Schiff Base Heterobimetallic Complex as Single-Source Precursor
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of the LCuCa Complex
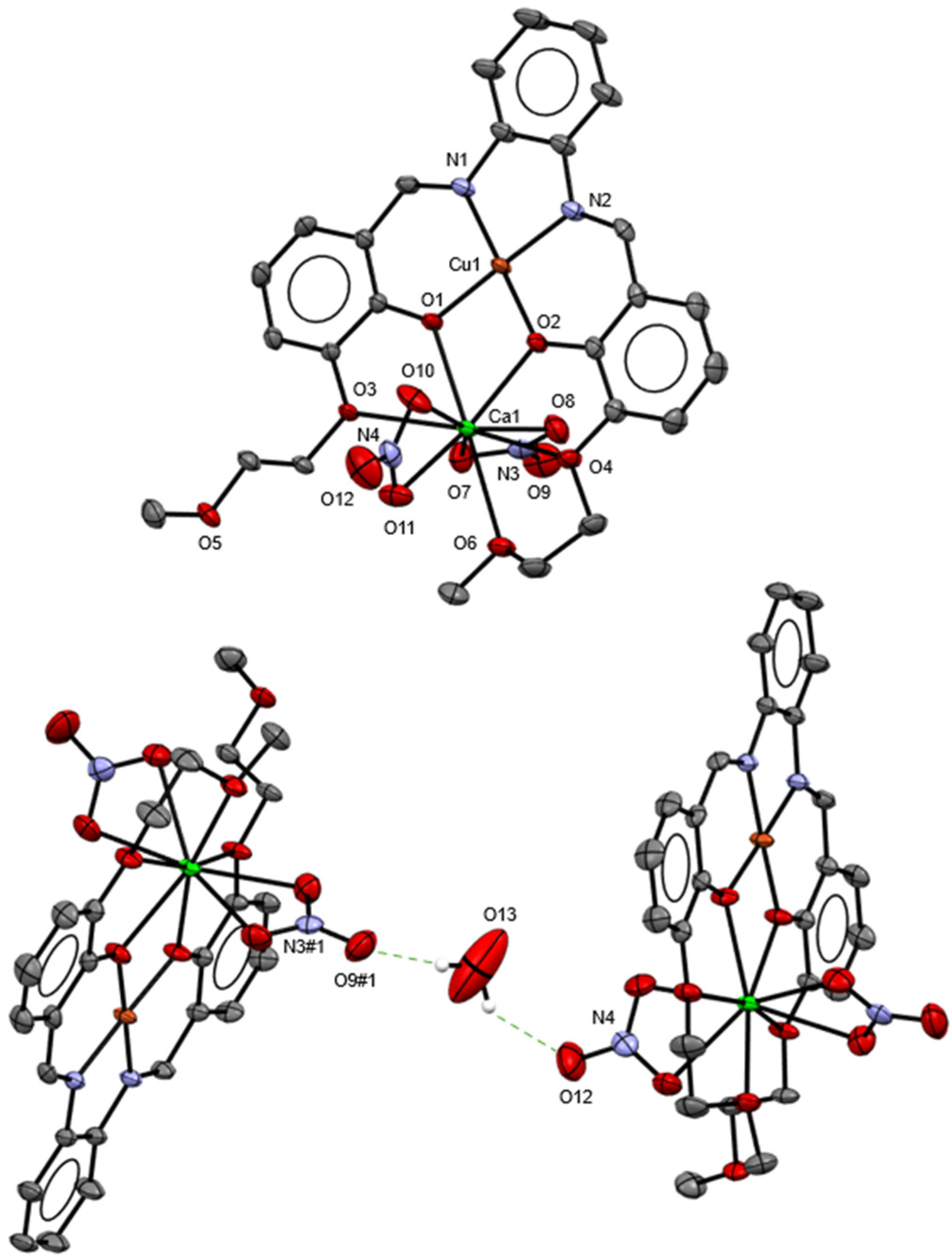
2.2. X-Ray Crystal Structure
2.3. Single-Source Precursor
3. Materials and Methods
3.1. Generalities
3.2. Synthesis of 2-methoxyethyl 4-methylbenzensulfonate, L′
3.3. Synthesis of 2-hydroxy-3-(2-methoxyethoxy)benzaldehyde, L″
3.4. Synthesis of LCu Complex
3.5. Synthesis of LCuCa Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asatkar, A.K.; Tripathi, M.; Asatkar, D. Stability and Applications of Coordination Compounds; Srivastva, A.N., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Khan, T.; Vaidya, S.; Mhatre, D.S.; Datta, A. The Prospect of Salophen in Fluorescence Lifetime Sensing of Al3+. J. Phys. Chem. B 2016, 120, 10319–10326. [Google Scholar] [CrossRef] [PubMed]
- Sahudin, M.A.; Su’ait, M.S.; Tan, L.L.; Lee, Y.H.; Karim, N.H.A. Zinc(II) salphen complex-based fluorescence optical sensor for biogenic amine detection. Anal. Bioanal. Chem. 2019, 411, 6449–6461. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Muduli, S.K.; Tran, P.D.; Soo, H.S. Enhancing electrocatalytic hydrogen evolution by nickel salicylaldimine complexes with alkali metal cations in aqueous media. Chem. Commun. 2016, 52, 2948–2951. [Google Scholar] [CrossRef] [PubMed]
- Van Staveren, C.J.; Van Eerden, J.; Van Veggel, F.C.J.M.; Harkema, S.; Reinhoudt, D.N. Cocomplexation of neutral guests and electrophilic metal cations in synthetic macrocyclic hosts. J. Am. Chem. Soc. 1988, 110, 4994–5008. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. B Struct. Sci. 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Teske, C.L.; Müller-Buschbaum, H. Über Erdalkalimetall—Oxocuprate. I. Zur Kenntnis von CaCu2 O3. Z. Anorg. Allg. Chem. 1969, 370, 134–143. [Google Scholar] [CrossRef]
