Abstract
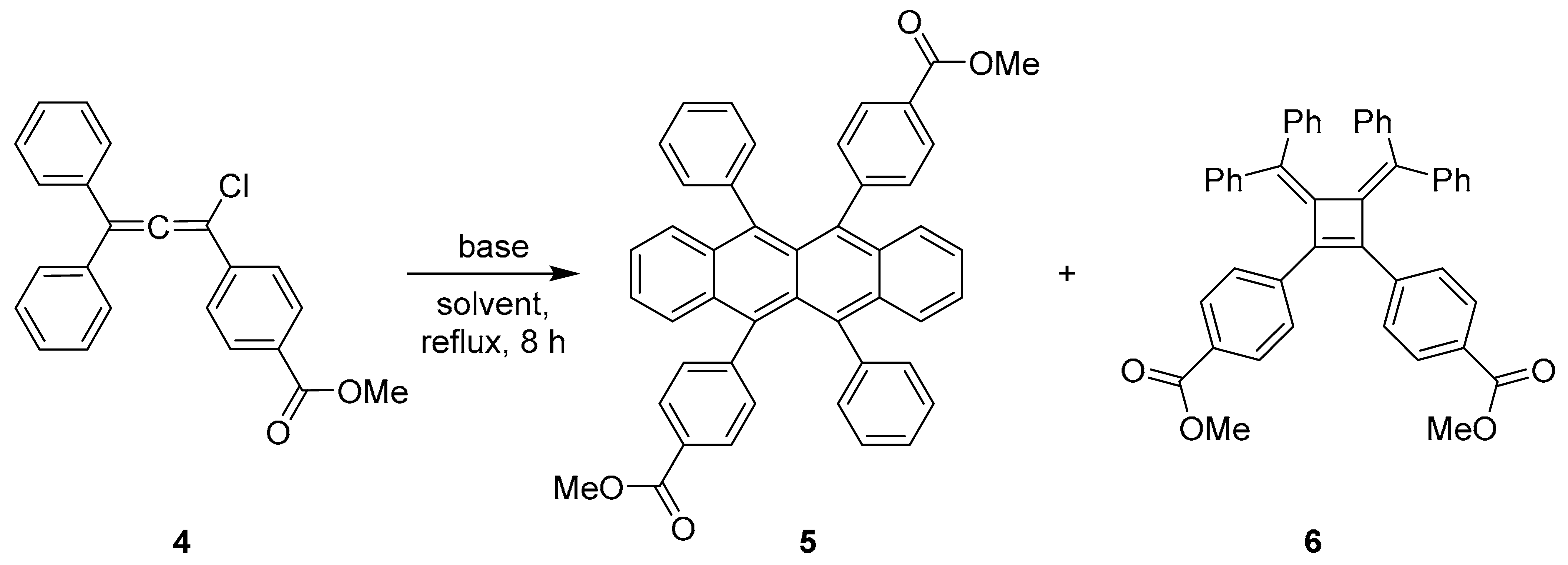
A symmetrical rubrene derivative, 5,6-bis(4-(methoxycarbonyl)phenyl)-11,12-diphenyltetracene, was synthesized via the thermal dimerization of 1,1-diphenyl-3-[4-(methoxycarbonyl)phenyl]-3-chloroallene. The reaction proceeded with the low selectivity typical of the classical “rubrenic synthesis” under these conditions, affording the target tetracene and the bis(alkylidene)cyclobutene by-product in nearly equal yields of 25% each. The optical characteristics of this rubrene derivative were investigated, revealing bright orange fluorescence in a CHCl3 solution (λem = 565 nm, ΦF = 0.81, τ = 11.41 ns), which is strongly quenched in the solid state (ΦF = 0.01) due to aggregation.
