Abstract
A tetraerbium cluster containing soft-base dianionic 4,8-difluorobenzo [1,2-d:5,4-d′]bisthiazole-2,6-dithiol (H2L) ligands, μ-OH, and coordinated 1,2-dimethoxyethane (DME) of the general formula {Er4(μ-L)4(μ-OH)4(DME)4} (1) was synthesized using a one-pot method. X-ray analysis revealed that 1 is an asymmetrical tetramer in which there are four μ2-bridging bisthiazole ligands and four μ2-bridging hydroxide anions per four erbium ions. The molecule of 1 has inherent chirality, and the geometry of intramolecular F…F short contacts implies the formation of a classical halogen bond. Upon excitation by a 375 nm diode laser, compound 1 shows the moderate metal-centered emission of Er3+ ions that peaked at 1530 nm.
1. Introduction
Lanthanide(III) clusters have attracted considerable attention in recent years due to their specific nuclear-related properties [1,2]. The ability to modify the magnetic and optical properties of Ln(III) ions determines the development of new optical [3,4], luminescent [5,6], upconversion [7,8], magnetic [9,10], and anticounterfeiting [11,12] materials based on clusters. It is well known that lanthanides are hard Lewis acids and form stable compounds with hard-base anions. This concept is also realized for lanthanide clusters, in which most of the anions are derivatives of β-diketonates [13], carboxylates [14,15], and other hard Lewis bases [5,16]. However, a number of Ln3+ complexes with soft-base chalcogenide ligands are known [17,18,19,20]. They may exhibit room temperature phosphorescence [20] and show intense and prolonged near-infrared emission when all protons in the ligand are replaced by halogens [21,22,23]. Moreover, lanthanide coordination polymers containing soft-base ditopic derivatives of 2-mercaptobenzothiazole have been reported recently [24]. Additionally, it was reported that benzothiazole-containing compounds are useful for pharmacological applications and exhibit antituberculosis [25], antitumor [26] and anticancer [27] activities. It is known that thiophenolate and selenophenolate ligands are the only organochalcogenide ligands capable of forming lanthanide clusters [28,29,30,31]. To the best of our knowledge, lanthanide clusters containing ditopic soft-base ligands are unknown to date. In order to prepare Ln3+ clusters with ditopic mercaptothiazole-4,8-difluorobenzo, [1,2-d:5,4-d′]bisthiazole-2,6-dithiol (H2L) was used as a ligand.
2. Results
One of the commonly used synthetic strategies for preparation of Ln3+ clusters is the exposure of hydrolyzing agents on lanthanide coordination compounds [1,32]. This strategy was also applied for the synthesis of a tetraerbium cluster based on the H2L ligand. The synthesis of the targeted compound was performed using a one-pot method in 1,2-dimethoxyethane (DME) media starting from tris(cyclopentadienyl)erbium(III) (Cp3Er) and the H2L ligand according to Scheme 1.
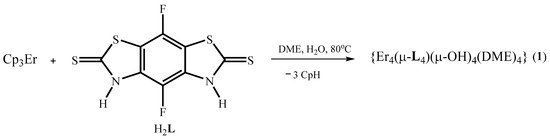
Scheme 1.
Synthesis of 1.
X-ray analysis revealed that complex 1 is an asymmetrical tetramer in which there are four μ2-bridging bisthiazole ligands and four μ2-bridging hydroxide anions per four erbium ions. In this case, each metal atom is bidentately bound to two SCN fragments of two different L ligands, which bind it to two other metal atoms. The bridging hydroxyl groups form, together with two erbium atoms not bound by L ligands, four-membered rings ErOErO. In addition, each metal atom additionally coordinates a neutral DME molecule (Figure 1a). Views along the crystallographic a, b, and c axes are additionally shown in Figure S1. The binding of erbium cations via benzothiazole ligands is shown in detail in Figure S2.
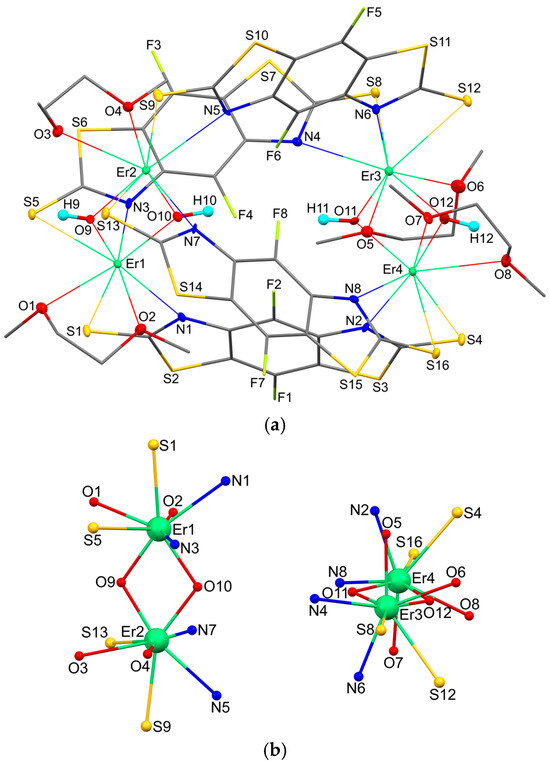
Figure 1.
