6,7,8,9-Tetrafluoro-11H-indeno[1,2-b]quinoxalin-11-one
Abstract
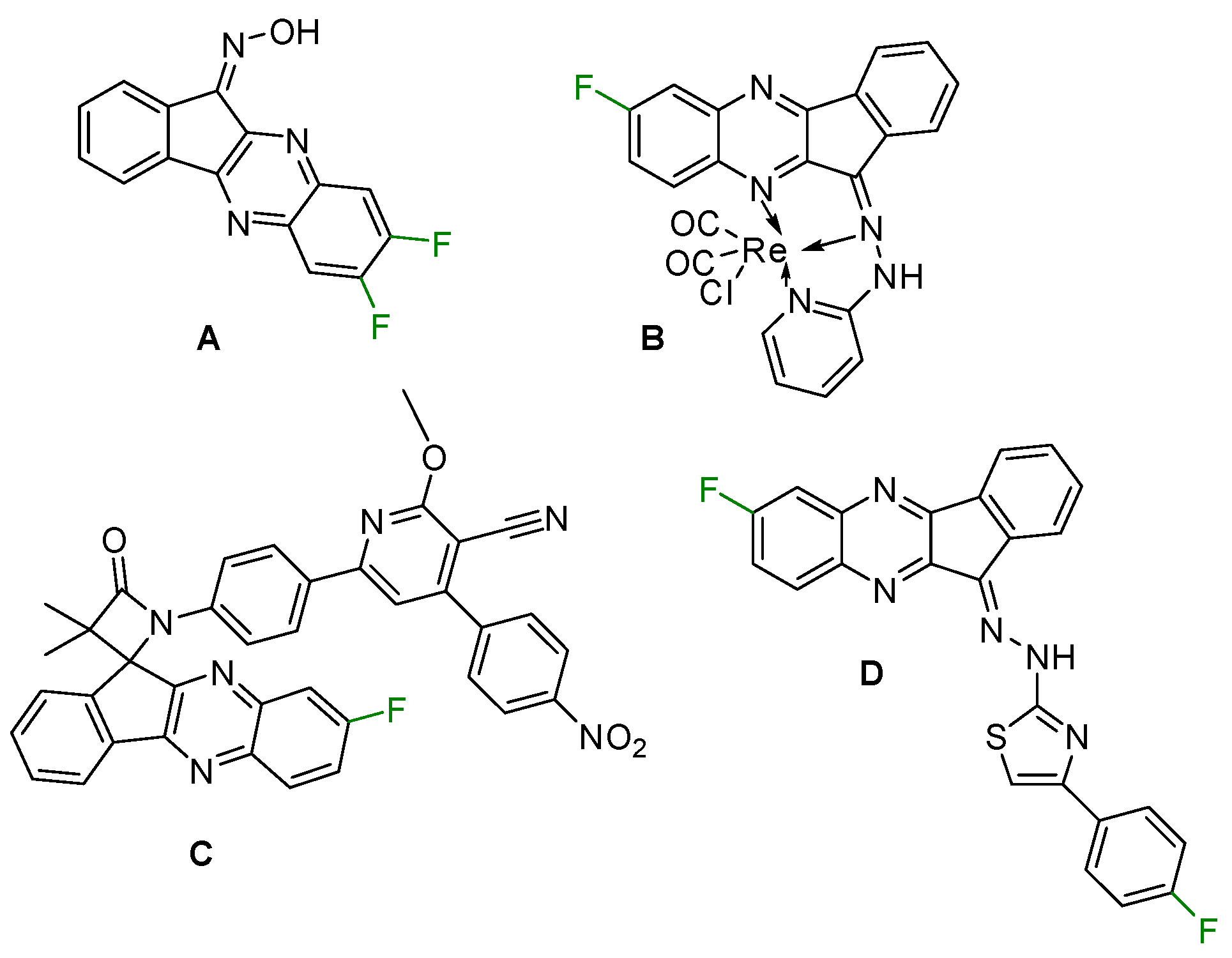
1. Introduction
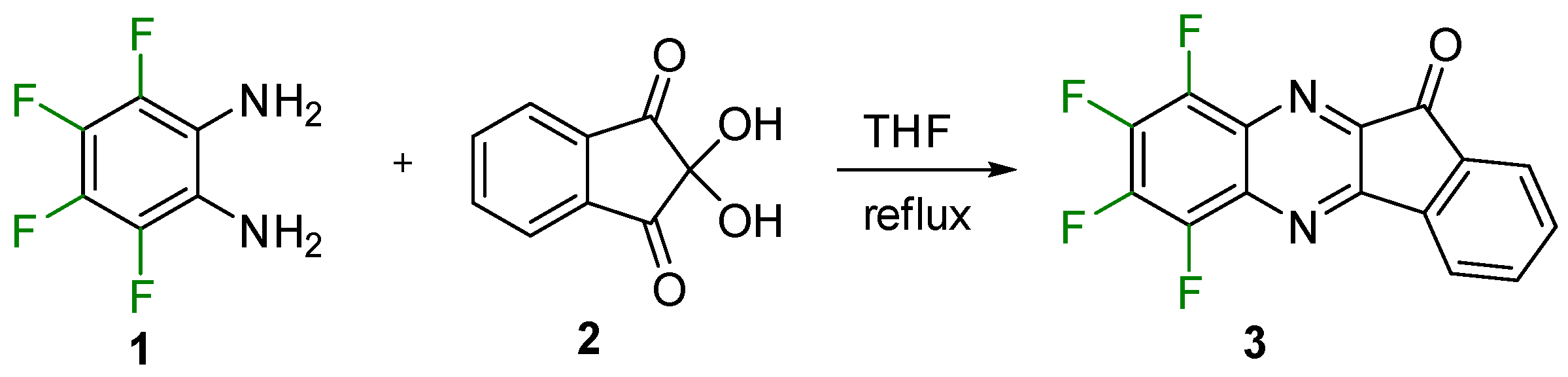
2. Results and Discussion
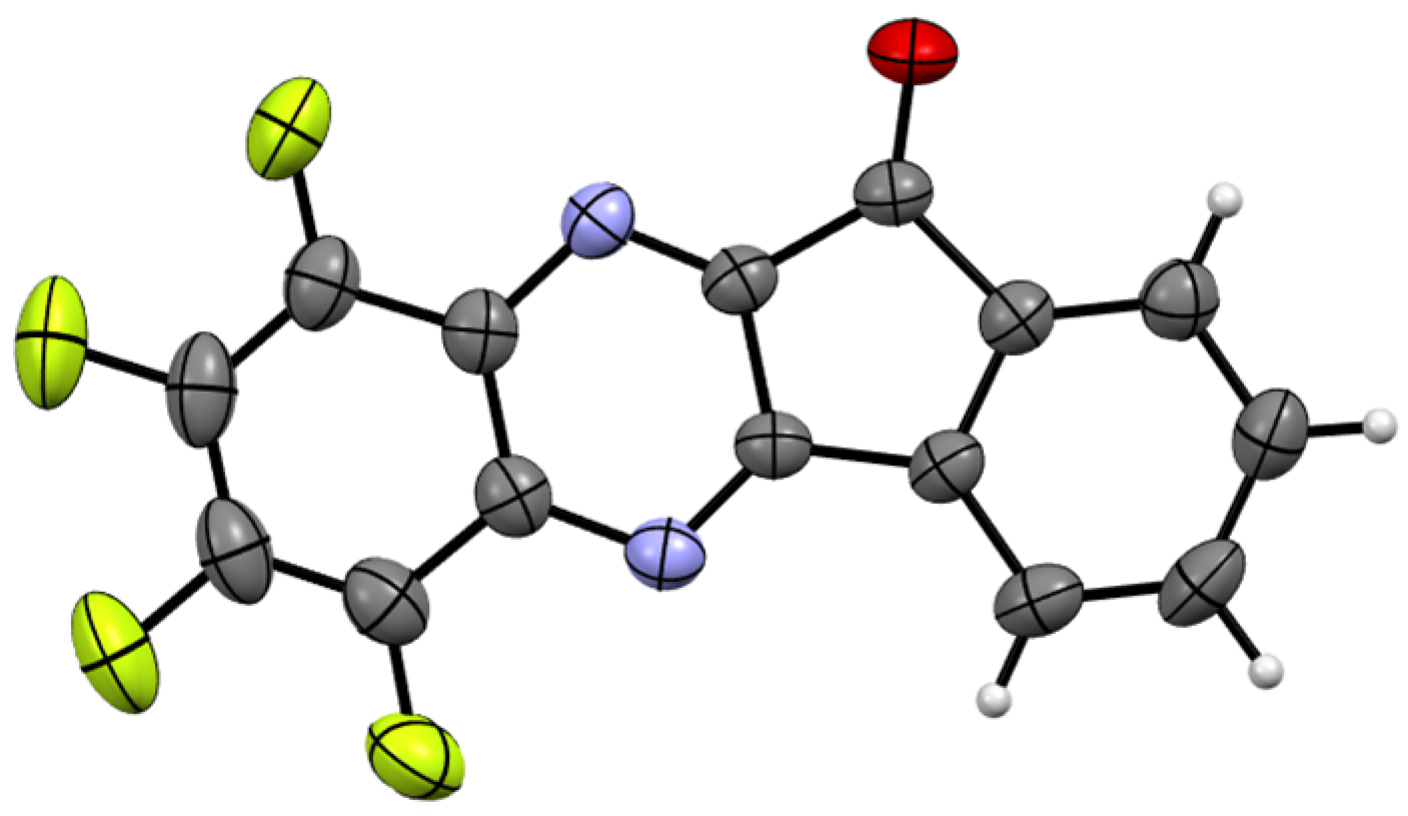
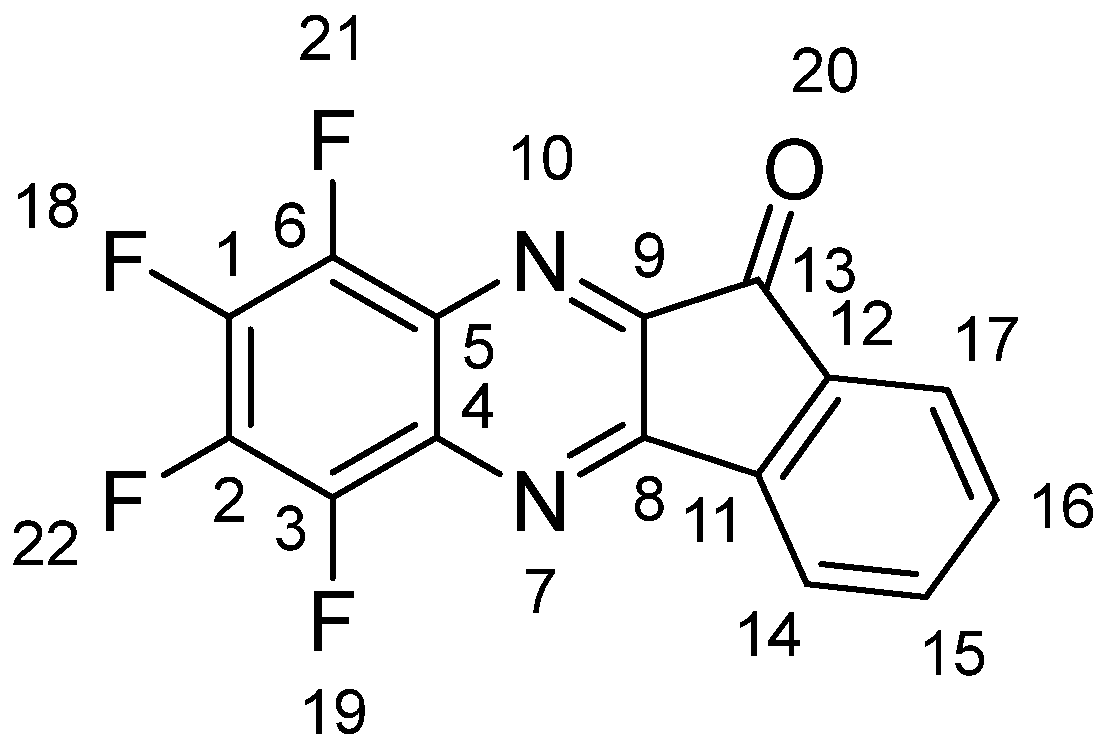
Synthesis and Molecular and Crystal Structure
3. Materials and Methods
3.1. General
3.2. Synthesis
3.3. X-Ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dehnen, S.; Schafer, L.; Lectka, T.; Togni, A. Fluorine: A very special element and its very special impacts on chemistry. J. Org. Chem. 2021, 86, 16213–16219. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Henary, E.; Casa, S.; Dost, T.L.; Sloop, J.C.; Henary, M. The role of small molecules containing fluorine atoms in medicine and imaging applications. Pharmaceuticals 2024, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.; Young, R.J. Future challenges and opportunities with fluorine in drugs? Med. Chem. Res. 2023, 32, 1231–1234. [Google Scholar] [CrossRef]
- Meanwell, N.A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 2018, 61, 5822–5880. [Google Scholar] [CrossRef]
- Petterson, M.; Hou, X.; Kuhn, M.; Wager, T.T.; Kauffman, G.M.; Verhoest, P.R. Quantitative assessment of the impact of fluorine substitution on P-glycoprotein (P-gp) mediated efflux, permitiability, lipophilicity, and metabolic stability. J. Med. Chem. 2016, 59, 5284–5296. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donelly, D.J.; Meanwell, N.A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Rizzo, C.; Amata, S.; Pibiri, I.; Pace, A.; Buscemi, S.; Piccionello, A.P. FDA-approved fluorinated heterocyclic drugs from 2016 to 2022. Int. J. Mol. Sci. 2023, 24, 7728. [Google Scholar] [CrossRef]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of organofluorine compounds in pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gu, Z.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Iwata, K.; Liu, H. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II-III clinical trials of major pharmaceutical companies: New structural trends and therapeutic areas. Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef]
- Suthar, S.K.; Chundawat, N.S.; Singh, G.P.; Pedrón, J.M.; Jhala, Y.K. Quinoxaline: A comprehension of current pharmacological advancements in medicinal chemistry. Eur. J. Med. Chem. Rep. 2022, 5, 100040. [Google Scholar] [CrossRef]
- Selvam, P.; Lakra, D.R.; Pannecouque, C.; De Clercq, E. Synthesis, anti-viral and cytotoxicity studies of novel N-substituted indophenazine derivatives. Indian J. Pharm. Sci. 2012, 74, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Ghalib, R.M.; Hashim, R.; Mehdi, S.H.; Sulaiman, O.; Silva, P.S.P.; Jassbi, A.R.; Firuzi, O.; Kawamura, F.; Chan, K.-L.; Murugaiyah, V. Synthesis of ninhydrin derivatives and their anticancer, antimicrobial and cholinesterase enzymes inhibitory activities. Lett. Drug Des. Discov. 2012, 9, 767–774. [Google Scholar] [CrossRef]