1. Introduction
Rubrene (5,6,11,12-tetraphenyltetracene) holds a legendary status in materials science due to its exceptional charge carrier mobility in single crystals [1,2,3]. However, despite its prominence and a history dating back to its first isolation by Moureu and Dufraisse in 1926 [4], the synthetic chemistry of rubrene has not kept pace with the interest in its physical properties. The structure of rubrene was initially misassigned before being correctly identified as a tetracene derivative by Dufraisse and Velluz [5]. As highlighted in a recent review by Douglas and co-workers [6], synthetic approaches to rubrene and its derivatives have remained surprisingly limited. Multi-step approaches, including Diels–Alder cycloadditions [7] and cross-coupling reactions with pre-formed tetracene cores [8], offer greater control but often involve lengthy sequences with specific limitations [6]. The most direct method remains the classical one-pot synthesis involving dimerization of a 1,1,3-triaryl-3-chloroallene intermediate generated from the corresponding propargyl alcohol [4,6]. While valued for its simplicity, this “rubrenic synthesis” is complicated by a competing pathway that leads to bis(alkylidene)cyclobutene by-products, a common impurity in commercial rubrene [9]. The mechanistic picture, refined by Rigaudy and Capdevielle [10], suggests a delicate equilibrium between the tetracene and cyclobutene frameworks. According to a pivotal study by Braga, the reaction outcome correlates with the electronic nature of the substituents on the chloroallene, such that strong electron-donating groups (e.g., diethylamino) favored the cyclobutene, whereas electron-withdrawing or neutral substituents afforded the desired tetracene framework [9].
Despite the development of modern methods, this classical pathway remains highly relevant for the straightforward preparation of symmetrical rubrene derivatives [6,11], driven by the need to tailor rubrene properties for applications in organic electronics and as an annihilator in triplet-triplet annihilation upconversion (TTA-UC) [12,13,14]. Functionalization of the rubrene core, particularly with electron-withdrawing groups (EWGs), is a recognized strategy to modulate its electronic structure and suppress detrimental processes like singlet fission [14,15]. While, for instance, nitro- or cyano-substituted rubrene derivatives have been synthesized via the 3-chloroallene route [11], this work focused exclusively on the tetracene product and did not report the formation of the cyclobutene byproduct, implying high selectivity. However, the behavior of moderate EWGs, such as esters, on the critical 3-chloroallene dimerization step remains underexplored. To the best of our knowledge, rubrene derivatives bearing ester substituents, whether on the peripheral phenyl rings or directly on the tetracene core, have not been described in the literature. The synthesis and photophysical characterization of a symmetrically disubstituted ester functionalized rubrene via the classical allene route reported herein therefore represent a novel addition to the family of functionalized tetraaryltetracenes.
Herein, we report the synthesis and comprehensive characterization of a novel symmetrically disubstituted rubrene derivative bearing methoxycarbonyl groups at the para-positions of its 5- and 11-phenyl moieties.
2. Results and Discussion
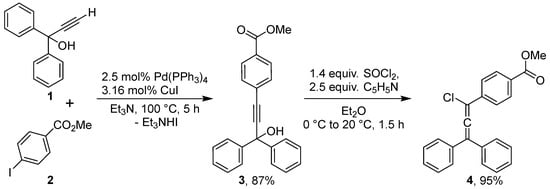
Our synthetic approach to the target methoxycarbonyl-substituted rubrene is outlined in Scheme 1. The synthesis commenced with a Sonogashira cross-coupling between commercially available 1,1-diphenylprop-2-yn-1-ol (1) and methyl 4-iodobenzoate (2) using a catalytic system of Pd(PPh3)4 and CuI in a solution of triethylamine. The reaction proceeded smoothly to afford the propargyl alcohol 3 in 87% yield. Subsequent treatment of alcohol 3 with thionyl chloride in the presence of pyridine in dry Et2O gave 3-chloroallene 4, which was isolated in 95% yield. It should be noted that the resulting 3-chloroallene turned out to be quite stable at room temperature and was even characterized by NMR spectroscopy and HRMS. This is evidence of the stabilization of its molecule due to the presence of the electron-withdrawing methoxycarbonyl group in the phenyl moiety at C-3 atom of the allene structure.
Scheme 1.
Synthesis of 3-chloroallene 4.
