Abstract
Complete assignments of the 1H NMR chemical shifts for monoterpenes, borneol, and isoborneol, and their derivatives in which the secondary hydroxy group is protected with an acetyl group or a benzoyl group, have been made in CDCl3 and C6D6. Upon the protection of the hydroxy group with the carbonyl functional groups, or acetyl or benzoyl groups, many protons constituting the bicyclic ring exhibited downfield and upfield shifts in the chemical shift values, aiding in the unambiguous assignments of protons and carbons.
1. Introduction
NMR spectroscopy is a valuable tool for the determination of structures as well as acquiring insight into the chemical environment of a wide range of organic molecules. It is also of great advantage that NMR spectroscopy can distinguish subtle differences in the structures of sample molecules based on the chemical shift values. Various factors, such as electron densities, steric congestions, electron circulations, and anisotropic effects are known to affect chemical shifts. Such effects are often applied to the elucidation of structures with slight differences. Some of these effects are also applied to the determination of the absolute configurations of enantiomerically enriched organic compounds [1,2,3,4,5,6,7,8,9,10,11,12,13].
Borneol (1), is a natural product and a chiral bicyclic monoterpene found in various plants and has been applied to medicine, insect repellant, fragrance, etc. [14,15,16,17]. It has a hydroxy group placed in the endo position. Isoborneol (2) is an epimer of 1 in which the hydroxy group is placed in the exo position, and its derivatives have often been utilized as chiral ligands for asymmetric organic synthesis [18,19,20]. Because of the rigid and strained bicyclic structures combined with the chirality, NMR spectroscopic behaviors of these epimers, which show fairly distinct chemical shifts for most protons and carbons, have been under close scrutiny for many decades [21,22,23,24,25,26,27]. Although attempts for the complete assignment of 1H NMR and 13C NMR chemical shifts for these epimers and their derivatives have been reported, isoborneol has a few ambiguities left due to some overlapping peaks [24,25,26,27]. Here, we report our complete assignments of the 1H NMR chemical shifts of borneol (1) and isoborneol (2), as well as their derivatives in which the hydroxy group is protected with an acetyl group or a benzoyl group (Scheme 1).
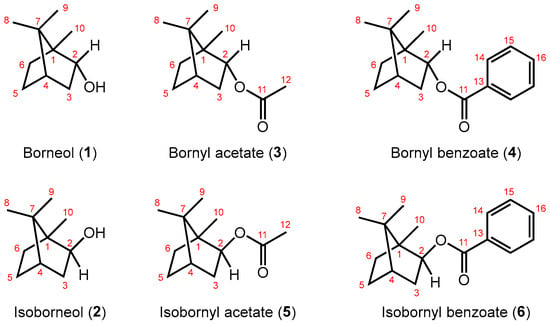
Scheme 1.
Borneol (1), isoborneol (2), and their derivatives 3–6.
2. Results and Discussion
2.1. Change in 1H NMR Chemical Shifts after Acetylation and Benzoylation in Borneol
Figure 1, Figure 2 and Figure 3 show 1H NMR spectra of borneol (1), bornyl acetate (3), and bornyl benzoate (4) in CDCl3 and C6D6. The 1H NMR chemical shifts of 1, 3, and 4 were assigned with the use of HMQC spectra based on cross peaks between separated 13C NMR and 1H NMR signals. Table S1 summarizes the 1H NMR chemical shift values for 1, 3, and 4. The spectra in Figure 1 are those obtained in CDCl3. The chemical shift of the proton attached to C-2 (labeled as H-2exo) exhibited downfield shifts by acetylation and benzoylation of the hydroxy group of 1. In addition, it was observed that the chemical shift values of the protons attached to the borneol ring appear in broader ranges after the protection of the hydroxy group. In particular, as depicted in Figure 3a, the chemical shift values of the methyl groups for H-8, H-9, and H-10 are close to each other, with the methyl group for H-10 being the most upfield in 1; however, these methyl groups showed more distinct chemical shifts after the acetylation as in 3, and the chemical shifts for these methyl groups changed considerably after the benzoylation as in 4. After the acetylation, both H-8 and H-10 exhibited noticeable upfield shifts, while benzylation led to downfield shifts for all these methyl protons. As for the protons directly attached to the bicyclic ring, the chemical shift ranges from the most downfield H-3exo proton to the most upfield H-3endo proton became broader, and each proton tended to exhibit more distinct chemical shift with less overlap as a result of the acetylation or benzylation. In particular, the chemical shifts for H-3exo exhibited downfield shifts similar to those of H-2exo. Also, the chemical shifts for H-6endo and H-6exo showed the downfield shifts.
