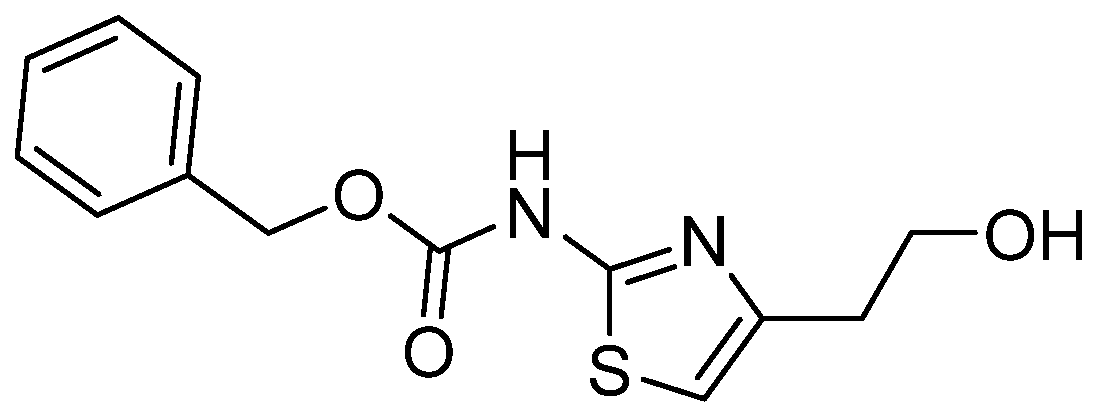
Benzyl-N-[4-(2-hydroxyethyl)-1,3-thiazol-2-yl]carbamate
Abstract
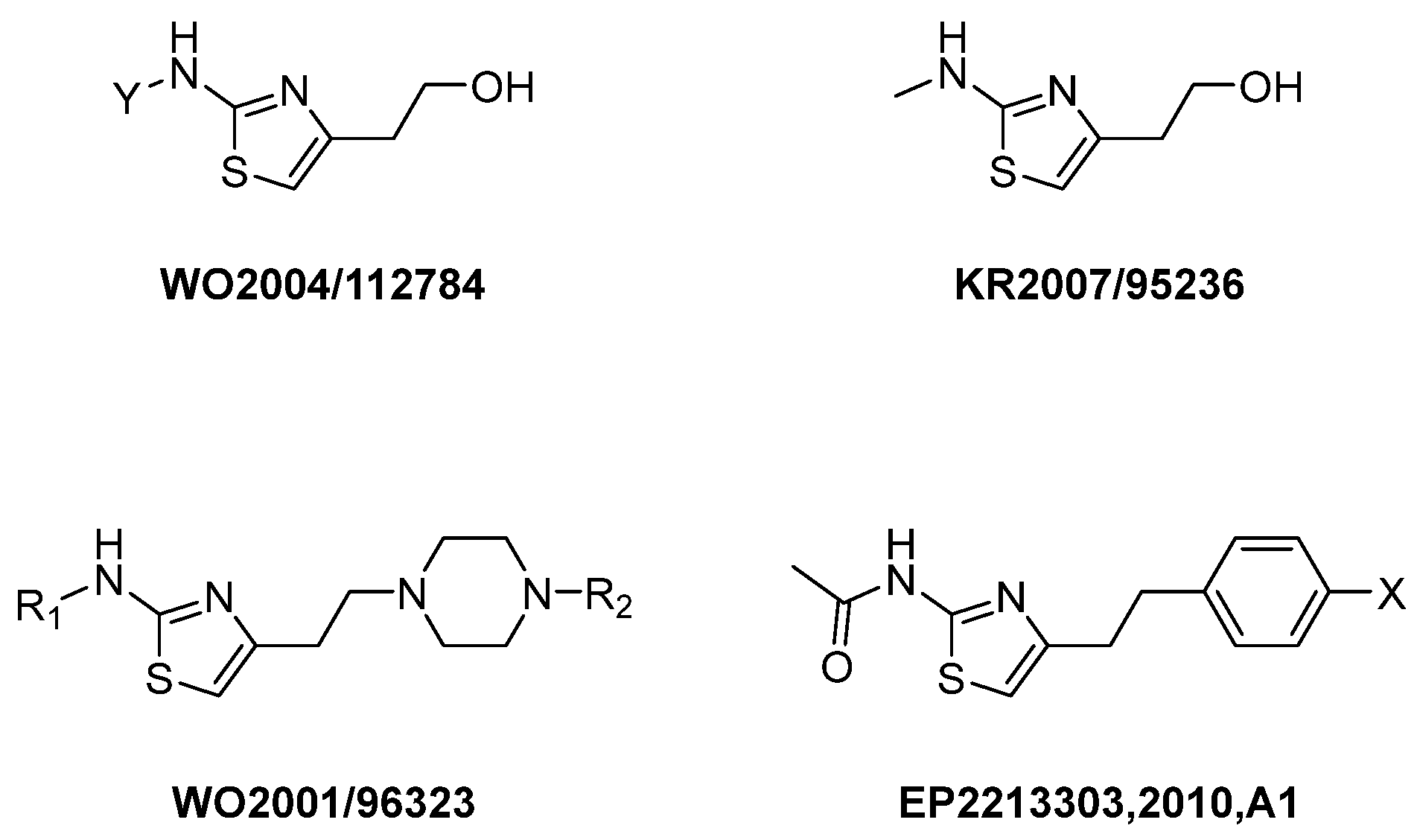
1. Introduction
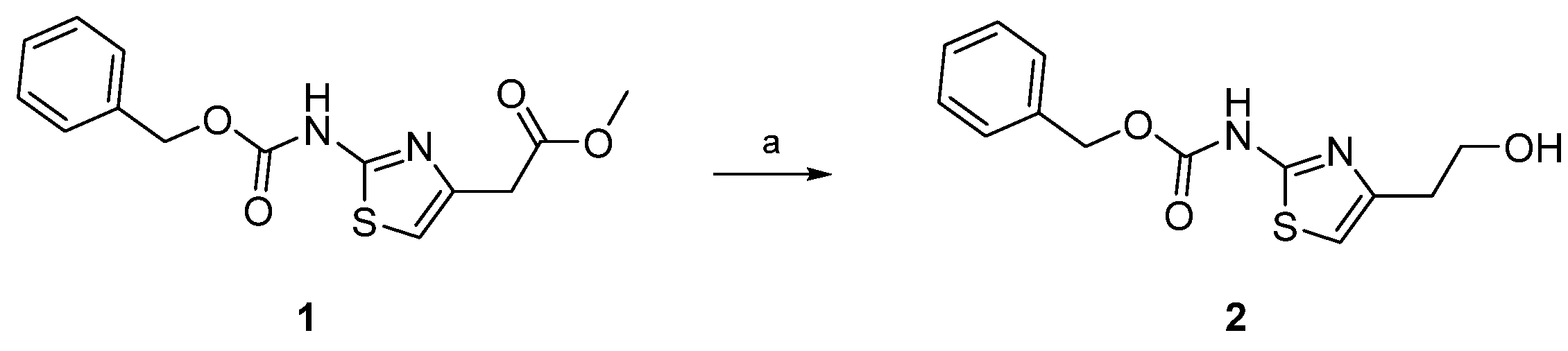
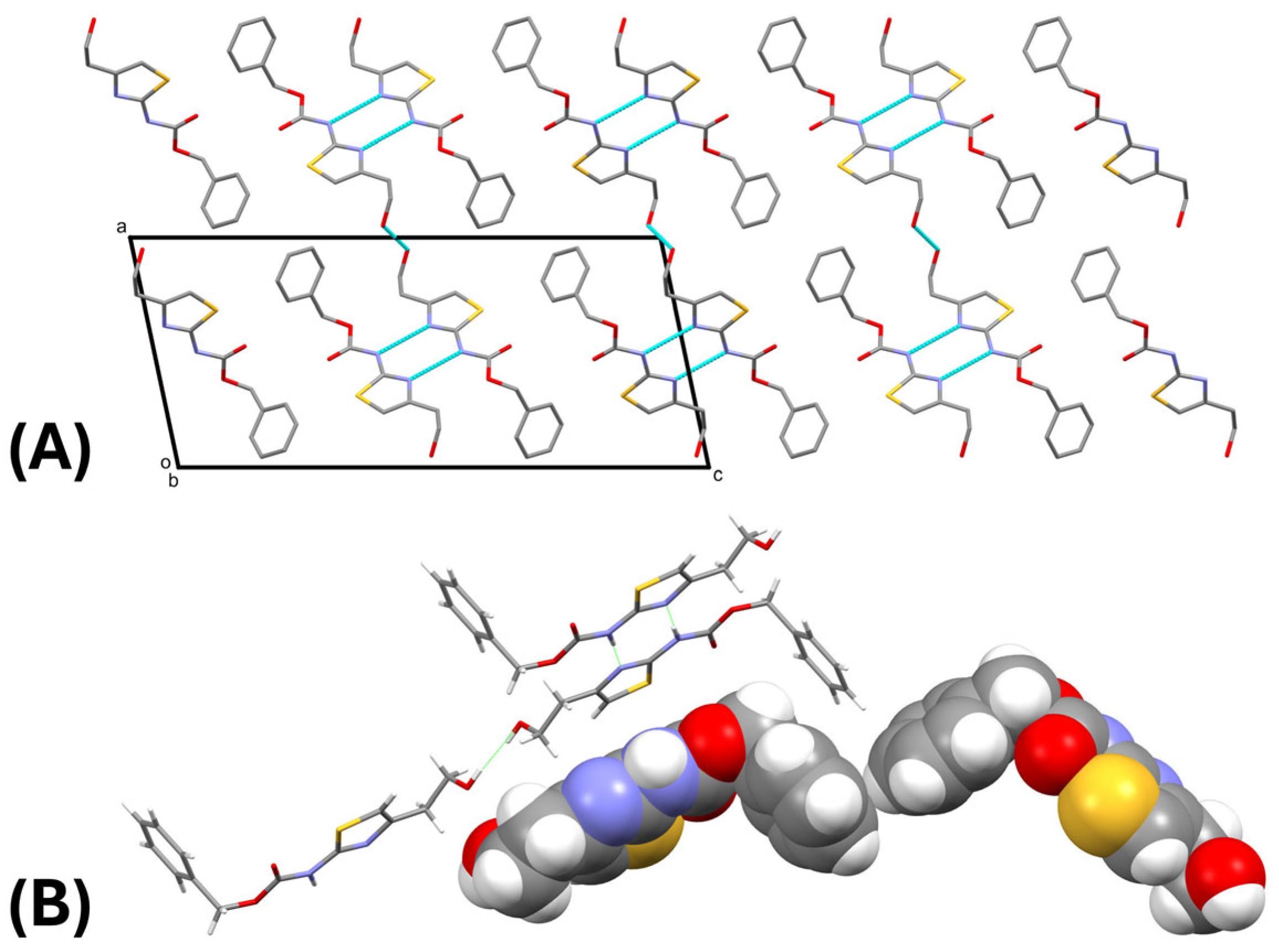
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis and Characterization of Benzyl-(4-(2-hydroxyethyl)thiazol-2-yl)carbamate
3.2. Single-Crystal X-Ray Diffraction
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cbz | Benzyloxycarbonyl |
| CCD | Charge-Coupled Device |
| NMR | Nuclear Magnetic Resonance |
| THF | Tetrahydrofuran |
References
