Abstract
The structure verification of 5′-oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione by NMR is reported. Toward this aim, 2D NMR techniques including 1H-1H COSY, HMQC, and HMBC experiments were used to assist with the assignment of the 1H and 13C chemical shifts for the corresponding structure. The mutual interpretation of the 1D and 2D NMR spectra ensured a complete and accurate 1H and 13C NMR data assignment for 5′-oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione.
1. Introduction
The diverse bioactivity of spirohydantoins has a significant role in numerous practical applications. The lipase catalysis of 5,5-spirohydantoins is often responsible for the production of specific amino acids that benefit the synthesis of mGluR agonists [1,2] and phosphotyrosyl mimetics [3] as well as an increase in the drug transport efficiency through lipophilic membranes [4]. In addition, 1-substituted 5-spirohydantoins have been found to be suitable for the treatment of tropical diseases [5] whereas some cyclopropanespirohydantoin derivatives have been tested as anticonvulsant agents [6,7]. The synthesis of spirohydantoins participates in the production of the drug sorbinil, which is used to treat chronic complications of diabetes mellitus [8]. Moreover, spiro[imidazolidine-4,3′-indole]2,2′,5′-(1H)-trione has been found to inhibit the function of the vanilloid receptor (VR1), which is needed in cases of pain treatment [9]. Another important property of the spirohydantoins is their complexation ability. Previously, 3-thiolanespiro-5′-hydantoin and 4-thio-1H-tetrahydropyranespiro-5′-hydantoin were used as ligands to form Pt(II) and Pt(IV) complexes [10]. Furthermore, the potential of 5′-oxospiro-(fluorene-9,4′-imidazolidine)-2′-one and 5′-oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione to participate as ligands in Pt(II) [11] and Cu(II) [12] complexes has been tested. The crystal structure and bioactivity of the 2′-thio derivative has also been additionally studied [13]. However, the structure of 5′-oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione was previously only supported by 1H NMR, 13C NMR, ATR, and Raman data [11]. Therefore, the aim of the current work was the structure verification of 5′-oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione (Figure 1) by using a combination of 1D (1H, 13C, DEPT 135) and 2D NMR techniques (1H-1H COSY, HMQC, HMBC). Thus, a complete and accurate assignment of the 1H and 13C chemical shifts were provided, increasing the degree of confidence with which the structure of 5′-oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione was verified.
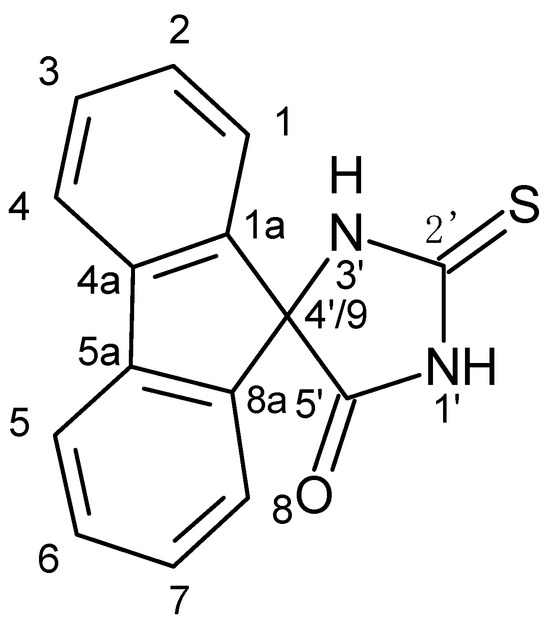
Figure 1.
The structure of 5′-oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione.
2. Results
The molecular formula of 5′-oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione is C15H10N2OS. There were nine signals in the 13C NMR spectrum as six of them corresponded to six pairs of chemically equivalent carbons (Table 1), respectively. The two 13C NMR signals with the highest chemical shifts, δC 183.60 ppm and δC 174.80 ppm, were assigned to the thiocarbonyl and carbonyl carbon atoms, C2′=S and C5′=O, respectively. The signal at δC 74.84 ppm was for the spiro-carbon, C-4′/9.

Table 1.
1H and 13C NMR spectral data and 1H–1H COSY and HMBC correlations for 5′-oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione [600.130 MHz (1H) and 150.903 MHz (13C)] a.
