Abstract
Nitrogen-containing heterocycles of 3-pyrrolin-2-one series are widely represented in natural and synthetic compounds, with a broad spectrum of pharmacological activity and considerable potential in medicinal and synthetic organic chemistry. In this communication, we report the previously unknown acid-catalyzed transformation of a N-substituted derivative of 3-pyrrolin-2-one that generates two types of heterocyclic moieties. The reflux of 1-benzyl-3-chloro-5-hydroxy-4-[(4-methylphenyl)sulfanyl]-1,5-dihydro-2H-pyrrol-2-one in toluene in the presence of catalytic amounts of H2SO4 resulted in the formation of a mixture of 1-benzyl-3-[(4-methylphenyl)sulfanyl]-1H-pyrrole-2,5-dione and 1-benzyl-7-methyl-1H-benzo[4,5]thieno[3,2-b]pyrrole-2,3-dione. The structures of four novel nitrogen-containing heterocycles were elucidated through IR, NMR spectroscopy and HRMS spectrometry. A new derivative of the fused tricyclic compounds, possessing benzo[b]thiophene and pyrrole-1,2-dione fragments, was also characterized by single-crystal X-ray diffraction.
1. Introduction
The chemistry of nitrogen-containing heterocycles is one of the most important research areas in modern organic chemistry and related fields. Due to their widespread occurrence in natural products and synthetic bioactive molecules, N-heterocycles have attracted significant attention from medicinal and synthetic organic chemists worldwide [1,2,3,4]. Various bioactive compounds, including pharmaceuticals, antibiotics, nucleic acids, vitamins, dyes and agrochemicals, contain a N-heterocyclic core.
Among these compounds, N-heterocycles of 3-pyrrolin-2-one series represent highly relevant pharmacophores, being widely investigated for their possible medicinal applications and utilization in organic synthesis. The development of efficient methods for the preparation and functionalization of 3-pyrrolin-2-ones is being actively explored due to their abundance in nature, broad spectrum of biological activity in γ-lactam-containing molecules and rich synthetic potential [5,6,7,8,9].
In the course of our study on the synthesis of novel sulfur heterocycles on the basis of 3-pyrrolin-2-one, we came across unexpected reactivity of 5-hydroxy-3-pyrrolin-2-one derivative in acidic medium. Thus, in the present communication, we report the previously unknown acid-catalyzed transformation of 1-benzyl-3-chloro-5-hydroxy-4-[(4-methylphenyl)sulfanyl]-1,5-dihydro-2H-pyrrol-2-one, which results in the formation of two types of heterocycles, namely N-substituted derivatives of 1H-pyrrole-2,5-dione and 1H-benzo[4,5]thieno[3,2-b]pyrrole-2,3-dione.
To the best of our knowledge, this is the first example of the construction of fused tricyclic systems possessing benzo[b]thiophene and pyrrole-1,2-dione fragments from the derivative of 3-pyrrolin-2-one. It was previously reported that the synthesis of benzo[b]thiophene-based molecules from arylsulfanyl-containing substrates can be achieved through the intramolecular arylation of the C–H bond of thioethers R1SR2 (R1 and/or R2-aryl moiety) in the presence of bis(triphenylphosphine) palladium(II)dichloride or palladium acetate catalysts [10,11,12,13,14,15,16]. Here, we present the formation of a benzothiophene scaffold from the p-tolylsulfanyl derivative of pyrrolinone without metal-based catalysts.
