Abstract
The title compound has been fully characterised by NMR for the first time. Fully assigned 1H and 13C NMR spectra, and the X-ray structure of two different polymorphs are presented. The polymorphs show similar molecular geometries but exhibit significantly different patterns of intermolecular interactions.
1. Introduction
A pioneering study in 1971 described the treatment of 2-ethoxydihydrobenzoxazinone 1 with boron trifluoride diethyl etherate in diethyl ether as a way of generating the reactive parent heterocycle 1,3-benzoxazin-4-one 2, which could be trapped in a Diels–Alder reaction with an added diene (Scheme 1) []. We have recently reinvestigated this chemistry and have been able to characterise a range of Diels–Alder adducts of 2, as well as obtain direct spectroscopic evidence for 2 itself for the first time. These studies will be described in detail elsewhere, but on a number of occasions a crystalline side-product was obtained in low yield from attempted cycloaddition reactions of 2, which turned out to be the title compound 3. This has only been described once before [], when it was formed by direct reaction of salicylamide with boron trifluoride diethyl etherate in diethyl ether and characterised by its melting point, elemental analysis, IR spectrum and low resolution 1H and 11B NMR data. The precursor 1 is prepared by condensation of salicylamide with triethyl orthoformate and is somewhat moisture sensitive []. We assume that in the attempted Diels–Alder reactions of 2, competing hydrolysis of 1 to salicylamide is followed by reaction of the latter with BF3 etherate to give 3. In this paper we present full NMR characterisation of 3 as prepared by the literature method, as well as X-ray structure determination of the crystals obtained from two separate reactions of 1, leading to two different polymorphs.
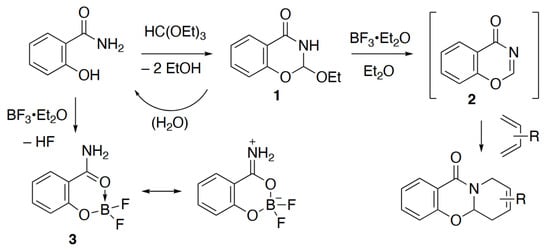
Scheme 1.
Formation of the title compound 3 in reactions of 1 and directly from salicylamide.
2. Results
The compound 3 was readily prepared for spectroscopic study by stirring a mixture of salicylamide and BF3·Et2O in diethyl ether at 0 °C. The resulting solid showed a melting point in good agreement with the literature value []. It was readily soluble in CDCl3, but since the only literature NMR data were recorded in CD3OD, we studied it also in that solvent. The simple one-dimensional spectra gave chemical shifts in the expected range for 1H, 13C, 11B and 19F and by obtaining COSY, HSQC and HMBC spectra (see Supplementary Materials) a complete unambiguous assignment was possible (Figure 1) showing good agreement between the data in the two different solvents.
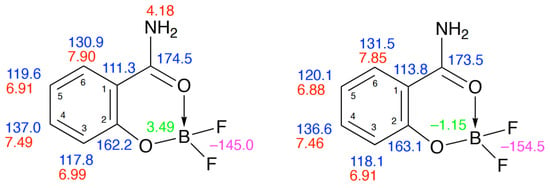
Figure 1.
1H (red), 13C (blue), 11B (green) and 19F (purple) NMR data for 3 in CDCl3 (left) and CD3OD (right).
Although the 1H NMR spectra showed fairly complex patterns, particularly in CD3OD due to peak overlap, simulation (see Supplementary Materials) allowed determination of all CH–CH coupling constants with good agreement between the values in the two solvents (Table 1).

Table 1.
Values of CH–CH coupling constants for 3 (Hz, numbering as in Figure 1).
Although it is difficult to find similar structures for comparison, the data for 3 agree very well with those for the silylated analogues 4–6 [], such that we are able to assign the signals for these also with a fair degree of certainty (Figure 2), something not done by the original authors.
