1,3,4,5-Tetra-O-benzoyl-α-d-tagatopyranose
Abstract
1. Introduction
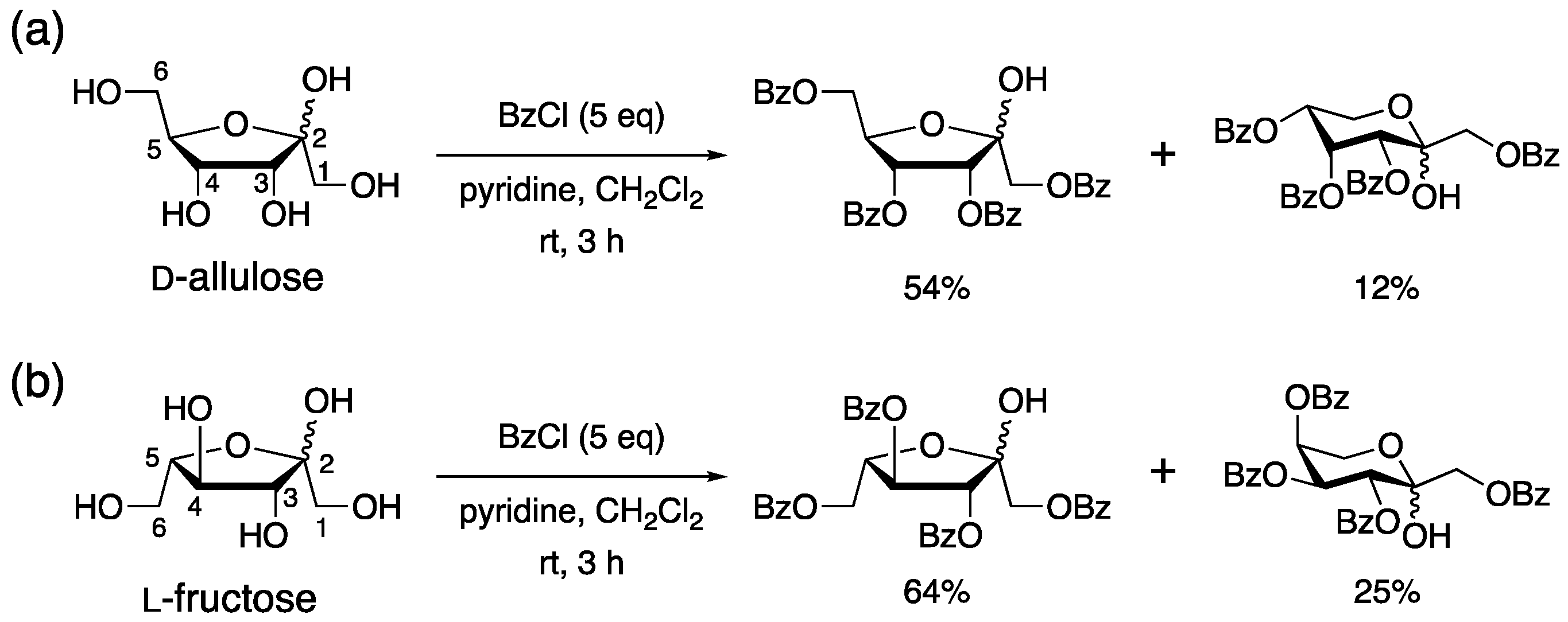
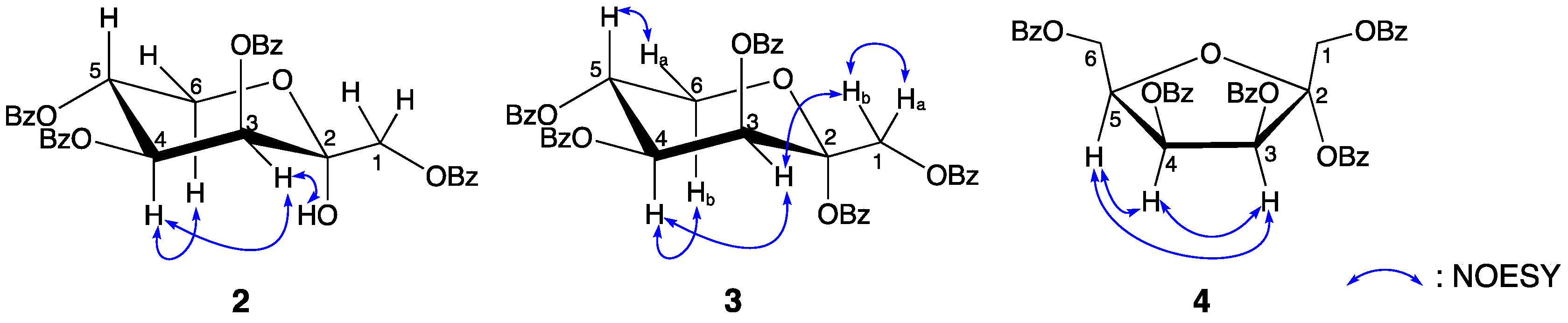
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure and Method
3.2. 1,3,4,5-Tetra-O-benzoyl-α-d-tagatopyranose (2), 1,2,3,4,5-penta-O-benzoyl-α-d-tagatopyranose (3), and 1,2,3,4,6-penta-O-benzoyl-α-d-tagatofuranose (4)
3.2.1. Synthesis of 2, 3, and 4 from 1 (Table 1, Entry 1)
3.2.2. Synthesis of 3 and 4 from 1 (Table 1, Entry 4)
3.2.3. Conversion of 2 to 3 (Table 2, Entry 3)
3.2.4. Compound Data of 1,3,4,5-Tetra-O-benzoyl-α-d-tagatopyranose (2)
3.2.5. Compound Data of 1,2,3,4,5-Penta-O-benzoyl-α-d-tagatopyranose (3) and 1,2,3,4,6-Penta-O-benzoyl-α-d-tagatofuranose (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bz | Benzoyl |
| DMAP | 4-Dimethylaminopyridine |
| THF | Tetrahydrofuran |
| NOESY | Nuclear Overhauser Effect Spectroscopy |
References
- Granstrom, T.B.; Takata, G.; Tokuda, M.; Izumori, K. Izumoring: A novel and complete strategy for bioproduction of rare sugars. J. Biosci. Bioeng. 2004, 97, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Izumori, K. Izumoring: A strategy for bioproduction of all hexoses. J. Biotechnol. 2006, 124, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Donner, T.W.; Wilber, J.F.; Ostrowski, D. d-tagatose, a novel hexose: Acute effects on carbohydrate tolerance in subjects with and without type 2 diabetes. Diabetes Obes. Metab. 1999, 1, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Levin, G.V.; Donner, T.W. Tagatose, a new antidiabetic and obesity control drug. Diabetes Obes. Metab. 2008, 10, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Brown, J.C. d-tagatose is a bulk sweetener with zero energy determined in rats. J. Nutr. 1996, 126, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.V. Tagatose, the new gras sweetener and health product. J. Med. Food 2002, 5, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, P.S.J.; Wootton, A.N. Bioconversion of d-galactose into d-tagatose. Enzyme Microb. Technol. 1993, 15, 105–108. [Google Scholar] [CrossRef]
