Abstract
A novel method employing a tandem Cadogan and Arbuzov reaction sequence has been developed, providing access to a series of previously unreported dimethyl (Z)-((3-oxoindolin-2-ylidene)(aryl)methyl)phosphonates. Restricted rotation of the aryl substituent, particularly in the presence of ortho substituents, gives axial chirality to these compounds.
Keywords:
Cadogan–Sundberg reaction; Arbuzov reaction; chalcones; reduction; indoles; phosphites; phosphonates 1. Introduction
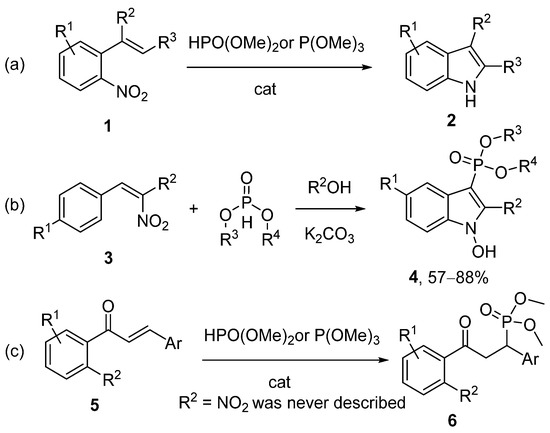
The indole moiety is prevalent in natural products and contributes to a significant number of biologically active compounds [1]. For instance, the plant family Cryptolepis sanguinolenta contains numerous secondary indole metabolites and has been historically employed in the treatment of malaria and cancer [2,3,4]. Consequently, the development of novel methods for assembling the indole core with specific characteristics and substitution patterns remains a crucial challenge for research groups worldwide. One of the prominent approaches to indole synthesis is the Cadogan–Sundberg reaction [5,6,7] (Scheme 1a). This reaction, based on the deoxygenation of the nitro group in 2-vinylnitrobenzenes 1, enables the formation of indoles 2 in good yields and with high substituent diversity without use of transition metals. However, in these transformations, phosphorus acts solely as a reducing agent and is not incorporated into the final molecule. The only exception is the cyclization of β-nitrostyrenes 3 to indoles 4, which cannot be regarded as a typical precedent for the Cadogan–Sundberg reaction [8] (Scheme 1b). We became interested in the possibility of synthesizing organophosphorus indoles and turned our attention to chalcones 5. The Arbuzov reaction involving chalcones 5 is well-documented in the literature [9,10,11,12] (Scheme 1c). At the same time, 2′-nitrochalcones have not been previously utilized in such transformations, which serves as the basis for this study.
Scheme 1.
Previously shown precedents of the Cadogan reaction (a,b); phospha-Michael on chalcones (c).
Herein, we wish to report the first tandem of Arbuzov and Cadogan–Sundberg reactions of 2′-nitrochalcones and P(OMe)3, providing access to a series of dimethyl (Z)-((3-oxoindolin-2-ylidene)(aryl)methyl)phosphonates possessing axial chirality.
2. Results and Discussion
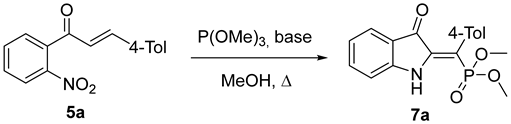
As a model reaction, we have investigated the interaction of 4-methyl-2′-nitrochalcone 5a with P(OMe)3 under reflux in methanol, employing various bases as catalysts (Table 1). An initial attempt with KOH yielded unsatisfactory results (entry 1). Similarly, other bases, including Et3N and DBU, failed to produce the desired product (entries 2 and 3). However, the use of sodium methoxide resulted in the formation of the target compound 7, albeit in a modest 14% yield (entry 4). Ultimately, conducting the reaction in methanol with K2CO3 as the base led to the desired product 7 in a 31% yield (entry 5). This result is not entirely unexpected, as K2CO3 has been previously reported as a catalyst for both the Cadogan–Sundberg [5] and Arbuzov [9] reactions. Despite our best efforts, a good yield remained elusive. The primary cause of this yield reduction appears to be the formation of a red polymeric material, which can be isolated via column chromatography using methanol as the eluent. In experiment 5, the isolated mass of this polymer was 238 mg.

Table 1.
Optimization of reaction of 4-methyl-2′-nitrochalcone 5a with P(OMe)3.