2. Results and Discussion
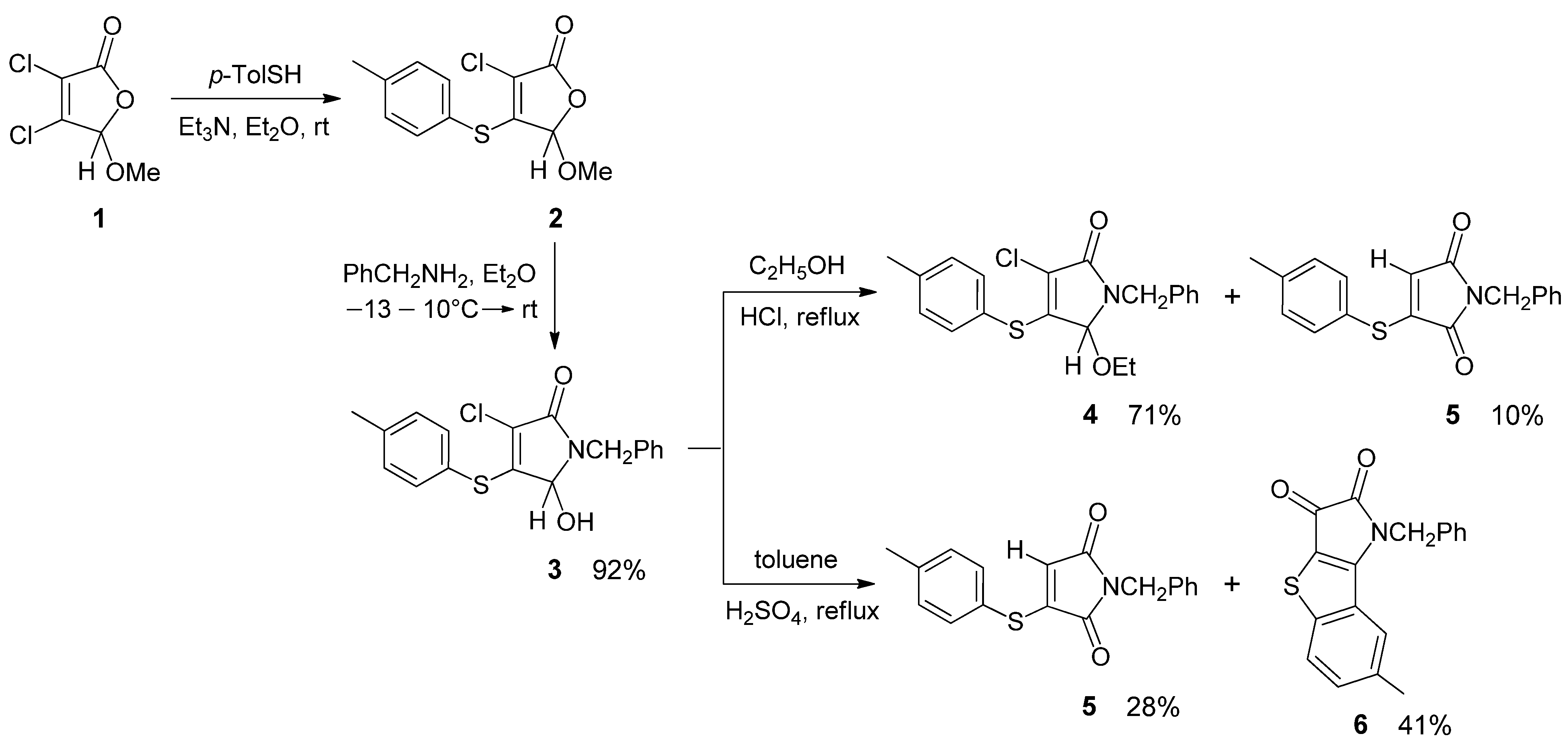
We previously conducted a structural study and the selective preparation of several thioethers of 3-pyrrolin-2-one from commercially available mucochloric and mucobromic acids [17,18,19,20,21,22]. A similar protocol was applied for the synthesis of 4-(p-tolylsulfanyl)-3-pyrrolin-2-one 3 (Scheme 1) served as a key compound for the current study. The methoxy derivative of 2(5H)-furanone 1 synthesized from mucochloric acid and methanol [23] was involved in a thiylation reaction with p-thiocresol in the presence of a base [19]. The furanone 2 obtained with a thio substituent in the fourth position of the unsaturated γ-lactone ring was allowed to react with benzylamine. As a result, the pyrrolinone thioether 3 was isolated as colorless crystals with a 92% yield and characterized by HRMS, IR and NMR spectroscopy.
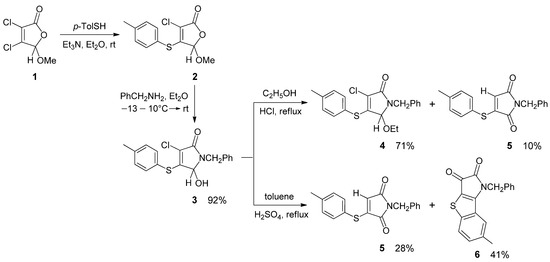
Scheme 1.
The synthesis of 3-pyrrolin-2-one 3 and its transformations in an acidic medium.
The IR spectrum of the heterocycle 3 shows characteristic absorption bands of OH stretching vibrations at 3450–3110 cm−1, stretching vibrations of the lactam carbonyl group at 1676 cm−1, and an aromatic ring at 1586, 1492 cm−1. The 1H NNR spectrum of pyrrolinone 3 in acetone-d6 displays a singlet for the methyl group at 2.32 ppm and multiplets in the range of 7.1–7.6 ppm, characteristic for aromatic protons. The signals of the methine proton and hydroxy group at carbon atom C-5 of the lactam ring appear as an AB-quadruplet in the range of 5.1–5.7 ppm (J 10.0 Hz). Another characteristic AB-quadruplet (δ 4.78, 4.31 ppm, JAB 15.6 Hz) corresponds to the diastereotopic methylene NCH2 protons.
During the study of pyrrolinone reactivity, interesting results were obtained for acid-catalyzed reactions with 5-hydroxypyrrolinone 3. In the reaction of compound 3 with ethanol performed in the presence of hydrochloric acid, together with the expected 5-ethoxypyrrolinone 4, a small amount of a side-product 5 was detected by NMR (Scheme 1). In addition, even after prolonged heating under reflux, incomplete conversion of the starting material 3 was achieved (12% recovery of 3), delivering reaction products 4 and 5 in 71% and 10% yields, respectively.
Isolation of the minor product by column chromatography and full characterization allowed us to identify it as a N-benzyl maleimide derivative 5 with a hydrogen atom instead of chlorine. IR and 13C{1H} NMR spectroscopic analyses indicated the presence of two carbonyl groups in the product 5. In the 1H NMR spectrum of compound 5, there are multiplets for the aromatic protons in the range of 7.2–7.6 ppm and three singlets at δ 5.73, 4.65 and 2.40 ppm, corresponding to the vinyl proton at carbon atom C-4 and protons of the NCH2 and CH3 groups, respectively.
