1H NMR Chemical Shift Changes as a Result of Introduction of Carbonyl-Containing Protecting Groups Observed in Bornol and Isoborneol
Abstract
1. Introduction
2. Results and Discussion
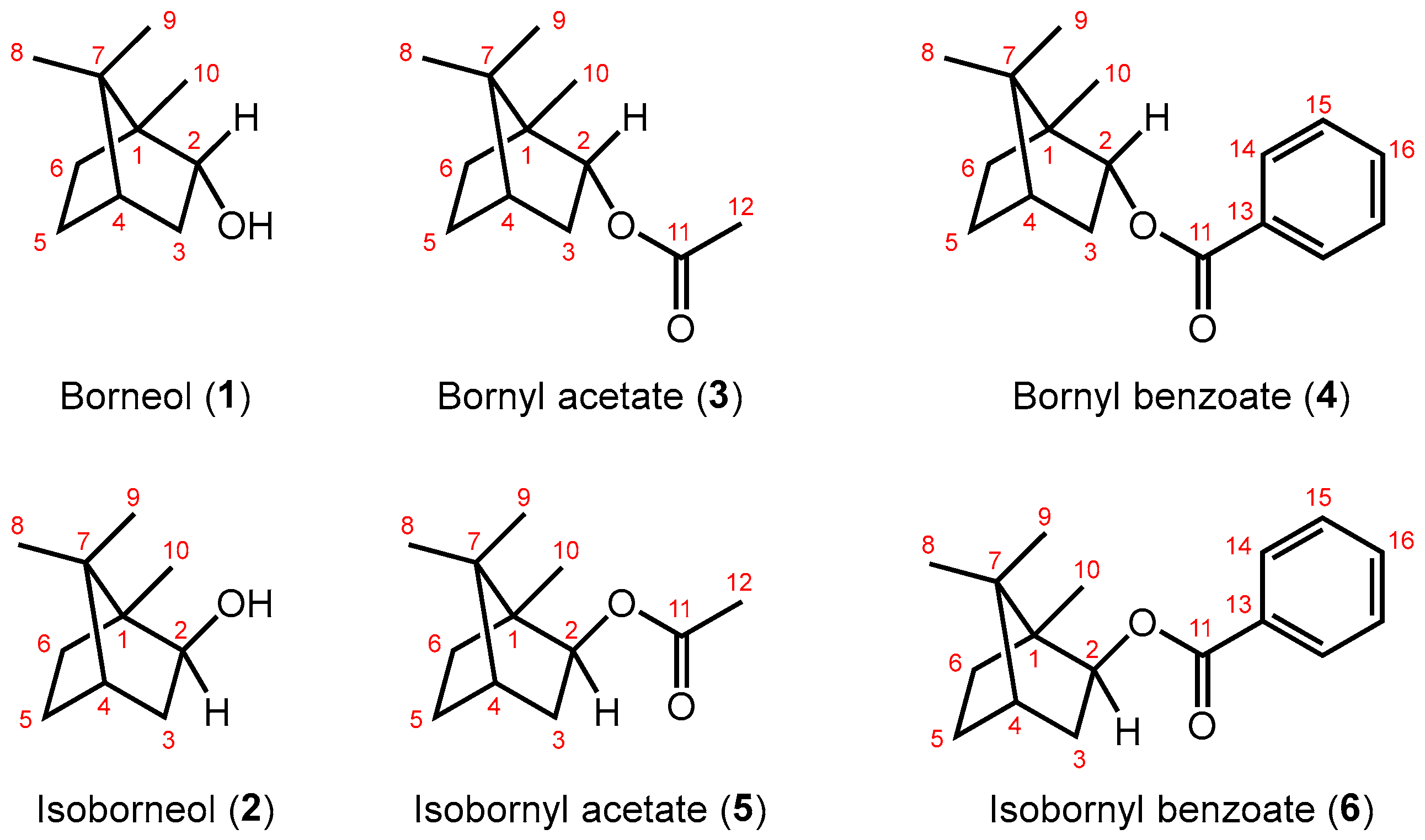
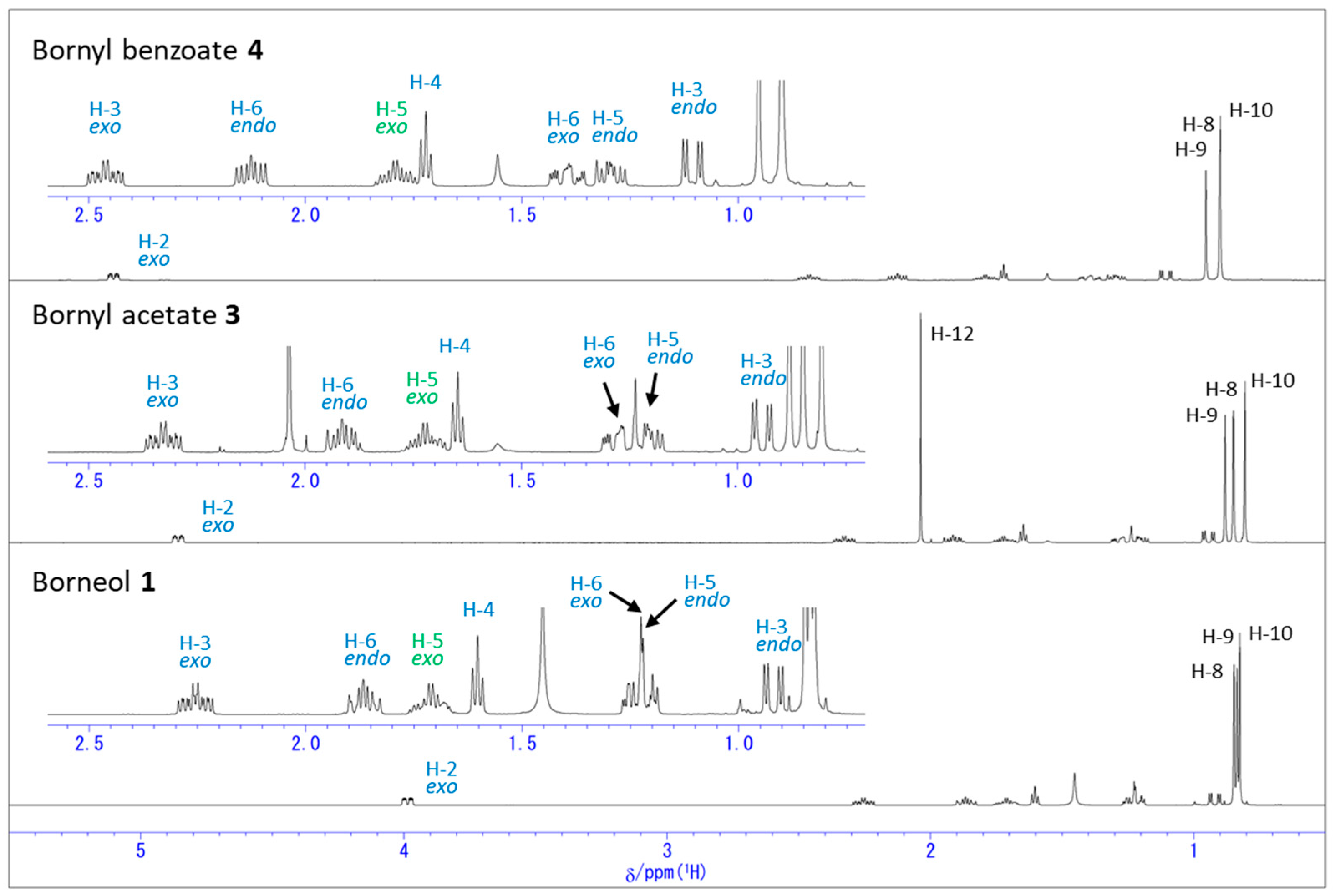
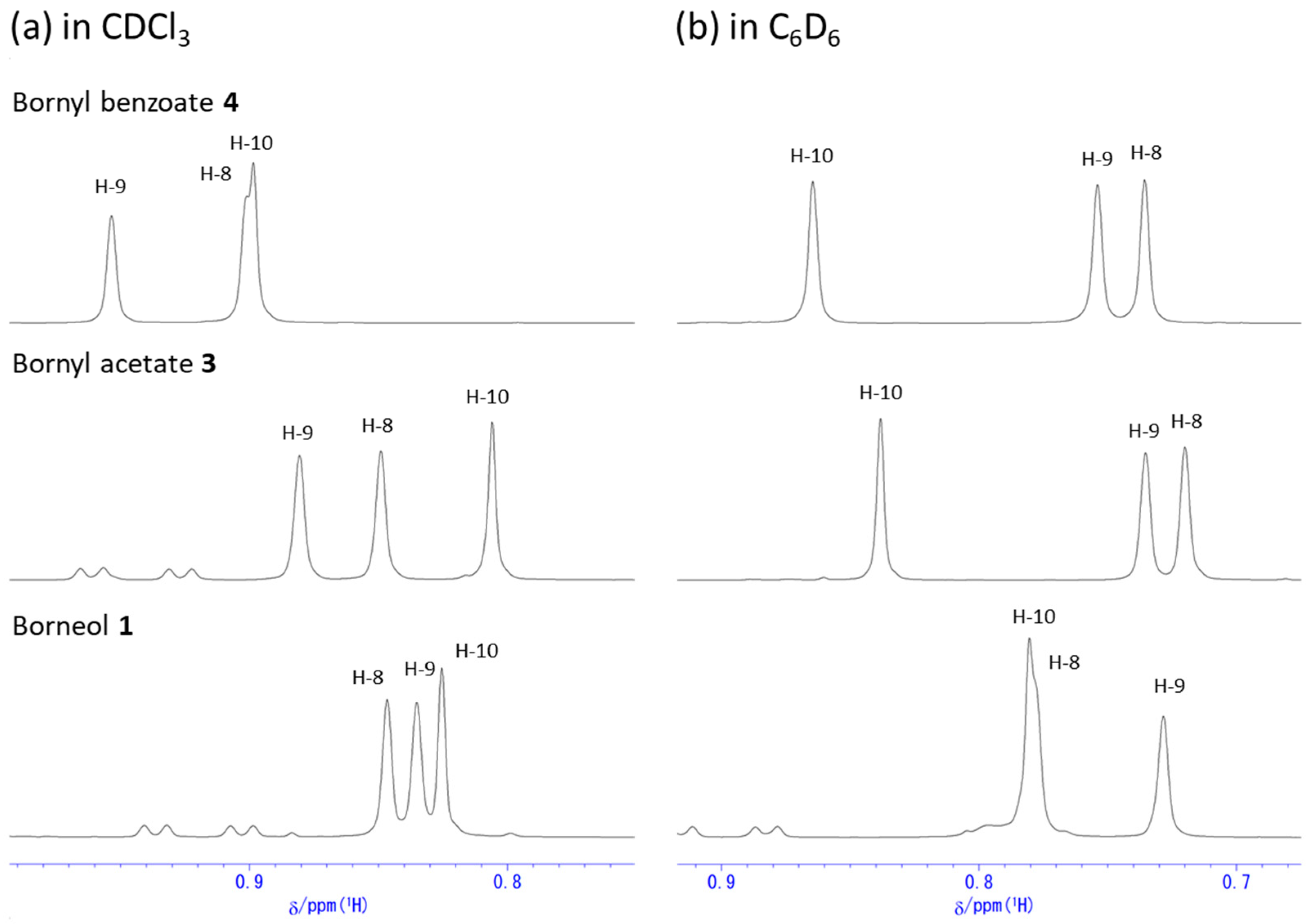
2.1. Change in 1H NMR Chemical Shifts after Acetylation and Benzoylation in Borneol
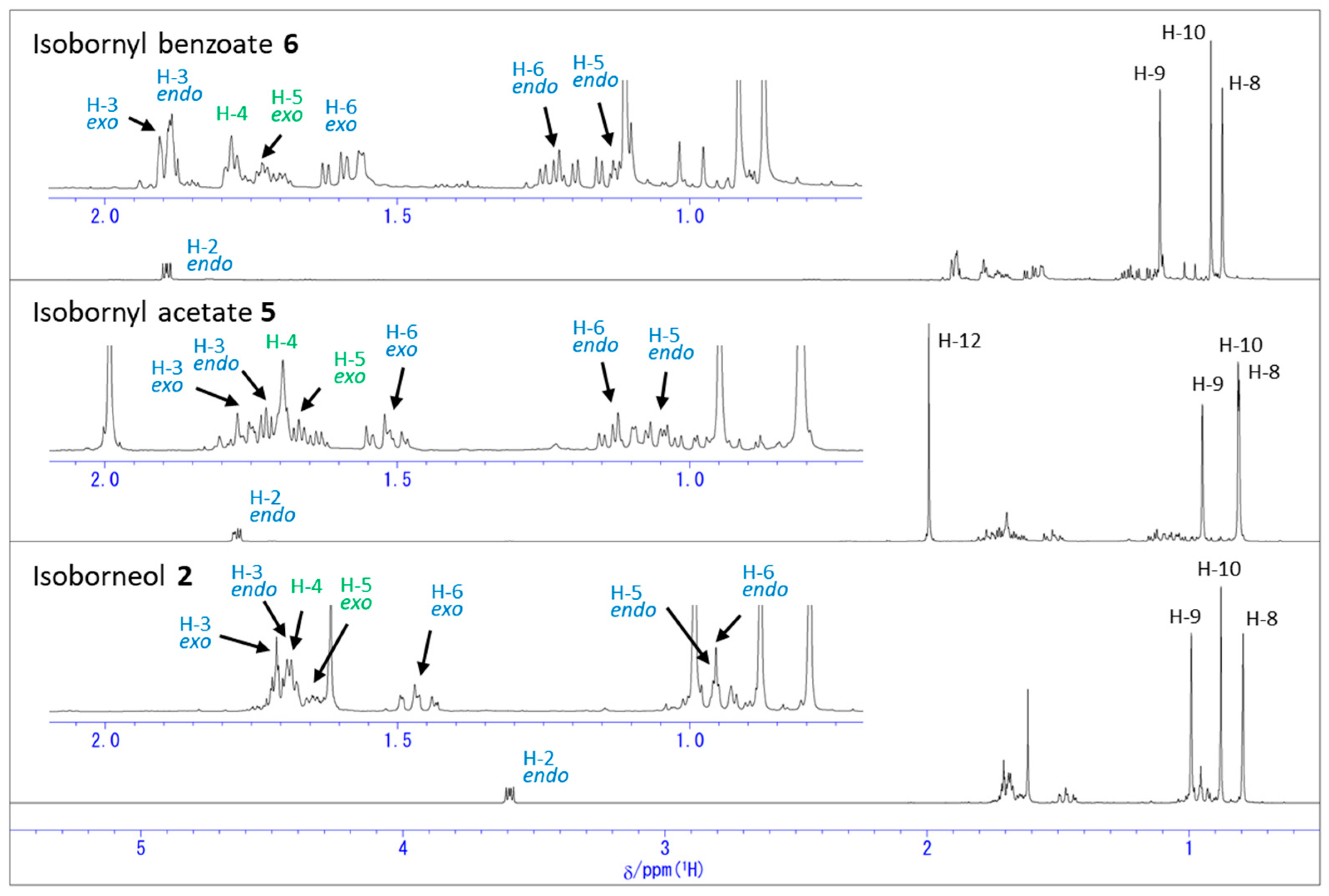
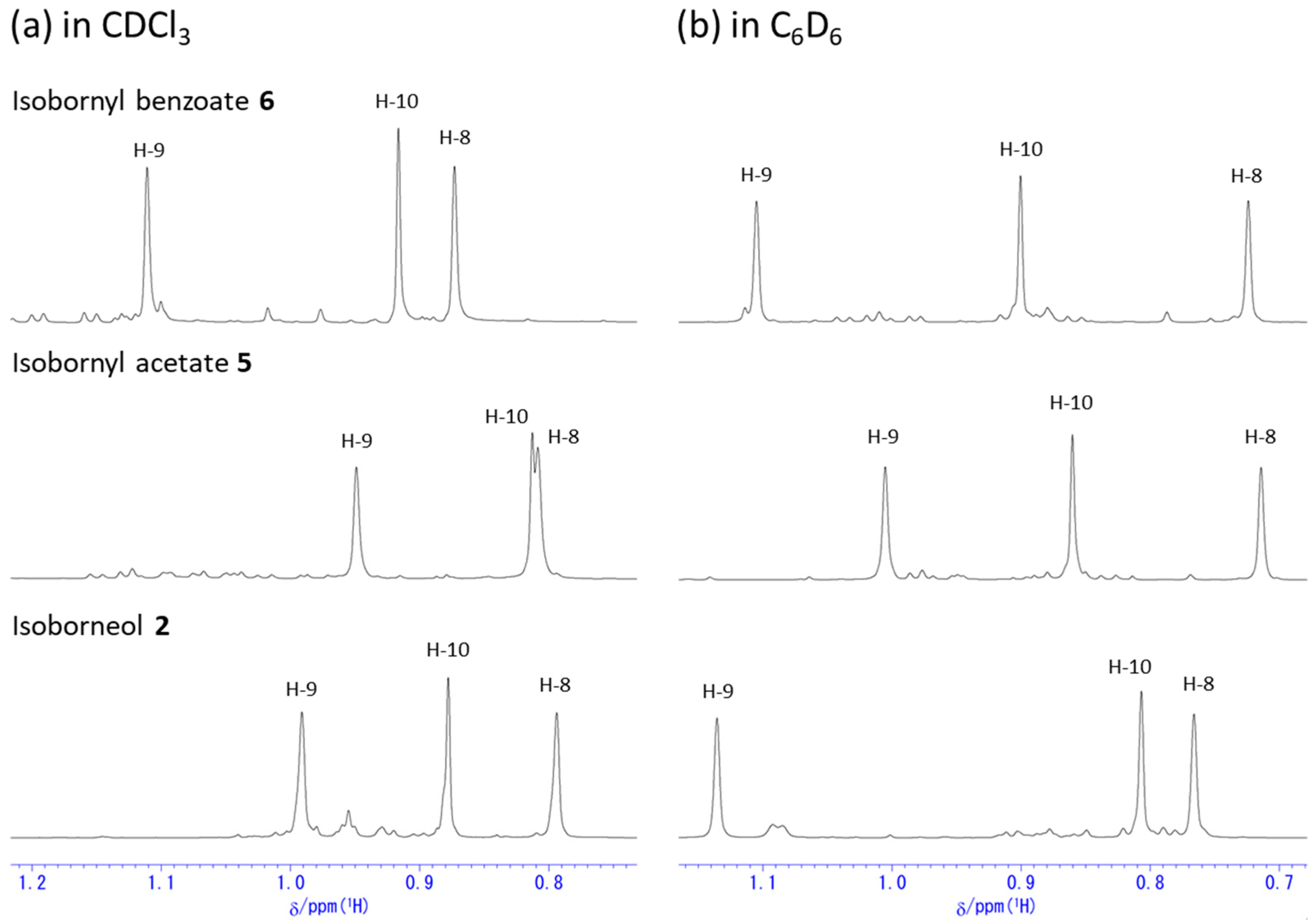
2.2. Change in 1H NMR Chemical Shifts after Acetylation and Benzoylation in Isoborneol
3. Materials and Methods
3.1. Chemicals
3.2. NMR Measurements
3.3. General Procedure for Acetylation of Borneol and Isoborneol
3.4. General Procedure for Benzoylation of Borneol and Isoborneol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Trost, B.M.; Belletire, J.L.; Godleski, S.; McDougal, P.G.; Balkovec, J.M.; Baldwin, J.J.; Christy, M.E.; Ponticello, G.S.; Varga, S.L.; Springer, J.P. On the use of the O-methylmandelate ester for establishment of absolute configuration of secondary alcohols. J. Org. Chem. 1986, 51, 2370–2374. [Google Scholar] [CrossRef]