The 1H NMR spectrum indicates the formation of compound 7a as a single geometric isomer. To definitively establish the stereochemistry of the reaction, X-ray analysis was conducted, confirming the (Z)-configuration of the product (Figure 1).
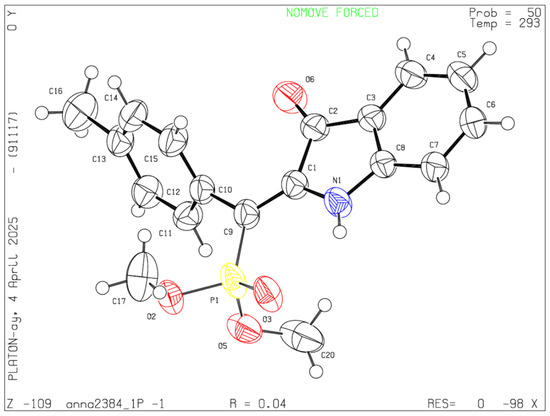
Figure 1.
ORTEP drawings of 7a (CCDC # 2441064) showing atom numbering schemes and 50% probability ellipsoids. The following color scheme is used: yellow—phosphorus, red—oxygen, blue—nitrogen, black—carbon. Hydrogen atoms are depicted as isotropic.
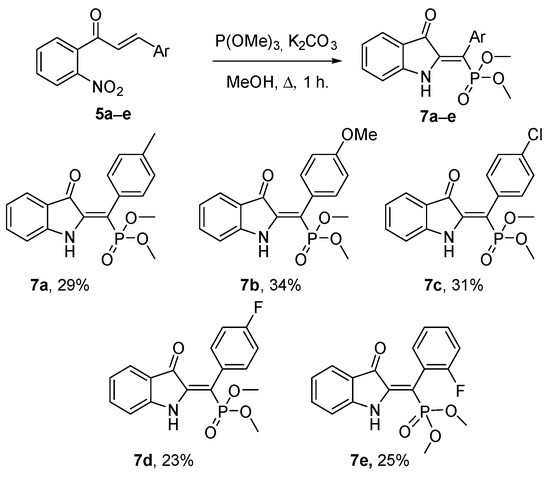
Next, a small library of dimethyl (Z)-((3-oxoindolin-2-ylidene)(aryl)methyl)phosphonates 7 was synthesized under optimized conditions. Notably, the protons at positions 2/6 and 3/5 in the para-substituted products 7a–d exhibited non-equivalence. This observation suggests restricted rotation of the aryl substituent, likely due to steric hindrance induced by the phosphonate moiety. In support of this, the X-ray data show a 60-degree rotation of the 4-tolyl fragment and an elongation of the C2-C3 bond of the pyrrole ring to 1.53 Å. It is worth noting that related indoxyls containing a cyano group had free rotation even of the ortho-substituted aryl substituents and typical bond lengths for 3-oxoindoles [13,14]. Consequently, ortho-substituted analog 7e was also prepared. The presence of axial chirality in this molecule rendered the methyl groups on the phosphorus atom diastereotopic and, therefore, non-equivalent in the NMR spectra (Scheme 2).
Scheme 2.
A library of dimethyl (Z)-((3-oxoindolin-2-ylidene)(aryl)methyl)phosphonates.
The proposed reaction mechanism initiates with a nucleophilic attack by P(OMe)3 on the double bond, resulting in the formation of phosphonium salt 8. The demethylation of this intermediate produces phosphonate 6. Subsequent deprotonation, resulting in the formation of benzyl anion 9, triggers a Cadogan-type cyclization. This cyclization starts from a nucleophilic attack on the nitro group, forming a six-membered ring intermediate 10, which then undergoes dehydration to yield the 1-oxo-4-quinolone anion 12. A second nucleophilic attack on the nitrogen atom generates an aziridine 13. This strained ring is readily cleaved under the reaction conditions to form intermediate 14, which is subsequently reduced by P(OMe)3 to furnish the desired product 7 (Scheme 3). Cadogan-type cyclizations involving a nitro group are relatively uncommon in the literature. But, based on existing research [13,14,15,16], it appears that the enolate anion lacks sufficient nucleophilicity to effectively participate in such a reaction. Moreover, our recent work demonstrated that the interaction of nitrochalcones with methylene-active compounds, including 1,3-diketones, nitroalkanes, and benzyl cyanide, failed to facilitate this transformation, which is further suggesting the necessity of forming a three-membered ring intermediate [17].