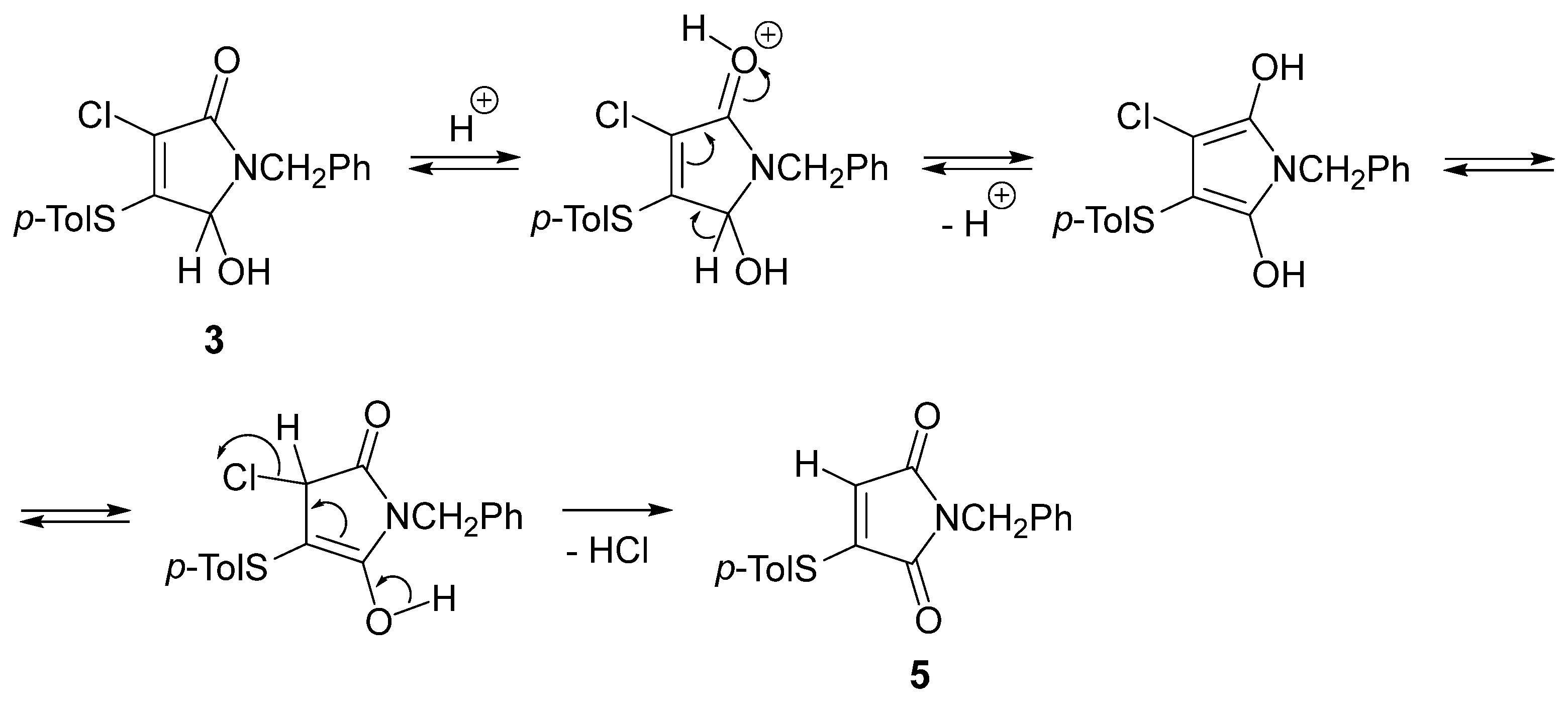
Regarding the mechanism for the observed formation of the unexpected product 5, we can propose initial protonation of the carbonyl oxygen atom of pyrrolinone 3, followed by deprotonation to give the enolic structure. The subsequent elimination of HCl from the corresponding keto-tautomer leads to the formation of a maleimide derivative 5 (Scheme 2).
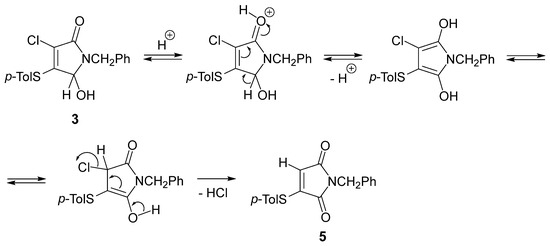
Scheme 2.
A possible mechanism for the formation of compound 5.
In a separate experiment, we showed, that maleimide derivative 5 can also be formed from 5-ethoxypyrrolinone 4. As evidenced by the 1H NMR analysis, the reaction mixture obtained after 10 h of heating under reflux of the ethanol solution of compound 4 in the presence of HCl contained unreacted 4 and maleimide 5 in a ratio of 8:1.
Further investigation of the transformations of pyrrolinone 3 in an acidic medium allowed us to discover another unexpected heterocyclic product. When compound 3 was refluxed in toluene in the presence of HCl, no reaction took place within 40 h; only the unreacted starting material 3 was detected in the reaction mixture by TLC and NMR spectroscopy. On the contrary, in the reaction carried out in the presence of sulfuric acid, complete consumption of pyrrolinone 3 occurred after 7 h. As a result, from this reaction, maleimide derivative 5 and novel heterocyclic compound 6 were isolated in 28% and 41% yields after purification by column chromatography on silica gel (Scheme 1).
The structure of compound 6 was confirmed by IR, 1H and 13C NMR spectroscopy, and HRMS. The 1H NMR spectrum of 6 in acetone-d6 displays two singlets at δ 2.33 and 5.33 ppm for the protons of the CH3 and NCH2 groups, respectively, and groups of signals in the range of 7.2–8.0 ppm corresponding to protons of two different aromatic rings.
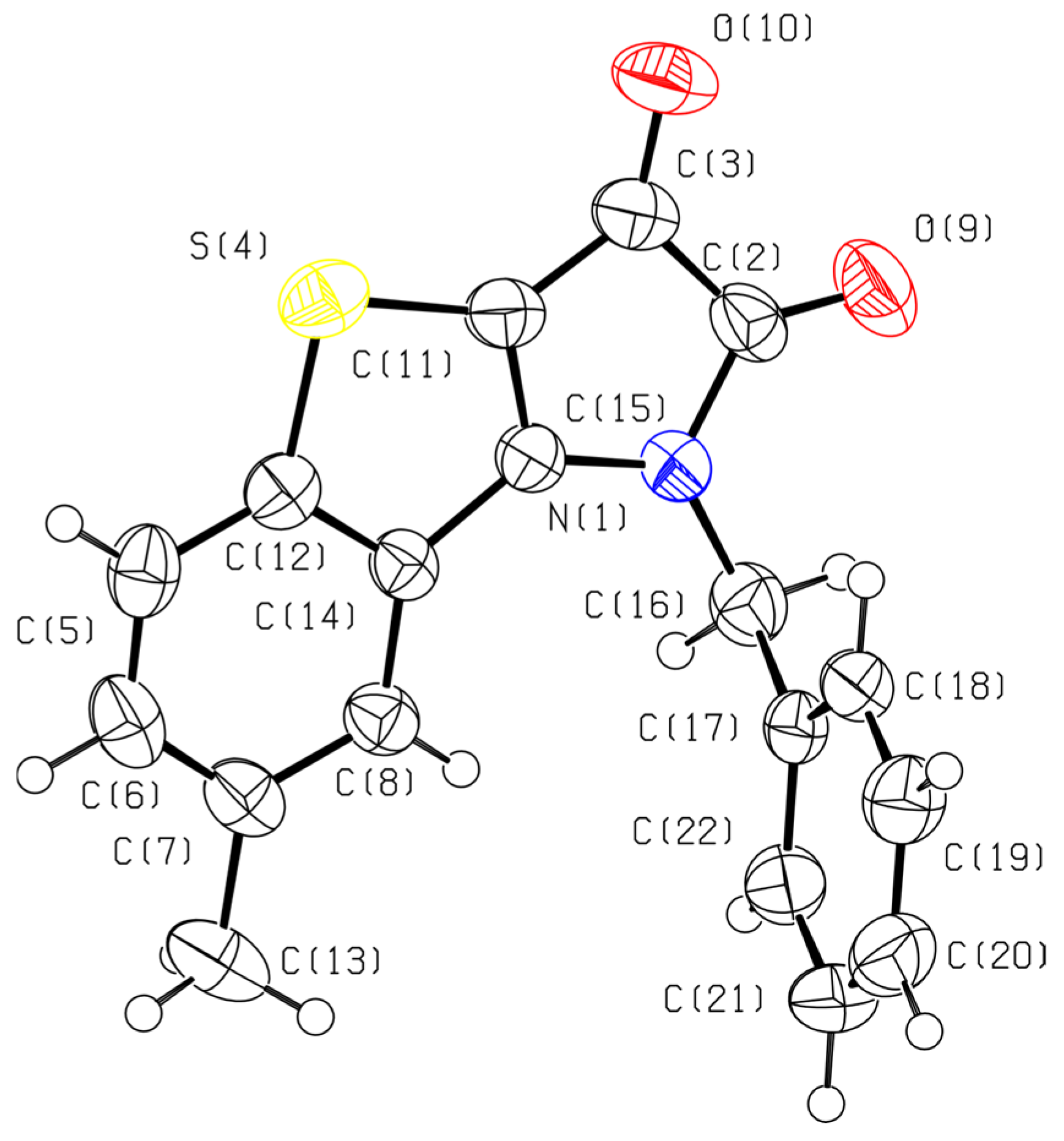
The structure of fused tricyclic compound 6 was determined by single-crystal X-ray diffraction using black crystals obtained from a CCl4–benzene solution (Figure 1). The structure of compound 6 was solved in the monoclinic space group P21/n, and the asymmetric unit contains a single crystallographically independent molecule (Z’ = 1). The fused tricyclic core consisting of benzothiophene and pyrroledione fragments is planar within 0.236(9) Å. The dihedral angle between the planes of the tricyclic system and phenyl ring is 88.02°. In the crystal, the molecules of 6 form chains along the glide plane.
