Abstract
3-Ethoxycarbonyl-1,4-benzodioxin-2-carboxylic acid, a novel 2,3-disubstituted benzodioxin, was prepared from readily available 1,4-benzodioxin-2-carboxylic acid by lithiation at C(3) and a reaction with the electrophile ethyl chloroformate. The analytical characterization of the product was performed via IR, 1H-NMR, 13C-NMR, HRMS, and HPLC-UV. Due to the unsymmetrically disubstituted unsaturation, the obtained monoester of 1,4-benzodioxin-2,3-dicarboxylic acid is a building block of great potential in the synthesis of a variety of compounds containing the benzodioxin or benzodioxane scaffold.
1. Introduction
The 1,4-benzodioxane motif has wide application in a variety of compounds, many of which exert bioactivity and have therapeutic potential [1,2,3,4]. Two main structural features critically condition the interaction capabilities of 1,4-benzodioxane-based compounds with biological targets, namely the substitutions at dioxane C(2), resulting in chirality and, frequently, high eudismic ratios, [1] and decoration of the benzene ring, mainly involved in receptor subtype selectivity [5,6]. Whilst many methods, based on different approaches, have been developed to prepare 1,4-benzodioxanes 2-substituted [7] and/or substituted at the benzene ring [8,9,10], the 2,3-disubstitution has received less attention from the literature, possibly due to lower demand by medicinal chemists. In this respect, unsaturation between C(2) and C(3) can be synthetically strategic in order to access symmetrically and unsymmetrically 2,3-disubstituted 1,4-benzodioxins and hence 1,4-benzodioxanes. Such an approach, exploiting the presence of the 2,3-unsaturation, is exemplified by the preparation reported here, in which 1,4-benzodioxin-2-carboxylic acid (2) is converted into the unsymmetrically 2,3-disubstituted benzodioxin 3-ethoxycarbonyl-1,4-benzodioxin-2-carboxylic acid (3) (Figure 1) and (Scheme 1). The dicarboxylic acid monoester 3 is a synthon of great potential due to the presence of unsaturation and two substituents, both susceptible to many useful chemical transformations, which can be selectively performed thanks to the differentiating mono-esterification.
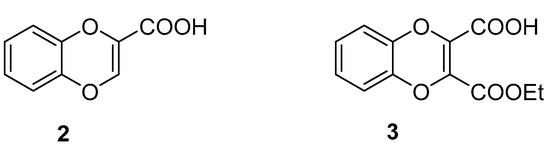
Figure 1.
Chemical structures of 1,4-benzodioxin-2-carboxylic acid and 3-ethoxycarbonyl-1,4-benzodioxin-2-carboxylic acid.
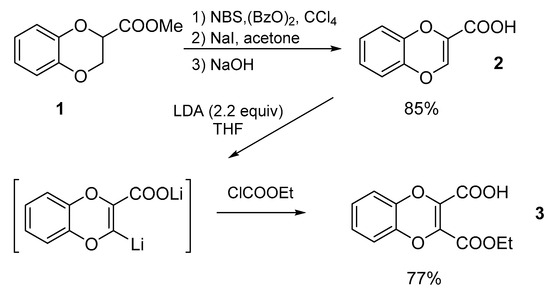
Scheme 1.
Synthesis of 3-ethoxycarbonyl-1,4-benzodioxin-2-carboxylic acid.
2. Results and Discussion
The starting α,β-unsaturated carboxylic acid 2 was easily prepared from readily accessible methyl ester of 1,4-benzodioxan-2-carboxylate (1) [11] with an almost quantitative yield according to previously reported procedures involving, in sequence, 2,3-dibromination with NBS, debromination to methyl 1,4-benzodioxin-2-carboxylate with NaI in acetone, and ester saponification [11,12].
To convert 2 into the 2,3-dicarboxylic acid monoester 3, we exploited the possibility of regioselective dioxin ring metalation by reaction with lithium diisopropylamide (LDA) to give the bis-anionic intermediate species lithium 3-lithio-1,4—benzodioxin-2-carboxylate, which reacts well with the electrophile ethyl chloroformate providing 3. In the literature, the lithiation of oxygenated heterocycles, followed by condensation with an electrophile, was first described for furan, 3-furoic acid, and 2-furoic acid which gave the nucleophiles 2-furyllithium, lithium 2-lithio-3-furoate, and lithium 5-lithio-2-furoate, respectively [13,14]. Shortly thereafter, the procedure was successfully applied to 2, and the resulting dianion condensed with several carbonyl compounds [15]. On the other hand, the use of ethyl chloroformate is reported in the ethoxycarbonylation of a wide variety of nucleophyles [16]. In our case, the lithiation/ethoxycarbonylation of 2, performed in THF at −78 °C under anhydrous conditions, provided 3, after standard work-up and purification by flash chromatography, with a high 77% yield.