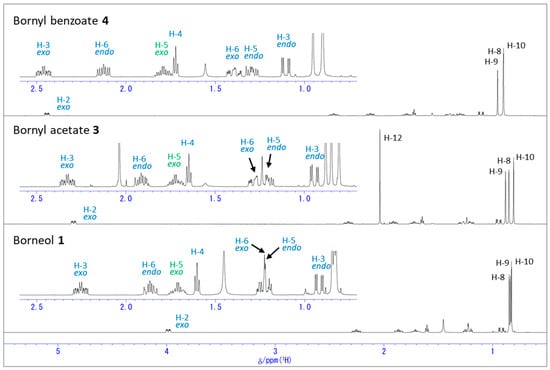
Figure 1.
1H NMR spectra of borneol (1), bornyl acetate (3), and bornyl benzoate (4) in CDCl3.
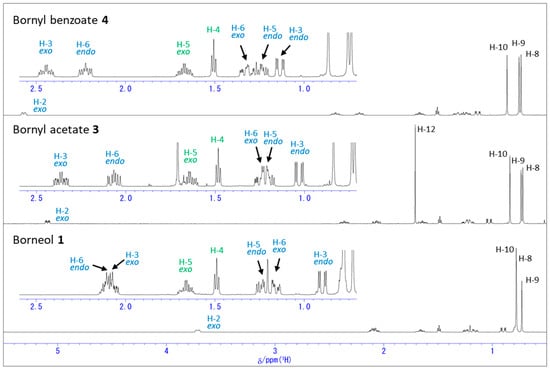
Figure 2.
1H NMR spectra of borneol (1), bornyl acetate (3), and bornyl benzoate (4) in C6D6.
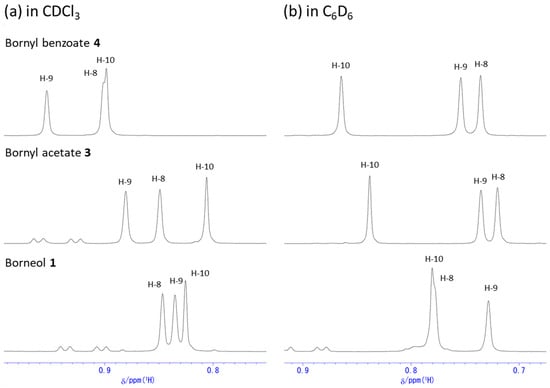
Figure 3.
Assignment of methyl groups in 1H NMR spectra of borneol (1), bornyl acetate (3), and bornyl benzoate (4) in different solvents.
In C6D6 (Figure 2), each compound again showed chemical shift ranges similar to those in CDCl3, but the extents of the downfield shifts were quite different from those in CDCl3. In particular, the chemical shifts in C6D6 for three methyl groups in 1 are fairly different from those in CDCl3, and the H-10 methyl protons appeared the most downfield while H-9 methyl protons appeared the most upfield as shown in Figure 3b. The acetylation and the benzoylation shifted the H-10 methyl protons further downfield. All the protons on the bicyclic ring exhibited even more separated chemical shifts than in CDCl3 as a result of the acetylation or the benzoylation. From these observations, it seems that C6D6 is a better solvent than CDCl3 for the assignment of each proton. These observations are likely due to generally known molecular interactions such as dipole–dipole interactions and/or vdW interactions with the benzene solvent as well as anisotropic effects by the benzene ring.
2.2. Change in 1H NMR Chemical Shifts after Acetylation and Benzoylation in Isoborneol
Next, we protected the hydroxy group of isoborneol (2), with an acetyl and a benzoyl group, and examined which protons were influenced. Figure 4, Figure 5 and Figure 6 and Table S2 show the 1H NMR spectra of isoborneol (2), isobornyl acetate (5), and isobornyl benzoate (6), observed in the CDCl3 and C6D6 solutions, and their chemical shift values. In the case of the 1H NMR spectra observed in CDCl3, the proton attached to C-2 bearing the hydroxy group, H-2, exhibited downfield shifts in CDCl3 after the acetylation of 2 or the benzoylation, which is similar to 1. Most of the other hydrogen atoms attached to the isoborneol ring, except for H-4 and H-5exo, also showed downfield shifts. In addition, among three methyl groups, H-9 and H-10 shifted upfield upon the acetylation while all these three methyl groups shifted downfield upon benzoylation as shown in Figure 6.
