1. Introduction
Synthetic peptides are extensively used in various fields, including diagnostics, treatment, vaccine development, and peptide antibody production, leading to a remarkable revival in peptide drug discovery over the last decades [
1,
2,
3,
4]. Furthermore, branched architecture peptide macromolecules have been employed in immunology to overcome several limitations associated with linear epitopes, such as poor antigenicity, immune response, and stability [
5,
6,
7,
8].
In addition, Sequential Oligopeptide Carriers (SOCn) with the moiety [Lys(antigen)-Aib-Gly]
4, (Aib: 2 aminoisobutyric acid), as well as the modified versions [Lys-Aib-Cys]
4 (C-SOC
4) and [Lys-Aib-Cys(3,9 Acm)]
4 (CPSOC(3,9 Acm)), have been developed for anchoring multiple copies of peptide epitopes, enhancing their antigenic/immunogenic potency [
9,
10,
11]. These carriers allow the construction of high molecular weight molecules (>3000 Da) capable of inducing a potent immune response. Their key advantage lies in their ability to produce antibodies recognizing different regions of the same protein.
Moreover, innovative strategies for snake antivenom include the immunization of animals with chemically synthesized toxin epitopes, which mitigates several challenges associated with traditional antivenom production, such as the maintenance and handling of dangerous animals [
12,
13,
14,
15].
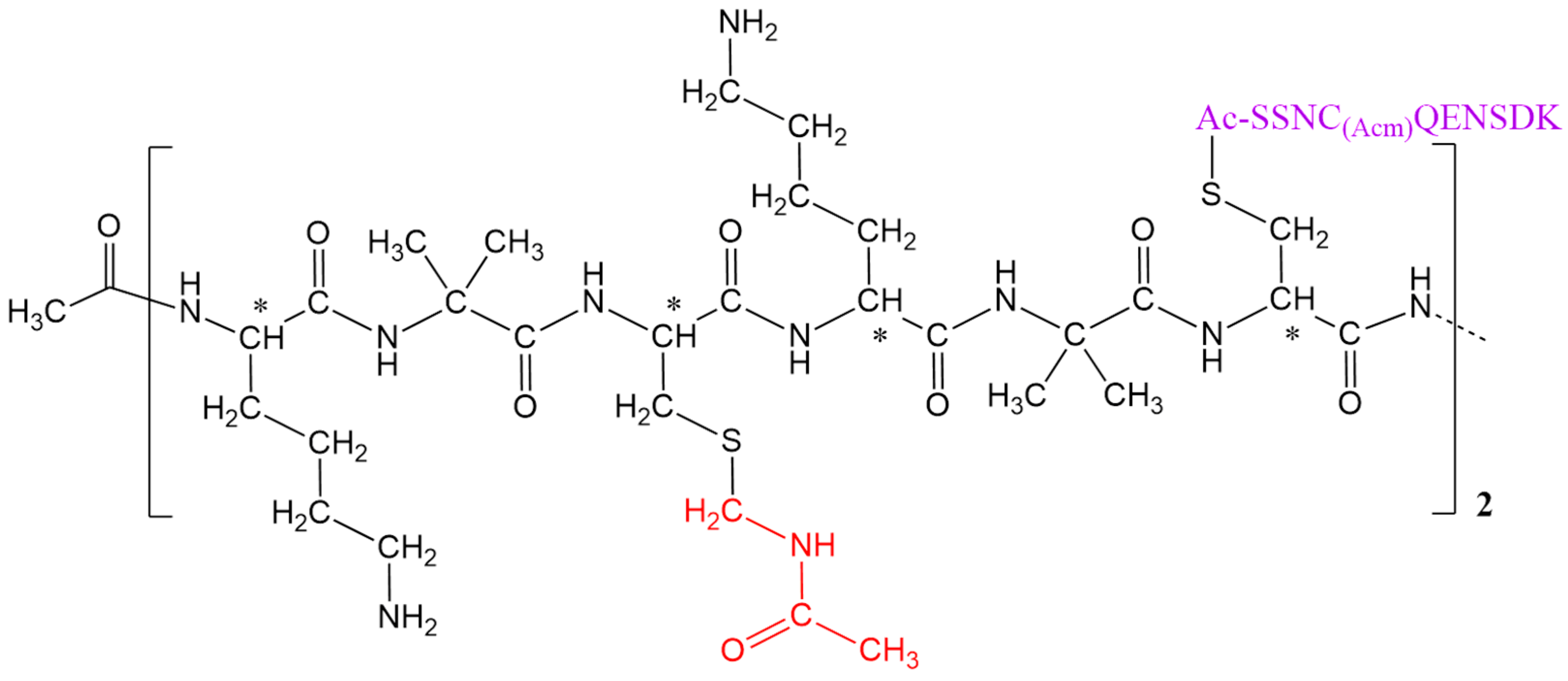
Based on the above, in this study, we designed and synthesized the Ac-[K-Aib-C(3,9-Acm; 6,12-SSNC(Acm)QENSDK)]4-NH2 (Acm: acetamidomethyl group) peptide conjugate of branched architecture. The peptide epitope S128SNCQENSDK137 belongs to the V. berus basic phospholipase A2, one of the most lethal snake toxins. This synthesis represents a pivotal step in developing alternative antivenom production methods, such as employing multiple peptide antigenic macromolecules to produce antibodies that cross-react with heterologous snake venoms.