- Jørgensen, F.; Hansen, O.C.; Stougaard, P. Enzymatic conversion of d-galactose to d-tagatose: Heterologous expression and characterisation of a thermostable L-arabinose isomerase from Thermoanaerobacter mathranii. Appl. Microbiol. Biotechnol. 2004, 64, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mao, X.; Lu, Z.; Gao, C.; Chen, Z.; Liu, J. advances in biological production of d-tagatose: A comprehensive overview. Fermentation 2025, 11, 46. [Google Scholar] [CrossRef]
- Hanessian, S. Approaches to the total synthesis of natural products using "chiral templates" derived from carbohydrates. Acc. Chem. Res. 1979, 12, 159–165. [Google Scholar] [CrossRef]
- Iyoshi, A.; Miyazaki, Y.; Tanaka, M.; Ueda, A. (S,Z)-1,4-Bis(benzyloxy)hexa-3,5-dien-2-ol. Molbank 2024, 2024, M1848. [Google Scholar] [CrossRef]
- Jenkinson, S.F.; Fleet, G.W.J.; Nash, R.J.; Koike, Y.; Adachi, I.; Yoshihara, A.; Morimoto, K.; Izumori, K.; Kato, A. Looking-glass synergistic pharmacological chaperones: DGJ and L-DGJ from the enantiomers of tagatose. Org. Lett. 2011, 13, 4064–4067. [Google Scholar] [CrossRef] [PubMed]
- Baráth, M.; Lin, C.-H.; Tvaroška, I.; Hirsch, J. Development of transition state analogue inhibitors for N-acetylglycosyltransferases bearing d-psico or d-tagatofuranose scaffolds. Chem. Pap. 2015, 69, 348–357. [Google Scholar] [CrossRef]
- Hunt-Painter, A.A.; Stocker, B.L.; Timmer, M.S.M. The synthesis of the molecular chaperone 2,5-dideoxy-2,5-imino-d-altritol via diastereoselective reductive amination and carbamate annulation. Tetrahedron 2018, 74, 1307–1312. [Google Scholar] [CrossRef]
- Makura, Y.; Ueda, A.; Matsuzaki, T.; Minamino, T.; Tanaka, M. α-Selective glycosidation of d-tagatofuranose with a 3,4-O-isopropylidene protection. Tetrahedron 2019, 75, 3758–3766. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, O.; Sabater, C.; Calvete-Torre, I.; Doyagüez, E.G.; Muñoz-Labrador, A.M.; Julio-Gonzalez, C.; de las Rivas, B.; Muñoz, R.; Ruiz, L.; Margolles, A.; et al. Tailoring the natural rare sugars d-tagatose and L-sorbose to produce novel functional carbohydrates. npj Sci. Food 2024, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Pi, J.; Makura, Y.; Tanaka, M.; Uenishi, J. Stereoselective synthesis of (+)-5-thiosucrose and (+)-5-thioisosucrose. RSC Adv. 2020, 10, 9730–9735. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Sato, Y.; Shibakami, M. Stereoselective glycosylations using benzoylated glucosyl halides with inexpensive promoters. Carbohydr. Res. 2008, 343, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Pareek, S.; Mandadi, P.; Rao Sanapala, S. Glycosyl esters as stable donors in chemical glycosylation. Eur. J. Org. Chem. 2025, 28, e202401330. [Google Scholar] [CrossRef]
- Han, Y.; Wang, X.; Tao, Q.; Yang, B.; Zhu, F. Switchable divergent photocatalytic c-glycosylation of glycosyl benzoates. Angew. Chem. Int. Ed. 2025, 64, e202504504. [Google Scholar] [CrossRef] [PubMed]
- Evtushenko, E.V. Regioselective benzoylation of glycopyranosides by benzoic anhydride in the presence of Cu(CF3COO)2. Carbohydr. Res. 2012, 359, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Yamanoi, T.; Ishiyama, T.; Oda, Y.; Matsuda, S.; Watanabe, M. L-Fructo- and d-psicofuranosylation reactions catalyzed by scandium triflate. Heterocycles 2010, 81, 1141–1147. [Google Scholar] [CrossRef]
- Angyal, S.J.; Bethell, G.S. Conformational analysis in carbohydrate chemistry. III* The 13C N.M.R. spectra of the hexuloses. Aust. J. Chem. 1976, 29, 1249–1265. [Google Scholar] [CrossRef]
- Miura, D.; Fujimoto, T.; Tashiro, M.; Machinami, T. Crystal structure of 1,3,4,5-Tetra-O-acetyl-alpha-d-tagatopyranose. X-Ray Struct. Anal. Online 2014, 30, 3–4. [Google Scholar] [CrossRef]
- Richter, C.; Berndt, F.; Kunde, T.; Mahrwald, R. Decarboxylative cascade reactions of dihydroxyfumaric acid: A preparative approach to the glyoxylate scenario. Org. Lett. 2016, 18, 2950–2953. [Google Scholar] [CrossRef] [PubMed]
- Yamanoi, T.; Saitoh, T.; Oda, Y.; Misawa, N.; Watanabe, M.; Ishikawa, J.; Koizumi, A. Efficient d-fructopyranosylation method catalyzed by scandium triflate and preparation of new sucrose analogs. Heterocycles 2017, 95, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.A.; Jenkinson, S.F.; Soengas, R.; Fanefjord, M.; Wormald, M.R.; Dwek, R.A.; Kiran, G.P.; Devendar, R.; Takata, G.; Morimoto, K.; et al. Synthesis of and NMR studies on the four diastereomeric 1-deoxy-d-ketohexoses. Tetrahedron: Asymmetry 2007, 18, 774–786. [Google Scholar] [CrossRef]