- Burdina, A.A.; Merkulov, O.V.; Markov, A.A.; Patrakeev, M.V. Evaluation of Ca2CuO3 as an oxygen carrier material. Mater. Lett. 2021, 297, 129968. [Google Scholar] [CrossRef]
- Fetisov, A.V.; Slobodin, B.V. Magnetic Properties of Ca2CuO3+β and YBa2Cu3O6+δ. Dokl. Phys. Chem. 2001, 379, 211–214. [Google Scholar] [CrossRef]
- Bordas, E.; De Graaf, C.; Caballol, R.; Calzado, C.J. Electronic structure of CaCu2O3: Spin ladder versus one-dimensional spin chain. Phys. Rev. B 2005, 71, 045108. [Google Scholar] [CrossRef]
- Liu, H.K.; Dou, S.X.; Li, X.G. Effect of Ca2CuO3 excess on superconducting properties in Bi-Pb-Sr-Ca-Cu-O system. Phys. C Supercond. 1991, 185–189, 2251–2252. [Google Scholar] [CrossRef]
- Plakida, N.M.; Vladimirov, A.; Drechsler, S.-L. Superconductivity in the doped “pseudo-ladder” compound CaCu2O3. Phys. C Supercond. 2004, 408–410, 232–233. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hooper, R.; Lyons, L.J.; Mapes, M.K.; Schumacher, D.; Moline, D.A.; West, R. Highly Conductive Siloxane Polymers. Macromolecules 2001, 34, 931–936. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradegan, J.; Crochet, A.; Fromm, K.M. Schiff Base Heterobimetallic Complex as Single-Source Precursor. Molbank 2025, 2025, M2092. https://doi.org/10.3390/M2092
Pradegan J, Crochet A, Fromm KM. Schiff Base Heterobimetallic Complex as Single-Source Precursor. Molbank. 2025; 2025(4):M2092. https://doi.org/10.3390/M2092
Chicago/Turabian StylePradegan, Jocelyn, Aurélien Crochet, and Katharina M. Fromm. 2025. "Schiff Base Heterobimetallic Complex as Single-Source Precursor" Molbank 2025, no. 4: M2092. https://doi.org/10.3390/M2092
APA StylePradegan, J., Crochet, A., & Fromm, K. M. (2025). Schiff Base Heterobimetallic Complex as Single-Source Precursor. Molbank, 2025(4), M2092. https://doi.org/10.3390/M2092