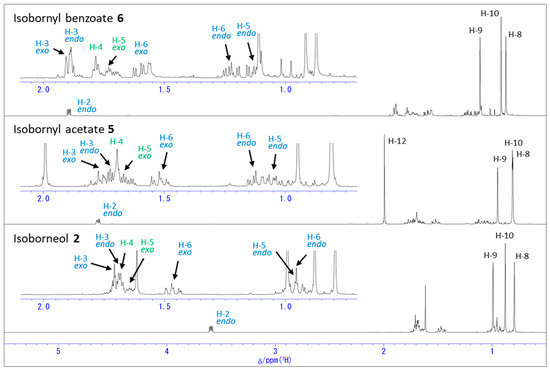
Figure 4.
1H NMR spectra of isoborneol (2), isobornyl acetate (5), and isobornyl benzoate (6) in CDCl3.
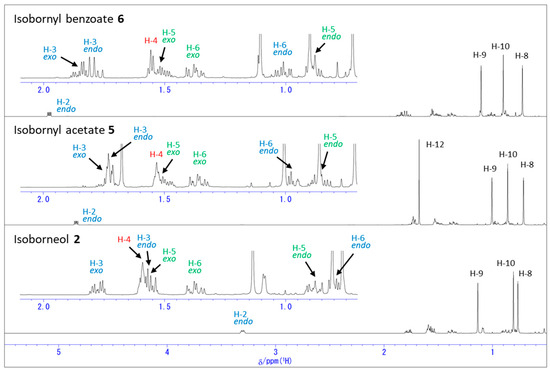
Figure 5.
1H NMR spectra of isoborneol (2), isobornyl acetate (5), and isobornyl benzoate (6) in C6D6.
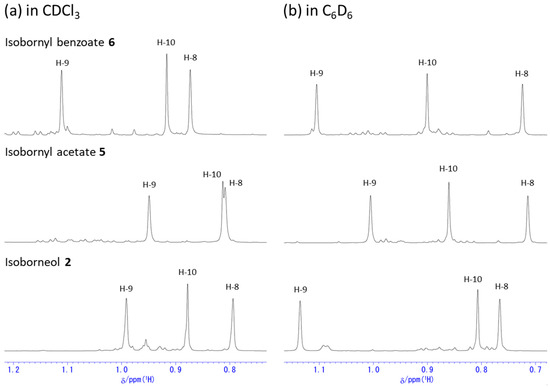
Figure 6.
Assignment of methyl groups in 1H NMR spectra of isoborneol (2), isobornyl acetate (5), and isobornyl benzoate (6) in different solvents.
Figure 5 shows the 1H NMR spectra of the same compounds 2, 5, and 6 in C6D6. The protons, H-3endo and H-6endo, exhibited significant downfield shifts, similar to the observation in the CDCl3 solution as shown in Figure 4. As shown in Figure 6b, both the methyl protons, H-8 and H-9, exhibited significant upfield shifts, while H-10 shifted downfield upon the acetylation and benzoylation. These different behaviors in different solvents are due to the intermolecular interactions with the solvent, similar to the 1H NMR chemical shift changes observed with the different solvents for 1 and its derivatives (Figure 3).
3. Materials and Methods
3.1. Chemicals
(–)-Borneol (1), purity > 95% (GC) and (±)-isobornel (2), purity > 90% (GC) were purchased from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan), and the compounds 1 and 2 were used without further purification. The deuterated solvents, CDCl3 and C6D6, and other reagents were purchased from Fujifilm Wako Chemicals Co. Ltd. (Osaka, Japan).
3.2. NMR Measurements
1H and 13C NMR spectra were recorded using JEOL JNM-ECZ400S NMR spectrometer operating at 400 MHz for 1H experiments at 298 K with 5 mm (o.d.) Pyrex glass tubes. 1H and 13C NMR chemical shifts were measured with reference to the residual signal of the incompletely deuterated solvent (CDCl3, δH 7.24, δC 77.0; C6D6, δH 7.16, δC 128.0). The spectra of 1H NMR, 13C NMR, DEPT-135, and 2D correlation experiments (DQF COSY, and HMQC) were obtained for these products in CDCl3 or C6D6. The numbers of accumulations for NMR were as follows: 1H NMR, 8 scans; 13C NMR, 5000 scans; DEPT 2500 scans; DQF COSY, 8 scans; HMQC, 16scans.
