Abstract
We present a recipe for the stereoselective conversion of commercial (cis)-4-hydroxy-L-proline into (4S)-1-methyl-4-propyl-L-proline, an analog of the amino acid fragment found in the clinically used antibacterial antibiotic lincomycin. The single-crystal X-ray diffraction analysis of the final target’s hydrochloride salt confirms its identity and absolute stereochemistry.
1. Introduction
Due to the rapid rise in bacterial resistance to clinically used natural product antibiotics [1,2], the preparation of non-natural analogs has become a vital pursuit [3,4,5,6,7]. Our laboratory has a programmatic focus on the development of new organic reactions to simplify the syntheses of anti-infective natural and non-natural compounds [8,9,10,11]. In line with this agenda, we were attracted to the lincosamide family of antibiotics, whose members include the clinically used lincomycin and clindamycin [12]. Because of their useful activity against Gram-positive bacterial pathogens [13], naturally occurring lincosamides continue to inspire the development of non-natural analogs for future clinical and agricultural use [6,14].
Lincomycin, a natural product and the flagship member of the lincosamides, is a fusion of two smaller compounds: (4R)-1-methyl-4-propyl-L-proline, an amino acid, and methyl 1-thiolincosaminide, an aza-monosaccharide (Figure 1A). (4R)-1-methyl-4-propyl-L-proline is commercially sold and can be obtained from the base-mediated hydrolysis of lincomycin [15,16,17,18]. It has served as the target of an elegant synthesis [19]. In sharp contrast, its epimer, (4S)-1-methyl-4-propyl-L-proline is not commercially available, and no literature syntheses exist. In this communication, we present a stereoselective preparation of (4S)-1-methyl-4-propyl-L-proline from the commercial and widely available (cis)-4-hydroxy-L-proline (Figure 1B).
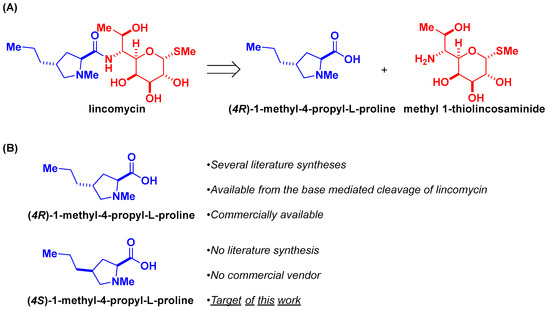
Figure 1.
(A) Lincomycin is a clinically used natural product antibacterial antibiotic and comprises two smaller natural products. (B) The focus of this work is the stereoselective preparation of (4S)-1-methy1-4-propyl-L-proline, an analog of the amino acid fragment of lincomycin.
2. Results and Discussion
Our synthesis of (4S)-1-methyl-4-propyl-L-proline hydrochloride started with attachment of a carboxybenzyl group to the nitrogen atom of commercial (cis)-4-hydroxy-L-proline (Scheme 1) [20]. The alcohol in 1 was oxidized to the corresponding ketone using a combination of trichloroisocyanuric acid (TCICA) and TEMPO in ethyl acetate [20]. Wittig olefination proceeded by heating 2 with a combination of propyltriphenylphosphonium bromide and sodium hydride in dimethyl sulfoxide (DMSO) [18]. Other common Wittig conditions, such as using sodium hydride in DMF, were much less successful. While the carboxylic acid was unstable during purification by silica gel chromatography or reversed-phase high-performance liquid chromatography, the dicyclohexylamine salt could be purified by repeated washes with diethyl ether [18]. The hydrogenation of the olefin using palladium on carbon (Pd/C) and 1 atmosphere of hydrogen gas yielded 4 with excellent diastereoselectivity. The high selectivity of this transformation can be explained using a simple steric argument; the palladium hydride species prefers to attack the less hindered face of the olefin, placing the propyl and carboxylic acid groups syn to each other. Finally, N-methylation proceeded smoothly using formalin, Pd/C, and 1 atmosphere of hydrogen gas [19]. Treatment of the product with a solution of hydrochloric acid in dioxane and washing the resulting salt with diethyl ether yielded (4S)-1-methyl-4-propyl-L-proline as its hydrochloride salt. Crystallization of this compound from an ethanolic solution gave single crystals suitable for X-ray diffraction analysis, confirming its identity and absolute stereochemistry (Figure 2).