The molecular structure of complex 1 (a) and coordination environment of metal atoms (b). Displacement ellipsoids are drawn at the 30% probability level. Hydrogen atoms except O–H hydrogen atoms are omitted.
Thus, the coordination number of each erbium atom is 8 (Figure 1b). It is interesting to note that the SCN fragments are coordinated differently. Each metal atom is involved in one short (2.777(3) − 2.802(3) Å) and one long (2.899(3) − 2.945(3) Å) Er-S bond. In the case of shortening of the Er-S bond, a simultaneous lengthening of the Er-N interaction is observed (Table 1). Despite this, the geometry of each of the eight thiazolethiolate fragments does not differ from that of the others. All C-S bonds with the thiolate sulfur atom lie in a narrow range of 1.693(12) − 1.712(12) Å. The C-N bonds in all eight thiazole heterocycles are not equivalent to each other and vary in the ranges of 1.312(14) − 1.341(15) and 1.385(14) − 1.402(14) Å. Similar geometric characteristics have been observed previously in related benzothiazolethiolate ligands [33,34,35,36].

Table 1.
The selected bond lengths [Å] in 1.
Analysis of the eight-vertex polyhedron using SHAPE v2.1 software [37] indicates that the geometry of the all erbium atoms could be best described as a triangular dodecahedron (Table 2).

Table 2.
SHAPE analysis for metal atoms in 1.
All bisthiazole ligands are essentially planar. The average deviation of atoms from the plane is 0.04–0.06 Å. The most significant deviations (0.10–0.17 Å) are observed for sulfur atoms bound to the erbium atom by elongated Er-S bonds in only three ligands. It is interesting that in the S1–S4 ligand the greatest deviation does not exceed 0.086 Å. This is probably due to the nonequivalent environment of the molecule in the crystal. The mutual arrangement of the bisthiazole ligands in complex 1 is presented in Table 3. The four-membered ErSCN metallacycles are not planar. The dihedral angles between the SErN and SCN planes vary in the range of 3.9–13.1°. The ErOErO metallocycles, conversely, are almost perfectly flat. The maximum deviation of atoms does not exceed 0.04 Å. In molecule 1, the ErOErO planes are located almost orthogonally to each other. The corresponding dihedral angle is 88.5°. The molecule has inherent chirality. Based on the value of the absolute Flack structural parameter (−0.006(9)), we conclude that the crystal is a single diastereomer.

Table 3.
Dihedral angles [°] between the planes of the bisthiazolate ligands in 1.
Four fluorine atoms of the bisthiazolate ligands are directed into the cavity formed by the ligands. It is interesting to note that the geometry of the F…F short contacts corresponds to the classical halogen bond (angle C1-Hal1…Hal2 ≈ 180°, angle Hal1…Hal2-C2 ≈ 90°) [38,39,40]. The geometric characteristics of the short F…F contacts are given in Table 4. Numerous studies demonstrate a decrease in the tendency for halogen bonds to be realized in the series I > Br > Cl > F [41,42,43]. This is due to the fact that the electrostatic potential of the fluorine atom is negative over the entire surface, in contrast to the heavier halogens in almost all compounds [44]. However, some studies show that interaction between two fluorine atoms is possible when the charge depletion region faces the charge concentration region [45,46,47,48]. The distances between the fluorine atoms in complex 1 (2.759(9) − 2.855(8) Å) are significantly shorter than the sum of the van der Waals radii (3.0 Å) [49], which also indicates the implementation of the F…F interaction.

Table 4.
The geometric characteristics of short F…F contacts in 1.
As a result, a four-membered ring FFFF is formed (Figure 2a). A similar structural motif was observed in the crystal of 5-fluorouracil [45]. The cycle consisting of fluorine atoms is not flat in both cases. The average deviation of atoms from the plane is 0.26 Å in molecule 1 and 0.22 Å in the 5-fluorouracil crystal. Note that the distances between fluorine atoms in the crystal of the uracil derivative (3.052–3.093 Å) exceed the corresponding distances observed in molecule 1. However, the study of the deformation electron density demonstrated a correspondence between the charge depletion region on one of the atoms and the charge concentration region on the other fluorine atom [45].