- Prandi, C.; Occhiato, E.G. From Synthetic Control to Natural Products: A Focus on N-heterocycles. Pest. Manag. Sci. 2019, 75, 2385–2402. [Google Scholar] [CrossRef] [PubMed]
- Favi, G. Modern Strategies for Heterocycle Synthesis. Molecules 2020, 25, 2476. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Pace, A.; Pibiri, I.; Buscemi, S.; Palumbo Piccionello, A. From Conventional to Sustainable Catalytic Approaches for Heterocycles Synthesis. ChemSusChem 2024, 17, e202301604. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.; Uzzaman, M. A Review on Biological and Medicinal Impact of Heterocyclic Compounds. Results Chem. 2022, 4, 100606. [Google Scholar] [CrossRef]
- Rusu, A.; Moga, I.-M.; Uncu, L.; Hancu, G. The Role of Five-Membered Heterocycles in the Molecular Structure of Antibacterial Drugs Used in Therapy. Pharmaceutics 2023, 15, 2554. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.F.; Alam, A.; Alshammari, A.A.; Alhazza, M.B.; Alzimam, I.M.; Alam, M.A.; Mustafa, G.; Ansari, M.S.; Alotaibi, A.M.; Alotaibi, A.A.; et al. Thiazole: A Versatile Standalone Moiety Contributing to the Development of Various Drugs and Biologically Active Agents. Molecules 2022, 27, 3994. [Google Scholar] [CrossRef] [PubMed]
- Liebeschuetz, J.W.; Murray, C.W.; Young, S.C.; Camp, N.P.; Jones, S.D.; Wylie, W.A.; Master, J.J. Serine Protease Inhibitors 2001. Eli Lilly and Company. WO 01/96323 A1.