- Sungsuwan, S.; Ruangsupapichart, N.; Prabpai, S.; Kongsaeree, P.; Thongpanchang, T. Tetrahydro-1,4-epoxynaphthalene-1-carboxylic acid: A chiral derivatizing agent for the determination of the absolute configuration of secondary alcohols. Tetrahedron Lett. 2010, 51, 4965–4967. [Google Scholar] [CrossRef]
- Seco, J.M.; Quiñoá, E.; Riguera, R. The Assignment of Absolute Configuration by NMR. Chem. Rev. 2004, 104, 17–118. [Google Scholar] [CrossRef]
- Seco, J.M.; Quiñoá, E.; Riguera, R. Assignment of the Absolute Configuration of Polyfunctional Compounds by NMR Using Chiral Derivatizing Agents. Chem. Rev. 2012, 112, 4603–4641. [Google Scholar] [CrossRef]
- Baranac-Stojanović, M. New insight into the anisotropic effects in solution-state NMR spectroscopy. RSC Adv. 2014, 4, 308–321. [Google Scholar] [CrossRef]
- Harriswangler, C.; Lucio-Martínez, F.; Godec, L.; Soro, L.K.; Fernández-Fariña, S.; Valencia, L.; Rodríguez-Rodríguez, A.; Esteban-Gómez, D.; Charbonnière, L.J.; Platas-Iglesias, C. Effect of Magnetic Anisotropy on the 1H NMR Paramagnetic Shifts and Relaxation Rates of Small Dysprosium(III) Complexes. Inorg. Chem. 2023, 62, 14326–14338. [Google Scholar] [CrossRef] [PubMed]