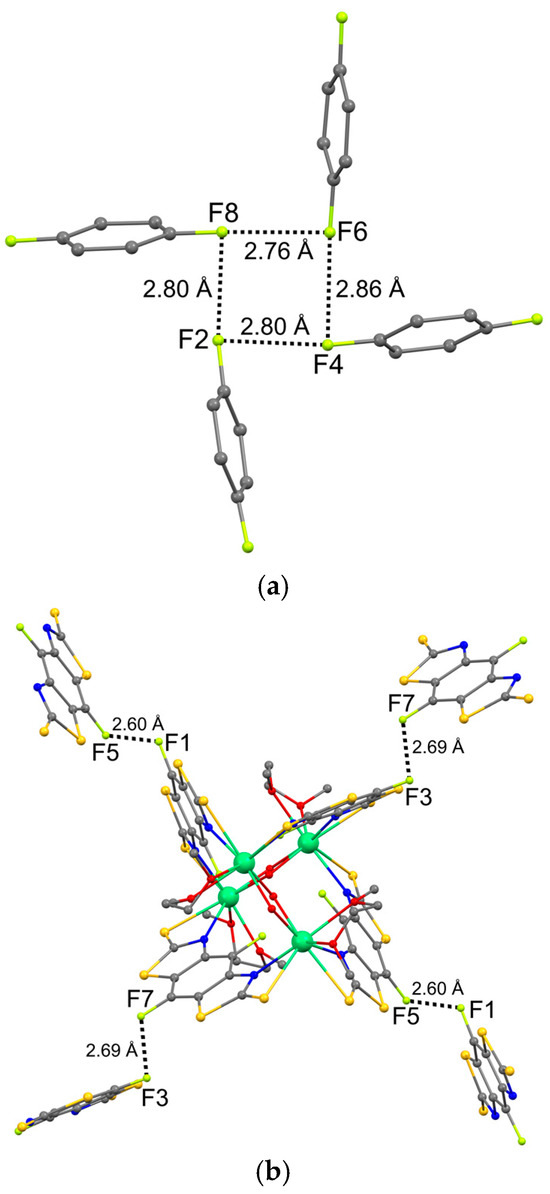
Figure 2.
Intramolecular (a) and intermolecular (b) F…F short contacts in the crystal of 1.
In crystal 1, intermolecular short F…F contacts are also observed. The geometric characteristics correspond to another type of halogen…halogen interactions, which is characterized by C1-Hal1…Hal2 ≈ Hal1…Hal2-C2 (Table 4). It is generally accepted that such contacts are related to weak van der Waals interactions arising under the influence of the packing effect. However, the intermolecular distances F…F in crystal 1 are significantly shorter than the intramolecular ones. One of the F…F distances (2.600(9) Å) is shorter than the boundary (2.65 Å) between the shortened (Van der Waals) and contracted contacts, and the second (2.688(9) Å) only slightly exceeds this boundary. Contracted contacts usually indicate the presence of specific interactions that are much stronger than ordinary van der Waals interactions; they are attractive in character and significantly affect the structure and properties of molecular crystals [50].
As expected from the triplet level energy of H2L (21,400 cm−1) [51], it acts as an antenna ligand in 1, sensitizing the metal-centered emission of Er3+ that peaked at 1530 nm, which originates from the 4I13/2 → 4I15/2 f-f ionic transition. This emission may be applicable in telecommunication systems because its wavelength matches perfectly to the third low-loss transmission window for silica optical fibers [52]. It should be noted that cluster 1 contains a large number of CH- and OH- quenching groups; so, high-power pumping with a 375 nm diode laser was used to excite erbium near-infrared photoluminescence (Figure 3).
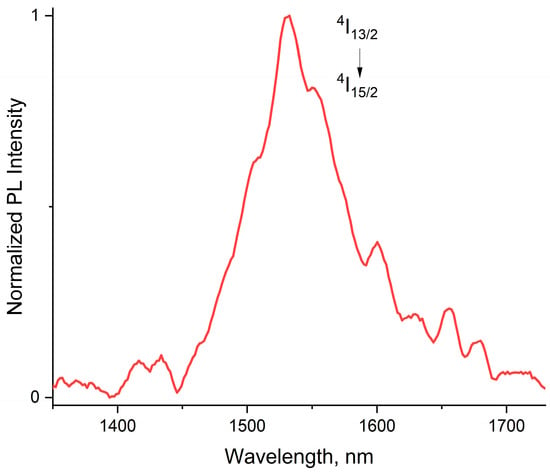
Figure 3.