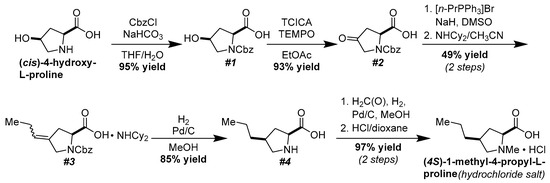
Scheme 1.
Conversion of commercial (cis)-4-hydroxy-L-proline into the hydrochloride salt of (4S)-1-methy1-4-propyl-L-proline.
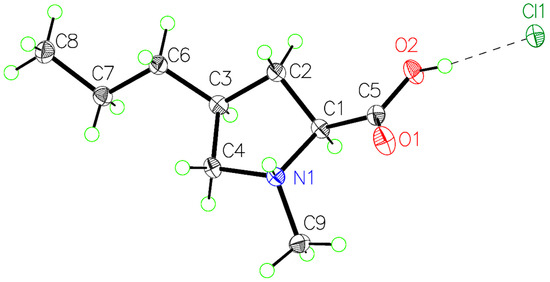
Figure 2.
Labeled, 50%-probability, ellipsoid plot of the formula unit of (4S)-1-methyl-4-propyl-L-proline hydrochloride (CCDC 2440605).
The geometry of the cation is very similar to N-methyl-L-proline hydrochloride [21]. C-C distances around the ring range from 1.501(2) Å to 1.539(3) Å. Bond distances involving the substituted carbon atom are slightly longer than the corresponding distances in N-methyl-L-proline hydrochloride. Interior ring angles are distorted to acute values ranging from 101.93(2)° to 105.95(2)°, with the corresponding angles in agreement in both structures. The shortest cation–anion contacts in (4S)-1-methyl-4-propyl-L-proline hydrochloride are 2.957(2) Å for the OH···Cl− donor–acceptor distance and 3.069(2) Å for the NH+···Cl− donor–acceptor distance. These correspond to strong hydrogen bonds and are very similar to those found in N-methyl-L-proline hydrochloride. The propyl chain is in the equatorial position, and its carbon atoms adopt an all-anti configuration.
(4S)-1-methyl-4-propyl-L-proline hydrochloride crystallizes neatly in the orthorhombic space group P212121 with one formula unit per asymmetric unit. Each cation makes short (less than the sum of the van der Waals radii) contacts with three neighboring anions and two cations, while each anion makes short contact with three cations (Figure 3). The cation–anion contact includes two strong hydrogen bonds and a third in which the anion interacts with the CH2 group alpha to the ammonium center and on which the formal positive charge is delocalized. These positively charged CH2 groups also donate hydrogen bonds to the carboxylic acid OH oxygen atoms. The strong hydrogen bonds link the ions into infinite, charge-ordered, head-to-tail chains parallel to the c axis, while the electrostatic interactions between the alpha CH2 groups and chloride anions link these along the a axis to form infinite layers with the propyl groups projecting into the interlayer space (Figure 4).
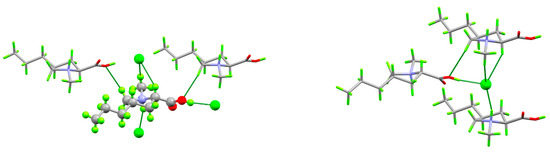
Figure 3.
Packing plots showing short contact environments around the cation (left) and anion (right). Element colors are the same as in Figure 2. Dashed green lines indicate short contacts.