 | |||||
|---|---|---|---|---|---|
| Entry | BzCl (eq) 1 | Additive | Solvent | Yield | |
| 2 | 3 + 4 (ratio) 2 | ||||
| 1 | 5 | none | pyridine/CH2Cl2 (1:1) | 88% | 5% (1.5:1) |
| 2 | 5 | none | pyridine | 82% | trace |
| 3 3 | 7 | DMAP (0.3 eq) | pyridine/CH2Cl2 (1:1) | 46% | 32% (1.3:1) |
| 4 3,4 | 7 | DMAP (0.3 eq) | pyridine/CH2Cl2 (1:1) | 13% | 50% (7:1) |
 | |||||
|---|---|---|---|---|---|
| Entry | Additive | Solvent | Temp | Time (h) | NMR Yield 1 |
| 1 | DMAP (0.3 eq) | pyridine/CH2Cl2 (1:1) | rt | 26 | 10% |
| 2 | DMAP (0.3 eq) | pyridine/CH2Cl2 (1:1) | 50 °C | 26 | 21% |
| 3 | n-BuLi (1.3 eq) | THF | −30 °C | 4 | 61% |
| Compound | J1a,1b | J3,4 | J4,5 | J5,6a | J5,6b | J6a,6b |
|---|---|---|---|---|---|---|
| 2 | 12.0 | 3.4 | 10.2 | 5.9 | 10.7 | 10.7 |
| 3 | 12.2 | 3.3 | 10.3 | 5.9 | 10.5 | 11.3 |
| 4 | 11.9 | 5.4 | 4.6 | 6.5 | 5.6 | 11.7 |
| 1-deoxy-α-d-tagatopyranose 2 | – | 3.3 | 9.6 | 5.6 | 10.8 | 10.8 |
| 1-deoxy-β-d-tagatopyranose 2 | – | 3.3 | 4.6 | 2.0 | 2.7 | 13.1 |
| 1-deoxy-α-d-tagatofuranose 2 | – | 5.3 | 5.3 | N.D. 3 | N.D. 3 | N.D. 3 |
| 1-deoxy-β-d-tagatofuranose 2 | – | 4.5 | 4.5 | N.D. 3 | N.D. 3 | N.D. 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Iyoshi, A.; Makura, Y.; Tanaka, M.; Ueda, A. 1,3,4,5-Tetra-O-benzoyl-α-d-tagatopyranose. Molbank 2025, 2025, M2041. https://doi.org/10.3390/M2041
Hu Y, Iyoshi A, Makura Y, Tanaka M, Ueda A. 1,3,4,5-Tetra-O-benzoyl-α-d-tagatopyranose. Molbank. 2025; 2025(3):M2041. https://doi.org/10.3390/M2041
Chicago/Turabian StyleHu, Yiming, Akihiro Iyoshi, Yui Makura, Masakazu Tanaka, and Atsushi Ueda. 2025. "1,3,4,5-Tetra-O-benzoyl-α-d-tagatopyranose" Molbank 2025, no. 3: M2041. https://doi.org/10.3390/M2041
APA StyleHu, Y., Iyoshi, A., Makura, Y., Tanaka, M., & Ueda, A. (2025). 1,3,4,5-Tetra-O-benzoyl-α-d-tagatopyranose. Molbank, 2025(3), M2041. https://doi.org/10.3390/M2041





