Abstract
The crystal structure of the hydrated form of 7-chloro-4-(4-methyl-1-piperazinyl)quinoline (BPIP) was determined by single-crystal X-ray diffraction analysis. This study revealed a one-dimensional supramolecular network stabilized by hydrogen bonding interactions between BPIP and water molecules. This compound represents one-half of a piperaquine molecule, a member of the 4-aminoquinoline class of antimalarial treatments, currently employed as a partner agent in modern combination therapies. As a simplified structural analog, BPIP can serve as a critical model system for probing the intermolecular interactions, physicochemical properties, and structural behavior of the parent compound. As a result, conducting a thorough solid-state characterization of BPIP is critical for gaining insight into its physical properties and verifying the material’s identity and purity.
1. Introduction
Many derivatives of 4-aminoquinoline are well-known antiparasitic medicines used in the treatment and prophylaxis of malaria. The same structural fragment has also been employed as building block in the design and synthesis of drugs that are highly effective against various diseases including viral infections and different types of cancer [1].
Pharmaceutical drugs can typically exist in different crystal or solid-state forms such as polymorphs, solvates/hydrates, and amorphous phases that can differ widely in their physical properties, including their solubility, dissolution rate, melting point, and tablet-forming ability. Such differences can significantly impact the performance and bioavailability of pharmaceutical products [2]. A thorough structural characterization is essential for assessing the relative stability of polymorphic and solvate forms. This plays a critical role in the development of new drugs as well as in optimizing crystallization processes and formulation strategies to ensure consistent, high-quality product performance [3].
Similarly, to ensure the reliability of activity studies for a drug or its analogous derivatives used for comparison, it is crucial to confirm the nature of the products in their solid state, also using straightforward analytical methods [4]. However, this is only possible when the necessary experimental data are readily available. In industrial drug production, structural identification is an essential part of rigorous quality control, ensuring that products meet expected specifications and maintain high standards of efficacy and consistency across batches.
In this article, the crystal structure of a monohydrate adduct of 7-chloro-4-(4-methyl-1-piperazinyl)quinoline (BPIP∙H2O), a truncated analog of the piperazinyl-quinoline class of active antimalarial compounds, is reported. BPIP corresponds to half a molecule of piperaquine (PQ), a drug previously used extensively to treat chloroquine-resistant falciparum malaria and recently rediscovered to be a promising partner in combination therapies, including artemisinin-based strategies [5]. Therefore, the title compound can serve as a simplified molecular entity of certain antimalarials, making it an effective model system for investigating drug mechanisms [6,7] and interactions with biologically relevant metal ions. Consequently, its solid-state characterization is crucial, complementing existing crystal structures of other antimalarial compounds [8,9,10,11].
2. Results
2.1. Solid-State Structure
Single-crystal data revealed that the BPIP∙H2O structure crystallizes in the monoclinic system with the space group P21/c. The asymmetric unit comprises one independent quinoline derivative and one water molecule (Figure 1).
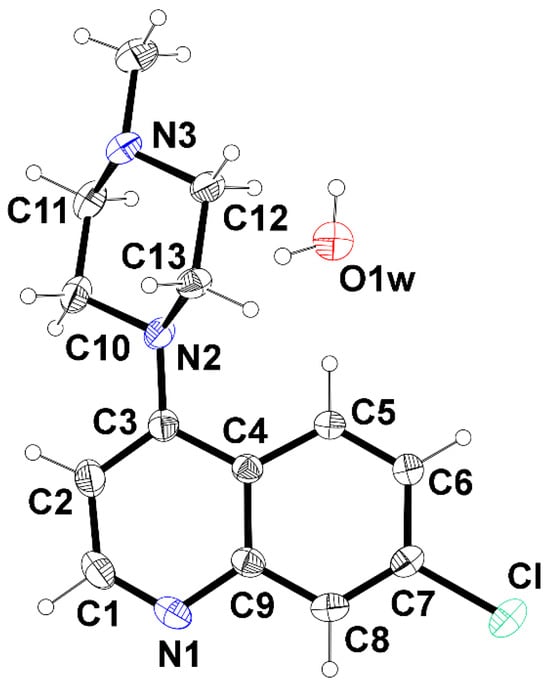
Figure 1.