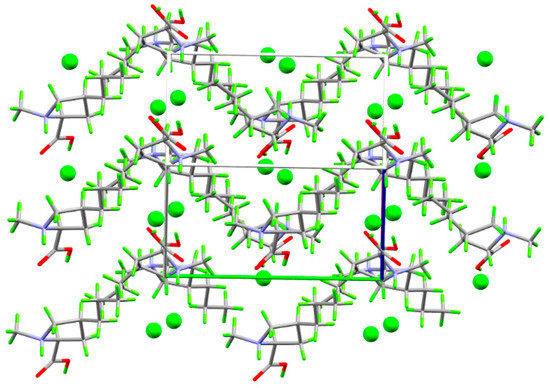
Figure 4.
Packing plot showing 2 × 2 × 2 unit cells viewed down the ac face diagonal. Axes are color coded: a = red; b = green; c = blue.
A search of the Cambridge Structural Database [22] for 4-substituted proline derivatives (including those functionalized at the N and OH positions) resulted in 240 hits, of which 187 were unique structures where the substituents could be clearly assigned cis or trans with respect to the α-carbonyl group. Structures with cis substituents are significantly underrepresented, accounting for only 51 of these entries, and many of these studies emphasize differences between both epimers in aspects such as prochirality [23,24], metal coordination [25,26], noncovalent interactions in biomacromolecules [27,28,29], and access to other natural products [30,31] and pharmaceuticals [32,33].
3. Conclusions
In summary, we present a concise preparation of (4S)-1-methyl-4-propyl-L-proline, which commences from the widely available chiron (cis)-4-hydroxy-L-proline. Single-crystal X-ray diffraction analysis confirms the identity of the final target as its hydrochloride salt. We expect this work to be of interest to medicinal chemists engaged in the discovery of new antibacterial antibiotics and to synthetic chemists tasked with the preparation of unusual amino acids.
Supplementary Materials
Additional experimental details include reaction procedures, tabulated characterization data, NMR spectra, IR spectra, high resolution mass spectra, and X-ray crystallographic tables.
Author Contributions
Conceptualization: S.S. and G.H.M.; Formal Analysis: G.H.M., S.C., S.P.K., and S.S.; Investigation and Data Curation: G.H.M., S.C., and S.P.K.; Writing: G.H.M., S.P.K., and S.S.; Supervision: S.S.; Project Administration: S.S.; Funding Acquisition: S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Institutes of Health grants R35GM142499, P20GM113117, and P20GM130448.
Data Availability Statement
The data underlying this study are available in the published article and its Supplementary Materials.
Acknowledgments
Justin Douglas and Sarah Neuenswander (KU NMR Lab) are acknowledged for their help with structural elucidation. Lawrence Seib and Anita Saraf (KU Mass Spectrometry Facility) are acknowledged for their help in acquiring HRMS data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.M.; Seiple, I.B.; Myers, A.G. The Evolving Role of Chemical Synthesis in Antibacterial Drug Discovery. Angew. Chem. Int. Ed. 2014, 53, 8840–8869. [Google Scholar] [CrossRef] [PubMed]
- Privalsky, T.M.; Soohoo, A.M.; Wang, J.; Walsh, C.T.; Wright, G.D.; Gordon, E.M.; Gray, N.S.; Khosla, C. Prospects for Antibacterial Discovery and Development. J. Am. Chem. Soc. 2021, 143, 21127–21142. [Google Scholar] [CrossRef]
- Wu, Z.-C.; Boger, D.L. Maxamycins: Durable Antibiotics Derived by Rational Redesign of Vancomycin. Acc. Chem. Res. 2020, 53, 2587–2599. [Google Scholar] [CrossRef]