PL spectrum of 1 in the near-infrared region. Excitation—375 nm diode laser.
3. Materials and Methods
3.1. General
All manipulations were carried out under vacuum using standard Schlenk techniques or in an argon glove box (H2O < 0.1 ppm; O2 < 1 ppm). Tris(cyclopentadienyl)erbium(III) was purchased from commercial sources (Sigma Aldrich, St. Louis, MO, USA) and used as received. The 4,8-difluorobenzo [1,2-d:5,4-d′]bisthiazole-2,6-dithiol (H2L) was synthesized as described elsewhere [51]. The solvent DME was purified by distillation from sodium/benzophenoneketyl. The C, H, N elemental analyses were performed on a Vario EL cube analyzer. The erbium content was determined by complexometric titration. IR spectra were obtained on a PerkinElmer 577 spectrometer (Waltham, MA, USA) and recorded from 4000 to 450 cm−1 as a Nujol oil mull on KBr plates. Lanthanide content was analyzed by complexometric titration. The photoluminescence of 1 evacuated in a quartz tube was excited by a 375 nm diode laser (CNI Laser, Changchun, China) and registered with an Ocean Optics NIR 512 fluorimeter (Orlando, FL, USA) in the range 900–1700 nm.
3.2. Synthesis of Tetra-μ-hydroxo-tetrakis{μ-[4,8-difluorobenzo [1,2-d:5,4-d′] bis(thiazole)-2,6-dithiolato-κ2N,S:κ2N′,S′]}-tetrakis{[1,2-dimethoxyethane-κ2O,O′]-tetraerbium(III)} (1)
A solution of Cp3Er (200 mg, 0.55 mmol) in DME (5 mL) was added to a 30 mL ampoule containing a suspension of H2L (161 mg, 0.55 mmol) and H2O (10 μL, 0.55 mmol) in DME (5 mL). The resulting mixture was sealed and stirred ultrasonically for 1 h. Then, the ampoule was kept at 80 °C for 100 h. The precipitated light pink crystals of 1 were decanted and dried in vacuum to receive mg (12% yield). FTIR (Nujol, cm−1): 472 (w), 532 (w), 557 (w), 644 (m), 659 (m), 818 (w), 922 (w), 1020 (m), 1047 (s), 1088 (m), 1190 (w), 1227 (m), 1264 (m), 1352 (s), 1422 (w), 1503 (w), 1538 (w), 1611 (m), 1636 (s), 3396 (m) (Figure S3). Elemental Analysis for C48H44Er4F8N8O12S16 calculated: C, 25.52; H, 1.96; N, 4.96; S, 22.71; Er, 29.62; found: C, 25.29; H, 1.94; N, 4.87; S, 22.14; Er, 29.27. Crystals suitable for XRD were taken directly from the reaction ampoule.
3.3. X-Ray Crystallography
The diffraction data for tetra-μ-hydroxo-tetrakis{μ-[4,8-difluorobenzo [1,2-d:5,4-d′] bis(thiazole)-2,6-dithiolato-κ2N,S:κ2N′,S′]}-tetrakis{[1,2-dimethoxyethane-κ2O,O′]-tetraerbium(III)} (1) were collected using a Bruker D8 Quest diffractometer (Billerica, MA, USA) (Mo-Kα radiation, ω-scan technique, λ = 0.71073 Å). The intensity data were integrated by the SAINT program (v.8.37A) [53]. The structure was solved by the dual method [54] and was refined on F2hkl using the SHELXTL package (SHELXT-2014/5 SHELXL-2019/3 XCIF-2014/2 XP-5) [55]. All non-hydrogen atoms were refined anisotropically. All hydrogen atoms except hydrogen atoms of hydroxyl groups were placed in calculated positions and were refined using the riding model (Uiso(H) = 1.5 Ueq(C) for CH3-groups and Uiso(H) = 1.2 Ueq(C) for all other groups). In turn, the O–H hydrogen atoms in 1 were located from difference Fourier maps and refined with distance (AFIX 147 instruction) and thermal parameter (Uiso(H) = 1.2 Ueq(O)) restraints. The SADABS program (2016/2) [56] was used to perform absorption corrections.