The asymmetric unit of the crystal structure of BPIP∙H2O showing the atom labeling. The displacement ellipsoids are drawn at the 30% probability level. Hydrogen atoms are shown as small spheres of arbitrary radii. BPP and water molecules are linked by multiple hydrogen bond interactions, forming a one-dimensional network (see Figure 2).
The quinoline double-ring shows a distortion from the planarity (rms deviation from the best fit plane 0.0567 Å), with the benzene and pyridine inclined at a dihedral angle of 5.83(3)° with respect to each other. The piperazinyl ring adopts a chair conformation with puckering parameters of Q = 0.5886(16) Å and θ = 178.43(16)° [12] and is oriented at a dihedral angle of about 39.1° with respect to the quinoline moiety.
In the crystal structure, BPIP and water molecules are alternatively linked via hydrogen bonding interactions into chains running in the direction generated by the glide plane c (Figure 2). The water molecule acts as a double donor to the pyridinic nitrogen and the terminal N atom of the piperazinyl group, generating quasi-linear hydrogen bonds (O⋯N distance (Å), O—H⋯N angle (°): 2.9194(19), 175(3); 2.9417(19), 166(2)) (Table S1).
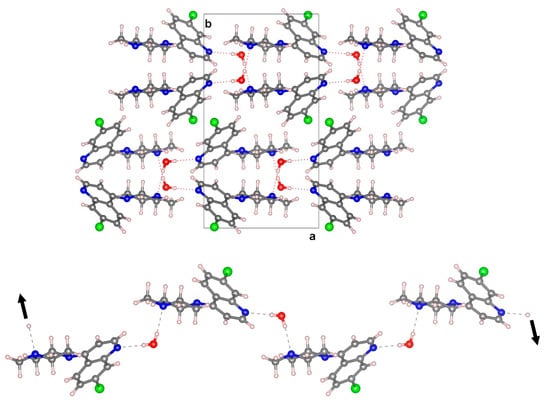
Figure 2.
The view in the c direction of the molecular arrangement of the title compound (top). Details of hydrogen bonding within the one-dimensional chains -H2O-BPIP-H2O- (bottom).
Volumetric analysis indicates that water molecules occupy 3.2% of the unit cell volume (probe radius of 1.0 Å) and reside within discrete, isolated pockets of the crystal framework without having discernible pathways to egress.
2.2. Intermolecular Interaction Analysis
The “Aromatics Analyser” functionality in the CSD-Materials [13] was employed to identify and quantify aromatic interactions within the crystal lattice. The analysis shows that the crystal structure is stabilized by a strong π–π interaction involving the chloro-benzene rings of the quinoline unit (score 9.5). Aromatic stacking dimers are formed in which the molecules are related by inversion symmetry. These dimers exhibit a parallel displaced arrangement with a centroid–centroid distance of 3.96 Å and a relative orientation angle of 0° (Table S2).
Hirshfeld surface (HS) and 2D fingerprint (FP) analysis were further performed by using CrystalExplorer [14] to investigate, visualize, and quantitatively evaluate the intermolecular interactions within the crystal packing of the title compound (Figure 3, Figures S1 and S2). The greatest contribution to the HS comes from the H⋯H contacts (51.3%) due to the large hydrogen content of the molecule. These contacts are responsible for the widespread, high-density blue point distribution in the FP with two beak-shaped tips at de + di ≈ 2.5 Å (c1 in Figure 3). The light blue stripe roughly along the diagonal reflects the fraction of points on the Hirshfeld surfaces that involve nearly head-to-head H∙∙∙H contacts with an almost linear orientation between two pairs of axial hydrogens of a neighboring piperazinyl ring (c2 in Figure 3).