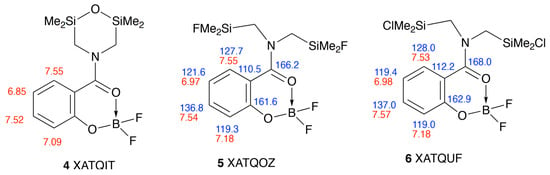
Figure 2.
Structure of literature analogues [] with NMR assignments and CSD Refcodes.
As mentioned in the introduction, crystals of 3 suitable for X-ray diffraction were obtained in low yield from reaction of 1 with BF3·Et2O in the presence of 1,3-dienes. Perhaps unexpectedly, the samples obtained with cyclohexa-1,3-diene (“Form A”) and 2,3-dimethylbuta-1,3-diene (“Form B”) gave different structures (Figure 3) with similar molecular dimensions (Table 2). Form B forms N-H···O (8) hydrogen-bonded dimers (Table 3), while Form A forms a series of weaker N-H···F hydrogen bonds. The stronger hydrogen bonding in Form B takes some of the electron density away from the O(2)–B(3) interaction, resulting in longer O(2)–B(3) and shorter B(3)–O(4) distances as compared to Form A.
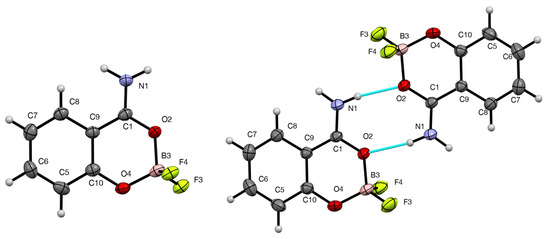
Figure 3.
Molecular structures of 3, Form A (left) and Form B (right), showing the numbering system used, 50% probability ellipsoids and hydrogen bonding in Form B.
A search of the Cambridge Structural Database (CSD) showed only very few comparable structures. In addition to the three silicon-containing analogues 4–6 [] already mentioned, the tricyclic analogue 7 [] and a dioxane adduct of the oxime analogue 8 [] show some structural similarities (Figure 4). Worthy of particular mention is what appears on the face of it to be an 18-crown-6 adduct of 3 [], but the structure of this is described both in the paper and the structure deposited at the CSD as 9, an ammonium cation hydrogen bonded to the crown ether but with no counter-ion. It seems clear that the authors have solved the structure with an extra proton on N, which is not in fact present. Unsurprisingly, the dimensions around the heterocyclic ring (Table 2) are remarkably similar to those for both forms of 3.



Table 2.
Molecular dimensions for 3 and 9 [] (Å, °).
Table 2.
Molecular dimensions for 3 and 9 [] (Å, °).
| Bond Lengths (Å) | 3 Form A | 3 Form B | 9 |
|---|---|---|---|
| C(1)–N(1) | 1.304(3) | 1.3049(14) | 1.297(3) |
| C(1)–O(2) | 1.298(3) | 1.2949(13) | 1.284(3) |
| O(2)–B(3) | 1.496(3) | 1.5163(15) | 1.505(4) |
| B(3)–O(4) | 1.446(4) | 1.4332(16) | 1.449(4) |
| O(4)–C(10) | 1.348(3) | 1.3444(14) | 1.340(3 |
| C(10)–C(9) | 1.409(4) | 1.4052(15) | 1.395(3) |
| C(9)–C(1) | 1.448(4) | 1.4476(16) | 1.461(4) |
| B(3)–F(3) | 1.383(3) | 1.3641(16) | 1.372(4) |
| B(3)–F(5) | 1.398(4) | 1.3926(16) | 1.375(4) |
| Angles (°) | |||
| C(9)–C(1)–O(2) | 120.7(2) | 120.03(9) | 119.6(2) |
| C(9)–C(1)–N(1) | 122.2(3) | 123.31(10) | 122.8(2) |
| O(2)–C(1)–N(1) | 117.1(3) | 116.66(10) | 117.6(2) |
| C(1)–O(2)–B(3) | 121.5(2) | 121.46(9) | 122.3(2) |
| O(2)–B(3)–O(4) | 112.1(2) | 110.77(9) | 111.5(2) |
| B(3)–O(4)–C(10) | 119.8(2) | 119.04(9) | 118.3(2) |
| O(4)–C(10)–C(9) | 121.0(3) | 121.57(10) | 121.8(2) |
| C(10)–C(9)–C(1) | 117.4(2) | 117.43(10) | 117.2(2) |

Table 3.