A colorless stick-shaped single crystal (0.14 × 0.03 × 0.03 mm) was selected for SC-XRD analysis. The crystal data for complex 1 were as follows: monoclinic crystal system, space group P21, unit cell dimensions: a = 15.0538(4) Å, b = 14.7273(4) Å, c = 15.7091(5) Å, β = 98.4400(10)°, V = 3445.02(17) Å3, Z = 2, dcalc = 2.178 g/cm3, μ = 5.390 mm−1, F(000) = 2168, reflection collected/unique = 33,113/11,447, Rint = 0.0339, S = 1.037, R1 = 0.0360 and wR2 = 0.0749 [I > 2σ(I)], R1 = 0.0399 and wR2 = 0.0768 (all data), largest diff. peak and hole 1.218 and −1.285 e·Å−3. The absolute structure parameter (Flack) was −0.006(9).
Supplementary Materials
Figure S1: Molecular structure of complex 1. Views along crystallographic a (a), b (b) and c (c) axes; Figure S2. The binding of erbium cations via benzothiazole ligands in different projections; Figure S3. FTIR spectrum of 1 as Nujol mull.
Author Contributions
Conceptualization, V.A.I.; methodology, G.K.F.; software, R.V.R. and G.K.F.; validation, G.K.F. and M.N.B.; investigation, A.F.R. and R.V.R.; resources, G.K.F. and M.N.B.; writing—original draft preparation, V.A.I., A.F.R. and R.V.R.; writing—review and editing, G.K.F. and M.N.B.; visualization, A.F.R. and R.V.R.; supervision, M.N.B.; project administration, V.A.I.; funding acquisition, V.A.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 20-73-10115.
Data Availability Statement
CCDC 2448255 contains the supplementary crystallographic data. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures (accessed on 1 May 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gálico, D.A.; Calado, C.M.S.; Murugesu, M. Lanthanide molecular cluster-aggregates as the next generation of optical materials. Chem. Sci. 2023, 14, 5827–5841. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-J.; Holmberg, R.J.; Lin, P.-H. Slight synthetic changes eliciting different topologies: Synthesis, structure and magnetic properties of novel dinuclear and nonanuclear dysprosium complexes. Dalton Trans. 2015, 44, 19758–19762. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Suzuki, Y.; Doi, Y.; Seki, T.; Koizumi, H.; Fushimi, K.; Fujita, K.; Hinatsu, Y.; Ito, H.; Tanaka, K.; et al. Enhancement of Optical Faraday Effect of Nonanuclear Tb(III) Complexes. Inorg. Chem. 2014, 53, 7635–7641. [Google Scholar] [CrossRef]
- Mavragani, N.; Gálico, D.A.; Kitos, A.A.; Murugesu, M. Near-Infrared Magnetic Circularly Polarized Luminescence and Slow Magnetic Relaxation in a Tetrazinyl-Bridged Erbium Metallocene. J. Am. Chem. Soc. 2025, 147, 1387–1391. [Google Scholar] [CrossRef]
- Gálico, D.A.; Ovens, J.S.; Murugesu, M. NIR-to-NIR emission on a water-soluble {Er6} and {Er3Yb3} nanosized molecular wheel. Nanoscale 2020, 12, 11435–11439. [Google Scholar] [CrossRef]
- Calado, C.M.S.; Gálico, D.A.; Murugesu, M. Intra-cluster energy transfer editing in a dual-emitting system to tap into lifetime thermometry. Chem. Commun. 2023, 59, 13715–13718. [Google Scholar] [CrossRef] [PubMed]
- Hamon, N.; Godec, L.; Sanchez, S.; Beyler, M.; Charbonnière, L.J.; Tripier, R. Upconversion Luminescence with Bis-pyclen Yb(III) Chelates: Crown vs. Linear Polyether Linkers in Discrete Heteropolynuclear Architectures. Angew. Chem. Int. Ed. 2025, 64, e202414608. [Google Scholar] [CrossRef]
- Gálico, D.A.; Kitos, A.A.; Ramdani, R.; Ovens, J.S.; Murugesu, M. Distortion Engineering: A Strategy to Modulate Molecular Upconversion with Molecular Cluster-Aggregates. J. Am. Chem. Soc. 2024, 146, 26819–26829. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.-D.; Ge, R.; Lv, Z.-H.; Tian, C.-B.; Wei, B.-W.; Li, X.-X.; Zhuang, N.-F.; Zheng, S.-T. A Stable 3d–4f Heterometallic Cluster with Magneto-Optical Activity. Inorg. Chem. 2022, 61, 8746–8751. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.; Sun, W. Sandglass-like Ln9 Nanoclusters with Magnetocaloric Effect and Lanthanide-Centered Luminescence. Cryst. Growth Des. 2024, 24, 8076–8084. [Google Scholar] [CrossRef]