2. Results and Discussion
2.1. Peptide and Conjugate Synthesis
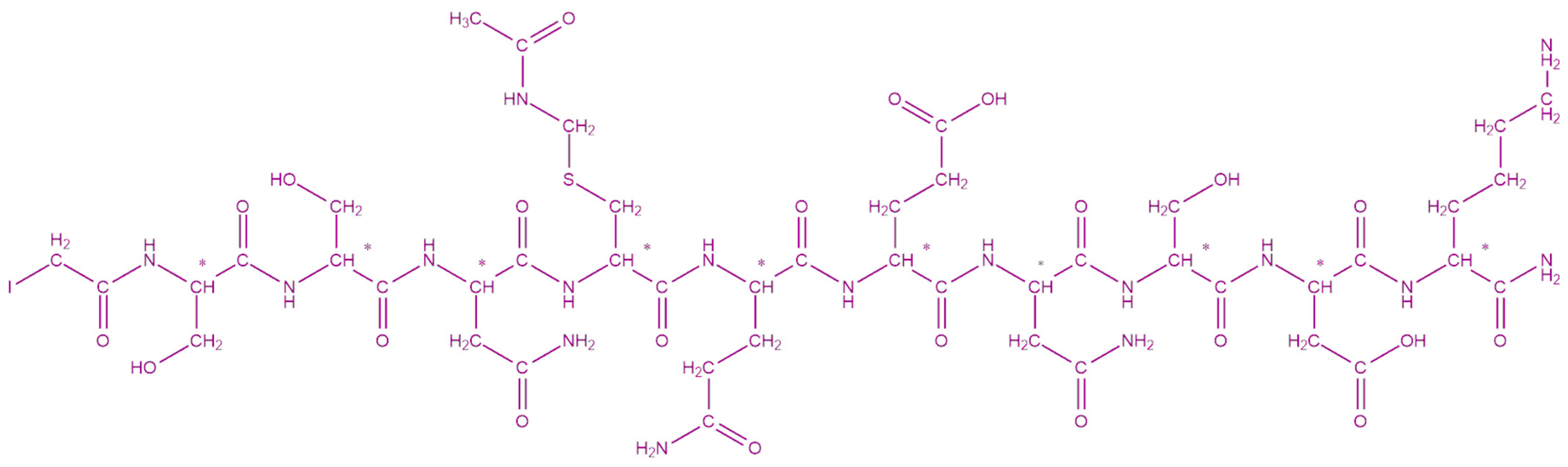
We successfully synthesized the desired peptide conjugate of branched architecture through the Solid Phase Peptide Synthesis (SPPS) and a chemoselective reaction for the thioether linkage. The characterization for both peptide (
Figure 1) and conjugate (
Figure 2) was accomplished via mass spectrometry (
Supplementary Materials Figures S3 and S4). HR-ESI-MS, positive (
m/
z): C
45H
73IN
16O
22S (IAc-S
128SNC
(Acm)QENSDK
137-NH
2): found 675.20; calc. 675.56 for the [C
45H
73IN
16O
22S]
+2; found 1349.39, calc. 1349.12 for the [C
45H
73IN
16O
22S]
+1. HR-ESI-MS, positive (
m/
z): C
150H
255N
510
59S
6 (Ac-[K-Aib-C(3,9-Acm; 6,12-SSNC
(Acm)QENSDK)]
4-NH
2) found 1955.35; calc. 1955.66 for the [C
150H
255N
510
59S
6]
+2; found 1303.91; calc. 1304.11 for the [C
150H
255N
510
59S
6]
+3; found 977.93; calc. 978.33 for the [C
150H
255N
510
59S
6]
+4. The crude recoveries were 70% and 64% for the peptide and the conjugate, respectively.