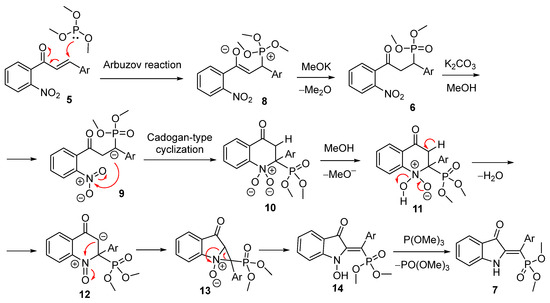
Scheme 3.
Proposed mechanism of reaction.
To elucidate the reaction mechanism, we conducted a series of control experiments (Scheme 4). Initially, we lowered the reaction temperature to 40 °C, enabling the isolation of intermediate compound 6e in an 8% yield (Scheme 4a). Subsequently, compound 6e was subjected to standard reaction conditions, yielding product 7e (Scheme 4b). To determine whether the (Z) product was kinetically or thermodynamically favored, we performed isomerization of (Z)-7c in the presence of H2SO4-H3PO4 with subsequent treatment with NaHCO3 (Scheme 4c). Upon concentrating the solution, the resulting product (E)-7c completely converted into the primary isomer (Z)-7c within half an hour. For this reason, we could only acquire a 1H NMR spectrum of an approximately 1:1 mixture of isomers (Figures S35 and S36). This observation confirms that the (Z) isomer represents the thermodynamically favored product of the reaction. Furthermore, we performed photoisomerization using two-dimensional thin-layer chromatography (TLC), which produced a product spot consistent with the previously observed (E) isomer (Figure S37). This outcome suggests a distortion of the double bond in compound (E)-7e, attributable to the repulsion between the phosphonate fragment and the lone pairs of electrons on the indoxyl oxygen. We also attempted to introduce triethylamine as a scavenger for the methyl group liberated during the conversion of 9 to 10. However, the addition of triethylamine did not result in a yield of the target product that was significantly higher than the statistical error (Scheme 4d).
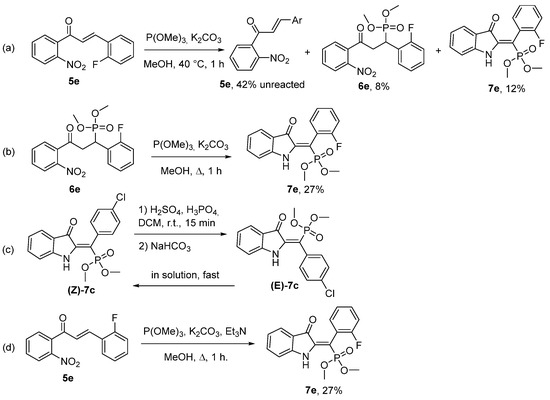
Scheme 4.
Control experiments. (a) Isolation of reaction intermediates; (b) Reaction of intermediate 6e under standard conditions; (c) Acid-catalyzed Z/E isomerization of product 7c; (d) Reaction conducted in the presence of Et3N.
3. Materials and Methods
3.1. General Information
NMR spectra, 1H, 13C, 19F, and 31P, were measured in solutions of CDCl3 on Bruker AVANCE-III HD instrument (at 400, 101, 376, and 162 MHz, respectively). Residual solvent signals were used as internal standards (7.26 ppm for 1H, and 77.16 ppm for 13C nuclei). HRMS spectra was measured on Bruker maXis impact (electrospray ionization, in MeCN solutions, employing HCO2Na–HCO2H for calibration). IR spectra was measured on FT-IR spectrometer Shimadzu IRAffinity-1S equipped with an ATR sampling module. Reaction progress, purity of isolated compounds, and Rf values were monitored with TLC on Silufol UV-254 plates. A two-dimensional thin-layer chromatography (2D TLC) experiment was performed using Convoy S2+ 365 nm UV flashlight. Column chromatography was performed on silica gel (32–63 μm, 60 Å pore size). Melting points were measured with Stuart SMP30 apparatus. 2′-Nitrochalcones 5a–e were synthesized according to the previously reported procedure and were identical to those described [14]. All reagents and solvents were purchased from commercial vendors and used as received.