- Gálico, D.A.; Kitos, A.A.; Ovens, J.S.; Sigoli, F.A.; Murugesu, M. Lanthanide-Based Molecular Cluster-Aggregates: Optical Barcoding and White-Light Emission with Nanosized {Ln20} Compounds. Angew. Chem. Int. Ed. 2021, 60, 6130–6136. [Google Scholar] [CrossRef] [PubMed]
- Gálico, D.A.; Murugesu, M. Dual-signalled magneto-optical barcodes with lanthanide-based molecular cluster-aggregates. Nanoscale 2023, 15, 18198–18202. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.-G.; Zuo, J.-L.; Yu, Z.; You, X.-Z.; Chen, W. Eu5(μ4-OH)(μ3-OH)4(μ-DBM)4(DBM)6 (DBM=dibenzoylmethide): A novel Eu5 square-pyramid polynuclear complex with a rare μ4-OH bridging mode. Inorg. Chem. Commun. 1999, 2, 490–494. [Google Scholar] [CrossRef]
- Grebenyuk, D.; Martynova, I.; Tsymbarenko, D. Self-Assembly of Hexanuclear Lanthanide Carboxylate Clusters of Three Architectures. Eur. J. Inorg. Chem. 2019, 26, 3103–3111. [Google Scholar] [CrossRef]
- Guo, X.-F.; Feng, M.-L.; Xie, Z.-L.; Li, J.-R.; Huang, X.-Y. The first examples of lanthanide selenite-carboxylate compounds: Syntheses, crystal structures and properties. Dalton Trans. 2008, 3101–3106. [Google Scholar] [CrossRef]
- Félix, G.; Kulakova, A.; Sene, S.; Charlot, C.; Bilyachenko, A.N.; Korlyukov, A.A.; Volodin, A.D.; Shubina, E.S.; Elizbarian, I.G.; Guari, Y.; et al. Lanthanide-Based Germsesquioxanes: Luminescence and Temperature Sensing. Organometallics 2023, 42, 2613–2622. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, X.; Kornienko, A.Y.; Kumar, G.A.; Yu, D.; Emge, T.J.; Riman, R.E.; Brennan, J.G. Efficient NIR Emission from Nd, Er, and Tm Complexes with Fluorinated Selenolate Ligands. Inorg. Chem. 2018, 57, 1912–1918. [Google Scholar] [CrossRef]
- Ilichev, V.A.; Balashova, T.V.; Polyakova, S.K.; Rogozhin, A.F.; Kolybalov, D.S.; Bashirov, D.A.; Konchenko, S.N.; Yablonskiy, A.N.; Rumyantcev, R.V.; Fukin, G.K.; et al. Synthesis, structure, and luminescence properties of sodium and ytterbium complexes with 2-(benzothiazol-2-yl)selenophenolate ligands. Russ. Chem. Bull. 2022, 71, 298–305. [Google Scholar] [CrossRef]
- Ilichev, V.A.; Blinova, L.I.; Rozhkov, A.V.; Balashova, T.V.; Rumyantcev, R.V.; Fukin, G.K.; Bochkarev, M.N. Fluorinated mercaptobenzothiazolates of lanthanides: Synthesis, structure and photoluminescence. J. Mol. Struct. 2017, 1148, 201–205. [Google Scholar] [CrossRef]
- Ilichev, V.A.; Rozhkov, A.V.; Rumyantcev, R.V.; Fukin, G.K.; Grishin, I.D.; Dmitriev, A.V.; Lypenko, D.A.; Maltsev, E.I.; Yablonskiy, A.N.; Andreev, B.A.; et al. LMCT facilitated room temperature phosphorescence and energy transfer in substituted thiophenolates of Gd and Yb. Dalton Trans. 2017, 46, 3041–3050. [Google Scholar] [CrossRef]
- Rogozhin, A.F.; Silantyeva, L.I.; Yablonskiy, A.N.; Andreev, B.A.; Grishin, I.D.; Ilichev, V.A. Near infrared luminescence of Nd, Er and Yb complexes with perfluorinated 2-mercaptobenzothiazolate and phosphine oxide ligands. Opt. Mater. 2021, 118, 111241. [Google Scholar] [CrossRef]
- Ilichev, V.A.; Rogozhin, A.F.; Belyakova, A.V.; Rozhkov, A.V.; Yablonskiy, A.N.; Rumyantcev, R.V.; Kozlova, E.A.; Fukin, G.K.; Bochkarev, M.N. Structural and Luminescent Features of Lanthanide Ate Complexes with Polychlorinated 2-Mercaptobenzothiazolate Ligands. Organometallics 2023, 42, 2792–2799. [Google Scholar] [CrossRef]