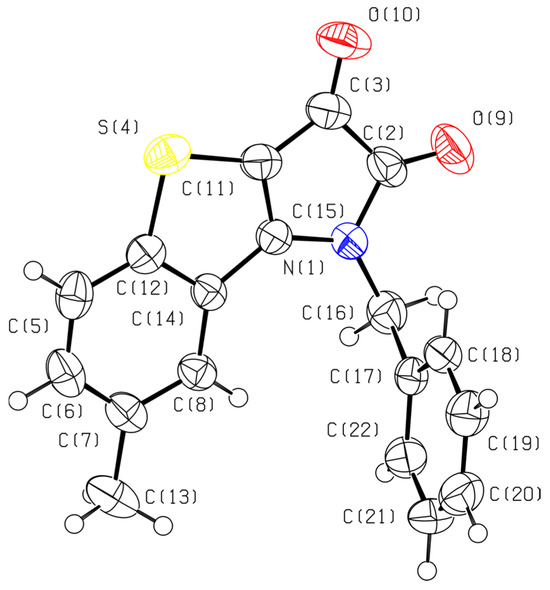
Figure 1.
The molecular structure of fused tricyclic compound 6 in the crystal. Carbon: grey, oxygen: red, nitrogen: blue, sulfur: yellow.
To the best of our knowledge, only a few examples of 1H-benzo[4,5]thieno[3,2-b]pyrrole-2,3-diones have been reported to date. N-Dodecyl [24,25], N-(2-octyldodecyl) [24], N-hexyl [26], N-benzyl [27] and N-(2-phenylethyl) [27] derivatives of this fused heterocycle were obtained in low to moderate yields (6–49%) from the reaction of the corresponding N-substituted benzo[b]thiophen-3-amines with oxalyl chloride in the presence of Me3N.
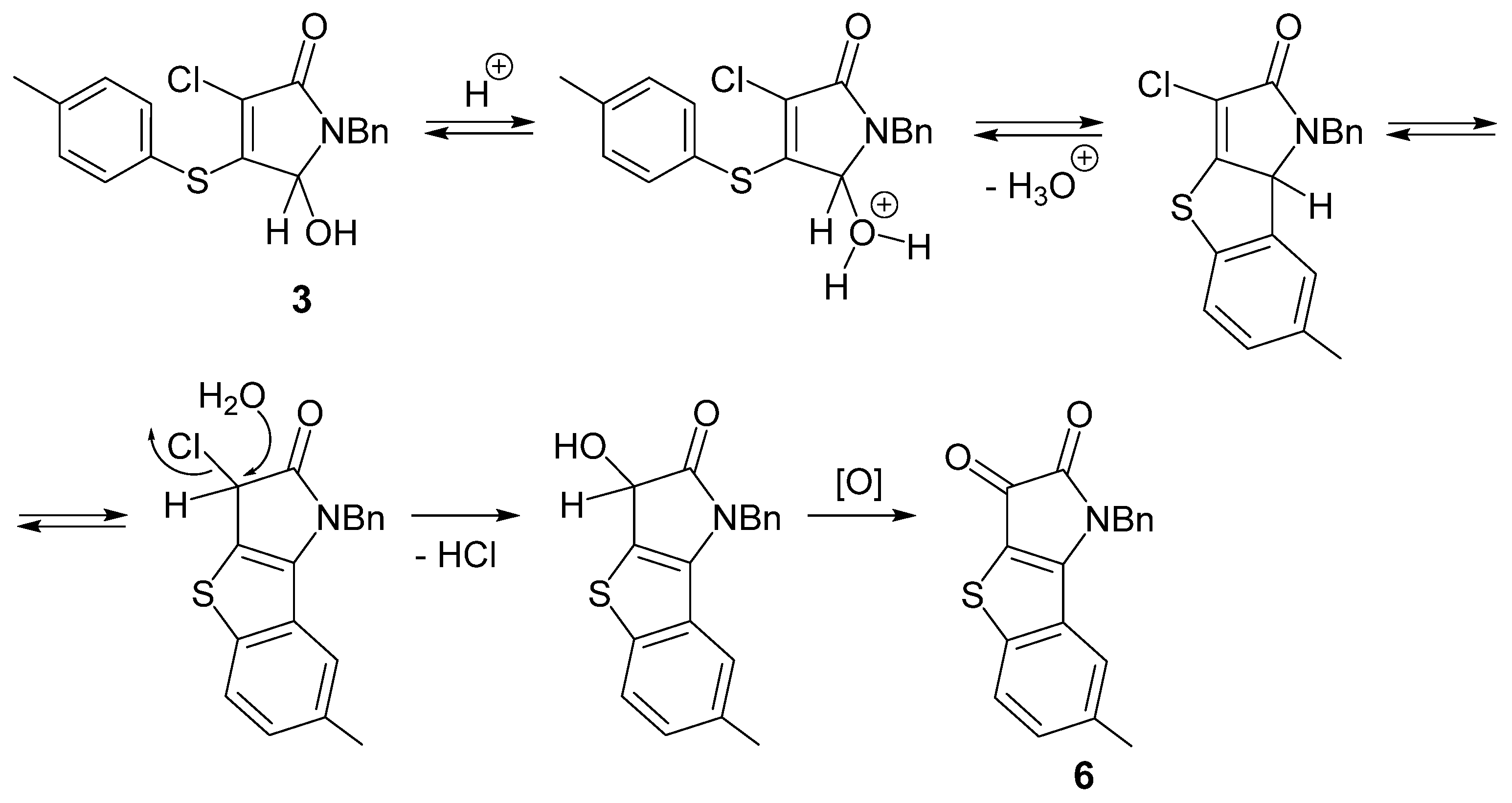
The observed formation of tricyclic compound 6 from pyrrolinone 3 in the presence of sulfuric acid represents an interesting result and requires further detailed study. We can tentatively propose some key steps for this acid-catalyzed transformation (Scheme 3). After the protonation of the OH group at carbon atom C-5, an intramolecular aromatic electrophilic substitution may occur that produces a tricyclic system. An isolated compound 6 may arise as a result of nucleophilic substitution of the chlorine atom and oxidation of the secondary hydroxyl group.
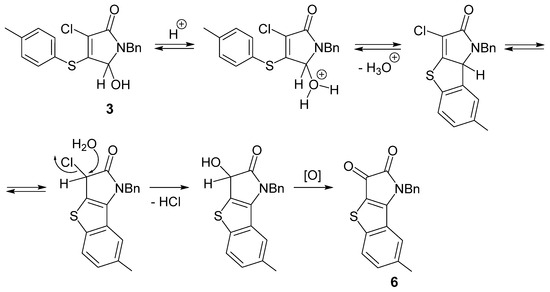
Scheme 3.
A possible mechanism for the formation of compound 6.
3. Materials and Methods
3.1. General Information
3,4-Dichloro-5-methoxy-2(5H)-furanone (1) [23] and 3-chloro-5-methoxy-4-[(4-methylphenyl)sulfanyl]-2(5H)-furanone (2) [19] were synthesized according to the known methods. Benzylamine (Acros Organics, Jinan, China) was used as received without further purification. All solvents were purified and distilled by standard procedures. Analytical thin-layer chromatography (TLC) was carried out on Sorbfil PTLC-AF-A-UF plates using UV light (254 nm) as the visualizing agent. Silica gel 60A (Acros Organics, Jinan, China; 70–230 mesh; 0.060–0.200 mm) was used for open column chromatography. The melting