3.3. General Procedure for Acetylation of Borneol and Isoborneol
Bornyl acetate (3) and isobornyl acetate (5) were prepared by acetylation of borneol (1) and isoborneol (2), respectively, with acetic anhydride with the use of a catalytic amount of 4-dimethylaminopyridine (DMAP), according to the reported procedure [28]. Yield: bornyl acetate (3): 43%, isobornyl acetate (5): 39%.
3.4. General Procedure for Benzoylation of Borneol and Isoborneol
Bornyl benzoate (4) and isobornyl benzoate (6) were prepared by acetylation of borneol (1) and isoborneol (2), respectively, with benzoic acid, N,N-dicyclohexylcarbodiimide (DCC) with the use of a catalytic amount of DMAP according to the reported procedure [29]. Yield: bornyl benzoate (4): 43%, isobornyl benzoate (6): 24%.
4. Conclusions
In conclusion, the complete assignments of 1H NMR chemical shifts for borneol (1), isoborneol (2), and their derivatives 3–6 were made in CDCl3 and C6D6. The fact that the chemical shift values were significantly influenced by the protection of the hydroxy group with the carbonyl-containing protecting groups helped the unambiguous assignments. To our knowledge, this is the first study reporting the complete assignments of 1H NMR chemical shifts of borneol (1), isoborneol (2), and their derivatives 3–6 in various solvents. We are currently studying the observed changes of the chemical shift values in more depth, in combination with quantum mechanical calculations, and the results will be reported in due course.
Supplementary Materials
The following materials are available online: Figure S1: 1H NMR, 13C NMR, DEPT-135, DQF-COSY, HMQC spectra of borneol (1) in CDCl3; Figure S2: 1H NMR, 13C NMR, DEPT-135, DQF-COSY, HMQC spectra of borneol (1) in C6D6; Figure S3: 1H NMR, 13C NMR, DEPT-135, DQF-COSY, HMQC spectra of isoborneol (2) in CDCl3; Figure S4: 1H NMR, 13C NMR, DEPT-135, DQF-COSY, HMQC spectra of isoborneol (2) in C6D6; Figure S5: 1H NMR, 13C NMR, DEPT-135, DQF-COSY, HMQC spectra of bornyl acetate (3) in CDCl3; Figure S6: 1H NMR, 13C NMR, DEPT-135, DQF-COSY, HMQC spectra of bornyl acetate (3) in C6D6; Figure S7: 1H NMR, 13C NMR, DEPT-135, DQF-COSY, HMQC spectra of bornyl benzoate (4) in CDCl3; Figure S8: 1H NMR, 13C NMR, DEPT-135, DQF-COSY, HMQC spectra of bornyl benzoate (4) in C6D6; Figure S9: 1H NMR, 13C NMR, DEPT-135, DQF-COSY, HMQC spectra of isobornyl acetate (5) in CDCl3; Figure S10: 1H NMR, 13C NMR, DEPT-135, DQF-COSY, HMQC spectra of isobornyl acetate (5) in C6D6; Figure S11: 1H NMR, 13C NMR, DEPT-135, DQF-COSY, HMQC spectra of isobornyl benzoate (6) in CDCl3; Figure S12: 1H NMR, 13C NMR, DEPT-135, DQF-COSY, HMQC spectra of isobornyl benzoate (6) in C6D6; Table S1: 1H NMR chemical shifts for borneol (1), bornyl acetate (3), and bornyl benzoate (4) in CDCl3 and C6D6; Table S2 1H NMR chemical shifts for isoborneol (2), isobornyl acetate (5), and isobornyl benzoate (6) in CDCl3 and C6D6;. Table S3: 13C NMR chemical shifts for borneol (1), bornyl acetate (3), and bornyl benzoate (4) in CDCl3 and C6D6; Table S4. 13C NMR chemical shifts for isoborneol (2), isobornyl acetate (5), and isobornyl benzoate (6) in CDCl3 and C6D6.
Author Contributions
Conceptualization, Y.H. and S.N.; methodology, Y.H. and S.N.; investigation, Y.H., S.N., B.L., H.S., M.S. and R.T.; writing—original draft preparation, Y.H., S.N. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the financial support from The Ogasawara Toshiaki Memorial Foundation Grant, and Grants-in-Aid for Scientific Research (22K05106).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, and further inquiries can be directed to the corresponding author.