The novel benzodioxindicarboxylic acid monoester was characterized by IR and NMR spectroscopy and high-resolution mass spectroscopy. The 1HNMR showed the disappearance of the singlet of the starting material, located at 7.09 ppm and ascribable to the olefinic hydrogen of 2, and the appearance of the signals of the ethyl ester group, due to the ethoxycarbonylation of the benzodioxin C(3), namely, a quartet at 4.45 and a triplet at 1.43. The 13CNMR showed twelve singlets in agreement with the number of unique carbon environments. The high-resolution mass spectrum confirmed the identity of the product.
3. Materials and Methods
1,4-benzodioxin-2-carboxylic acid (2) was prepared from methyl 1,4-benzodioxan-2-carboxylate (1), in turn obtained from methyl 2,3-dibromopropionate and catechol as previously reported [11,12]. Methyl 2,3-dibromopropionate and catechol were purchased from Sigma-Aldrich S.A. (St. Louis, MO, USA). ATR-FT-IR spectra were acquired with a Perkin Elmer Spectrum One FT-IR (Perkin Elmer, Waltham, MA, USA), equipped with a Perkin Elmer Universal ATR sampling accessory consisting of a diamond crystal. The 1H and 13C NMR spectra were measured on a Varian Mercury 300 FT-NMR spectrometer operating at 300 MHz for 1H and 75 MHz for 13C. NMR data were recorded in CDCl3 at 25 °C, with chemical shifts reported in parts per million (ppm) and coupling constants J in Hertz. Melting points were recorded with a Buchi B-540 apparatus (Flawil, Switzerland). An HRMS analysis was performed on a Sciex QTOF X500b system. The purity of the title compound was assessed by HPLC-UV analysis using an HP1050 system. A chromatographic run was performed with a Vision HT C18 Classic column (particle size 5 μm, 250 × 4.6 mm dimensions) using a solvent gradient elution (flux rate 1.5 mL/min) with a mobile phase consisting of A = 0.1% TFA in water and B = 0.1% TFA in acetonitrile; the linear gradient started with a 30% B and rose to 50% in 9 min. The detection wavelength was set to 280 nm.
3-Ethoxycarbonyl-1,4-benzodioxin-2-carboxylic Acid (3)
A stirred solution of 2 (4 g, 22.45 mmol) in anhydrous THF (40 mL) was cooled at −78 °C under an inert atmosphere. Then, a 2 M solution of LDA in THF (24.7 mL, 49.39 mmol) was slowly added, and the obtained solution was stirred for 4 h at −78 °C. Ethyl chloroformate (2.6 mL, 26.94 mmol) was added, and the mixture was stirred for an additional 4 h at −78 °C, then warmed to room temperature and added to a saturated solution of NH4Cl (30 mL). The suspension was extracted with diethyl ether (3 × 40 mL), and the collected organic layers were dried over sodium sulphate and filtered and concentrated under vacuum. The resulting crude was purified by flash chromatography on silica gel (gradient elution: from 9/1 to 8/2 DCM/MeOH) to give 3 as a white solid (4.30 g, 77%): m.p. 100 °C; Rf = 0.45 (8/2 DCM/MeOH); 1H NMR (300 MHz, CDCl3) δ 6.94 (m, 3H), 6.81 (m, 1H), 4.47 (q, J = 7.1 Hz, 2H), 1.45 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, CDCl3) ppm 163,38, 160.97, 141.11, 140.86, 135.96, 134.68, 125.95, 125.68, 116.86, 116.35, 64.09, 13.87. FTIR (ATR): ν = 3420, 2985, 1742, 1666 cm−1. HRMS (ESI-) m/z calcd. for C12H9O6 [M-H]− 249.0405, found 249.0403. HPLC-UV purity: 94.02%.
4. Conclusions
In summary, we have developed an efficient and simple procedure to prepare 3-ethoxycarbonyl-1,4-benzodioxin-2-carboxylic acid with high yield from the readily available 1,4-benzodioxin-2-carboxylic acid. The title compound is an intermediate of great interest because the two substituents are susceptible to selective transformations, paving the way to other 2,3-substituted benzodioxin and benzodioxane derivatives and benzodioxins and benzodioxanes 2,3-fused with lactones, anhydrides, and lactams.
Supplementary Materials
The following supporting information can be downloaded online: IR, 1H NMR and 13C NMR spectra of 3, HRMS spectrum of 3.
Author Contributions
Conceptualization, C.B.; methodology, E.A.; investigation, E.A., A.G. and C.M.; writing—review and editing, M.P.; supervision, C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data for the compound presented in this study are available in Supplementary Materials of this article.