points were measured on a Boetius hot stage and were not corrected. The IR spectra were recorded on a Bruker Tensor-27 spectrometer fitted with a Pike MIRacle ATR accessory (diamond/ZnSe crystal plate). The absorption maxima (νmax) are reported in wavenumbers (cm−1). The NMR spectra were measured on a Bruker Avance III 400 spectrometer at 400.17 MHz (1H) and 100.62 MHz (13C) at 20 °C in acetone-d6 and DMSO-d6. The chemical shifts (δ) are expressed in parts per million (ppm) and were calibrated using residual undeuterated solvent peak as an internal reference (acetone-d6: δH 2.05; DMSO-d6: δH 2.50, δC 39.5). All coupling constants (J) are reported in Hertz (Hz), and multiplicities are indicated as s (singlet), d (doublet), t (triplet), q (quadruplet), and m (multiplet). High-resolution mass spectra (HRMS) were obtained through electrospray ionization (ESI) with positive (+) ion detection on a Waters Xevo G2-XS quadrupole time-of-flight mass spectrometer (compounds 3, 5, 6) and a Bruker micrOTOF–QIII quadrupole time-of-flight mass spectrometer (compound 4).
The X-ray diffraction (XRD) data for the single crystal of compound 6 were obtained on a Bruker D8 QUEST automated three-circle diffractometer with a PHOTON III area detector and an IμS DIAMOND microfocus X-ray tube: λ (MoKα) = 0.71073 Å, ω/ϕ scanning mode with a step of 0.5°. Data collection and the indexing, determination and refinement of unit cell parameters were carried out using the APEX3 software package (version 2018.1–9, Bruker AXS, Madison, WI, USA). Numerical absorption correction based on the crystal shape, additional spherical absorption correction, and systematic error correction were performed using SADABS-2016/2 software [28]. Using OLEX2 (v1.5) [29], structures were solved by direct methods using the SHELXT-2018/3 program [30] and refined by full-matrix least-squares on F2 using the SHELXL-2018/3 program [31]. Nonhydrogen atoms were refined anisotropically. The positions of hydrogen atoms of the methyl group were determined using the rotation of the group with idealized bond angles; the remaining hydrogen atoms were refined using a riding model. Most calculations were performed using the WinGX-2021.3 software package [32].