3.2. Preparation of Dimethyl (Z)-((3-oxoindolin-2-ylidene)(aryl)methyl)phosphonates 7 (General Procedure)
A 10 mL round-bottomed flask was charged with 2′-nitrochalcone 5 (2.00 mmol), MeOH (1 mL), P(OMe)3 (471 µL, 496 mg, 4.0 mmol), and K2CO3 (280 mg, 2 mmol) and heated at reflux temperature upon vigorous stirring for 1 h. After consumption of starting material (TLC control) the reaction mixture was concentrated in vacuo and obtained residue was purified by column chromatography (EtOAc/hexane, 1:2, v/v). Alternatively, products can be recrystallized from benzene/hexane 1:5.
Dimethyl (Z)-((3-oxoindolin-2-ylidene)(p-tolyl)methyl)phosphonate (7a): red solid, m.p. 159.9–161.1 °C, Rf 0.4 (EtOAc/hexane, 1:2, v/v). Yield 199 mg (0.58 mmol, 29%). 1H NMR (400 MHz, CDCl3) δ 9.42 (br. s, 1H), 7.49 (d, J = 7.6 Hz, 1H), 7.44 (ddd, J = 8.1, 7.3, 1.3 Hz, 1H), 7.23–7.18 (m, 2H), 7.14 (dd, J = 8.1, 1.9 Hz, 2H), 6.92–6.82 (m, 2H), 3.74 (s, 3H), 3.72 (s, 3H), 2.39 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 185.3 (d, J = 17.9 Hz), 152.2, 143.8 (d, J = 7.1 Hz), 137.9 (d, J = 2.6 Hz), 137.2, 129.6, 129.5, 129.5, 129.5, 125.3, 120.7 (d, J = 3.6 Hz), 120.6, 111.5, 105.8 (d, J = 174.6 Hz), 53.1, 53.0, 21.5; 31P NMR (162 MHz, CDCl3) δ 21.29; FTIR, vmax: 3257, 2949, 1705, 1514, 1578, 1463, 1239 cm−1; HRMS (ESI TOF) m/z calc’d. for C18H18NNaO4P [M + Na]+: 366.0866, found: 366.0875 (−2.6 ppm).
Dimethyl (Z)-((4-methoxyphenyl)(3-oxoindolin-2-ylidene)methyl)phosphonate (7b): red solid, m.p. 166.4–167.2 °C, Rf 0.34 (EtOAc/hexane, 1:2, v/v). Yield 244 mg (0.68 mmol, 34%). 1H NMR (400 MHz, CDCl3) δ 9.43 (s, 1H), 7.52–7.47 (m, 1H), 7.43 (ddd, J = 8.5, 7.3, 1.4 Hz, 1H), 7.19 (dd, J = 8.7, 2.0 Hz, 2H), 6.93 (d, J = 8.5 Hz, 2H), 6.89–6.82 (m, 2H), 3.83 (s, 3H), 3.74 (s, 3H), 3.71 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 185.3 (d, J = 17.7 Hz), 159.5 (d, J = 2.4 Hz), 152.1, 143.8 (d, J = 7.4 Hz), 137.2, 131.0, 131.0, 125.3, 124.5 (d, J = 3.3 Hz), 120.7 (d, J = 3.7 Hz), 120.6, 114.1, 114.1, 111.5, 105.6 (d, J = 174.6 Hz), 55.3, 53.1, 53.0; 31P NMR (162 MHz, CDCl3) δ 21.38; FTIR, vmax: 3268, 2926, 2851, 1701, 1624, 1590, 1510, 1471, 1289, 1236, 1175, 1020 cm−1; HRMS (ESI TOF) m/z calc’d. for C18H18NNaO5P [M + Na]+: 382.0815, found: 382.0819 (−1.0 ppm).