Hydrogen bonding parameters for 3 (Å, °).
Table 3.
Hydrogen bonding parameters for 3 (Å, °).
| D–H···A | D–H | H···A | D···A | D–H···A | Symmetry Operator |
|---|---|---|---|---|---|
| Form A | |||||
| N(1)–H(1B)···F(4) | 0.93(3) | 1.91(3) | 2.829(3) | 170(3) | 2 − x, 1⁄2 + y, 3⁄2 − z |
| N(1)–H(1A)···F(3) | 0.95(3) | 2.02(3) | 2.919(3) | 159(3) | x, 3⁄2 − y, 1⁄2 − z |
| Form B | |||||
| N(1)–H(1A)···O(2) | 0.929(15) | 2.088(15) | 3.0074(14) | 170.1(13) | 1 − x, −y, 1 − z |
| N(1)–H(1B)···F(4) | 0.910(14) | 2.009(14) | 2.8690(12) | 156.9(14) | 1 + x, y, z |
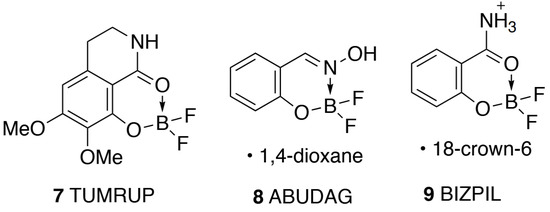
Figure 4.
Other comparable structures with CSD Refcodes.
The crystal structure of Form A consists of slip-stacked sheets in the (1 0 0) plane (Figure 5) formed by (12) rings of N–H···F hydrogen bonds (Table 3). In contrast, Form B’s hydrogen-bonded dimers link together through non-classical C–H···O hydrogen bonds to form sheets in the (1 0 1) plane, which stack together through N–H···F hydrogen bonds (Figure 5, Table 3).
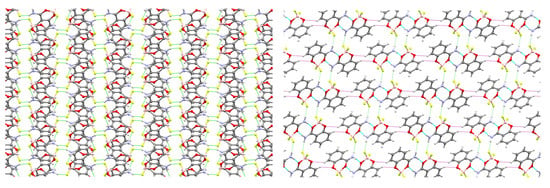
Figure 5.
Structures of 3 showing packing in the crystal: (left) Form A viewed along the a-axis, (right) Form B viewed along the b-axis. N–H···O (blue), N–H···F (green), and C–H···O (violet) hydrogen bonds shown as dashed lines.
In summary, we have been able to record and fully assign the 1H and 13C NMR spectra for compound 3 for the first time. The X-ray structures of two quite different polymorphs have been determined, with only one of them consisting of N–H···O hydrogen-bonded dimers.
3. Experimental
3.1. General Experimental Details
Melting points were recorded on a Reichert hot-stage microscope (Reichert, Vienna, Austria) and are uncorrected. NMR spectra were obtained using a Bruker AV II 400 instrument (Bruker, Billerica, MA, USA). Spectra were run in CDCl3 or CD3OD and chemical shifts are reported in ppm to high frequency of the reference and are referenced to internal Me4Si (δ 0.00) for 1H, to the solvent signal (δ 77.0 or 49.0) for 13C, to external CFCl3 for 19F and external BF3·Et2O for 11B, and coupling constants are in Hz. NMR spectra were processed using iNMR reader, version 6.3.3 (Mestrelab Research, Santiago de Compostela, Spain). Salicylamide was prepared by heating a mixture of methyl salicylate and aqueous ammonia under reflux for 18 h and filtering off the crystalline product obtained upon cooling [].