- Kleinpeter, E.; Lämmermann, A.; Kühn, H. The anisotropic effect of functional groups in 1H NMR spectra is the molecular response property of spatial nucleus independent chemical shifts (NICS)—Conformational equilibria of exo/endo tetrahydrodicyclopentadiene derivatives. Org. Biomol. Chem. 2011, 9, 1098–1111. [Google Scholar] [CrossRef]
- Kleinpeter, E.; Szatmári, I.; Lázár, L.; Koch, A.; Heydenreich, M.; Fülöp, F. Visualization and quantification of anisotropic effects on the 1H NMR spectra of 1,3-oxazino[4,3-a]isoquinolines—Indirect estimates of steric compression. Tetrahedron 2009, 65, 8021–8027. [Google Scholar] [CrossRef]
- Kleinpeter, E.; Koch, A. Antiaromaticity Proved by the Anisotropic Effect in 1H NMR Spectra. J. Phys. Chem. A 2012, 116, 5674–5680. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J. Ab initio hybrid DFT–GIAO calculations of the shielding produced by carbon–carbon bonds and aromatic rings in 1H NMR spectroscopy. New J. Chem. 1998, 22, 381–385. [Google Scholar] [CrossRef]
- Venianakis, T.; Oikonomaki, C.; Siskos, M.G.; Varras, P.C.; Primikyri, A.; Alexandri, E.; Gerothanassis, I.P. DFT Calculations of 1H- and 13C-NMR Chemical Shifts of Geometric Isomers of Conjugated Linoleic Acid (18:2 ω-7) and Model Compounds in Solution. Molecules 2020, 25, 3660. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.J.; Edgar, M.E.; Glover, R.P.; Warne, M.A.; Griffiths, L. Substituent chemical shifts in NMR spectroscopy. Part 6. A model for the calculation of proton chemical shifts in substituted alkanes. J. Chem. Soc., Perkin Trans. 1996, 2, 333–341. [Google Scholar] [CrossRef]
- Rajput, A.; Kasar, A.; Thorat, S.; Kulkarni, M. Borneol: A Plant-Sourced Terpene with a Variety of Promising Pharmacological Effects. Nat. Prod. J. 2023, 13, 13–28. [Google Scholar]
- Kulkarni, M.; Sawant, N.; Kolapkar, A.; Huprikar, A.; Desai, N. Borneol: A Promising Monoterpenoid in Enhancing Drug Delivery Across Various Physiological Barriers. AAPS Pharm. Sci. Tech. 2021, 22, 145. [Google Scholar] [CrossRef]
- Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on borneol. Food Chem. Toxicol. 2008, 46, S77–S80. [Google Scholar] [CrossRef]
- Li, Y.; Ren, M.; Wang, J.; Ma, R.; Chen, H.; Xie, Q.; Li, H.; Li, J.; Wang, J. Progress in Borneol Intervention for Ischemic Stroke: A Systematic Review. Front Pharmacol. 2021, 12, 606682. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.G.; Vilar, E.T.; Fraile, A.G.; de la Moya, C.S.; Maroto, B.L. Synthesis and catalytic activity of 10-(aminomethyl)isoborneol-based catalysts: The role of the C(2)-group on the asymmetric induction. Tetrahedron Asymmetry 2003, 14, 1959–1963. [Google Scholar] [CrossRef]