- Ilichev, V.A.; Silantyeva, L.I.; Yablonskiy, A.N.; Andreev, B.A.; Rumyantcev, R.V.; Fukin, G.K.; Bochkarev, M.N. Synthesis, structure and long-lived NIR luminescence of lanthanide ate complexes with perfluorinated 2-mercaptobenzothiazole. Dalton Trans. 2019, 48, 1060–1066. [Google Scholar] [CrossRef]
- Ilichev, V.A.; Rogozhin, A.F.; Rumyantcev, R.V.; Kozlova, E.A.; Fukin, G.K.; Yablonskiy, A.N.; Andreev, B.A.; Bochkarev, M.N. Lanthanide Coordination Polymers with Soft-Base Ditopic Bisthiazolate Ligands. Inorg. Chem. 2023, 62, 12625–12629. [Google Scholar] [CrossRef] [PubMed]
- Klimešová, V.; Kočí, J.; Waisser, K.; Kaustová, J.; Möllmann, U. Preparation and in vitro evaluation of benzylsulfanyl benzoxazole derivatives as potential antituberculosis agents. Eur. J. Med. Chem. 2009, 44, 2286–2293. [Google Scholar] [CrossRef]
- Aiello, S.; Wells, G.; Stone, E.L.; Kadri, H.; Bazzi, R.; Bell, D.R.; Stevens, M.F.G.; Bradshaw, T.D.; Westwell, A.D. Synthesis and Biological Properties of Benzothiazole, Benzoxazole, and Chromen-4-one Analogues of the Potent Antitumor Agent 2-(3,4-Dimethoxyphenyl)-5-fluorobenzothiazole (PMX 610, NSC 721648). J. Med. Chem. 2008, 51, 5135–5139. [Google Scholar] [CrossRef]
- Mitra, R.; Samuelson, A.G. Substitution-Modulated Anticancer Activity of Half-Sandwich Ruthenium(II) Complexes with Heterocyclic Ancillary Ligands. Eur. J. Inorg. Chem. 2014, 2014, 3536–3546. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Emge, T.J.; Brennan, J.G. Chalcogen-Rich Lanthanide Clusters with Fluorinated Thiolate Ligands. Inorg. Chem. 2002, 41, 3528–3532. [Google Scholar] [CrossRef] [PubMed]
- Melman, J.H.; Emge, T.J.; Brennan, J.G. Cubic lanthanide sulfido clusters: Ln8S6(SPh)12(thf)8 (Ln = Pr, Nd, Gd). Chem. Commun. 1997, 2269–2270. [Google Scholar] [CrossRef]
- Banerjee, S.; Huebner, L.; Romanelli, M.D.; Kumar, G.A.; Riman, R.E.; Emge, T.J.; Brennan, J.G. Oxoselenido Clusters of the Lanthanides: Rational Introduction of Oxo Ligands and Near-IR Emission from Nd(III). J. Am. Chem. Soc. 2005, 127, 15900–15906. [Google Scholar] [CrossRef]
- Huebner, L.; Kornienko, A.; Emge, T.J.; Brennan, J.G. Lanthanide Clusters with Internal Ln: Fragmentation and the Formation of Dimers with Bridging Se2- and Se22- Ligands. Inorg. Chem. 2005, 44, 5118–5122. [Google Scholar] [CrossRef] [PubMed]
- Roitershtein, D.M.; Vinogradov, A.A.; Lyssenko, K.A.; Nifant’ev, I.E. Self-assembly of heteroleptic tetranuclear carboxylate complexes of yttrium and lanthanides during hydrolysis and oxidation of rare earth homoleptic carboxylates. Inorg. Chem. Commun. 2017, 84, 225–228. [Google Scholar] [CrossRef]
- Radha, A. Crystal and molecular structure of 2-mercaptobenzothiazole—A redetermination. Z. Kristallogr. 1985, 171, 225–228. [Google Scholar]
- Watts, S.; Peloquin, A.J.; Bandara, M.; McMillen, C.D.; Pennington, W.T. Halogen, chalcogen, and hydrogen bonding in organoiodine cocrystals of heterocyclic thiones: Imidazolidine-2-thione, 2-mercaptobenzimidazole, 2-mercapto-5-methylbenzimidazole, 2-mercaptobenzoxazole, and 2-mercaptobenzothiazole. Acta Crystallogr. Sect. C 2022, 78, 702–715. [Google Scholar] [CrossRef]
- Politanskaya, L.; Duan, Z.; Bagryanskaya, I.; Eltsov, I.; Tretyakov, E.; Xi, C. Highly efficient synthesis of polyfluorinated 2-mercaptobenzothiazole derivatives. J. Fluor. Chem. 2018, 212, 130–136. [Google Scholar] [CrossRef]
- Zhong, H.-P.; Long, L.-S.; Huang, R.-B.; Zheng, L.-S.; Ng, S.W. 5-Amino-1,3-benzothiazole-2(3H)-thione. Acta Crystallogr. Sect. E 2003, 59, o1599–o1600. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE Is a Free Software. Available online: http://www.ee.ub.edu/ (accessed on 1 April 2020).