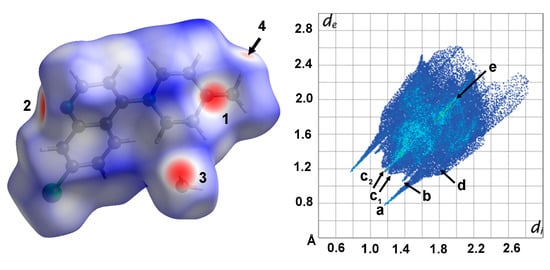
Figure 3.
(Left) Hirshfeld surface representations for the compound BPIP∙H2O with the function dnorm plotted onto the surface. Color code: −0.54 Å (red) to 1.41 Å (blue). The red spots are the result of the Owater—H⋯Npyridinic hydrogen bonds (labeled 1, 2 and 3) and Cmethyl—H⋯Owater contacts (labeled 4). (Right) Overall two-dimensional fingerprint plots for the title compound plot de versus di. The labels and arrows represent interactions between different atoms: (a) (O—)H⋯N, (b) (C—)H⋯O, (c) H···H, (d) C···H and Cl⋯H, and (e) Cl⋯Cl. Given that the plots are symmetrical, the arrows can be mirrored through the diagonal. The di and de values are the distances from the Hirshfeld surface (Å) to the nearest atom internal and external, respectively, to the surface.
The characteristic pair of long outer spikes with their tips at de + di ∼1.8 Å (a in Figure 3) are due to the presence of the Owater—H⋯Npyridinic hydrogen bond, which covers 9.6% of the overall crystal packing. The O⋯H contacts make a 5.9% contribution to the HS and are represented by a second shorter pair of spikes in the region de + di ≃ 2.2 Å (b in Figure 3). This feature underscores the role of the water oxygen atom as an acceptor in a weak intermolecular C—H⋯O hydrogen bond, with the terminal methyl group of the piperazinyl ring (2.567 Å). The short interatomic C···H/H···C and Cl⋯H/H⋯Cl contacts are overlapped in the FP and appear as a forceps-like distribution with parabolic tips at de + di ∼2.8 Å (C⋯H) and 3.0 Å (Cl⋯H), respectively (d in Figure 3). Their overall contributions are 14.4% for C···H/H···C and 10.7% for Cl⋯H/H⋯Cl. The contribution of C···H/H···C contacts largely corresponds to the aromatic interactions described above. The decomposition of fingerprint plots into individual atom–atom interactions is depicted in Figures S1 and S2.
A comparison of these results with the Hirshfeld surface analyses conducted separately on BPIP and the water molecule reveals that hydrogen bonding dominates the water–BPIP interactions, while the supramolecular assembly primarily arises from other intermolecular contacts, predominantly involving the primary molecule.
3. Discussion
Knowledge of the solid-state structure of a molecule is crucial for evaluating its properties and reactivity using both experimental and computational methods. However, until now, crystallographic data have been available for some compounds analogous to BPIP such as 7-chloro-4-(piperazin-1-yl)quinoline [15] but not for the BPIP system. Thus, to determine the crystal structure and the packing architecture of the title compound, a single-crystal X-ray diffraction study was performed. The structure determination revealed that the solid phase is the monohydrate form of the substance showing an extended hydrogen bonding network involving water–BPIP interactions.
To understand if the hydration water was already present in the source material or was incorporated during the crystallization process, a powder diffraction experiment was carried out on the starting bulk material. The nature of the reactive was assessed by taking and testing the whole content of a freshly opened 100 mg vial of the reactive as supplied by the manufacturer.
The experimentally observed PXRD pattern shown in Figure 4 was found to be in agreement with the simulated pattern obtained from the single-crystal data, confirming the hydration state of the commercial product and the bulk homogeneity of the samples. The melting point of the compound was measured and compared with the literature data to establish a reliable, easily obtainable, and cost-effective reference value for assessing the substance’s identity and purity. The measurement, conducted using a standard melting point apparatus, yielded a range of 82.8–83.5 °C. This result shows reasonable consistency with the findings reported in [16] (86 °C), where isopropanol was used as recrystallization solvent. Other available melting point data report somewhat different values, i.e., 80 °C [17] for a pale-brown solid precipitated from an aqueous solution and subsequently dried and 88–89 °C [6] for white crystals obtained from methanol, with a mass corresponding to the anhydrous form as determined by high-resolution mass spectrometry (HRMS).
