 1408 | 777 | 1340 | |
| Pardosa montgomeryi Gertsch | 32 | 15 | 39 | 8 | 102 | 5 | 166 | 12 | 170 | 36 | |
| Pardosa xerophila Vogel | 16 | 8 | 0 | 0 | 0 | 2 | 15 | 1 | 38 | 0 | |
| Schizocosa mccooki (Montgomery) | 1 | 3 | 0 | 0 | 4 | 3 | 0 | 0 | 0 | 0 | |
| Philodromidae | Ebo immatures | 0 | 1 | 1 | 11 | 1 | 6 | 0 | 0 | 4 | 13 |
| Ebo pepinensis Gertsch | 3 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 1 | 11 | |
| Ebo punctatus Sauer & Platnick | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 12 | 0 | 0 | |
| Thanatus immatures | 79 | 22 | 21 | 27 | 28 | 37 | 16 | 29 | 18 | 9 | |
| Thanatus coloradensis Keyserling | 48 | 14 | 33 | 42 | 89 | 75 | 63 | 31 | 27 | 18 | |
| Thanatus formicinus (Clerck) | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Thanatus vulgaris Simon | 0 | 0 | 0 | 11 | 0 | 44 | 0 | 3 | 2 | 4 | |
| Salticidae | Pellenes sp. | 3 | 4 | 0 | 1 | 1 | 4 | 0 | 0 | 1 | 1 |
| Phidippus olympus Edwards | 6 | 0 | 6 | 4 | 3 | 2 | 0 | 0 | 1 | 0 | |
| Theridiidae | Euryopis immatures | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Euryopis saukea Levi | 1 | 2 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 3 | |
| Euryopis scriptipes Banks | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Thomisidae | Xysticus immatures | 36 | 58 | 61 | 40 | 59 | 80 | 50 | 49 | 60 | 49 |
| Xysticus apachecus Gertsch | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Xysticus cunctator Thorell | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | |
| Xysticus ellipticus Turnbull, Dondale & Redner | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Xysticus emertoni Keyserling | 0 | 0 | 1 | 0 | 2 | 5 | 0 | 0 | 0 | 1 | |
| Xysticus ferox (Hentz) | 6 | 8 | 57 | 61 | 16 | 24 | 26 | 90 | 8 | 49 | |
| Xysticus montanensis Keyserling | 3 | 13 | 7 | 31 | 1 | 24 | 1 | 60 | 3 | 38 | |
| Xysticus paiutus Gertsch | 0 | 7 | 9 | 14 | 15 | 1 | 27 | 13 | 26 | 9 | |
| Xysticus triguttatus Keyserling | 102 | 12 | 67 | 57 | 70 | 32 | 124 | 43 | 33 | 13 | |
| Opiliones Sclerosomatidae | Leiobunum sp. | 0 | 0 | 0 | 14 | 0 | 27 | 0 | 5 | 0 | 0 |
| Togwoteeus biceps (Thorell) | 0 | 1 | 0 | 22 | 0 | 3 | 1 | 2 | 1 | 2 | |
| Acari Mesostigmata | 5 | 2 | 3 | 17 | 28 | 176 | 91 | 227 | 482 | 356 | |
| Oribatida | 1 | 0 | 1 | 0 | 2 | 5 | 1 | 3 | 0 | 0 | |
| Trombidiformes Anystidae | 32 | 10 | 0 | 2 | 10 | 15 | 1 | 0 | 17 | 9 | |
| Erythraeidae | 2170 | 1698 | 1269 | 382 | 2730 | 678 | 732 | 536 | 798 | 401 |
References
- Southwest Fire Consortium. Las Conchas Fact Sheet. Available online: https://www.swfireconsortium.org/wp-content/uploads/2012/11/Las-Conchas-Factsheet_bsw.pdf (accessed on 11 August 2020).
- Dennison, P.E.; Brewer, S.C.; Arnold, J.D.; Moritz, M.A. Large wildfire trends in the western United States, 1984–2011. Geophys. Res. Lett. 2014, 41, 2928–2933. [Google Scholar] [CrossRef]
- Rother, M.T.; Veblen, T.T. Limited conifer regeneration following wildfires in dry ponderosa pine forests of the Colorado Front Range. Ecosphere 2016, 7, e01594. [Google Scholar] [CrossRef]
- Westerling, A.L.R. Increasing western US forest wildfire activity: Sensitivity to changes in the timing of spring. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [PubMed]
- National Forests Website for CFLRP. Available online: www.nationalforests.org/collaboration-research/learning-tools/ (accessed on 1 March 2020).