The critical thermal dimerization of 3-chloroallene 4 was then investigated. This reaction is known to proceed via a complex mechanism that can lead to the desired rubrene derivative 5 and a bis(alkylidene)cyclobutene by-product 6 [9,10]. We examined this transformation under various conditions. When the reaction was conducted in p-xylene with collidine as a base under reflux (Table 1, entry 1), the desired rubrene derivative 5 was obtained in a mere 3% yield after work-up and purification, while the cyclobutene 6 was isolated in 28% yield. Omitting the base and switching to chlorobenzene under reflux (Table 1, entry 2) led to a marked improvement for the tetracene, yielding rubrene 5 in 23%, with the yield of cyclobutene 6 being 25%. The best result for the target compound was achieved under reflux in o-dichlorobenzene without base (Table 1, entry 3), affording compound 5 in 25% isolated yield; cyclobutene 6 was also obtained in 25% yield. A striking and consistent feature across all experiments was the formation of substantial amounts of by-product 6, which was separated from the target rubrene by crystallization from acetone. Thus, all our attempts to improve selectivity toward the tetracene product resulted in comparable formation of rubrene 5 and cyclobutene 6, indicating that the methoxycarbonyl substituent does not notably affect the competition between the two reaction pathways under the investigated conditions.

Table 1.
Thermal dimerization–cyclization of 3-chloroallene 4.
The 1H NMR spectra of synthesized compounds 5 and 6 were recorded in both CDCl3 and DMSO-d6, providing a consistent set of diagnostic signals for structural elucidation (for full spectra, see Supplementary Materials). The spectrum of rubrene 5 in CDCl3 was in full agreement with the proposed highly symmetric tetracene framework. It showed two characteristic doublets for the ortho-protons of the methoxycarbonyl-substituted phenyl rings at 7.74 and 6.96 ppm (each J = 8.1 Hz, 4H each), and a sharp singlet for the methoxy groups at 3.98 ppm. The protons of the tetracene core and the unsubstituted phenyl rings gave rise to a series of complex multiplets in the range of 7.40–6.84 ppm. The corresponding spectrum in DMSO-d6 confirmed this assignment, with the key doublets observed at 7.68 and 6.99 ppm (J = 8.1 Hz) and the methoxy singlet at 3.91 ppm. In clear contrast, the spectrum of the cyclobutene 6 in CDCl3 exhibited a distinct pattern: two characteristic doublets for the ester-substituted rings at 7.52 and 6.58 ppm (each J = 8.4 Hz, 4H each), a methoxy singlet at 3.85 ppm, and the aromatic protons of the diphenylmethylene moieties appearing as multiplets between 7.08 and 6.71 ppm. The spectrum in DMSO-d6 showed a corresponding profile with doublets at 7.44 and 6.60 ppm (J = 8.5 Hz), a methoxy singlet at 3.78 ppm, and a set of multiplets for the diphenylmethylene protons between 7.13 and 6.72 ppm. The distinct and reproducible 1H NMR patterns observed for compounds 5 and 6 in both solvents were fully consistent with the proposed tetracene and cyclobutene structures, respectively, enabling their clear identification.
The optical characteristics of the new rubrene derivative 5 were examined in a CHCl3 solution and in the solid state (Table 2). In solution, compound 5 exhibits well-defined vibronic absorption bands with a dominant maximum at 529 nm, typical for tetracene-based chromophores with extended conjugation, and emits at 565 nm with a moderate Stokes shift, indicative of limited excited-state relaxation (Figure 1). The high fluorescence quantum yield of 0.81 and the long excited-state lifetime of 11.41 ns confirm efficient radiative deactivation and a relatively low non-radiative decay rate, showing that moderately electron-withdrawing methoxycarbonyl substituents preserve the emissive character of the tetracene core. A comparison with cyano-functionalized rubrene 2CN-Rub [15] reveals a clear structure–property contrast: while both substituent types reduce electron density, the strong acceptor CN group significantly enhances non-radiative decay and leads to a considerable loss of emission efficiency in solution (Φ = 0.56 reported for 2CN-Rub). This difference suggests that derivative 5 maintains a more favorable balance between radiative and non-radiative processes. Although triplet–triplet annihilation behavior was not yet explored for compound 5, its moderate acceptor strength may lead to a distinct influence on triplet energy levels and annihilation pathways compared to strongly electron-deficient CN analogs, which were shown to increase the statistical probability of singlet formation via TTA in solid-state photonic applications [15].