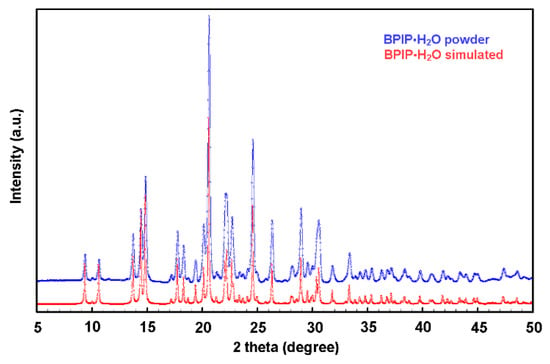
Figure 4.
The comparison between the simulated PXRD pattern from the single-crystal X-ray data of hydrate BPIP∙H2O (in red) and experimental PXRD data of BPIP reactive (in blue) as supplied by the manufacturer. The result indicates that the experimental PXRD profile matches well with the simulated one.
The identification of anhydrous and hydrated forms of a drug substance is of great importance for ensuring that products meet expected specifications in terms of bioavailability [18]. The hydrate form with a well-defined stoichiometric water content exhibits a unique molecular arrangement in the solid state generated by a complex and extended hydrogen bond network, which is responsible for the characteristic physical and chemical properties of the compound.
4. Materials and Methods
4.1. Materials
The 7-chloro-4-(4-methylpiperazin-1-yl)quinoline (BPIP) (CAS number: 84594-63-8) was purchased from Merk Life Science S.r.l., Milano, Italy. The reactive is a Sigma-Aldrich product sold without any assurance as to the identity and purity of the product. 2-propanol was supplied by Sigma-Aldrich (Merck SpA), Milano, Italy and was of ACS reagent grade. The materials were used as received, without any further purification.
4.2. Single-Crystal X-Ray Diffraction
Materials X-ray-quality crystals were obtained by the slow evaporation of an isopropanol solution of BPIP at room temperature.
The single-crystal X-ray data collection for BPIP-H2O was performed on an air-stable single crystal mounted on a glass fiber at a random orientation on a Bruker SMART-APEX II diffractometer [19] equipped with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). The data were collected by the ω-scan method (width of 0.5° frame−1, exposure time of 20 s per frame) within the limits 2.2°< θ < 31.7°.
Cell parameters were retrieved and refined using SAINT software v8.34A [20] on 1624 reflections (19% of the observed reflections). Data processing and space group determination were performed with the SAINT and XPREP (2013) programs [20]. The structures were solved by direct methods (SIR2019) and refined by full-matrix least squares on F2 (SHELX 2018) [21] with the WINGX (2023.1) interface [22]. The collected intensities were corrected for Lorentz and polarization effects and for absorption effects by applying a multi-scan method by using the SADABS (2013) program [20]. The structure pictures were generated using the ORTEPIII (v1.0.3) [22] and VESTA(v3.5.8) programs [23].
All non-H atoms were refined with full occupancies and anisotropic displacement parameters. No disorder was observed. All hydrogen atoms were located from the difference-Fourier map and were refined using a riding model of Uiso = 1.5 Ueq (parent atom) for -CH3 and -OH and Uiso = 1.2 Ueq (parent atom) for -CH- and -CH2-. Bond lengths and angles are summarized in Table S3.
Crystal data and refinement details for BPIP∙H2O: C14H16ClN3·H2O—Mr = 279.76, T = 296(2) K, λ = 0.71073 Å, monoclinic, space group P21/c, a = 9.4737 (11), b = 17.650 (2), c = 8.5451 (10) Å, β = 90.156 (2)°, V = 1428.9 (3) Å3, Z = 4, ρcalc = 1.300 g cm−3, µcalc = 0.264 mm−1, F(000) = 592, crystal size 0.42 × 0.30 × 0.04 mm, θ range = 2.2–31.7°, 22429 reflections collected, 4534 reflections unique, Rint = 0.0287, observed reflections [I > 2σ(I)] 2831, 0 restraints, 226 parameters, R1 (all data) = 0.0857, wR2 (all data) = 0.1247, R1 (observed) = 0.0434, wR2 (observed) = 0.1052, ∆ρmax = 0.25 eÅ−3, and ∆ρmin = −0.21 eÅ−3.