Dimethyl (Z)-((4-chlorophenyl)(3-oxoindolin-2-ylidene)methyl)phosphonate (7c): red solid, m.p. 131.1–132.3°C, Rf 0.3 (EtOAc/hexane, 1:2, v/v). Yield 225 mg (0.62 mmol, 31%). 1H NMR (400 MHz, CDCl3) δ 9.41 (s, 1H), 7.51–7.48 (m, 1H), 7.45 (td, J = 7.8, 1.3 Hz, 1H), 7.39–7.32 (m, 2H), 7.21–7.15 (m, 2H), 6.92–6.84 (m, 2H), 3.74 (s, 3H), 3.72 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 185.3 (d, J = 17.6 Hz), 152.2, 143.9 (d, J = 6.6 Hz), 137.4, 134.1 (d, J = 3.2 Hz), 131.3, 131.2, 128.9, 128.9, 125.4, 120.9, 120.5 (d, J = 3.7 Hz), 111.6, 104.1 (d, J = 176.7 Hz), 53.1, 53.1; 31P NMR (162 MHz, CDCl3) δ 20.64; FTIR, vmax: 3314, 1697, 1588, 1463, 1317, 1299, 1243 cm−1; HRMS (ESI TOF) m/z calc’d. for C17H15ClNNaO4P [M + Na]+: 386.0319, found: 386.0310 (2.5 ppm).
Dimethyl (Z)-((4-fluorophenyl)(3-oxoindolin-2-ylidene)methyl)phosphonate (7d): red solid, m.p. 171.8–173.2°C, Rf 0.29 (EtOAc/hexane, 1:2, v/v). Yield 159 mg (0.46 mmol, 23%). 1H NMR (400 MHz, CDCl3) δ 9.41 (s, 1H), 7.49 (d, J = 7.6 Hz, 1H), 7.45 (ddd, J = 8.4, 7.4, 1.3 Hz, 1H), 7.21 (ddd, J = 8.8, 5.4, 2.1 Hz, 2H), 7.12–7.03 (m, 2H), 6.91–6.84 (m, 2H), 3.75 (s, 3H), 3.72 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 185.4 (d, J = 17.6 Hz), 162.7 (dd, J = 247.2, 2.8 Hz), 152.2, 144.0 (d, J = 6.9 Hz), 137.4, 131.6 (d, J = 5.6 Hz), 131.6 (d, J = 5.8 Hz), 128.4 (t, J = 3.4 Hz), 125.4, 120.8, 120.5 (d, J = 3.7 Hz), 115.8 (d, J = 2.0 Hz), 115.6 (d, J = 2.0 Hz), 111.6, 104.3 (d, J = 176.5 Hz), 53.1, 53.0; 19F NMR (376 MHz, CDCl3) δ -113.97; 31P NMR (162 MHz, CDCl3) δ 20.81; FTIR, vmax: 3321, 1703, 1591, 1505, 1474, 1329, 1214 cm−1; HRMS (ESI TOF) m/z calc’d. for C17H15FNNaO4P [M + Na]+: 370.0615, found: 370.0609 (1.6 ppm).
Dimethyl (Z)-((2-fluorophenyl)(3-oxoindolin-2-ylidene)methyl)phosphonate (7e): orange solid, m.p. 160.5–162.4 °C, Rf 0.34 (EtOAc/hexane, 1:2, v/v). Yield 174 mg (0.5 mmol, 25%). 1H NMR (400 MHz, CDCl3) δ 9.47 (s, 1H), 7.51 (d, J = 7.5 Hz, 1H), 7.45 (t, J = 7.7 Hz, 1H), 7.36 (q, J = 7.7, 6.8 Hz, 1H), 7.20 (p, J = 7.4 Hz, 2H), 7.11 (t, J = 9.0 Hz, 1H), 6.94–6.85 (m, 2H), 3.80 (d, J = 11.2 Hz, 3H), 3.72 (d, J = 11.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 185.3 (d, J = 17.6 Hz), 160.9 (dd, J = 245.2, 6.6 Hz), 152.3, 144.7 (d, J = 6.5 Hz), 137.4, 131.5, 131.5, 131.5, 131.4, 131.0, 130.0, 129.9, 129.9, 124.4 (dd, J = 3.6, 2.2 Hz), 125.7, 124.4, 120.3 (td, J = 8.6, 8.2, 3.2 Hz), 115.6 (d, J = 1.8 Hz), 115.4 (d, J = 1.8 Hz), 111.6, 96.9 (d, J = 180.3 Hz), 53.2 (dd, J = 4.7, 2.7 Hz), 53.0 (d, J = 5.8 Hz); 19F NMR (376 MHz, CDCl3) δ -114.34; 31P NMR (162 MHz, CDCl3) δ 20.83; FTIR, vmax: 3253, 1708, 1596, 1461, 1242 cm−1; HRMS (ESI TOF) m/z calc’d. for C17H15FNNaO4P [M + Na]+: 370.0615, found: 370.0629 (−3.8 ppm).