Crystal data for C18H13NO2S (M = 307.35 g/mol): monoclinic; space group P21/n (no. 14), a = 7.7862(11) Å, b = 19.405(3) Å, c = 9.8436(14) Å, β = 100.773(2)°, V = 1461.1(4) Å3, Z = 4, T = 296.15 K, μ(Mo Kα) = 0.228 mm−1, Dcalc = 1.397 g/cm3; 12,320 reflections measured (2.099° ≤ 2θ ≤ 28.743°), 3467 unique (Rint = 0.0509, Rsigma = 0.0554), which were used in all calculations. The final R1 was 0.0533 (I > 2σ(I)) and wR2 was 0.1306 (all data).
CCDC 2442740 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/ (accessed on 28 April 2025) (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk). XRD data were obtained from the Collective Spectro-Analytical Center of FRC Kazan Scientific Center of RAS, supported by the State Assignment of the Federal Research Center “Kazan Scientific Center”, Russian Academy of Sciences.
3.2. Synthesis
3.2.1. 1-Benzyl-3-Chloro-5-Hydroxy-4-[(4-Methylphenyl)Sulfanyl]-1,5-Dihydro-2H-Pyrrol-2-One (3)
A cooled solution of benzylamine (1.84 mL; 17.0 mmol) in diethyl ether (25 mL) was added dropwise to a solution of furanone 2 (3.05 g; 11.3 mmol) in diethyl ether (25 mL) cooled to −13–10 °C with vigorous stirring. The mixture was stirred under cooling for an hour and then at room temperature until the reaction was complete (monitored by TLC, 48 h). The white precipitate was filtered off, washed with diethyl ether and recrystallized from benzene. Yield 92% (3.60 g); colorless crystals; mp 163–164 °C; Rf 0.49 (acetone—benzene, 1:6); IR (ATR) νmax 3450−3110 br. (O–H), 1676 (C=O), 1586, 1492 (C=C arom) cm−1; 1H NMR (acetone-d6, 400 MHz, ppm) δ 7.31–7.23 (5H, m, Ar-H), 7.48, 7.20 (4H, AA′BB′, N = 3JAB + 5JAB′ = 8.0 Hz, SC6H4), 5.68 (1H, d, 3J = 10.0 Hz, OH), 5.18 (1H, d, 3J = 10.0 Hz, H-5), 4.78, 4.31 (both 1H, AB q, 2JAB = 15.6 Hz, CH2), 2.32 (3H, s, CH3); 1H NMR (dmso-d6, 300 MHz, ppm) δ 7.71–6.99 (9H, m, Ar-H), 6.85 (1H, br.s, OH), 5.09 (1H, br.s, H-5), 4.65, 4.24 (both 1H, AB q, 2JAB = 15.6 Hz, CH2), 2.30 (3H, s, CH3); 13C{1H} NMR (acetone-d6, 100 MHz, ppm) δ 163.57 (C-2), 150.60 (C-4), 140.58, 138.41, 135.31, 130.91, 129.33, 128.71, 128.11, 125.34, 123.86 (C-3, C-Ar), 82.07 (C-5), 44.24 (CH2N), 21.16 (CH3); HRMS (ESI) m/z 368.0490 (calcd for C18H16ClNNaO2S [M + Na]+, 368.0482).
3.2.2. Reaction of Pyrrolinone 3 with Ethanol in the Presence of Hydrochloric Acid
A solution of pyrrolinone 3 (0.40 g; 1.16 mmol) and concentrated hydrochloric acid (0.154 mL; 1.75 mmol) was heated under reflux in ethanol (20 mL; 0.33 mol) for 87 h. According to 1H NMR spectroscopy, the reaction mixture contained starting compound 3, 5-ethoxypyrrolinone 4, and maleimide 5 in a ratio of 2:12:1. After cooling, the reaction mixture was evaporated to dryness under reduced pressure, and the dark oily residue was purified by column chromatography (eluent: acetone/toluene, 1:12). The insoluble in the eluent residue contained unreacted starting material 3 (48 mg, 12% recovery). Two different column eluates were collected separately and evaporated to dryness to produce products 4 and 5.
1-Benzyl-3-chloro-5-ethoxy-4-[(4-methylphenyl)sulfanyl]-1,5-dihydro-2H-pyrrol-2-one (4). Yield 71% (0.31 g); beige solid; mp 61–62 °C; Rf = 0.57 (acetone/toluene, 1:12); IR (ATR) νmax 1708 (C=O), 1582, 1495 (C=C arom) cm−1; 1H NMR (acetone-d6, 400 MHz, ppm) δ 7.51, 7.26 (4H, AA′BB′, N = 3JAB + 5JAB′ = 8.0 Hz, SC6H4), 7.36–7.17 (5H, m, Ar-H), 5.26 (1H, s, H-5), 4.62, 4.36 (both 1H, AB q, 2JAB = –15.3 Hz, CH2), 3.27, 2.97 (both 1H, m, AB part of ABX3 system, 2JAB = −8.3 Hz, 3JAX = 3JBX = 7.1 Hz, OCH2), 2.34 (3H, s, CH3), 0.90 (3H, t, X part of ABX3 system, 3JAX = 3JBX = 7.1 Hz, CH3); 13C{1H} NMR (acetone-d6, 100 MHz, ppm) δ 163.73 (C-2), 148.41 (C-4), 141.01, 138.16, 135.42, 131.01, 129.31, 128.85, 128.22, 124.55, 123.43 (C-3, C-Ar), 87.04 (C-5), 58.82 (CH2O), 45.20 (CH2N), 21.18 (CH3Ar), 14.82 (CH3); HRMS (ESI) m/z 374.0981 (calcd for C20H21ClNO2S [M + H]+, 374.0982).
1-Benzyl-3-[(4-methylphenyl)sulfanyl]-1H-pyrrole-2,5-dione (5). Yield 10% (36 mg); light yellow oil; Rf = 0.63 (acetone/toluene, 1:12); IR (ATR) νmax 1769, 1706 (C=O), 1560 (C=C pyr), 1598, 1494 (C=C arom) cm−1; 1H NMR (acetone-d6, 400 MHz, ppm) δ 7.53, 7.38 (4H, AA′BB′, N = 3JAB + 5JAB′ = 8.1 Hz, SC6H4), 7.36–7.24 (5H, m, Ar-H), 5.73 (1H, s, H-4), 4.66 (2H, s, CH2N), 2.40 (3H, s, CH3); 13C{1H} NMR (acetone-d6, 100 MHz, ppm) δ 169.53, 168.28 (C-2), 153.17 (C-3), 141.78, 137.79, 134.98, 131.89, 129.41, 128.68, 128.39, 124.48 (Ar-C), 119.71 (C-4), 42.02 (CH2N), 21.27 (CH3); HRMS (ESI) m/z 310.0888 (calcd for C18H16NO2S [M + H]+, 310.0896).