Table 2.
Photophysical properties of the compound in solution (CHCl3) and solid state.
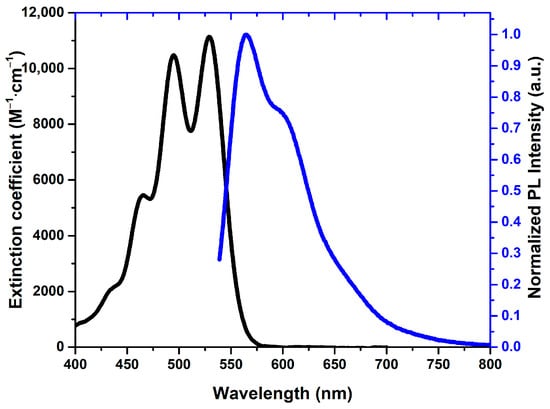
Figure 1.
Absorption (black line) and fluorescence (blue line) spectra of rubrene 5 in a CHCl3 solution.
In the solid state, compound 5 demonstrates a markedly different behavior. Both absorption and emission spectra undergo pronounced bathochromic shifts (547 and 618 nm, respectively), consistent with strong π–π interactions in the aggregated phase. However, this packing causes severe aggregation-induced quenching: the quantum yield sharply drops to 0.01 and the lifetime decreases to 2.17 ns, indicating highly efficient non-radiative decay triggered by exciton coupling. This result stands in contrast to certain cyano-functionalized rubrenes, which can retain significant solid-state emission. However, it should be noted that such differences in quenching behavior may be dominated by structural packing effects rather than purely electronic influences of the substituents. While the methoxycarbonyl groups in rubrene 5 allow the material to exhibit high photoluminescence efficiency in solution, they likely lead to a packing motif in the condensed phase that facilitates exciton-loss processes. Overall, the data show that compound 5 is a highly efficient emitter in solution but becomes limited by aggregation-induced non-radiative decay in the solid state. This suggests that optimizing the emissive performance of such derivatives for solid-state applications requires careful control over crystal packing and intermolecular distances to suppress non-radiative pathways.
3. Materials and Methods
1H and 13C NMR spectra were recorded on Bruker AVANCE-500 (500 MHz) (Bruker BioSpin, Ettlingen, Germany), in CDCl3 and DMSO-d6 with SiMe4 as an internal standard. High-resolution mass spectra were obtained on a Bruker maXis Impact HD spectrometer (Bruker BioSpin, Germany). Unless otherwise stated, all reagents were purchased from commercial sources and used without further purification. Melting points were determined on combined heating stages and were uncorrected.
Synthesis of methyl 4-(3-hydroxy-3,3-diphenylprop-1-yn-1-yl)benzoate (3). A mixture of 1,1-diphenylprop-2-yn-1-ol (1, 1.05 g, 5.05 mmol), methyl 4-iodobenzoate (2, 1.31 g, 5.0 mmol), CuI (30 mg, 0.16 mmol), and Pd(PPh3)4 (150 mg, 0.125 mmol) in triethylamine (22 mL) was placed in a 50 mL Schlenk tube equipped with a reflux condenser. The mixture was deoxygenated by four cycles of vacuum/argon flushing, then stirred and heated at 100 °C under an argon atmosphere for 6 h. After cooling, diethyl ether (30 mL) was added, and the mixture was filtered through a short pad of silica gel (approx. 4 × 1 cm), eluting with additional ether. The filtrate was concentrated under reduced pressure, and the residue was crystallized from isopropanol to afford 3 as a beige powder.
Yield: 1.49 g (87%); m.p. 140–141 °C. 1H NMR (500 MHz, CDCl3) δ 8.00 (d, J = 8.4 Hz, 2H), 7.69–7.63 (m, 4H), 7.57 (d, J = 8.4 Hz, 2H), 7.40–7.33 (m, 4H), 7.32–7.27 (m, 2H), 3.92 (s, 3H), 2.96 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 166.6, 144.8, 131.9, 130.1, 129.6, 128.5, 128.0, 127.2, 126.2, 94.8, 86.5, 75.0, 52.4. HRMS (ESI) m/z calcd for C23H18O3Na [M + Na]+: 365.1148; found: 365.1148.