- Magesh, R.; Devadoss, T.; Pandey, D.K.; Bhatt, S. Discovery of new antidepressants from structurally novel 5-HT3 receptor antagonists: Design, synthesis and pharmacological evaluation of 3-ethoxyquinoxaline-2-carboxamides. Bioorg. Med. Chem. Lett. 2011, 21, 1253–1256. [Google Scholar] [CrossRef]
- Mani, K.S.; Kaminski, W.; Rajendron, S.P. A facile atom-economic one pot multicomponent synthesis of bioactive spiro-indenoquinoxaline pyrrolizines as potent antioxidants and anti-cancer agents. New J. Chem. 2018, 42, 301–310. [Google Scholar] [CrossRef]
- Liakhov, S.A.; Schepetkin, I.A.; Karpenko, O.S.; Duma, H.I.; Haidarzhy, N.M.; Kirpotina, L.N.; Kovrizhina, A.R.; Khlebnikov, A.I.; Bagryanskaya, I.Y.; Quinn, M.T. Novel c-Jun N-terminal kinase (JNK) inhibitors with an 11H-indeno[1,2-b]quinoxalin-11-one scaffold. Molecules 2021, 26, 5688. [Google Scholar] [CrossRef]
- Padariya, A.D.; Savaliya, N.K.; Dhaduk, M.P.; Dabhi, P.A.; Bhatt, B.S.; Bhatt, V.D.; Patel, M.N. Synthesis and characterization of quinoxaline-based rhenium(I) organometallic compounds: Biological and computational applications. J. Mol. Struct. 2024, 1302, 137477. [Google Scholar] [CrossRef]
- Dabhi, R.A.; Daduk, M.P.; Savaliya, N.K.; Padariya, A.D.; Patil, A.P.; Desai, R.A.; Bhatt, V.D.; Bhatt, B.S. Influence of pyridine entangled novel hybrid quinoxaline spirane on the fluorescence and absorption spectra of biomolecules: Molecular docking, pharmacokinetics and in-vitro biological investigations. J. Fluoresc. 2024, 1–18. [Google Scholar] [CrossRef]