- Mason, J.D.; Terwilliger, D.W.; Pote, A.R.; Myers, A.G. Practical Gram-Scale Synthesis of Iboxamycin, a Potent Antibiotic Candidate. J. Am. Chem. Soc. 2021, 143, 11019–11025. [Google Scholar] [CrossRef]
- Mandhapati, A.R.; Yang, G.; Kato, T.; Shcherbakov, D.; Hobbie, S.N.; Vasella, A.; Böttger, E.C.; Crich, D. Structure-Based Design and Synthesis of Apramycin–Paromomycin Analogues: Importance of the Configuration at the 6′-Position and Differences between the 6′-Amino and Hydroxy Series. J. Am. Chem. Soc. 2017, 139, 14611–14619. [Google Scholar] [CrossRef]
- Sathyamoorthi, S. Fun with Unusual Functional Groups: Sulfamates, Phosphoramidates, and Di-tert-butyl Silanols. Eur. J. Org. Chem. 2024, 27, e202301283. [Google Scholar] [CrossRef]
- Joshi, H.; Nirpal, A.K.; Paul, D.; Kelley, S.P.; Mague, J.T.; Sathyamoorthi, S. The Development of a Sulfamate-Tethered Aza-Michael Cyclization Allows for the Preparation of (−)-Negamycin tert-Butyl Ester. J. Org. Chem. 2024, 89, 5911–5916. [Google Scholar] [CrossRef]
- Mandal, G.H.; Kelley, S.P.; Sathyamoorthi, S. Enantioselective Total Syntheses of (+)-Kasugamycin and (+)-Kasuganobiosamine Highlighting a Sulfamate-Tethered Aza-Wacker Cyclization Strategy. Org. Lett. 2024, 26, 5463–5466. [Google Scholar] [CrossRef]
- Mandal, G.H.; Sathyamoorthi, S. Sulfamate Tethered Aza-Wacker Strategy for a Kasugamine Synthon. J. Org. Chem. 2024, 89, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Abe, I. Lincosamide Antibiotics: Structure, Activity, and Biosynthesis. ChemBioChem 2024, 25, e202300840. [Google Scholar] [CrossRef] [PubMed]
- Macleod, A.J.; Ross, H.B.; Ozere, R.L.; Digout, G.; Van, R. Lincomycin: A New Antibiotic Active Against Staphylococci And Other Gram-Positive Cocci: Clinical And Laboratory Studies. Can. Med. Assoc. J. 1964, 91, 1056–1060. [Google Scholar]
- Pyörälä, S.; Baptiste, K.E.; Catry, B.; van Duijkeren, E.; Greko, C.; Moreno, M.A.; Pomba, M.C.M.F.; Rantala, M.; Ružauskas, M.; Sanders, P.; et al. Macrolides and lincosamides in cattle and pigs: Use and development of antimicrobial resistance. Vet. J. 2014, 200, 230–239. [Google Scholar] [CrossRef]
- Collin, M.-P.; Hobbie, S.N.; Böttger, E.C.; Vasella, A. Synthesis of 1,2,3-Triazole Analogues of Lincomycin. Helv. Chim. Acta 2008, 91, 1838–1848. [Google Scholar] [CrossRef]
- Brahme, N.M.; Gonzalez, J.E.; Rolls, J.P.; Hessler, E.J.; Mizsak, S.; Hurley, L.H. Biosynthesis of the lincomycins. 1. Studies using stable isotopes on the biosynthesis of the propyl- and ethyl-L-hygric acid moieties of lincomycins A and B. J. Am. Chem. Soc. 1984, 106, 7873–7878. [Google Scholar] [CrossRef]
- Slomp, G.; MacKellar, F.A. Lincomycin. IV. Nuclear magnetic resonance studied on the structure of lincomycin, its degradation products, and some analogs. J. Am. Chem. Soc. 1967, 89, 2454–2459. [Google Scholar] [CrossRef]
- Magerlein, B.J.; Birkenmeyer, R.D.; Herr, R.R.; Kagan, F. Lincomycin. V. Amino acid fragment. J. Am. Chem. Soc. 1967, 89, 2459–2464. [Google Scholar] [CrossRef]