The 1H NMR spectrum showed two singlets at δH 12.39 ppm and δH 10.64 ppm that were assigned to the NH protons. The fact that there were no HMQC correlations for the signals at δH 12.39 ppm and δH 10.64 ppm indicated that there were protons that were not bonded to carbons. The proton in the N1′H group was located between the thiocarbonyl and carbonyl groups, C2′=S and C5′=O, therefore, it would be more strongly deshielded by their magnetic anisotropy [14,15,16]. Consequently, the singlet at δH 12.39 ppm was assigned to the N1′H proton while the signal at δH 10.64 ppm was for the N3′H proton. There were three HMBC correlations for each of the imido (N1′H) and amide (N3′H) protons (δH 10.64 ppm–δC 174.80 ppm), (δH 10.64 ppm–δC 183.60 ppm), (δH 10.64 ppm–δC 74.84 ppm) for the N3′H proton, and (δH 12.39 ppm–δC 174.80 ppm), (δH 12.39 ppm–δC 183.60 ppm), (δH 12.39 ppm–δC 74.84 ppm) for the N1′H proton. Additionally, one COSY correlation was found between the NH protons.
HMBC correlations of the H-1/8, H-2/7, H-3/6, and H-4/5 protons with the spiro-carbon, C-4′/9, were found. In this case, the strongest HMBC correlation included the chemical shift of the H-1/8 protons, (δH 7.42 ppm–δC 74.84 ppm), which were only three bonds away from C-4′/9. Additionally, there was an extremely weak HMBC correlation (δH 7.42 ppm–δC 174.80) indicating the 4-bond coupling of H-1/8 with the carbonyl carbon, C-5′. The other protons H-2/7, H-3/6, and H-4/5 were at a greater bond distance from C-5′, thus, it was very unlikely to find such an HMBC correlation for them.
Due to the fact that the H-3/6 protons were farther from the spiro-carbon, C-4′/9, than the other protons, H-1/8, H-2/7, and H-4/5, the weakest HMBC correlation (δH 7.52 ppm–δC 74.84 ppm) was most probably a result of the coupling of H-3/6 with C-4′/9. The HMBC correlation (δH 7.39 ppm–δC 74.84 ppm) indicated that δH 7.39 ppm corresponded to the H-2/7 protons that were closer to C-4′/9 than H-3/6. Thus, the weak HMBC correlation (δH 7.93 ppm–δC 74.84 ppm) indicated the 4-bond coupling of the H-4/5 protons with C-4′/9. The HMQC spectrum showed four correlations—(δH 7.42 ppm–δC 123.76 ppm), (δH 7.39 ppm–δC 128.64 ppm), (δH 7.52 ppm–δC 130.27 ppm) and (δH 7.93 ppm–δC 121.07 ppm)—thus, the signals at 121.07 ppm, 123.76 ppm, 128.64 ppm, and 130.27 ppm were assigned to the carbons C-4/5, C-1/8, C-2/7, and C-3/6, respectively. In support of these assignments, there were only four signals in the DEPT 135 spectrum that could be found correspondingly at 121.07 ppm, 123.76 ppm, 128.64 ppm, and 130.27 ppm. There were two COSY correlations found for each of the proton pairs, H-2/7 and H-3/6, indicating the vicinal coupling (3JHH) with their respective neighboring protons in each benzene ring.
The chemical shifts of the quaternary carbons, C-1a/8a and C-4a/5a, were determined following the fact that the meta (vicinal) coupling (3JCH) in benzene rings is usually resolved [14, page 27]. The lack of HMQC correlations involving the signals at δc 140.79 ppm and δc 141.40 ppm as well as the fact that there were no signals at δc 140.79 ppm and δc 141.40 ppm in the DEPT 135 spectrum indicated that these shifts probably correspond to quaternary carbons. Based on the following HMBC correlations—(δH 7.42 ppm–δC 140.79 ppm), (δH 7.52–δc 140.79 ppm), (δH 7.39 ppm–δC 141.40 ppm) and (δH 7.93 ppm–δC 141.40 ppm)—the chemical shifts δc 140.79 ppm and δc 141.40 ppm were assigned correspondingly to the signals of C-4a/5a and C-1a/8a, respectively.