- Břehová, P.; Chaloupecká, E.; Česnek, M.; Skácel, J.; Dračínský, M.; Tloušťová, E.; Mertlíková-Kaiserová, H.; Soto-Velasquez, M.P.; Watts, V.J.; Janeba, Z. Acyclic Nucleoside Phosphonates with 2-Aminothiazole Base as Inhibitors of Bacterial and Mammalian Adenylate Cyclases. Eur. J. Med. Chem. 2021, 222, 113581. [Google Scholar] [CrossRef] [PubMed]
- Decuyper, L.; Jukič, M.; Sosič, I.; Žula, A.; D’hooghe, M.; Gobec, S. Antibacterial and Β-Lactamase Inhibitory Activity of Monocyclic Β-Lactams. Med. Res. Rev. 2018, 38, 426–503. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Morita, M.; Tojo, T.; Yoshihara, K.; Nagashima, A.; Moritomo, A.; Ohkubo, M.; Miyake, H. Synthesis and SAR Study of New Thiazole Derivatives as Vascular Adhesion Protein-1 (VAP-1) Inhibitors for the Treatment of Diabetic Macular Edema. Bioorg. Med. Chem. 2013, 21, 1219–1233. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Kessler, M.; Luo, J.; Motherwell, W.D.S.; Purkis, L.H.; Smith, B.R.; Taylor, R.; Cooper, R.I.; Harris, S.E.; et al. Retrieval of Crystallographically-Derived Molecular Geometry Information. J. Chem. Inf. Comput. Sci. 2004, 44, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; Akhtar, T.; Yousuf, M.; Baig, M.W.; Tahir, M.N.; Rasheed, L. Synthesis, Spectroscopic Characterization and Crystallographic Behavior of Ethyl 2-(4-Methyl-(2-Benzylidenehydrazinyl))Thiazole-4-Carboxylate: Experimental and Theoretical (DFT) Studies. J. Mol. Struct. 2018, 1167, 154–160. [Google Scholar] [CrossRef]
- Cazzaniga, G.; Mori, M.; Meneghetti, F.; Chiarelli, L.R.; Stelitano, G.; Caligiuri, I.; Rizzolio, F.; Ciceri, S.; Poli, G.; Staver, D.; et al. Virtual Screening and Crystallographic Studies Reveal an Unexpected γ-Lactone Derivative Active against MptpB as a Potential Antitubercular Agent. Eur. J. Med. Chem. 2022, 234, 114235. [Google Scholar] [CrossRef] [PubMed]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal Structure Determination and Refinement via SIR2014. J. Appl. Crystallogr. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Nardelli, M. PARST 95—An Update to PARST: A System of Fortran Routines for Calculating Molecular Structure Parameters from the Results of Crystal Structure Analyses. J. Appl. Crystallogr. 1995, 28, 659. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