4.3. X-Ray Powder Diffraction
Ambient X-ray Powder Diffraction measurements were performed using a Miniflex-600 diffractometer (Rigaku, Japan) with Cu Kα (λ = 1.540598 Å) radiation over an angular range of 5°–60° (2θ), with an incremental step size of 0.02° (2θ) and a counting time of 2 s∙step−1. Phase identification was performed by comparing the experimental powder pattern with that simulated from single-crystal data. The analysis of PXRD data was carried out by using FullProf suite software [24].
4.4. Melting Point Measurements
Melting point measurements were conducted using thin glass capillary tubes with internal diameters of 1 mm and wall thicknesses of 0.1–0.2 mm, employing a standard melting point apparatus (FALC Instruments, vs. 1.11). The specimens were initially heated at a rapid rate (20 °C·min−1) up to 75 °C, after which the heating rate was reduced to 1 °C·min−1 until the melting point was reached.
Supplementary Materials
The following supporting information are available online. Table S1: Hydrogen-bond geometry (Å, °) for BPIP∙H2O; Table S2: Aromatic analyser score in BPIP∙H2O (distances and angles of π⋯π and C–H⋯π stacking with X as the centre of the aromatics); Table S3: Geometric parameters (Å, º) for BPIP∙H2O; Figure S1: The decomposed fingerprint plots (atom–atom interactions) of the Hirshfeld surface for BPIP∙H2O; Figure S2: Relative contributions of various intermolecular interactions to the Hirshfeld surface area in BPIP∙H2O.
Author Contributions
Conceptualization; methodology; validation; formal analysis, S.R.; investigation, S.R. and F.M.; resources, S.R.; data curation, S.R. and F.M.; writing—original draft preparation, S.R.; writing—review and editing, S.R. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
Università degli Studi di Milano is acknowledged for funding this work through the Research Support Plan 2024 (Piano di Sostegno alla Ricerca 2024), grant number PSR2023_DIP_005_PI_FTESS.
Data Availability Statement
CCDC 2443163 contains the supplementary crystallographic data for this paper. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures (accessed on 11 April 2025).
Acknowledgments
We would like to thank Pietro Colombo for providing technical assistance with the single-crystal X-ray experiment.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; the collection, analyses, or interpretation of data; the writing of the manuscript; or the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| BPIP | 7-chloro-4-(4-methyl-1-piperazinyl)quinoline |
| PQ | Piperaquine |
| PXRD | X-ray Powder Diffraction |
References
- Zhou, W.; Wang, H.; Yang, Y.; Chen, Z.-S.; Zou, C.; Zhang, J. Chloroquine against Malaria, Cancers and Viral Diseases. Drug. Discov. Today 2020, 25, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Espeau, P. Special Issue “Pharmaceutical Solid Forms: From Crystal Structure to Formulation”. Pharmaceutics 2025, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, E.; Mazurek, A.H.; Szeleszczuk, Ł.; Pisklak, D.M.; Zielińska-Pisklak, M. Pharmaceutical Hydrates Analysis—Overview of Methods and Recent Advances. Pharmaceutics 2020, 12, 959. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker, R.; Blatter, F.; von Raumer, M. Relevance of Solid-state Properties for Pharmaceutical Products. In Polymorphism; Wiley: Hoboken, NJ, USA, 2006; pp. 1–19. [Google Scholar]