Acknowledgments
We appreciate the financial support from The Ogasawara Toshiaki Memorial Foundation Grant, and Grants-in-Aid for Scientific Research (22K05106).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Trost, B.M.; Belletire, J.L.; Godleski, S.; McDougal, P.G.; Balkovec, J.M.; Baldwin, J.J.; Christy, M.E.; Ponticello, G.S.; Varga, S.L.; Springer, J.P. On the use of the O-methylmandelate ester for establishment of absolute configuration of secondary alcohols. J. Org. Chem. 1986, 51, 2370–2374. [Google Scholar] [CrossRef]
- Sungsuwan, S.; Ruangsupapichart, N.; Prabpai, S.; Kongsaeree, P.; Thongpanchang, T. Tetrahydro-1,4-epoxynaphthalene-1-carboxylic acid: A chiral derivatizing agent for the determination of the absolute configuration of secondary alcohols. Tetrahedron Lett. 2010, 51, 4965–4967. [Google Scholar] [CrossRef]
- Seco, J.M.; Quiñoá, E.; Riguera, R. The Assignment of Absolute Configuration by NMR. Chem. Rev. 2004, 104, 17–118. [Google Scholar] [CrossRef]
- Seco, J.M.; Quiñoá, E.; Riguera, R. Assignment of the Absolute Configuration of Polyfunctional Compounds by NMR Using Chiral Derivatizing Agents. Chem. Rev. 2012, 112, 4603–4641. [Google Scholar] [CrossRef]
- Baranac-Stojanović, M. New insight into the anisotropic effects in solution-state NMR spectroscopy. RSC Adv. 2014, 4, 308–321. [Google Scholar] [CrossRef]
- Harriswangler, C.; Lucio-Martínez, F.; Godec, L.; Soro, L.K.; Fernández-Fariña, S.; Valencia, L.; Rodríguez-Rodríguez, A.; Esteban-Gómez, D.; Charbonnière, L.J.; Platas-Iglesias, C. Effect of Magnetic Anisotropy on the 1H NMR Paramagnetic Shifts and Relaxation Rates of Small Dysprosium(III) Complexes. Inorg. Chem. 2023, 62, 14326–14338. [Google Scholar] [CrossRef] [PubMed]
- Kleinpeter, E.; Lämmermann, A.; Kühn, H. The anisotropic effect of functional groups in 1H NMR spectra is the molecular response property of spatial nucleus independent chemical shifts (NICS)—Conformational equilibria of exo/endo tetrahydrodicyclopentadiene derivatives. Org. Biomol. Chem. 2011, 9, 1098–1111. [Google Scholar] [CrossRef]
- Kleinpeter, E.; Szatmári, I.; Lázár, L.; Koch, A.; Heydenreich, M.; Fülöp, F. Visualization and quantification of anisotropic effects on the 1H NMR spectra of 1,3-oxazino[4,3-a]isoquinolines—Indirect estimates of steric compression. Tetrahedron 2009, 65, 8021–8027. [Google Scholar] [CrossRef]
- Kleinpeter, E.; Koch, A. Antiaromaticity Proved by the Anisotropic Effect in 1H NMR Spectra. J. Phys. Chem. A 2012, 116, 5674–5680. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J. Ab initio hybrid DFT–GIAO calculations of the shielding produced by carbon–carbon bonds and aromatic rings in 1H NMR spectroscopy. New J. Chem. 1998, 22, 381–385. [Google Scholar] [CrossRef]
- Venianakis, T.; Oikonomaki, C.; Siskos, M.G.; Varras, P.C.; Primikyri, A.; Alexandri, E.; Gerothanassis, I.P. DFT Calculations of 1H- and 13C-NMR Chemical Shifts of Geometric Isomers of Conjugated Linoleic Acid (18:2 ω-7) and Model Compounds in Solution. Molecules 2020, 25, 3660. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.J.; Edgar, M.E.; Glover, R.P.; Warne, M.A.; Griffiths, L. Substituent chemical shifts in NMR spectroscopy. Part 6. A model for the calculation of proton chemical shifts in substituted alkanes. J. Chem. Soc., Perkin Trans. 1996, 2, 333–341. [Google Scholar] [CrossRef]
- Rajput, A.; Kasar, A.; Thorat, S.; Kulkarni, M. Borneol: A Plant-Sourced Terpene with a Variety of Promising Pharmacological Effects. Nat. Prod. J. 2023, 13, 13–28. [Google Scholar]