3.3. Control Experiments: Preparation of Dimethyl (1-(2-fluorophenyl)-3-(2-nitrophenyl)-3-oxopropyl)phosphonate 6e (Scheme 4a)
A 10 mL round-bottomed flask was charged with 2-fluoro-2′-nitrochalcone 5e (542 mg, 2.00 mmol), MeOH (1 mL), P(OMe)3 (471 µL, 496 mg, 4.0 mmol), and K2CO3 (280 mg, 2 mmol) and heated at 40 °C upon vigorous stirring for 1 h. After consumption of starting material (TLC control) the reaction mixture was concentrated in vacuo and obtained residue was purified by column chromatography (gradient, EtOAc/hexane, from 1:2 to 1:0 v/v). This compound was isolated among with unreacted 2-fluoro-2′-nitrochalcone 5e (227 mg, 42%) and dimethyl (Z)-((2-fluorophenyl)(3-oxoindolin-2-ylidene)methyl)phosphonate 7e (83 mg, 12%).
Colorless liquid, Rf 0.27 (EtOAc). Yield 61 mg (0.16 mmol, 8%). 1H NMR (400 MHz, CDCl3) δ 8.11 (d, J = 8.1 Hz, 1H), 7.69 (t, J = 7.4 Hz, 1H), 7.64–7.55 (m, 1H), 7.45 (tt, J = 7.5, 2.2 Hz, 1H), 7.33–7.22 (m, 2H), 7.17 (t, J = 7.6 Hz, 1H), 7.10 (t, J = 9.2 Hz, 1H), 4.35 (ddd, J = 23.0, 9.6, 4.5 Hz, 1H), 3.79 (d, J = 10.8 Hz, 3H), 3.73–3.50 (m, 2H), 3.59 (d, J = 10.7 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 198.6 (d, J = 16.2 Hz), 160.8 (dd, J = 247.2, 7.3 Hz), 145.5, 137.2 (d, J = 1.4 Hz), 134.3, 130.8, 129.8 (dd, J = 5.0, 3.2 Hz), 129.3 (dd, J = 8.4, 3.3 Hz), 127.5, 124.5, 124.4 (t, J = 3.2 Hz), 122.4 (dd, J = 14.4, 7.3 Hz), 115.7 (dd, J = 22.7, 2.6 Hz), 53.6 (d, J = 7.0 Hz), 53.2 (d, J = 7.0 Hz), 42.2 (t, J = 1.7 Hz), 30.8 (dd, J = 142.0, 2.9 Hz); 19F NMR (376 MHz, CDCl3) δ -116.08; 31P NMR (162 MHz, CDCl3) δ 28.93, 28.90; FTIR, vmax: 2957, 2850, 1711, 1527, 1492, 1456, 1347, 1249, 1233, 1183, 1025 cm−1; HRMS (ESI TOF) m/z calc’d. for C17H17FNNaO6P [M + Na]+: 370.0670, found: 404.0674 (−1.0 ppm).
3.4. Control Experiments: Preparation of Dimethyl (Z)-((2-fluorophenyl)(3-oxoindolin-2-ylidene)methyl)phosphonate 7e from Dimethyl (1-(2-fluorophenyl)-3-(2-nitrophenyl)-3-oxopropyl)phosphonate 6e (Scheme 4b)
A 10 mL round-bottomed flask was charged with dimethyl (1-(2-fluorophenyl)-3-(2-nitrophenyl)-3-oxopropyl)phosphonate 6e (50 mg, 0.13 mmol), MeOH (0.5 mL), P(OMe)3 (30 µL, 31 mg, 0.25 mmol), and K2CO3 (280 mg, 2 mmol) and heated at reflux upon vigorous stirring for 1 h. After consumption of starting material (TLC control) the reaction mixture was concentrated in vacuo and obtained residue was purified by column chromatography (EtOAc/hexane, 1:2, v/v). Yield 12 mg (0.035 mmol, 27%). The analytical data are in agreement with those obtained by the general method.