- Davis, T.M.E.; Hung, T.-Y.; Sim, I.-K.; Karunajeewa, H.A.; Ilett, K.F. Piperaquine. Drugs 2005, 65, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Warhurst, D.C.; Craig, J.C.; Adagu, I.S.; Guy, R.K.; Madrid, P.B.; Fivelman, Q.L. Activity of Piperaquine and Other 4-Aminoquinoline Antiplasmodial Drugs against Chloroquine-Sensitive and Resistant Blood-Stages of Plasmodium Falciparum. Biochem. Pharmacol. 2007, 73, 1910–1926. [Google Scholar] [CrossRef] [PubMed]
- Lackovic, K.; Parisot, J.P.; Sleebs, N.; Baell, J.B.; Debien, L.; Watson, K.G.; Curtis, J.M.; Handman, E.; Street, I.P.; Kedzierski, L. Inhibitors of Leishmania GDP-Mannose Pyrophosphorylase Identified by High-Throughput Screening of Small-Molecule Chemical Library. Antimicrob. Agents Chemother. 2010, 54, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Courseille, C.; Busetta, B.; Hospital, M. 7-Chloro-4(4-Diethylamino-1-Methylbutyl-Amino) Quinoline. Cryst. Struct. Commun. 1973, 2, 283. [Google Scholar]
- Kaiser, C.R.; Pais, K.C.; de Souza, M.V.N.; Wardell, J.L.; Wardell, S.M.S.V.; Tiekink, E.R.T. Assessing the Persistence of the N–H⋯N Hydrogen Bonding Leading to Supramolecular Chains in Molecules Related to the Anti-Malarial Drug, Chloroquine. CrystEngComm 2009, 11, 1133. [Google Scholar] [CrossRef]
- Hisaki, I.; Hiraishi, E.; Sasaki, T.; Orita, H.; Tsuzuki, S.; Tohnai, N.; Miyata, M. Crystal Structure of Quinine: The Effects of Vinyl and Methoxy Groups on Molecular Assemblies of Cinchona Alkaloids Cannot Be Ignored. Chem. Asian J. 2012, 7, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, P.; Loconte, L.; Macetti, G.; Rizzato, S.; Lo Presti, L. Correlations of Crystal Structure and Solubility in Organic Salts: The Case of the Antiplasmodial Drug Piperaquine. Cryst. Growth Des. 2019, 19, 1399–1410. [Google Scholar] [CrossRef]
- Cremer, D.; Pople, J.A. General Definition of Ring Puckering Coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.A.; King, C.L.; Fortunak, J.M.D.; Butcher, R.J. 7-Chloro-4-(Piperazin-1-Yl)Quinoline. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o1497. [Google Scholar] [CrossRef] [PubMed]
- Dann, O.; Steuding, W.; Lisson, K.G.; Seidel, H.R.; Fink, E.; Nickel, P. [Antimalarial 6-aminoquinolines XV. 6- and 4-aminoquinolines with a tertiary basic alkylated amino group]. Arzneimittelforschung 1982, 32, 1219–1223. [Google Scholar] [PubMed]
- Staderini, M.; Cabezas, N.; Bolognesi, M.L.; Menéndez, J.C. Solvent- and Chromatography-Free Amination of π-Deficient Nitrogen Heterocycles under Microwave Irradiation. A Fast, Efficient and Green Route to 9-Aminoacridines, 4-Aminoquinolines and 4-Aminoquinazolines and Its Application to the Synthesis of the Drugs Amsacrine and Bistacrine. Tetrahedron 2013, 69, 1024–1030. [Google Scholar] [CrossRef]
- Khankari, R.K.; Grant, D.J.W. Pharmaceutical Hydrates. Thermochim. Acta 1995, 248, 61–79. [Google Scholar] [CrossRef]
- Bruker APEX2 V2014.1-1, Bruker AXS Inc.: Madison, WI, USA, 2015.
- Bruker SAINT v8.34A, XPREP V2013/3, SADABS 2013, Bruker AXS Inc.: Madison, WI, USA, 2013.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. FULLPROF: A Program for Rietveld Refinement and Pattern-Matching Analysis. In Proceedings of the Abstracts of the Meeting Powder Diffraction, Toulouse, France, 16–19 July 1990; pp. 127–128. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).