Acknowledgments
The authors acknowledge support from the University of Milan.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bolchi, C.; Bavo, F.; Appiani, R.; Roda, G.; Pallavicini, M. 1,4-Benzodioxane, an evergreen, versatile scaffold in medicinal chemistry: A review of its recent applications in drug design. Eur. J. Med. Chem. 2020, 200, 112419. [Google Scholar] [CrossRef] [PubMed]
- Muszak, D.; Surmiak, E.; Plewka, J.; Magiera-Mularz, K.; Kocik-Krol, J.; Musielak, B.; Sala, D.; Kitel, R.; Stec, M.; Weglarczyk, K.; et al. Terphenyl-based small-molecule inhibitors of programmed cell death-1/programmed death-ligand 1 protein-protein interaction. J. Med. Chem. 2021, 64, 11614–11636. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Vibhute, S.; Li, L.; Okumu, A.; Ratigan, S.C.; Nolan, S.; Papa, J.L.; Mann, C.A.; English, A.; Chen, A.; et al. Optimization of TopoIV potency, ADMET properties, and hERG inhibition of 5-amino-1,3-dioxane-linked novel bacterial topoisomerase inhibitors: Identification of a lead with in vivo efficacy against MRSA. J. Med. Chem. 2021, 64, 15214–15249. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, A.E.; Sharp, S.Y.; Chessum, N.E.A.; Hayes, A.; Pellegrino, L.; Tucker, M.J.; Miah, A.; Wilding, B.; Evans, L.E.; Rye, C.S.; et al. HSF1pahway inhibitor clinical candidate (CCT361814/NXP800) developed from a phenotypic screen as a potential treatment for refractory ovarian cancer and other malignancies. J. Med. Chem. 2023, 66, 5907–5936. [Google Scholar] [CrossRef] [PubMed]
- Bavo, F.; Pallavicini, M.; Gotti, C.; Appiani, R.; Moretti, M.; Colombo, S.F.; Pucci, S.; Viani, P.; Budriesi, R.; Renzi, M.; et al. Modifications at C(5) of 2-(2-pyrrolidinyl)-substituted 1,4-benzodioxane elicit potent α4β2 nicotinic acetylcholine receptor partial agonism with high selectibity over the α3β4 subtype. J. Med. Chem. 2020, 63, 15668–15692. [Google Scholar] [CrossRef] [PubMed]
- Bavo, F.; Pallavicini, M.; Appiani, R.; Bolchi, C. Determinants for α4β2 vs. α3β4 subtype selectivity of pyrrolidine-based nAChRs ligands: A computational perspective with focus on recent cryo-EM receptor structures. Molecules 2021, 26, 3603. [Google Scholar] [CrossRef] [PubMed]
- Bolchi, C.; Valoti, E.; Straniero, V.; Ruggeri, P.; Pallavicini, M. From 2-aminomenthyl-1,4-benzodioxane enantiomers to unichiral 2-cyano- and 2-carbonyl-substituted benzodioxanes via dichloroamine. J. Org. Chem. 2014, 79, 6732–6737. [Google Scholar] [CrossRef] [PubMed]
- Bouissane, L.; Khouili, M.; Coudert, G.; Pujol, M.D.; Guillaumet, G. New and promising type of leukotriene B4 (LTB4) antagonists based on the 1,4-benzodioxine structure. Eur. J. Med. Chem. 2023, 254, 115332. [Google Scholar] [CrossRef] [PubMed]
- Armano, E.; Giraudo, A.; Pallavicini, M.; Bolchi, C. Methyl 8- and 5-bromo-1,4-benzodioxane-2-carboxylate: Unambiguous identification of the two regioisomers. Molbank 2023, 2023, M1623. [Google Scholar] [CrossRef]
- Armano, E.; Giraudo, A.; Morano, C.; Pallavicini, M.; Bolchi, C. Methyl 8- and 5-nitro-1,4-benzodioxane-2-carboxylate. Molbank 2023, 2023, M1661. [Google Scholar] [CrossRef]
- Yin, X.; Huang, Y.; Chen, Z.; Hu, Y.; Tao, L.; Zhao, Q.; Dong, X.Q.; Zhang, X. Enantioselective access to chiral 2-substituted 2,3-dihydrobenzo[1,4]dioxane derivatives through Rh-catalyzed asymmetric hydrogenation. Org. Lett. 2018, 20, 4173–4177. [Google Scholar] [CrossRef] [PubMed]
- Lalloz, L.; Loppinet, V.; Coudert, G.; Guillaumet, G.; Loubinoux, B.; Labrid, C.; Beaughard, M.; Dureng, G.; Lamar, J.C. 2-Benzodioxinylaminoethanols: A new class of β-adrenergic blocking and antihypertensive agents. J. Med. Chem. 1981, 24, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, V.; Levine, R. Some reactions of 2-furyllithium. J. Org. Chem. 1962, 27, 1216–1219. [Google Scholar] [CrossRef]
- Knight, D.W. Formation and reactivity of bis-anions derived from furoic acids. Tetrahedron Lett. 1979, 20, 469–472. [Google Scholar] [CrossRef]
- Guillaumet, G.; Coudert, G.; Loubinoux, B. Synthese de benzodioxines-1,4 disubstituees sur l’heterocycle. Tetrahedron Lett. 1979, 20, 4379–4382. [Google Scholar] [CrossRef]
- Pearson, A.J.; Roush, W.R. Activating Agents and Protecting Groups; John Wiley & Sons: Chichester, UK, 1999; pp. 183–184. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).