3.2. Synthesis of 2-(Difluoroboryloxy)benzamide 3
A solution of salicylamide (1.37 g, 10 mmol) in diethyl ether (20 mL) was stirred at 0 °C while boron trifluoride diethyl etherate (1.13 mL, 1.42 g, 10 mmol) was added. The mixture was stirred and allowed to warm to RT over 18 h. The resulting precipitate was filtered off to give the product (1.25 g, 68%) as colourless crystals, mp 175–178 °C (lit. [] 176–178 °C). 1H NMR (400 MHz, CDCl3): δ 7.90 (1H, dd, J 8.0, 1.6, 6-H), 7.49 (1H, ddd, J 8.4, 7.2, 1.6, H-4), 6.99 (1H, dd, J 8.4, 0.8, H-3), 6.91 (1H, ddd, J 8.0, 7.2, 0.8, H-5) and 4.18 (2H, br s, NH2); 13C NMR (100 MHz, CDCl3): δ 174.5 (C=O), 162.2 (O–C=), 137.0 (4-CH), 130.9 (6-CH), 119.6 (5-CH), 117.8 (3-CH) and 111.3 (1-C); 19F NMR (376 MHz, CDCl3): δ –145.0; 11B NMR (128 MHz, CDCl3): δ 3.48; 1H NMR (400 MHz, CD3OD): δ 7.85 (1H, ddd, J 8.0, 2.0, 0.4, 6-H), 7.46 (1H, ddd, J 8.4, 7.2, 2.0, H-4), 6.91 (1H, ddd, J 7.2, 1.2, 0.4, H-3) and 6.885 (1H, ddd, J 8.4, 8.0, 1.2, H-5); 13C NMR (100 MHz, CD3OD): δ 173.5 (C=O), 163.1 (O–C=), 136.6 (4-CH), 131.5 (6-CH), 120.1 (5-CH), 118.1 (3-CH) and 113.8 (1-C); 19F NMR (376 MHz, CD3OD): δ −154.5; 11B NMR (128 MHz, CD3OD): δ −1.15.
3.3. X-Ray Structure Determination of 3
X-ray diffraction data for compound 3 (Form A) were collected at 100 K using a Rigaku MM-007HF High Brilliance RA generator/confocal optics with XtaLAB P200 diffractometer (Rigaku Corporation, Tokyo, Japan) [Cu Kα radiation (λ = 1.54187 Å)]. X-ray diffraction data for compound 3 (Form B) were collected at 173 K using a Rigaku FR-X Ultrahigh Brilliance Microfocus RA generator/confocal optics with XtaLAB P200 diffractometer (Rigaku Corporation, Tokyo, Japan) [Mo Kα radiation (λ = 0.71073 Å)]. Data for both forms were collected (using a calculated strategy) and processed (including correction for Lorentz, polarisation and absorption) using CrysAlisPro []. The data for Form A was processed as a two-component non-merohedral twin with the second component rotated 179.9770° around [0.00 0.00 1.00] (reciprocal), or [0.35 0.00 0.94] (direct), giving a twin law of [−0.9998 −0.0006 −0.0005 0.0003 −1.0002 0.0002 0.7356 0.0020 1.0002] and a refined twin fraction of 47.0(2)%. Structures were solved by dual-space methods (SHELXT []) and refined by full-matrix least-squares against F2 (SHELXL-2019/3 []). Non-hydrogen atoms were refined anisotropically and hydrogen atoms were refined using a riding model except for the hydrogen atoms on N1, in both structures, which were located from the difference Fourier map and refined isotropically subject to a distance restraint. All calculations were performed using the Olex2 [] interface. Selected crystallographic data are presented below. CCDC 2,494,454 (Form A) and 2,494,455 (Form B) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/structures.
Form A
Crystal data for C7H6BF2NO2, M = 184.94 g mol−1, colourless platy-needle, crystal dimensions 0.05 × 0.03 × 0.01 mm, monoclinic, space group P21/c (No. 14), a = 11.5161(14), b = 5.0928(4), c = 13.5062(17) Å, β = 108.478(13)°, V = 751.29(16) Å3, Z = 4, Dcalc = 1.635 g cm−3, T = 100 K, Goodness of fit on F2 1.093, 18,479 reflections measured, 2788 unique (Rint = 0.0333), which were used in all calculations. The final R1 [I > 2σ (I)] was 0.0515 and wR2 (all data) was 0.1549.