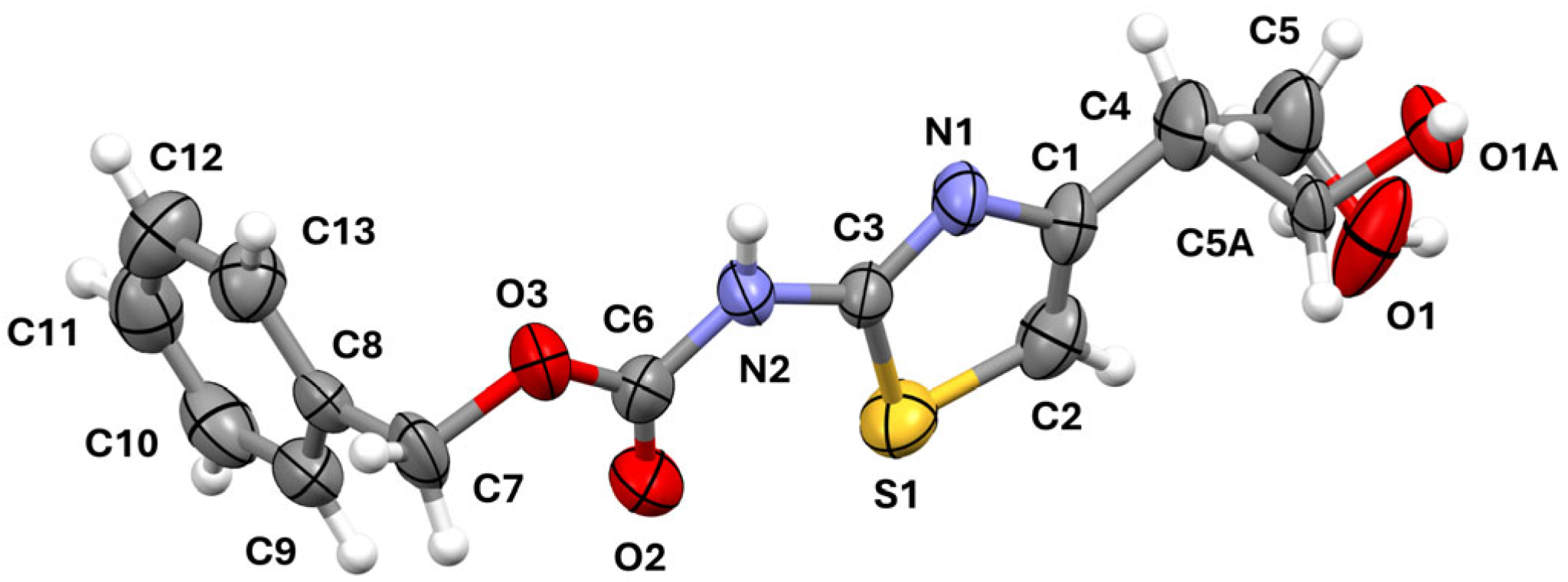
| D-H/Å | H∙∙∙A/Å | D∙∙∙A/Å | D-H∙∙∙A/° | |
|---|---|---|---|---|
| N2-H2···N1 I | 0.92 (5) | 2.09 (5) | 3.012 (6) | 177 (5) |
| O1A-H1A···O1 II | 0.82 (2) | 2.23 (1) | 2.79 (2) | 125 (1) |
| C13-H13···O1 III | 0.93 (1) | 2.76 (1) | 3.52 (1) | 139.8 (5) |
| O1-H1···O1A IV | 0.82 (1) | 1.92 (2) | 2.73 (2) | 167.9 (8) |
| O1-H1···O1 IV | 0.82 (1) | 2.29 (1) | 3.02 (2) | 148.7 (7) |
| Identification Code | 2 | |
|---|---|---|
| Empirical formula | C13H14N2O3S | |
| Formula weight | 278.32 | |
| Temperature (K) | 293(2) | |
| Wavelength (Å) | 0.71073 | |
| Crystal system | Monoclinic | |
| Space group | P21/c | |
| Unit cell dimensions (Å/°) | a = 11.0837 (11) | |
| b = 4.9184 (5) | β = 101.858 (10) | |
| c = 24.941 (3) | ||
| Volume (Å3) | 1330.6 (2) | |
| Z | 4 | |
| Density calcd. (Mg/m3) | 1.389 | |
| Abs. coefficient (mm−1) | 0.249 | |
| F(000) | 584 | |
| Crystal size (mm3) | 0.12 × 0.05 × 0.01 | |
| θ range data collection (°) | 2.757 to 23.257 | |
| Index ranges | −12 ≤ h ≤ 12, −5 ≤ k ≤ 5, −27 ≤ l ≤ 27 | |
| Reflections collected | 20,820 | |
| Independent reflections | 1910 [Rint = 0.0698] | |
| Completeness to θmax (%) | 99.4% | |
| Refinement method | Full-matrix least-squares on F2 | |
| Data/restraints/parameters | 1910/2/195 | |
| Goodness-of-fit on F2 | 1.090 | |
| Final R indices [I > 2σ(I)] | R1 = 0.0738, wR2 = 0.1421 | |
| R indices (all data) | R1 = 0.1142, wR2 = 0.1587 | |
| Extinction coefficient | 0.0040 (7) | |
| Largest diff. peak/hole (e·Å−3) | 0.199 and −0.214 | |
| CCDC deposition number | 2467715 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinelli, L.; Mori, M.; Fumagalli, L. Benzyl-N-[4-(2-hydroxyethyl)-1,3-thiazol-2-yl]carbamate. Molbank 2025, 2025, M2040. https://doi.org/10.3390/M2040
Spinelli L, Mori M, Fumagalli L. Benzyl-N-[4-(2-hydroxyethyl)-1,3-thiazol-2-yl]carbamate. Molbank. 2025; 2025(3):M2040. https://doi.org/10.3390/M2040
Chicago/Turabian StyleSpinelli, Lucrezia, Matteo Mori, and Laura Fumagalli. 2025. "Benzyl-N-[4-(2-hydroxyethyl)-1,3-thiazol-2-yl]carbamate" Molbank 2025, no. 3: M2040. https://doi.org/10.3390/M2040
APA StyleSpinelli, L., Mori, M., & Fumagalli, L. (2025). Benzyl-N-[4-(2-hydroxyethyl)-1,3-thiazol-2-yl]carbamate. Molbank, 2025(3), M2040. https://doi.org/10.3390/M2040