Synthesis of methyl 4-(1-chloro-3,3-diphenylprop-1,2-dien-1-yl)benzoate (4). Propargyl alcohol 3 (2.05 g, 6.00 mmol) was dissolved in dry diethyl ether (75 mL) and cooled in an ice bath. Dry pyridine (1.20 mL, 14.9 mmol) was added, followed by dropwise addition of freshly distilled thionyl chloride (0.60 mL, 8.3 mmol). The mixture was stirred for 1 h at ambient temperature. The precipitated pyridine hydrochloride was removed by filtration and washed with hexane (2 × 5 mL). The combined organic filtrates were washed with 6 M aqueous HCl (40 mL), followed by water (2 × 15 mL). The organic layer was dried over anhydrous CaCl2, filtered through a small silica gel pad, and concentrated under reduced pressure. The product was obtained as a gray powder, was found to be pure by NMR analysis, and was used in the next step without further purification.
Yield: 2.057 g (95%); m.p. 121–122 °C. 1H NMR (500 MHz, CDCl3) δ 8.04 (d, J = 8.7 Hz, 2H), 7.71 (d, J = 8.7 Hz, 2H), 7.52–7.32 (m, 10H), 3.92 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 166.7, 138.2, 134.8, 130.0, 130.0, 129.2, 129.0, 128.9, 126.3, 120.1, 107.4, 52.3. HRMS (ESI) m/z calcd for C23H18ClO2 [M + H]+: 361.0990; found: 361.0990.
Procedure for the thermal dimerization of 3-chloroallene 4 to give rubrene 5 and cyclobutene 6. 3-Chloroallene 4 (1.80 g, 5.00 mmol) was dissolved in o-dichlorobenzene (30 mL). The solution was heated at reflux for 8 h and then left to stand overnight at room temperature. The solvent was removed by evaporation under reduced pressure. The resulting crude residue was dissolved in benzene (10 mL), and acetone (50 mL) was added. The volume of the solution was reduced to approximately 30 mL by evaporation on air in a fume hood with gentle heating. The mixture was then allowed to stand at ambient temperature for 10 min, and the precipitated rubrene 5 was collected by filtration, washed with cold acetone (10 mL) and dried at 120 °C. The mother liquor was concentrated under reduced pressure. The resulting solid residue was dissolved in toluene (5 mL), and methanol (15 mL) was added to precipitate cyclobutene 6, which was collected by filtration. If necessary, the products were further purified by recrystallization from acetone (for 5) or a toluene/methanol mixture (for 6) to afford analytically pure samples.
Dimethyl 4,4′-(5,6,11,12-tetraphenyltetracene-5,11-diyl)dibenzoate (5). Isolated as reddish microcrystals.
Reddish crystals, yield 0.405 g (25%), m.p. 301–302 °C. 1H NMR (500 MHz, CDCl3) δ 7.74 (d, J = 8.1 Hz, 4H), 7.39–7.33 (m, 2H), 7.28–7.23 (m, 2H), 7.17–7.08 (m, 6H), 7.06–7.01 (m, 4H), 6.96 (d, J = 8.1 Hz, 4H), 6.87 (d, J = 7.0 Hz, 4H), 3.98 (s, 6H). 1H NMR (500 MHz, DMSO-d6) δ 7.68 (d, J = 8.1 Hz, 4H), 7.29–7.13 (m, 10H), 7.11–7.04 (m, 4H), 6.99 (d, J = 8.1 Hz, 4H), 6.89–6.83 (m, 4H), 3.91 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 167.4, 147.1, 141.5, 137.5, 136.1, 132.5, 132.3, 130.5, 130.1, 128.9, 128.6, 127.6, 127.5, 126.8, 126.5, 126.2, 125.6, 125.3, 52.2. HRMS (ESI) m/z calcd for C46H32O4Na [M + Na]+: 671.2193; found: 671.2193.