2.2. NMR Spectroscopy
Further characterization of the synthesized peptide was accomplished by NMR spectroscopy. 1D and 2D NMR spectra of the IAc-S
128SNC
(Acm)QENSDK
137-NH
2 peptide are shown in
Figures S5–S8 (
Supplementary Materials) and
1H (δ, ppm) chemical shifts are listed in
Table S1 in the
Supplementary Materials. The assignment started from the terminal part of the lysine side chain, which exhibits the characteristic signal sequence of the side chain at 1.35/1.65/1.58/2.79/7.42 ppm from TOCSY/NOESY spectra and following the signals through NOE correlations between CH and NH protons. In this specific peptide, the protons of the two neighboring serine residues appear at almost identical values (Ser 1: 4.38/3.83 ppm, Ser 2: 4.38/3.82 ppm), while the protons of Ser 3 display a different chemical shift (4.46/3.84 ppm) due to the distinct chemical environment (N, D residues). The absence of NOE signals between hydroxyl or amine groups or the side chains of amino acids, such as lysine with other protons of distant amino acids, at expected values suggests that there are no intramolecular hydrogen bond interactions leading to the bending of the molecule or any particular conformation.
2.3. Purification
Both the peptide and conjugate were purified via RP-HPLC. Chromatograms from analytical RP-HPLC are shown in
Figures S1 and S2 in the
Supplementary Materials. Purification yields were 50% for the peptide and 15% for the conjugate.
3. Materials and Methods
3.1. Materials
Fmoc amino acid derivatives, Rink amide AM resin, and 1-hydroxybenzotriazole (HOBt) were obtained from GL Biochem (Shanghai, China). Trifluoroacetic acid (TFA) and piperidine were purchased from Honeywell, Riedel-de Haen (Seelze, Germany). Dichloromethane (DCM), Dimethylformamide (DMF), N,N′-Diisopropylcarbodiimide (DIC), and dimethylbenzene (DMB) were purchased from Fluka (Seelze, Germany), while acetonitrile (HPLC grade) and n-hexane were purchased from Labscan (Dublin, Ireland). Acetic acid and iodoacetic acid were obtained from Sigma-Aldrich (Steinheim, Germany) and TIS from Acros Organics (Waltham, MA, USA).
3.2. Peptide Synthesis
The peptide synthesis was carried out by employing the stepwise solid-phase peptide synthesis on the Rink Amide AM resin (substitution 0.58 mmol/g) using the Fmoc/tBu methodology [
16,
17]. Amino acids were introduced as Na-Fmoc protected and were dissolved in DMF/DCM (1/1,
v/
v) using a molar ratio of Fmoc-amino acids/DIC/HOBt/peptide-resin: 3/3/3/1. The Fmoc protecting group was removed using 20% (
v/
v) piperidine in DMF. The coupling reactions and Fmoc-deprotection steps were monitored by the Kaiser test. For the iodoacetyl group introduction to the N-terminal, after completing the desired peptide sequence, iodoacetic acid (ICH
2COOH) was dissolved in DMF/DCM (1/1,
v/
v) and added to the vessel. The reaction was carried out for 3 h in the dark and tested by the Kaiser test.
3.3. Peptide Cleavage from the Resin
The peptide cleavage and side groups’ deprotection were accomplished using the following mixture of solvents: 95% TFA, 2.5% TIS, 2.5% DMB. The reaction was left for 3 h, in the dark, at RT. Then, the resin was filtered, DCM/hexane (1/1 v/v) was added, and the mixture was concentrated in a flash evaporator (Heidolph, Germany). The peptide was precipitated using cold diethyl ether and finally dissolved in 2N acetic acid, lyophilized (Martin Christ, Germany), and stored at 4 °C.
3.4. Thioether Bond Formation
The thioether bonds were formed between the CPSOC (3,9 Acm) carrier and the antigenic epitope IAc-S
128SNC
(Acm)QENSDK
137-NH
2. The iodoacetyl-peptide was dissolved in H
2O/AcN (1:1,
v/
v) and the pH was adjusted to 8.2 using diisopropylethylamine (DIPEA). The peptide carrier CPSOC (3,9 Acm) was added to the solution in solid form in small portions with a ~20 min interval. The reaction was performed under inert conditions (N
2 atmosphere) for 4 h and was terminated by adding formic acid to the solution until pH reached 2–3 [
9].