Form B
Crystal data for C7H6BF2NO2, M = 184.94 g mol−1, colourless prism, crystal dimensions 0.15 × 0.06 × 0.04 mm, monoclinic, space group P21/n (No. 14), a = 6.8478(2), b = 5.3517(2), c = 20.9843(7) Å, β = 93.369(3)°, V = 767.69(5) Å3, Z = 4, Dcalc = 1.600 g cm−3, T = 173 K, Goodness of fit on F2 1.052, 13,784 reflections measured, 1913 unique (Rint = 0.0281), which were used in all calculations. The final R1 [I > 2σ (I)] was 0.0333 and wR2 (all data) was 0.0899.
Supplementary Materials
The following are available online: 1H, 13C, 19F and 11B NMR spectra for 3.
Author Contributions
D.M. prepared the compound and recorded the NMR spectra; H.E.M.A., D.B.C. and A.P.M. collected the X-ray data and solved the structures; and R.A.A. designed the study, analysed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The research data supporting this publication (original NMR files) can be accessed at https://doi.org/10.17630/7e8debd8-4d51-4824-9432-c153360e7238.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ben-Ishai, D.; Warshawsky, A. The reactions of 2-ethoxy-2,3-dihydro-4H-1,3-benzoxazin-4-one with conjugated dienes. J. Heterocycl. Chem. 1971, 8, 865–866. [Google Scholar] [CrossRef]
- Dey, K.; Gangopadhyay, A.; Biswas, A.K. Synthesis and characterisation of some new fluoroboron complexes with monobasic and dibasic bidentate and dibasic tridentate ligands. Proc. Nat. Acad. Sci. India 1989, 59, 5–15. [Google Scholar]
- Runti, C.; D’Osualdo, V.; Ulian, F. Reazioni fra ortoformiato di etile e composti organici azotati. Nota II. Amidi. Ann. Chim. 1959, 59, 1668–1676. [Google Scholar]
- Korlyukov, A.A.; Lyssenko, K.A.; Antipin, M.Y.; Shipov, A.G.; Zamyshlyaeva, O.A.; Kramarova, E.P.; Negrebetsky, V.V.; Pogozhikh, S.A.; Ovchinnikov, Y.E.; Baukov, Y.I. Synthesis, molecular and crystal structures, and characteristic features of electronic structures of salicylamide-based B, Si-containing chelates. Russ. Chem. Bull. 2004, 53, 1924–1931. [Google Scholar] [CrossRef]
- Judd, K.E.; Mahon, M.F.; Caggiano, L. Efficient synthesis of tetrahydro-β-carbolin-1-one and dihydroisoquinolin-1-one derivatives as versatile intermediates. Synthesis 2009, 2009, 2809–2817. [Google Scholar] [CrossRef]
- Wang, H.; Schrage, B.R.; Takematsu, K.; Ziegler, C.J. Photophysical properties of a boron analogue of coumarin. Phys. Chem. Chem. Phys. 2021, 23, 18855–18862. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-H.; Wu, D.-E.; Song, W.-T.; Ge, S.-W.; Sun, B.-W. Positions of amino groups on ammonium salts tunes the conformations of crown ethers: Crystal structures, Hirshfeld surfaces and spectroscopic studies. CrystEngComm 2014, 16, 5319–5330. [Google Scholar] [CrossRef]
- Spilker, A. Ueber neue stickstoffhaltige Abkömmlinge der Salicylsäure. Ber. Dtsch. Chem. Ges. 1889, 22, 2767–2789. [Google Scholar] [CrossRef]
- Rigaku Corporation. CrysAlisPro, v1.171.42.94a, 43.142a and 43.144a; Rigaku Oxford Diffraction: Tokyo, Japan, 2023, 2024.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).