- Higgins, J.W.; Cobb, N.S.; Sommer, S.; Delph, R.J.; Brantley, S.L. Ground-dwelling arthropod responses to succession in a pinyon-juniper woodland. Ecosphere 2014, 5, 5. [Google Scholar] [CrossRef]
- Brantley, S.L.; Chapman, C.A.; Cobb, N.S. Influence of Habitat and Region on Spider Communities Along Two Elevation Gradients in the Southwestern USA. In Proceedings of the 12th Biennial Conference on Research on the Colorado River Plateau, Flagstaff, AZ, USA, 16–19 September 2013; Ralston, B.E., Ed.; Scientific Investigations Report; U.S. Geological Survey: Reston, VA, USA, 2016; pp. 59–72. [Google Scholar] [CrossRef]
- Symbiota Collections of Arthropods Network (SCAN). Available online: http://scan-bugs.org/portal/ (accessed on 11 May 2020).
- Wise, D.H. Spiders in Ecological Webs; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar] [CrossRef]
- Ubick, D.; Paquin, P.; Cushing, P.E.; Roth, V. Spiders of North America: An Identification Manual; American Arachnological Society: Keene, NH, USA, 2017. [Google Scholar]
- Uetz, G.W. Habitat Structure and Spider Foraging. In Habitat Structure: The Physical Arrangement of Objects in Space; Bell, S.S., McCoy, E.D., Mushinsky, H.R., Eds.; Chapman and Hall: London, UK, 1991; pp. 325–348. [Google Scholar] [CrossRef]
- Niwa, C.G.; Peck, R.W. Influence of prescribed fire on carabid beetle (Carabidae) and spider (Araneae) assemblages in forest litter in southwestern Oregon. Environ. Entomol. 2002, 31, 785–796. [Google Scholar] [CrossRef]
- Gillette, N.E.; Vetter, R.S.; Mori, S.R.; Rudolph, C.R.; Welty, D.R. Response of ground-dwelling spider assemblages to prescribed fire following stand structure manipulation in the southern Cascade Range. Can. J. For. Res. 2008, 38, 969–980. [Google Scholar] [CrossRef]
- Malmstrom, A.; Persson, T.; Ahlstrom, K.; Gongalsky, K.B.; Bengtsson, J. Dynamics of soil meso- and macrofauna during a 5-year period after clear-cut burning in a boreal forest. Appl. Soil Ecol. 2009, 43, 61–74. [Google Scholar] [CrossRef]
- Koponen, S. Early succession of a boreal spider community after forest fire. J. Arachnol. 2005, 33, 230–235. [Google Scholar] [CrossRef]
- Larrivee, M.; Fahrig, L.; Drapeau, P. Effects of a recent wildfire and clearcuts on ground-dwelling boreal forest spider assemblages. Can. J. For. Res. 2005, 35, 2575–2588. [Google Scholar] [CrossRef]
- Samu, F.; Kadarl, F.; Onodi, G.; Kertesz, M.; Sziranyi, A.; Szita, E.; Fetyko, K.; Neidert, D.; Botos, E.; Altbacker, V. Differential ecological responses of two generalist arthropod groups, spiders and carabid beetles (Araneae, Carabidae), to the effects of wildfire. Community Ecol. 2010, 11, 129–139. [Google Scholar] [CrossRef]
- Smith DiCarlo, L.A.; de Bano, S.J.; Burrows, S. Short-term response of two beneficial invertebrate groups to wildfire in an arid grassland system, United States. Rangel. Ecol. Manag. 2019, 72, 551–560. [Google Scholar] [CrossRef]
- Allen, C.D.; Savage, M.; Falk, D.A.; Suckling, K.F.; Swetnam, T.W.; Schulke, T.; Stacey, P.B.; Morgan, P.; Hoffman, M.; Klingel, J.T. Ecological restoration of southwestern ponderosa pine ecosystems: A broad perspective. Ecol. Appl. 2002, 12, 1418–1433. [Google Scholar] [CrossRef]
- Williams, A.P.; Allen, C.D.; Millar, C.I.; Swetnam, T.W.; Michaelsen, J.; Still, C.J.; Leavitt, S.W. Forest responses to increasing aridity and warmth in the southwestern United States. Proc. Natl. Acad. Sci. USA 2010, 107, 21289–21294. [Google Scholar] [CrossRef] [PubMed]
- Goff, F. Valles Caldera: A Geologic History; University of New Mexico Press: Albuquerque, NM, USA, 2009. [Google Scholar]
- Suazo, M.M.; Collins, S.L.; Parmenter, R.R.; Muldavin, E. Montane valley grasslands are highly resistant to summer wildfire. J. Veg. Sci. 2018, 29, 1017–1028. [Google Scholar] [CrossRef]
- Western Regional Climate Center (WRCC). Available online: https://wrcc.dri.edu (accessed on 11 May 2020).