3.5. Control Experiments: Z/E Isomerisation of Dimethyl (Z)-((4-chlorophenyl)(3-oxoindolin-2-ylidene)methyl)phosphonate (7c) in Acidic Medium (Scheme 4c)
A 10 mL round-bottomed flask was charged with dimethyl (Z)-((4-chlorophenyl)(3-oxoindolin-2-ylidene)methyl)phosphonate 7c (72 mg, 0.2 mmol), CH2Cl2 (0.5 mL), H3PO4 (0.5 mL), and H2SO4 (0.5 mL) and stirred at room temperature for 15 min. After consumption of starting material (TLC control), the reaction mixture was diluted with water, neutralized with NaHCO3 (10 g), and extracted with CH2Cl2 (4 × 20 mL). The organic phase was concentrated in vacuo and obtained residue was purified by column chromatography (EtOAc). Yield, (E)-7c 49 mg (0.13 mmol, 67%). Rf, 0.3 (EtOAc). Due to rapid reverse isomerization, the remaining data for the resulting mixture are given in the Supplementary Materials.
3.6. Control Experiments: 2D TLC Observation of Z/E Isomerisation of Dimethyl (Z)-((4-chlorophenyl)(3-oxoindolin-2-ylidene)methyl)phosphonate (7c)
Dimethyl (Z)-((4-chlorophenyl)(3-oxoindolin-2-ylidene)methyl)phosphonate 7c was applied to the lower left corner of a chromatographic plate (5 × 5 cm). The plate was eluted using a mixture of ethyl acetate and hexane (1:2, v/v) as the eluent. While the solvent was evaporating, the resulting spot was irradiated with ultraviolet light at a wavelength of 365 nm for 2 min. Subsequently, the plate was subjected to perpendicular elution using the same solvent mixture.
3.7. Control Experiments: Preparation of Dimethyl (Z)-((2-fluorophenyl)(3-oxoindolin-2-ylidene)methyl)phosphonate 7e in Presense of Et3N (Scheme 4d)
A 10 mL round-bottomed flask was charged with 2′-nitrochalcone 5 (2.00 mmol), MeOH (1 mL), P(OMe)3 (471 µL, 496 mg, 4.0 mmol), K2CO3 (280 mg, 2 mmol), and Et3N (277 µL, 202 mg, 2.0 mmol) and heated at reflux temperature upon vigorous stirring for 1 h. After consumption of starting material (TLC control) the reaction mixture was concentrated in vacuo and obtained residue was purified by column chromatography (EtOAc/hexane, 1:2, v/v). Yield 187 mg (0.54 mmol, 27%). The analytical data are in agreement with those obtained by the general method.
4. Conclusions
A method employing a tandem Cadogan and Arbuzov reaction has been developed, providing access to a series of previously unreported dimethyl (Z)-((3-oxoindolin-2-ylidene)(aryl)methyl)phosphonates. The restricted rotation of the aryl substituent, particularly in the presence of ortho substituents, determines axial chirality in these compounds. The resulting compounds hold potential as polydentate chiral ligands.
Supplementary Materials
Figures S1–S38 of 13C NMR, 19F, 31P, HRMS, and FTIR spectral charts, Xray diffraction data. References [18,19,20] are cited in the supplementary materials.