3. Materials and Methods
All chemicals used were purchased from Merck (Darmstadt, Germany) and Sigma-Aldrich (Burlington, MA, USA). A Koffler apparatus was used to determine the melting point of the compound. Thin layer chromatography was performed on Kieselgel 60 F254, 0.2 mm Merck plates, utilizing the following eluent systems (vol/vol ratio): (a) chloroform:acetone = 9:1 and (b) ethylacetate:petroleum ether = 1:5, in order to determine the purity of the compound. The synthesis of 5′-oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione was based on the one-hour reflux of a mixture containing 0.70 g (0.0023 mol) of 4-(2-hydroxyethylimino)-(9′-fluorene)-spiro-5-(2-thiohydantoin) and 8 mL 20% hydrochloric acid.ur. The product was cooled and after 24 h, it was filtered off and recrystallized from hot water. Yield: 0.5 g (83%); m. p. 302–303 °C; Rfa = 0.63; Rfb = 0.11. A VERTEX 70 spectrometer (Bruker Optics, Billerica, MA, USA) was used to measure the ATR spectrum of 5′-oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione in the range from 4000 to 600 cm−1 at a resolution of 2 cm−1 with 25 scans. MIRacleTM with one-reflection ZnSe element (Pike) was utilized as an ATR accessory while the stirred crystals were pressed by an anvil to the reflection element. RAM II (Bruker Optics) with a focused laser beam of 500 mW power of Nd:YAG laser (1064 nm) was applied to register the Raman spectrum of the compound (the stirred crystals were placed in an aluminum disc) in the range from 4000 to 100 cm−1 at a resolution of 2 cm−1 with 25 scans. ATR (υmax, cm−1): 3244 (sh., υNH), 3153 (υNH), 3091 (υCH), 2909, 1751, 1729 (υCO), 1606, 1530, 1503, 1475, 1449, 1401, 1374, 1288, 1249 (υCS), 1191, 1167, 1150, 1110, 1072, 1006, 944, 923, 879, 866, 793, 775, 756, 740, 733, 724, 711, 675, 657, 636, 625, 619. Raman (υmax, cm−1): 3067 (υCH), 3050 (υCH), 1728 (υCO), 1624, 1607, 1486, 1448, 1359, 1296, 1233 (υCS), 1211, 1157, 1151, 1113, 1074, 1022, 1006, 942, 786, 760, 743, 726, 675, 624, 537, 515, 475, 418, 362, 296, 271, 254, 165, 115. A Bruker Avance II + 600 MHz NMR spectrometer working with a frequencies of 600.130 MHz (1H) and 150.903 MHz (13C) was utilized to register the 1H, 13C, and 2D NMR spectra of 5′-oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione. TMS and DMSO-d6 were used as the internal standard and solvent, respectively. All NMR measurements were conducted at ambient temperature (293.0 K). Chemical shifts were expressed in ppm and coupling constants (J) in Hertz. 1D and 2D NMR spectra were recorded using the standard Bruker pulse programs.
4. Conclusions
The structure of 5′-oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione was completely verified by using a set of 1D and 2D NMR experiments. The interpretation of the 1H-1H COSY, HMQC, and HMBC spectra assisted with the assignment of the 1H and 13C chemical shifts, which additionally enhanced the reliability of the performed structure verification.
Supplementary Materials
Figure S1: 1H NMR. Spectrometer frequency is 600.13 MHz. DMSO-d6 is the used solvent; Figure S2: 13C NMR.Spectrometer frequency is 150.90 MHz. DMSO-d6 is the used solvent; Figure S3: DEPT 135. Spectrometer frequency is 150.90 MHz. DMSO-d6 is the used solvent; Figure S4: HMQC. The horizontal and vertical traces show 1H NMR and DEPT 135 spectra, respectively. Spectrometer frequencies for 1H NMR and DEPT 135 spectra are correspondingly 600.13 MHz and 150.90 MHz. DMSO-d6 is the used solvent; Figure S5: 1H-1H COSY. The horizontal and vertical traces show the 1H NMR spectrum, respectively. Spectrometer frequency for 1H NMR is correspondingly 600.13 MHz. DMSO-d6 is the used solvent; Figure S6: Expansion of 1H-1H COSY spectrum, showing the 1H-1H COSY correlations of the protons H-1/8, H-2/7, H-3/6 and H-4/5; Figure S7: HMBC spectrum. The horizontal and vertical traces show 1H and 13C NMR spectra, respectively. Spectrometer frequencies for 1H and 13C NMR are correspondingly 600.13 MHz and 150.90 MHz. DMSO-d6 is the used solvent; Figure S8: Expansion of the HMBC spectrum, showing the HMBC correlations of both NH protons; Figure S9: Expansion of the HMBC spectrum, showing the HMBC correlations of the protons H-1/8, H-2/7, H-3/6 and H-4/5; Figure S10: ATR; Figure S11: Raman.