- Kulkarni, M.; Sawant, N.; Kolapkar, A.; Huprikar, A.; Desai, N. Borneol: A Promising Monoterpenoid in Enhancing Drug Delivery Across Various Physiological Barriers. AAPS Pharm. Sci. Tech. 2021, 22, 145. [Google Scholar] [CrossRef]
- Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on borneol. Food Chem. Toxicol. 2008, 46, S77–S80. [Google Scholar] [CrossRef]
- Li, Y.; Ren, M.; Wang, J.; Ma, R.; Chen, H.; Xie, Q.; Li, H.; Li, J.; Wang, J. Progress in Borneol Intervention for Ischemic Stroke: A Systematic Review. Front Pharmacol. 2021, 12, 606682. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.G.; Vilar, E.T.; Fraile, A.G.; de la Moya, C.S.; Maroto, B.L. Synthesis and catalytic activity of 10-(aminomethyl)isoborneol-based catalysts: The role of the C(2)-group on the asymmetric induction. Tetrahedron Asymmetry 2003, 14, 1959–1963. [Google Scholar] [CrossRef]
- Arrayás, R.G.; Adrio, J.; Carretero, J.C. Recent Applications of Chiral Ferrocene Ligands in Asymmetric Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7674–7715. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Khine, A.A.; Liu, J.-W.; Cheng, H.-C.; Hu, A.; Chen, H.-P.; Shih, T.-L. Resolution of isoborneol and its isomers by GC/MS to identify “synthetic” and “semi-synthetic” borneol products. Chirality 2018, 30, 1233–1239. [Google Scholar] [CrossRef]
- Baldovini, N.; Tomi, F.; Casanova, J. Enantiomeric Differentiation of Bornyl Acetate by 13C-NMR Using a Chiral Lanthanide Shift Reagent. Phytochem. Anal. 2003, 14, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Simova, S. Application of to the measurement of homonuclear HSQC coupling constants, J(H,H). Magn. Reson. Chem. 1998, 36, 505–510. [Google Scholar] [CrossRef]
- Khandelwal, D.; Hooda, S.; Brar, A.S.; Shankar, R. Stereochemical assignments of the nuclear magnetic resonance spectra of isobornyl acrylate/methacrylonitrile copolymers. J. Appl. Polym. Sci. 2012, 126, 916–928. [Google Scholar] [CrossRef]
- Gansow, O.A.; Willcott, M.R.; Lenkinski, R.E. Carbon magnetic resonance. Signal assignment by alternately pulsed nuclear magnetic resonance and lanthanide-induced chemical shifts. J. Am. Chem. Soc. 1971, 93, 4295–4297. [Google Scholar] [CrossRef]
- Briggs, J.; Hart, F.A.; Moss, G.P.; Randall, E.W. A ready method of assignment for 13C nuclear magnetic resonance spectra: The complete assignment of the 13C spectrum of borneol. J. Chem. Soc. D. Chem. Commun. 1971, 364–365. [Google Scholar] [CrossRef]
- Hawkes, G.E.; Leibfritz, D.; Roberts, D.W.; Roberts, J.D. Nuclear magnetic resonance shift reagents. Question of the orientation of the magnetic axis in lanthanide-substrate complexes. J. Am. Chem. Soc. 1973, 95, 1659–1661. [Google Scholar] [CrossRef]
- Levy, G.C.; Komoroski, R.A. Paramagnetic relaxation reagents. Alternatives or complements to lanthanide shift reagents in nuclear magnetic resonance spectral analysis. J. Am. Chem. Soc. 1974, 96, 678–681. [Google Scholar] [CrossRef]
- Nguyen, K.; Nguyen, V.; Trana, H.; Pham, P. Organo-photocatalytic C–H bond oxidation: An operationally simple and scalable method to prepare ketones with ambient air. RSC Adv. 2023, 13, 7168–7178. [Google Scholar] [CrossRef]
- Matsumura, A.; Fan, Y.; Goto, H. Asymmetric electrochemical polymerization in cholesteric liquid crystalline media: Effect of isomeric structures of chiral inducers containing bornyl group. Synth. Met. 2015, 202, 157–164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).