3.2.3. The Heating Under Reflux of Pyrrolinone 3 in Toluene in the Presence of Sulfuric Acid
A solution of pyrrolinone 3 (0.60 g; 1.74 mmol) and concentrated sulfuric acid (6.5 μL; 0.12 mmol) was heated under reflux in toluene (25 mL) for 7 h. According to 1H NMR spectroscopy, the reaction mixture contained products 5 and 6 in a ratio of 4:5. After cooling, the mixture was washed with water until it reached a neutral pH. The organic layer was dried over MgSO4, filtered and evaporated to dryness under reduced pressure. The resulting black oily residue was purified by column chromatography (eluent: acetone/toluene, 1:9). Two different column eluates were collected separately and evaporated to dryness to produce products 5 and 6.
From the fraction with Rf 0.68 (acetone/toluene, 1:9), maleimide derivative 5 was obtained in 28% yield (0.15 g) as a light yellow oil.
1-Benzyl-7-methyl-1H-benzo[4,5]thieno[3,2-b]pyrrole-2,3-dione (6). The fraction with Rf 0.55 (acetone/toluene, 1:9) was evaporated and further recrystallized from a CCl4–benzene mixture (3:2). Yield 41% (0.22 g); black crystals; mp 195–197 °C; IR (ATR) νmax 2924, 2855 (C–H), 1752, 1700 (C=O), 1604, 1510, 1480 (C=C arom) cm−1; 1H NMR (acetone-d6, 400 MHz, ppm) δ 7.94 (1H, d, 3J = 8.5 Hz, H-5), 7.72 (1H, br s, H-8), 7.57–7.26 (6H, m, Ar-H), 5.33 (2H, s, CH2N), 2.33 (3H, s, CH3); 13C{1H} NMR (acetone-d6, 100 MHz, ppm) δ 174.96, 162.70, 160.78 (C=O, C8b), 145.84, 137.81, 136.88, 133.18, 129.78, 128.52, 127.36, 127.03, 125.46, 125.12, 110.74 (Ar-C, C3a), 45.56 (CH2N), 21.18 (CH3); HRMS (ESI) m/z 308.0744 (calcd for C18H14NO2S [M + H]+, 308.0740).
4. Conclusions
In this study, we observed a novel acid-catalyzed reaction of a 4-(p-tolylsulfanyl) derivative of 3-pyrrolin-2-one that leads to the formation of two unexpected N-heterocycles. 1-Benzyl-3-chloro-5-hydroxy-4-[(4-methylphenyl)sulfanyl]-1,5-dihydro-2H-pyrrol-2-one under reflux conditions in toluene in the presence of sulfuric acid was transformed into N-substituted derivatives of 1H-pyrrole-2,5-dione and 1H-benzo[4,5]thieno[3,2-b]pyrrole-2,3-dione. This reaction caused previously unknown non-metal-catalyzed formation of the fused tricyclic system, possessing benzo[b]thiophene and pyrrole-1,2-dione moieties from the easily accessible pyrrolinone substrate.
Supplementary Materials
The supplementary materials contain information on 1H, 13C{1H} NMR, the IR spectra, and HRMS for compounds 3–6.
Author Contributions
Conceptualization, L.Z.L. and A.R.K.; methodology, L.S.K. and E.S.S.; investigation, L.S.K., E.S.S. and M.F.V.; resources, L.Z.L.; software, L.S.K., E.S.S. and D.P.G.; writing—original draft preparation, E.S.S. and L.Z.L.; writing—review and editing, A.R.K.; visualization, E.S.S., L.Z.L. and D.P.G.; funding acquisition, L.Z.L.; project administration, A.R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the financial support of the Russian Science Foundation (project No. 23-73-10182, https://rscf.ru/en/project/23-73-10182/, accessed on 21 April 2025).
Data Availability Statement
All data are included in the manuscript and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.K.; Singh, H.; Vijayan, V.; Kumar, D.; Naik, J.; Thareja, S.; Yadav, J.P.; Pathak, P.; Grishina, M.; et al. Nitrogen containing heterocycles as anticancer agents: A medicinal chemistry perspective. Pharmaceuticals 2023, 16, 299. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Liu, Y.; Qin, H.; Zhao, Z.; Wang, S.; He, W.; Tang, S.; Peng, J. Nitrogen-containing heterocyclic drug products approved by the FDA in 2023: Synthesis and biological activity. Eur. J. Med. Chem. 2024, 279, 116838. [Google Scholar] [CrossRef]
- Caruano, J.; Muccioli, G.G.; Robiette, R. Biologically active γ-lactams: Synthesis and natural sources. Org. Biomol. Chem. 2016, 14, 10134–10156. [Google Scholar] [CrossRef]
- López-Francés, A.; del Corte, X.; Serna-Burgos, Z.; Martínez de Marigorta, E.; Palacios, F.; Vicario, J. Exploring the synthetic potential of γ-lactam derivatives obtained from a multicomponent reaction—Applications as antiproliferative agents. Molecules 2022, 27, 3624. [Google Scholar] [CrossRef]
- Pelkey, E.T.; Pelkey, S.J.; Greger, J.G. Reactions of 3-pyrrolin-2-ones. In Advances in Heterocyclic Chemistry; Scriven, E.F.V., Ramsden, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 128, pp. 433–565. [Google Scholar] [CrossRef]
- Mardjan, M.I.D.; Parrain, J.-L.; Commeiras, L. Strategies to access γ-hydroxy-γ-butyrolactams. Synthesis 2018, 50, 1175–1198. [Google Scholar] [CrossRef]
- Aksenov, A.V.; Aksenov, D.A.; Kurenkov, I.A.; Leontiev, A.V.; Aksenov, N.A. A new, convenient way to fully substituted α,β-unsaturated γ-hydroxy butyrolactams. Int. J. Mol. Sci. 2023, 24, 10213. [Google Scholar] [CrossRef]