Dimethyl 4,4′-(3,4-bis(diphenylmethylene)cyclobut-1-ene-1,2-diyl)dibenzoate (6).
Yellowish needles, yield 0.408 g (25%), m.p. 238–239 °C. m.p. 238–239 °C. 1H NMR (500 MHz, CDCl3) δ 7.52 (d, J = 8.4 Hz, 4H), 7.08–6.90 (m, 14H), 6.87–6.80 (m, 2H), 6.78–6.71 (m, 4H), 6.58 (d, J = 8.4 Hz, 4H), 3.85 (s, 6H). 1H NMR (400 MHz, DMSO-d6) δ 7.44 (d, J = 8.5 Hz, 4H), 7.13–7.04 (m, 2H), 7.03–6.92 (m, 8H), 6.92–6.81 (m, 6H), 6.81–6.72 (m, 4H), 6.60 (d, J = 8.5 Hz, 4H), 3.78 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 167.0, 154.3, 141.4, 140.8, 139.6, 137.4, 131.8, 131.7, 131.5, 128.5, 128.1, 127.9, 127.6, 127.4, 127.3, 127.2, 52.1. HRMS (ESI) m/z calcd for C46H35O4Na [M + Na]+: 673.2349; found: 673.2349.
4. Conclusions
In summary, we have presented a full characterization of a new methoxycarbonyl-substituted rubrene derivative, a noteworthy addition to the limited family of functionalized (tetraaryl)tetracenes. The synthetic study revealed that the ester group, a moderate electron-withdrawing substituent, disrupts the expected selectivity of the classical dimerization, leading to the competitive formation of both the tetracene and cyclobutene frameworks. This finding refines the mechanistic understanding of rubrenic synthesis and serves as a practical guide for the design of similar derivatives. The distinct photophysical profile of the new compound, with its high emission efficiency in solution and strong quenching in the solid state, defines its potential niche for future applications. Its excellent solubility and bright fluorescence make it a promising candidate as a solution processable emissive dopant or a sensitizer in liquid phase systems, such as in triplet–triplet annihilation upconversion where high solution quantum yields are critical. The severe aggregation-induced quenching observed in the solid state, while limiting for thin film light emitting devices, could be turned into an advantage. This property, combined with the synthetic accessibility of both the tetracene and its cyclobutene isomer, positions this system as an interesting model for fundamental studies of solid-state exciton dynamics and for developing strategies to control molecular packing. Further work could focus on modifying the ester group or the molecular core to engineer crystal structures that mitigate quenching, potentially unlocking the material’s utility in solid state photonics and organic electronics.
Supplementary Materials
1H and 13C NMR spectra copies of synthesized compounds can be found in the File Supplementary Materials.
Author Contributions
Conceptualization, R.A.I. and A.R.T.; Methodology, R.A.I. and A.S.S.; Investigation, R.A.I., A.S.S., G.L.R., I.R.S., A.E.A. and A.R.T.; Resources, G.L.R. and A.R.T.; Data Curation, R.A.I., A.S.S., I.R.S. and A.E.A.; Writing—Original Draft Preparation, R.A.I. (synthesis and mechanistic discussion) and A.S.S. (photophysical analysis); Writing—Review and Editing, Visualization, R.A.I. and A.R.T.; Supervision, G.L.R. and A.R.T.; Project Administration, R.A.I.; Funding Acquisition, A.R.T. All authors have read and agreed to the published version of the manuscript.