Author Contributions
N.A.A.—funding acquisition, supervision, writing (original draft, review and editing); D.A.A.—investigation, data analysis; D.D.G.—investigation; A.E.K.—investigation; A.P.B.—investigation; P.S.K.—investigation; A.V.A.—conceptualization, supervision, data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation No. 24-73-10027, https://rscf.ru/project/24-73-10027/ (accessed on 1 July 2024).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DBU | 1,8-Diazabicyclo [5.4.0]undec-7-en |
| DCM | Dichloromethane |
| 2D TLC | Two-dimensional thin-layer chromatography |
References
- Omar, F.; Tareq, A.M.; Alqahtani, A.M.; Dhama, K.; Sayeed, M.A.; Emran, T.B.; Simal-Gandara, J. Plant-Based Indole Alkaloids: A Comprehensive Overview from a Pharmacological Perspective. Molecules 2021, 26, 2297. [Google Scholar] [CrossRef] [PubMed]
- Tudu, C.K.; Bandyopadhyay, A.; Kumar, M.; Radha; Das, T.; Nandy, S.; Ghorai, M.; Gopalakrishnan, A.V.; Proćków, J.; Dey, A. Unravelling the pharmacological properties of cryptolepine and its derivatives: A mini-review insight. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Osafo, N.; Mensah, K.B.; Yeboah, O.K. Phytochemical and Pharmacological Review of Cryptolepis sanguinolenta (Lindl.). Schlechter. Adv. pharmacol. Pharm. Sci. 2017, 2017, 3026370. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, N.A.; Aksenov, A.V.; Kornienko, A.; De Carvalho, A.; Mathieu, V.; Aksenov, D.A.; Ovcharov, S.N.; Griaznov, G.D.; Rubin, M. A nitroalkane-based approach to one-pot three-component synthesis of isocryptolepine and its analogs with potent anti-cancer activities. RSC Adv. 2018, 8, 36980–36986. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J. Cadogan-Sundberg indole synthesis. In Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications, 5th ed.; Springer International Publishing: New York, NY, USA, 2014; pp. 102–103. [Google Scholar]
- Kaur, M.; Kumar, R. C-N and N-N bond formation via Reductive Cyclization: Progress in Cadogan /Cadogan-Sundberg Reaction. ChemistrySelect 2018, 3, 5330. [Google Scholar] [CrossRef]
- Reis, M.C.; Marín-Luna, M.; López, C.S.; Faza, O.N. Mechanism of the Molybdenum-Mediated Cadogan Reaction. ACS Omega 2018, 3, 7019–7026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liao, X.; Gao, Z. A Facile One-Pot Synthesis of 3-Dialkoxyphosphoryl- and 3-[Alkoxy(phenyl)phosphoryl]-1-hydroxyindoles. Synthesis 1990, 1990, 801–802. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, W.; Li, S.; Yang, Y.; Guo, S.; Cai, H. Potassium Carbonate Promoted Nucleophilic Addition of Alkenes with Phosphites. Synlett 2020, 31, 1295–1297. [Google Scholar]
- Song, H.; Sun, Y.; Lu, C.; Zhao, B. Asymmetric Hydrophosphonylation of α,β-Unsaturated Ketones Catalyzed by Rare-Earth Metal Complexes Bearing Trost Ligands. J. Org. Chem. 2022, 87, 7747–7762. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, J.; Yan, K.; Zhuang, X.; Yu, J.; Song, X.; Zhang, F.; Li, B.; Wen, J. Hydrophosphorylation of electron-deficient alkenes and alkynes mediated by convergent paired electrolysis. Chem. Comm. 2022, 58, 8238–8241. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shao, N.; Li, F.-Z.; Yang, X.-C.; Wang, M.-C. Azetidine-derived dinuclear zinc catalyzed asymmetric phospha-Michael addition of dialkyl phosphite to α,β-unsaturated carbonyl compounds. Org. Biomol. Chem. 2017, 15, 9465–9474. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, N.A.; Aksenov, A.V.; Kurenkov, I.A.; Kirillov, N.K.; Aksenov, D.A.; Arutiunov, N.A.; Aksenova, D.S.; Rubin, M. One-Pot Synthesis of (E)-2-(3-Oxoindolin-2-ylidene)-2-arylacetonitriles. Molecules 2022, 27, 2808. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, N.A.; Aksenov, D.A.; Arutiunov, N.A.; Aksenova, D.S.; Aksenov, A.V.; Rubin, M. Unexpected cyclization of ortho-nitrochalcones into 2-alkylideneindolin-3-ones. RSC Adv. 2020, 10, 18440–18450. [Google Scholar] [CrossRef] [PubMed]
- Trawczyński, A.; Bujok, R.; Wróbel, Z.; Wojciechowski, K. Simple synthesis of pyrrolo[3,2-e]indole-1-carbonitriles. Beilstein J. Org. Chem. 2013, 9, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Hu, Z.; Zhang, X.; Dong, J.; Liu, J.-B.; Chen, D.-Z.; Xu, X. Tandem Synthesis of Pyrrolo[2,3-b]quinolones via Cadogen-Type Reaction. Org. Lett. 2017, 19, 5284–5287. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, N.A.; Aksenov, D.A.; Ganusenko, D.D.; Kurenkov, I.A.; Aksenov, A.V. A Diastereoselective Assembly of Tetralone Derivatives via a Tandem Michael Reaction and ipso-Substitution of the Nitro Group. J. Org. Chem. 2023, 88, 5639–5651. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).