- Arrayás, R.G.; Adrio, J.; Carretero, J.C. Recent Applications of Chiral Ferrocene Ligands in Asymmetric Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7674–7715. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Khine, A.A.; Liu, J.-W.; Cheng, H.-C.; Hu, A.; Chen, H.-P.; Shih, T.-L. Resolution of isoborneol and its isomers by GC/MS to identify “synthetic” and “semi-synthetic” borneol products. Chirality 2018, 30, 1233–1239. [Google Scholar] [CrossRef]
- Baldovini, N.; Tomi, F.; Casanova, J. Enantiomeric Differentiation of Bornyl Acetate by 13C-NMR Using a Chiral Lanthanide Shift Reagent. Phytochem. Anal. 2003, 14, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Simova, S. Application of to the measurement of homonuclear HSQC coupling constants, J(H,H). Magn. Reson. Chem. 1998, 36, 505–510. [Google Scholar] [CrossRef]
- Khandelwal, D.; Hooda, S.; Brar, A.S.; Shankar, R. Stereochemical assignments of the nuclear magnetic resonance spectra of isobornyl acrylate/methacrylonitrile copolymers. J. Appl. Polym. Sci. 2012, 126, 916–928. [Google Scholar] [CrossRef]
- Gansow, O.A.; Willcott, M.R.; Lenkinski, R.E. Carbon magnetic resonance. Signal assignment by alternately pulsed nuclear magnetic resonance and lanthanide-induced chemical shifts. J. Am. Chem. Soc. 1971, 93, 4295–4297. [Google Scholar] [CrossRef]
- Briggs, J.; Hart, F.A.; Moss, G.P.; Randall, E.W. A ready method of assignment for 13C nuclear magnetic resonance spectra: The complete assignment of the 13C spectrum of borneol. J. Chem. Soc. D. Chem. Commun. 1971, 364–365. [Google Scholar] [CrossRef]
- Hawkes, G.E.; Leibfritz, D.; Roberts, D.W.; Roberts, J.D. Nuclear magnetic resonance shift reagents. Question of the orientation of the magnetic axis in lanthanide-substrate complexes. J. Am. Chem. Soc. 1973, 95, 1659–1661. [Google Scholar] [CrossRef]
- Levy, G.C.; Komoroski, R.A. Paramagnetic relaxation reagents. Alternatives or complements to lanthanide shift reagents in nuclear magnetic resonance spectral analysis. J. Am. Chem. Soc. 1974, 96, 678–681. [Google Scholar] [CrossRef]
- Nguyen, K.; Nguyen, V.; Trana, H.; Pham, P. Organo-photocatalytic C–H bond oxidation: An operationally simple and scalable method to prepare ketones with ambient air. RSC Adv. 2023, 13, 7168–7178. [Google Scholar] [CrossRef]