3.5. High-Performance Liquid Chromatography
The crude, lyophilized peptide and conjugate were purified by semipreparative RP-HPLC (Shimadzu, Germany, Discovery C18 column (25 cm × 10 mm)) with a flow rate of 4.7 mL/min. The wavelength was set at 214 nm and the gradient elution system was H2O (0.1% TFA)/acetonitrile (0.1% TFA) from 90/10% to 60/40%. Peptide purity was monitored by analytical RP-HPLC associated with a C18 Supelco column (25 cm × 3 mm, 5 μm), (Shimadzu, Germany).
3.6. Mass Spectroscopy
HR-ESI-MS (Thermo Scientific LTQ Orbitrap XL™system, Thermo Fisher Scientific, Bremen, Germany) was used to record the mass spectra. The pure lyophilized peptide and conjugate were dissolved in a concentration of 2 ppm in H2O/0.1% formic acid (FA).
3.7. NMR Spectroscopy
NMR experiments for the peptide epitope were conducted on a Bruker Avance spectrometer at a frequency of 500.13 MHz. and were processed using Topspin 4.2 (Bruker Analytik GmbH, Bremen, Germany). Both 1D and 2D experiments (1H, 1H–1H-TOCSY, 1H–1H COSY, 1H–1H NOESY) were acquired in a 9:1 (v/v) H2O:D2O solution at a concentration of 5 mM, T = 298 K).
4. Conclusions
In summary, we successfully synthesized the peptide macromolecule Ac-[K-Aib-C(3,9-Acm; 6,12-SSNC(Acm)QENSDK)]4-NH2 of branched architecture by linking two copies of the peptide epitope IAc-S128SNC(Acm)QENSDK137-NH2 to the CPSOC (3,9 Acm) carrier. The conjugation was achieved through a chemoselective thioether bond formation, specifically targeting the two available cysteine residues on the carrier. Both the peptide epitope and the final conjugate were synthesized with satisfactory yields and high purity.
This synthesis serves as the first step towards the development of alternative antivenom production strategies, as this macromolecule shows potential as a candidate for generating antibodies capable of cross-reacting with venoms from various snake species.
Supplementary Materials
The following supporting information can be downloaded online. Figure S1: Analytical chromatogram of the synthesized peptide IAc-S128SNC(Acm)QENSDK137-NH2; Figure S2: Analytical chromatogram of the conjugate Ac-[K-Aib-C(3,9-Acm; 6,12-SSNC(Acm)QENSDK)]4-NH2; Figure S3: Mass spectrum of the synthesized peptide IAc-S128SNC(Acm)QENSDK137-NH2; Figure S4: Mass spectrum of the conjugate Ac-[K-Aib-C(3,9-Acm; 6,12-SSNC(Acm)QENSDK)]4-NH2; Figure S5: 1H NMR spectrum of the IAc-S128SNC(Acm)QENSDK137-NH2; Figure S6: 1H 1H COSY NMR spectrum of IAc-S128SNC(Acm)QENSDK137-NH2; Figure S7: 1H 1H NOESY NMR spectrum of the IAc-S128SNC(Acm)QENSDK137-NH2; Figure S8: 1H 1H TOCSY NMR spectrum of the IAc-S128SNC(Acm)QENSDK137-NH2; Table S1: Chemical shifts of the synthesized peptide IAc-S128SNC(Acm)QENSDK137-NH2.