- National Centers for Environmental Information (Formerly the National Climatic Data Center). Palmer Drought Severity Index. Available online: https://ncdc.noaa.gov/temp-and-precip/drought/historical-palmers/ (accessed on 29 September 2020).
- Topping, C.J.; Sunderland, K.D. Limitations to the use of pitfall traps in ecological studies exemplified by a study of spiders in a field of winter wheat. J. Appl. Ecol. 1992, 29, 485–491. [Google Scholar] [CrossRef]
- Norris, K.C. Quantifying change through time in spider assemblages: Sampling methods, indices and sources of error. J. Insect Conserv. 1999, 3, 309–325. [Google Scholar]
- Krantz, G.W.; Walter, D.E. A Manual of Acarology; Texas Tech University Press: Lubbock, TX, USA, 2009. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA for PRIMER: Guide to Software and Statistical Methods; PRIMER-E. Ltd.: Plymouth, UK, 2008. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Bergeron, J.A.C.; Spence, J.R.; Volney, W.J.A.; Pinzon, J.; Hartley, D.J. Effect of habitat type and pitfall trap installation on captures of epigaeic arthropod assemblages in the boreal forest. Can. Entomol. 2013, 145, 547–565. [Google Scholar] [CrossRef]
- Lowrie, D.C. The microhabitats of western wolf spiders of the genus Pardosa. Entomol. News 1973, 84, 103–116. [Google Scholar]
- Guiterman, C.H.; Margolis, E.Q.; Allen, C.D.; Falk, D.A.; Swetnam, T.W. Long-term persistence and fire resilience of oak shrubfields in dry conifer forests of northern New Mexico. Ecosystems 2018, 21, 943–959. [Google Scholar] [CrossRef]
- Abbott, I.; Burbridge, T.; Strehlow, K.; Mellican, A.; Wills, A. Logging and burning impacts on cockroaches, crickets and grasshoppers, and spiders in Jarrah forest, Western Australia. For. Ecol. Manag. 2003, 174, 383–399. [Google Scholar] [CrossRef]
- Platnick, N.I.; Shadab, M.U. A revision of the American spiders of the genus Micaria (Araneae, Gnaphosidae). Am. Mus. Novit. 1988, 2916, 1–64. [Google Scholar]
- Knudsen, J. Effects of Wildland Fire on ant Community Structure and Colonization of the Valles Caldera National Preserve, New Mexico, USA. Master’s Thesis, Texas Tech University, Lubbock, TX, USA, 2018. [Google Scholar]
- Buddle, C.M.; Spence, J.R.; Langor, D.W. Succession of boreal forest spider assemblages following wildfire and harvesting. Ecography 2000, 23, 424–436. [Google Scholar] [CrossRef]
- Langlands, P.R.; Brennan, K.E.C.; Ward, B. Is the reassembly of an arid spider assemblage following fire deterministic? Austral Ecol. 2012, 37, 429–439. [Google Scholar] [CrossRef]
- Sauer, R.J.; Platnick, N.I. The crab spider genus Ebo (Araneida: Thomisidae) in the United States and Canada. Can. Entomol. 1972, 104, 35–60. [Google Scholar] [CrossRef]
- Ziesche, T.M.; Roth, M. Influence of environmental parameters on small-scale distribution of soil-dwelling spiders in forests: What makes the difference, tree species or microhabitat? For. Ecol. Manag. 2008, 25, 738–752. [Google Scholar] [CrossRef]
- Holmberg, R.G.; Cokendolpher, J.C. Re-description of Togwoteeus biceps (Arachnida, Opiliones, Sclerosomatidae) with notes on its morphology, karyology and phenology. J. Arachnol. 1997, 25, 229–244. [Google Scholar]
- Camann, M.A.; Gillette, N.E.; Lamoncha, K.L.; Mori, S.R. Response of forest soil Acari to prescribed fire following stand structure manipulation in the southern Cascade Range. Can. J. For. Res. 2008, 38, 956–968. [Google Scholar] [CrossRef]
- Platnick, N.I. The spider family Anyphaenidae in America north of Mexico. Bull. Mus. Comp. Zool. 1974, 146, 205–266. [Google Scholar]
- Ferrenberg, S.; Wickey, P.; Coop, J.D. Ground-dwelling arthropod community responses to recent and repeated wildfires in conifer forest of Northern New Mexico, USA. Forests 2019, 10, 667. [Google Scholar] [CrossRef]
- Molina, R.; Marcot, B.G. Definitions and Attributes of Little-Known Species. In Conservation of Rare or Little-Known Species: Biological, Social, and Economic Considerations; Raphael, M.G., Molina, R., Eds.; Island Press: Washington, DC, USA, 2007; pp. 67–92. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).