- Ryu, C.-K.; Choi, I.H.; Park, R.E. Synthesis of benzo[b]benzo[2,3-d]thiophen-6,9-diones via palladium(ii) acetate–mediated cyclization of 5-arylthiocyclohexa-2,5-diene-1,4-diones. Synth. Commun. 2006, 36, 3319–3328. [Google Scholar] [CrossRef]
- Mori, T.; Nishimura, T.; Yamamoto, T.; Doi, I.; Miyazaki, E.; Osaka, I.; Takimiya, K. Consecutive thiophene-annulation approach to π-extended thienoacene-based organic semiconductors with [1]benzothieno[3,2-b][1]benzothiophene (BTBT) substructure. J. Am. Chem. Soc. 2013, 135, 13900–13913. [Google Scholar] [CrossRef]
- Saravanan, P.; Anbarasan, P. Palladium catalyzed aryl(alkyl)thiolation of unactivated arenes. Org. Lett. 2014, 16, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Shinamura, S.; Sadamitsu, Y.; Arai, S.; Horiuchi, S.; Yoneya, M.; Takimiya, K.; Hasegawa, T. Extended and modulated thienothiophenes for thermally durable and solution-processable organic semiconductors. Chem. Mater. 2018, 30, 5050–5060. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, Y.; Ma, Y.; Yang, X.; Szostak, M. Palladium-catalyzed synthesis of benzothiophenes via cross-dehydrogenative coupling of 4-arylthiocoumarins and pyrones. Adv. Synth. Catal. 2019, 361, 5709–5714. [Google Scholar] [CrossRef]
- Ejaz, S.; Zubair, M.; Rizwan, K.; Karakaya, I.; Rasheed, T.; Rasool, N. An updated coverage on the synthesis of benzo[b]thiophenes via transition-metal catalyzed reactions: A review. Curr. Org. Chem. 2021, 50, 40–67. [Google Scholar] [CrossRef]
- Takuto, F.; Keiichi, Y.; Maiko, I.; Naohito, M. Compound, Material for Organic Electroluminescent Device, Organic Electroluminescent Device, and Electronic Apparatus. Patent JP2024143397, 11 October 2024. [Google Scholar]
- Gerasimova, D.P.; Zakharychev, D.V.; Saifina, A.F.; Fayzullin, R.R.; Kurbangalieva, A.R.; Lodochnikova, O.A. Homochiral versus heterochiral crystallization of 3-pyrrolin-2-one thioether results in the score 2:1 in favor of homochirality. Cryst. Growth Des. 2022, 22, 7273–7284. [Google Scholar] [CrossRef]
- Gerasimova, D.P.; Saifina, A.F.; Zakharychev, D.V.; Fayzullin, R.R.; Kurbangalieva, A.R.; Lodochnikova, O.A. The second example of doubly enantiophobic behavior during crystallization: A detailed crystallographic, thermochemical and spectroscopic study. CrystEngComm 2021, 23, 3907–3918. [Google Scholar] [CrossRef]
- Kosolapova, L.S.; Kurbangalieva, A.R.; Valiev, M.F.; Lodochnikova, O.A.; Berdnikov, E.A.; Chmutova, G.A. Synthesis and structure of the products of the reactions of 3-chloro-5-methoxy-4-[(4-methylphenyl)sulfanyl]-2(5H)-furanone with N,N-binucleophilic agents. Rus. Chem. Bull. Int. Ed. 2013, 62, 456–463. [Google Scholar] [CrossRef]
- Lodochnikova, O.A.; Kosolapova, L.S.; Saifina, A.F.; Gubaidullin, A.T.; Fayzullin, R.R.; Khamatgalimov, A.R.; Litvinov, I.A.; Kurbangalieva, A.R. Structural aspects of partial solid solution formation: Two crystalline modifications of a chiral derivative of 1,5-dihydro-2H-pyrrol-2-one under consideration. CrystEngComm 2017, 19, 7277–7286. [Google Scholar] [CrossRef]
- Lodochnikova, O.A.; Samigullina, A.I.; Zaripova, A.R.; Fayzullin, R.R.; Vandyukova, I.I.; Potapova, L.N.; Kurbangalieva, A.R. “Doubly enantiophobic” behavior during crystallization of racemic 1,5-dihydro-2H-pyrrol-2-one thioether. CrystEngComm 2018, 20, 3218–3227. [Google Scholar] [CrossRef]
- Kosolapova, L.S.; Kurbangalieva, A.R.; Kozyakov, D.A.; Lodochnikova, O.A.; Berdnikov, E.A.; Chmutova, G.A. 5-Methoxy-3,4-di[(4-methylphenyl)sulfanyl]-2(5H)-furanone in the reactions with nitrogen-containing nucleophiles. Butlerov. Commun. 2013, 36, 1–11. [Google Scholar]
- Mowry, D.T. Mucochloric acid. II. Reactions of the aldehyde group. J. Am. Chem. Soc. 1953, 75, 1909–1910. [Google Scholar] [CrossRef]
- Nanson, L.; Blouin, N.; Mitchell, W. Organic Semiconducting Compounds. Patent WO2015139802, 24 September 2015. [Google Scholar]
- Mok, Y.; Kim, Y.; Moon, Y.; Park, J.-J.; Choi, Y.; Kim, D.-Y. Quinoidal small molecule containing ring-extended termini for organic field-effect transistors. ACS Omega 2021, 6, 27305–27314. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Ashizawa, M.; Matsumoto, H. Design and structure–property relationship of benzothienoisoindigo in organic field effect transistors. RSC Adv. 2015, 5, 61035–61043. [Google Scholar] [CrossRef]
- Yoo, D.; Kohara, A.; Ashizawa, M.; Kawamoto, T.; Masunaga, H.; Ohta, N.; Matsumoto, H.; Mori, T. Bulky phenylalkyl substitutions to bisthienoisatins and thienoisoindigos. Cryst. Growth Des. 2020, 20, 3293–3303. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).