- Matsumura, A.; Fan, Y.; Goto, H. Asymmetric electrochemical polymerization in cholesteric liquid crystalline media: Effect of isomeric structures of chiral inducers containing bornyl group. Synth. Met. 2015, 202, 157–164. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, B.; Sako, H.; Sugiura, M.; Hiraga, Y.; Takagi, R.; Niwayama, S. 1H NMR Chemical Shift Changes as a Result of Introduction of Carbonyl-Containing Protecting Groups Observed in Bornol and Isoborneol. Molbank 2024, 2024, M1899. https://doi.org/10.3390/M1899
Lyu B, Sako H, Sugiura M, Hiraga Y, Takagi R, Niwayama S. 1H NMR Chemical Shift Changes as a Result of Introduction of Carbonyl-Containing Protecting Groups Observed in Bornol and Isoborneol. Molbank. 2024; 2024(4):M1899. https://doi.org/10.3390/M1899
Chicago/Turabian StyleLyu, Baohe, Honoka Sako, Mio Sugiura, Yoshikazu Hiraga, Ryukichi Takagi, and Satomi Niwayama. 2024. "1H NMR Chemical Shift Changes as a Result of Introduction of Carbonyl-Containing Protecting Groups Observed in Bornol and Isoborneol" Molbank 2024, no. 4: M1899. https://doi.org/10.3390/M1899
APA StyleLyu, B., Sako, H., Sugiura, M., Hiraga, Y., Takagi, R., & Niwayama, S. (2024). 1H NMR Chemical Shift Changes as a Result of Introduction of Carbonyl-Containing Protecting Groups Observed in Bornol and Isoborneol. Molbank, 2024(4), M1899. https://doi.org/10.3390/M1899