- Gandi, V.R.; Doan, B.N.D.; Kasinathan, S.; Bates, R.W. Stereocontrol in the synthesis of cyclic amino acids: A new ligand for directed hydrogenation through hydrogen bonding. Org. Biomol. Chem. 2019, 17, 2753–2758. [Google Scholar] [CrossRef]
- Xie, Y.; Luo, D.; Wiener, J.; Tang, S.; Chepyshev, S.; Schafmeister, C. Development of Fmoc-Protected Bis-Amino Acids toward Automated Synthesis of Highly Functionalized Spiroligomers. Org. Lett. 2022, 24, 3421–3425. [Google Scholar] [CrossRef]
- Jones, G.P.; Naidu, B.P.; Paleg, L.G.; Tiekink, E.R.T. Hygric acid (I) and stachydrine (II) as their hydrochlorides. Acta Crystallogr. Sect. C 1988, 44, 1669–1671. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Newberry, R.W.; Raines, R.T. n→π* Interactions Engender Chirality in Carbonyl Groups. Org. Lett. 2014, 16, 3421–3423. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.C.F.; Howard, K.J.; Snaith, J.S.; Blake, A.J.; Li, W.-S.; Steel, P.J. An enantioselective route to pyrrolidines: Removal of the chiral template from homochiral pyrroloimidazoles. Tetrahedron 2011, 67, 8925–8936. [Google Scholar] [CrossRef]
- de C. T. Carrondo, M.A.A.F.; Duarte, M.T.L.S.; Gonçalves, M.L.S.S.; O’Brien, P.; Hursthouse, M.B. Molecular and crystal structure of cis-bis(L-hydroxyprolinato)-copper(II) tetrahydrate and trans-bis(D-allohydroxyprolinato)-copper(II)–water(2/5). J. Chem. Soc. Dalton Trans. 1990, 213–217. [Google Scholar] [CrossRef]
- Yin, D.; Wang, L.; Yuan, Z. Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5. Z. Kristallogr. NCS 2023, 238, 1055–1056. [Google Scholar] [CrossRef]
- Costantini, N.V.; Ganguly, H.K.; Martin, M.I.; Wenzell, N.A.; Yap, G.P.A.; Zondlo, N.J. The Distinct Conformational Landscapes of 4S-Substituted Prolines That Promote an endo Ring Pucker. Chem. Eur. J. 2019, 25, 11356–11364. [Google Scholar] [CrossRef]
- Pinalli, R.; Brancatelli, G.; Pedrini, A.; Menozzi, D.; Hernández, D.; Ballester, P.; Geremia, S.; Dalcanale, E. The Origin of Selectivity in the Complexation of N-Methyl Amino Acids by Tetraphosphonate Cavitands. J. Am. Chem. Soc. 2016, 138, 8569–8580. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Kotch, F.W.; Choudhary, A.; Guzei, I.A.; Raines, R.T. The Aberrance of the 4S Diastereomer of 4-Hydroxyproline. J. Am. Chem. Soc. 2010, 132, 10857–10865. [Google Scholar] [CrossRef][Green Version]
- Mycock, D.K.; Glossop, P.A.; Lewis, W.; Hayes, C.J. A formal synthesis of (+)-lactacystin from 4-hydroxyproline. Tetrahedron Lett. 2013, 54, 55–57. [Google Scholar] [CrossRef]
- Jones, G.P.; Naidu, B.P.; Paleg, L.G.; Tiekink, E.R.T. Structures of three N-methylated 4-hydroxyproline derivatives. Acta Crystallogr. Sect. C 1988, 44, 2208–2211. [Google Scholar] [CrossRef]
- Yuan, J.; Cai, Z.-Q.; Huang, C.-J.; Xu, W.-R. (2R,4R)-1-(tert-Butoxycarbonyl)-4-methoxypyrrolidine-2-carboxylic acid. Acta Crystallogr. Sect. E 2010, 66, o3258. [Google Scholar] [CrossRef]
- Tamaki, M.; Han, G.; Hruby, V.J. Synthesis of 4-cis-Phenyl-l-proline via Hydrogenolysis. J. Org. Chem. 2001, 66, 3593–3596. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).