Author Contributions
Conceptualization, V.T. and V.M. (Vassilios Moussis); methodology, V.M. (Vasiliki Moulasioti) and E.F.; software, V.M. (Vasiliki Moulasioti) and E.F.; validation, V.M. (Vasiliki Moulasioti) and E.F.; formal analysis, V.M. (Vasiliki Moulasioti); investigation, V.M. (Vasiliki Moulasioti); resources, V.T.; data curation, V.M. (Vasiliki Moulasioti) and E.F.; writing—original draft preparation, V.M. (Vasiliki Moulasioti); writing—review and editing, V.M. (Vasiliki Moulasioti), E.F., V.M. (Vassilios Moussis) and V.T.; visualization, V.M. (Vasiliki Moulasioti); supervision, V.T.; project administration, V.T., V.M. (Vasiliki Moulasioti) and V.M. (Vassilios Moussis); funding acquisition, V.M. (Vasiliki Moulasioti). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hellenic Foundation for Research and Innovation (HFRI) under the 3rd Call for HFRI PhD Fellowships (Fellowship Number: 5428).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We would like to thank The Network of Research Supporting Laboratories, University of Ioannina, for providing access to the NMR and HR-ESI-MS facilities.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Maecke, H.R. 3 Radiolabeled Peptides in Nuclear Oncology: Influence of Peptide Structure and Labeling Strategy on Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 43–72. [Google Scholar]
- Trier, N.H.; Holm, B.E.; Heiden, J.; Slot, O.; Locht, H.; Lindegaard, H.; Svendsen, A.; Nielsen, C.T.; Jacobsen, S.; Theander, E.; et al. Antibodies to a Strain-Specific Citrullinated Epstein-Barr Virus Peptide Diagnoses Rheumatoid Arthritis. Sci. Rep. 2018, 8, 3684. [Google Scholar] [CrossRef] [PubMed]
- Trier, N.H.; Hansen, P.R.; Houen, G. Production and Characterization of Peptide Antibodies. Methods 2012, 56, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Hos, B.J.; Tondini, E.; van Kasteren, S.I.; Ossendorp, F. Approaches to Improve Chemically Defined Synthetic Peptide Vaccines. Front. Immunol. 2018, 9, 884. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.J.; Brown, L.E.; Kronin, V.; Jackson, D.C. The Geometry of Synthetic Peptide-Based Immunogens Affects the Efficiency of T Cell Stimulation by Professional Antigen-Presenting Cells. Int. Immunol. 2000, 12, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, K.S. Immune Peptide Enhancement of Peptide Based Vaccines. Front Biosci. 2005, 10, 478–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schott, M.E.; Wells, D.T.; Schlom, J.; Abrams, S.I. Comparison of Linear and Branched Peptide Forms (MAPs) in the Induction of T Helper Responses to Point-Mutated Ras Immunogens. Cell. Immunol. 1996, 174, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Moyle, P.M.; Toth, I. Self-Adjuvanting Lipopeptide Vaccines. Curr. Med. Chem. 2008, 15, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, C.; Fotou, E.; Moussis, V.; Ntoyhaniari, A.; Zografou, S.; Maltabe, V.; Kouklis, P.; Christoforidis, S.; Tsikaris, V. Intracellular Targets: A Multiple Cargo Transporting Molecule. J. Pept. Sci. 2021, 27, e3359. [Google Scholar] [CrossRef] [PubMed]
- Sakarellos-Daitsiotis, M.; Tsikaris, V.; Sakarellos, C. A New Circular Helicoid-Type Sequential Oligopeptide Carrier for Assembling Multiple Antigenic Peptides. In Self-Assembling Peptide Systems in Biology, Medicine and Engineering; Aggeli, A., Boden, N., Zhang, S., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 257–271. ISBN 978-0-306-46890-2. [Google Scholar]
- Alexopoulos, C.; Krikorian, D.; Panou-Pomonis, E.; Sakarellos-Daitsiotis, M.; Sakarellos, C. Innovative, Multifunctional Sequential Oligopeptide Carriers Soc n-I and SOC n-II: Functions-Technology-Perspectives. Protein Pept. Lett. 2005, 12, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Méndez, E.; Fuglsang-Madsen, A.; Føns, S.; Lomonte, B.; Gutiérrez, J.M.; Laustsen, A.H. Innovative Immunization Strategies for Antivenom Development. Toxins 2018, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Dolimbek, B.Z.; Atassi, M.Z. Protection against A-Bungarotoxin Poisoning by Immunization with Synthetic Toxin Peptides. Mol. Immunol. 1996, 33, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Curin Serbec, V.; Délot, E.; Faure, G.; Saliou, B.; Franc, G.; Bon’t, C.; Choumet’, V. Antipeptide Antibodies Directed to the C-Terminal Part of Ammodytoxin A React with the PLA2 Subunit of Crotoxin and Neutralize Its Pharmacological Activity. Toxicon 1994, 32, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.N.; Machado de Avila, R.A.; Sanchez, E.F.; Maria, W.S.; Molina, F.; Granier, C.; Chávez-Olórtegui, C. Antibodies against Synthetic Epitopes Inhibit the Enzymatic Activity of Mutalysin II, a Metalloproteinase from Bushmaster Snake Venom. Toxicon 2006, 48, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Chandrudu, S.; Simerska, P.; Toth, I. Chemical Methods for Peptide and Protein Production. Molecules 2013, 18, 4373–4388. [Google Scholar] [CrossRef] [PubMed]
- Albericio, F. (Ed.) Solid-Phase Synthesis: A Practical Guide, 1st ed.; CRC Press: New York, NY, USA, 2000. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).