- Abusaif, M.S.; Ragab, A.; Fayed, E.A.; Ammar, Y.A.; Gowitel, A.M.H.; Hassanin, S.O.; Ahmed, G.E.; Gohar, N.A. Exploring a novel triazole derivatives hybrid with fluorinated indenoquinoxaline as dual inhibitors targeting VEGFR2/AKT and apoptosis inducers against hepatocellular carcinoma with docking simulation. Bioorg. Chem. 2025, 154, 108023. [Google Scholar] [CrossRef]
- Basseto, M.; Ferla, S.; Pertusati, F. Polyfluorinated groups in medicinal chemistry. Future Med. Chem. 2015, 7, 527–546. [Google Scholar] [CrossRef]
- Prima, D.O.; Vorontsova, E.V.; Makarov, A.G.; Makarov, A.Y.; Bagryanskaya, I.Y.; Mikhailovskaya, T.F.; Slizhov, Y.G.; Zibarev, A.V. Halogenated (F, Cl) 1,3-benzodiazoles, 1,2,3-benzotriazoles, 2,1,3-benzothia/selenadiazoles and 1,4-benzodiazines inducing Hep2 cell apoptosis. Mendeleev Commun. 2017, 27, 439–442. [Google Scholar] [CrossRef]
- Shchegolkov, E.V.; Burgart, Y.V.; Shchur, I.V.; Saloutin, V.I. Polyfluorinated benzoic acids as promising reagents for organic synthesis and medicinal chemistry. Russ. Chem. Rev. 2024, 93, RCR5131. [Google Scholar] [CrossRef]
- Prima, D.O.; Makarov, A.G.; Bagryanskaya, I.Y.; Kolesnikov, A.E.; Zargarova, L.V.; Baev, D.S.; Eliseeva, T.F.; Politanskaya, L.V.; Makarov, A.Y.; Slizhov, Y.G.; et al. Fluorine-containing n-6 and angular and linear n-6-n′ (n, n′ = 5, 6, 7) diaza-heterocyclic scaffolds assembled on benzene core in unified way. ChemistrySelect 2019, 4, 2383–2386. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 1987, 2, S1–S19. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef]
- Rybalova, T.V.; Bagryanskaya, I.Y. C–F···π, F···H, and F···F intermolecular interactions and F-aggregations: Role in crystal engineering of fluoroorganic compounds. J. Struct. Chem. 2009, 50, 741–753. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure determination with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, v. 2008-1; Bruker AXS: Madison, WI, USA, 2008.
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovrizhina, A.R.; Bagryanskaya, I.Y.; Zibarev, A.V.; Khlebnikov, A.I. 6,7,8,9-Tetrafluoro-11H-indeno[1,2-b]quinoxalin-11-one. Molbank 2025, 2025, M2001. https://doi.org/10.3390/M2001
Kovrizhina AR, Bagryanskaya IY, Zibarev AV, Khlebnikov AI. 6,7,8,9-Tetrafluoro-11H-indeno[1,2-b]quinoxalin-11-one. Molbank. 2025; 2025(2):M2001. https://doi.org/10.3390/M2001
Chicago/Turabian StyleKovrizhina, Anastasia R., Irina Yu. Bagryanskaya, Andrey V. Zibarev, and Andrei I. Khlebnikov. 2025. "6,7,8,9-Tetrafluoro-11H-indeno[1,2-b]quinoxalin-11-one" Molbank 2025, no. 2: M2001. https://doi.org/10.3390/M2001
APA StyleKovrizhina, A. R., Bagryanskaya, I. Y., Zibarev, A. V., & Khlebnikov, A. I. (2025). 6,7,8,9-Tetrafluoro-11H-indeno[1,2-b]quinoxalin-11-one. Molbank, 2025(2), M2001. https://doi.org/10.3390/M2001