Funding
The elaboration and synthesis of compounds were performed with the financial support of the Russian Science Foundation (Project no. 23-19-00884). The analytical study of compounds was carried out with the financial support of the Ministry of Education and Science of the Russian Federation within the framework of the state assignment (subject no. state. reg. 124020100137-7).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
Analytical studies were carried out using equipment of the Center for Joint Use “Spectroscopy and Analysis of Organic Compounds” at the Postovsky Institute of Organic Synthesis of the Russian Academy of Sciences (Ural Division).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Podzorov, V.; Menard, E.; Rogers, J.A.; Gershenson, M.E. Hall Effect in the Accumulation Layers on the Surface of Organic Semiconductors. Phys. Rev. Lett. 2005, 95, 226601. [Google Scholar] [CrossRef] [PubMed]
- Sundar, V.C.; Zaumseil, J.; Podzorov, V.; Menard, E.; Willett, R.L.; Someya, T.; Gershenson, M.E.; Rogers, J.A. Elastomeric Transistor Stamps: Reversible Probing of Charge Transport in Organic Crystals. Science. 2004, 303, 1644–1646. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.E. Functionalized Acenes and Heteroacenes for Organic Electronics. Chem. Rev. 2006, 106, 5028–5048. [Google Scholar] [CrossRef] [PubMed]
- Moureu, C.; Dufraisse, C.; Dean, P.M. Sur un hydrocarbure coloré: Le rubrène. C. R. Acad. Sci. 1926, 182, 1440. [Google Scholar]
- Dufraisse, C.; Velluz, L. La nouvelle rubrène et la constitution des rubrènes. C. R. Acad. Sci. 1935, 201, 1394. [Google Scholar]
- Clapham, M.L.; Murphy, E.C.; Douglas, C.J. Synthesis and Crystal Engineering of Rubrene and Its Derivatives. Synthesis 2021, 53, 461–474. [Google Scholar] [CrossRef]
- Dodge, J.A.; Bain, J.D.; Chamberlin, A.R. Regioselective synthesis of substituted rubrenes. J. Org. Chem. 2002, 55, 4190–4198. [Google Scholar] [CrossRef]
- McGarry, K.A.; Xie, W.; Sutton, C.; Risko, C.; Wu, Y.; Young, V.G.; Brédas, J.L.; Frisbie, C.D.; Douglas, C.J. Rubrene-based single-crystal organic semiconductors: Synthesis, electronic structure, and charge-transport properties. Chem. Mater. 2013, 25, 2254–2263. [Google Scholar] [CrossRef]
- Braga, D.; Jaafari, A.; Miozzo, L.; Moret, M.; Rizzato, S.; Papagni, A.; Yassar, A. The rubrenic synthesis: The delicate equilibrium between tetracene and cyclobutene. European J. Org. Chem. 2011, 2011, 4160–4169. [Google Scholar] [CrossRef]
- Rigaudy, J.; Capdevielle, P. Dimerisation des polyphenyl-allenes—I: Chloropolyphenyl-allenes: Mecanisme de la reaction “Rubrenique”. Tetrahedron 1977, 33, 767–773. [Google Scholar] [CrossRef]
- Uttiya, S.; Miozzo, L.; Fumagalli, E.M.; Bergantin, S.; Ruffo, R.; Parravicini, M.; Papagni, A.; Moret, M.; Sassella, A. Connecting molecule oxidation to single crystal structural and charge transport properties in rubrene derivatives. J. Mater. Chem. C 2014, 2, 4147–4155. [Google Scholar] [CrossRef]
- Ravetz, B.D.; Pun, A.B.; Churchill, E.M.; Congreve, D.N.; Rovis, T.; Campos, L.M. Photoredox catalysis using infrared light via triplet fusion upconversion. Nature 2019, 565, 343–346. [Google Scholar] [CrossRef]
- Musser, A.J.; Clark, J. Triplet-Pair States in Organic Semiconductors. Annu. Rev. Phys. Chem. 2019, 70, 323–351. [Google Scholar] [CrossRef]
- Fallon, K.J.; Churchill, E.M.; Sanders, S.N.; Shee, J.; Weber, J.L.; Meir, R.; Jockusch, S.; Reichman, D.R.; Sfeir, M.Y.; Congreve, D.N.; et al. Molecular Engineering of Chromophores to Enable Triplet–Triplet Annihilation Upconversion. J. Am. Chem. Soc. 2020, 142, 19917–19925. [Google Scholar] [CrossRef] [PubMed]
- Radiunas, E.; Naimovičius, L.; Baronas, P.; Jozeliūnaitė, A.; Orentas, E.; Kazlauskas, K. CN-Tuning: A Pathway to Suppress Singlet Fission and Amplify Triplet-Triplet Annihilation Upconversion in Rubrene. Adv. Opt. Mater. 2025, 13, 2403032. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.