- Ramasubbu, N.; Parthasarathy, R.; Murray-Rust, P. Angular preferences of intermolecular forces around halogen centers: Preferred directions of approach of electrophiles and nucleophiles around carbon-halogen bond. J. Am. Chem. Soc. 1986, 108, 4308–4314. [Google Scholar] [CrossRef]
- Sakurai, T.; Sundaralingam, M.; Jeffrey, G.A. A Nuclear Quadrupole Resonance and X-ray Study of the Crystal Structure of 2,5-Dichloroaniline. Acta Crystallogr. 1963, 16, 354–363. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: The Design of Organic Solids; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Walsh, P.L.; Ma, S.; Obst, U.; Rebek, J., Jr. Nitrogen-Halogen Intermolecular Forces in Solution. J. Am. Chem. Soc. 1999, 121, 7973–7974. [Google Scholar]
- Messina, M.T.; Metrangolo, P.; Panzeri, W.; Ragg, E.; Resnati, G. Perfluorocarbon-Hydrocarbon Self-Assembly. Part 3.1 Liquid Phase Interactions between Perfluoroalkylhalides and Heteroatom Containing Hydrocarbons. Tetrahedron Lett. 1998, 39, 9069–9072. [Google Scholar] [CrossRef]
- Metrangolo, P.; Panzeri, W.; Recupero, F.; Resnati, G. Perfluorocarbon–Hydrocarbon Self–Assembly. J. Fluor. Chem. 2002, 114, 27–33. [Google Scholar] [CrossRef]
- Bartashevich, E.V.; Tsirelson, V.G. A Comparative View on the Potential Acting on an Electron in a Molecule and the Electrostatic Potential through the Typical Halogen Bonds. J. Comput. Chem. 2018, 39, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Jarzembska, K.N.; Kubsik, M.; Kamiński, R.; Woźniak, K.; Dominiak, P.M. From a Single Molecule to Molecular Crystal Architectures: Structural and Energetic Studies of Selected Uracil Derivatives. Cryst. Growth Des. 2012, 12, 2508–2524. [Google Scholar] [CrossRef]
- Pavan, M.S.; Prasad, K.D.; Row, T.N.G. Halogen bonding in fluorine: Experimental charge density study on intermolecular F⋯F and F⋯S donor–acceptor contacts. Chem. Commun. 2013, 49, 7558–7560. [Google Scholar] [CrossRef]
- Rumyantsev, R.V.; Fukin, G.K. Intramolecular Nonvalent Interactions in the EuIIEuIII(μ-ORF)2(μ2-ORF)3(μ3-ORF)2(DME)2 Complex. Russ. J. Coord. Chem. 2019, 45, 767–775. [Google Scholar] [CrossRef]
- Rumyantsev, R.V.; Fukin, G.K.; Baranov, E.V. Application of the Molecular Invariom Model for the Study of Interactions Involving Fluorine Atoms in the {YbII(μ2-OCH(CF3)2)3(μ3-OCH(CF3)2)2YbIII(OCH(CF3)2)2(THF)(Et2O)} Complex. Russ. J. Coord. Chem. 2021, 47, 235–243. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Zefirov, Y.V.; Zorky, P.M. New applications of van der Waals radii in chemistry. Russ. Chem. Rev. 1995, 64, 415–428. [Google Scholar] [CrossRef]
- Rogozhin, A.F.; Ilichev, V.A.; Fagin, A.A.; Rumyantcev, R.V.; Fukin, G.K.; Yablonskiy, A.N.; Andreev, B.A.; Bochkarev, M.N. Novel ditopic 2-mercaptothiazoles and their sodium salts: Synthesis, structural diversity and luminescence. New J. Chem. 2022, 46, 13987–13995. [Google Scholar] [CrossRef]
- Hu, J.X.; Karamshuk, S.; Gorbaciova, J.; Ye, H.Q.; Lu, H.; Zhang, Y.P.; Zheng, Y.X.; Liang, X.; Hernández, I.; Wyatt, P.B.; et al. High sensitization efficiency and energy transfer routes for population inversion at low pump intensity in Er organic complexes for IR amplification. Sci. Rep. 2018, 8, 3226. [Google Scholar] [CrossRef]
- SAINT, Data Reduction and Correction Program, v.8.37A; Bruker AXS: Madison, WI, USA, 2014.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).