Author Contributions
Conceptualization, M.M. and P.P.; Formal analysis, D.S.; Writing—original draft preparation, D.S.; Writing—review and editing, M.M., P.P., N.S., D.S. and P.M.; Supervision, P.P. and M.M.; Funding acquisition, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fund for Scientific Research of Plovdiv University, project CП 23-XФ-006.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lee, W.; Miller, M.J. Concise Synthesis of 4-Acylamino Analogues of 2-Aminobicyclo[3.1.0]hexane-2,6-dicarboxylic Acids (LY354740) from an Acylnitroso Diels-Alder Cycloadduct. J. Org. Chem. 2004, 69, 4516–4519. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, K.; Kumagai, T.; Taguchi, T.; Nakazato, A. Scalable Synthesis of (+)-2-Amino-3-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic Acid as a Potent and Selective Group II Metabotropic Glutamate Receptor Agonist. Chem. Pharm. Bull. 2007, 55, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Oishi, S.; Kang, S.-U.; Liu, H.; Zhang, M.; Yang, D.; Deschamps, J.R.; Burke, T.R. Synthesis of α,α-Disubstituted 4-Phosphonophenylalanine Analogs as Conformationally-constrained Phosphotyrosyl Mimetics. Tetrahedron 2004, 60, 2971–2977. [Google Scholar] [CrossRef]
- Martins, F.J.C.; van der Hoven, H.; Viljoen, A.M. Synthesis of Exo-3-amino-10-hydroxy-hexacyclo[10.2.1.02,11.04,10.04,14.09,13]-pentadecane-5,7-diene-endo-3-carboxyclic Acid and Endo-3-amino-10-hydroxy-hexacyclo[10.2.1.02,11.04,10.04,14.09,13]pentadecane-5,7-dieneexo-3-carboxylic Acid. Tetrahedron 2009, 65, 2921–2926. [Google Scholar] [CrossRef]
- Goebel, T.; Ulmer, D.; Projahn, H.; Kloeckner, J.; Heller, E.; Glaser, M.; Ponte-Sucre, A.; Specht, S.; Sarite, S.R.; Hoerauf, A.; et al. In Search of Novel Agents for Therapy of Tropical Diseases and Human Immunodeficiency Virus. J. Med. Chem. 2008, 51, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lou, J.; Wang, Z.; Wang, T.; Xiao, Y.; Hu, X.; Liu, P.; Hong, X. Design, Synthesis and Pharmacological Evaluation of Novel N-(2-(1,1-Dimethyl-5,7-Dioxo-4,6-diazaspiro[2.4]heptan-6-yl)ethyl) Sulfonamide Derivatives as Potential Anticonvulsant Agents. Eur. J. Med. Chem. 2015, 92, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Pan, Y.; Xu, Z.; Li, R.; Qiu, G.; Xu, W.; Ke, X.; Wu, L.; Hu, X. Synthesis and Potential Anticonvulsant Activity of New N-3- Substituted 5,5-Cyclopropanespirohydantoins. Eur. J. Med. Chem. 2009, 44, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Suzuki, M.; Kanai, M.; Shibasaki, M. General and Practical Catalytic Enantioselective Strecker Reaction of Keto-imines: Significant Improvement through Catalyst Tuning by Protic Additives. Tetrahedron Lett. 2004, 45, 3147–3151. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Zhou, J. Organocatalytic Asymmetric Cyanation of Isatin Derived N-Boc ketoimines. Chem. Commun. 2013, 49, 4421–4423. [Google Scholar] [CrossRef] [PubMed]
- Bakalova, A.; Buyukliev, R.; Momekov, G. Synthesis, DFT Calculations and Cytotoxic Investigation of Platinum Complexes with 3-Thiolanespiro-5′-hydantoin and 4-Thio-1H-tetrahydropyranespiro-5′-hydantoin. J. Mol. Struct. 2015, 1091, 118–124. [Google Scholar] [CrossRef]
- Marinova, P.; Marinov, M.; Kazakova, M.; Feodorova, Y.; Penchev, P.; Sarafian, V.; Stoyanov, N. Synthesis and in vitro activity of platinum(II) complexes of two fluorenylspirohydantoins against a human tumour cell line. Biotechnol. Biotechnol. Equip. 2014, 28, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Ahmedova, A.; Marinova, P.; Marinov, M.; Stoyanov, N. An integrated experimental and quantum chemical study on the complexation properties of (9′-fluorene)-spiro-5-hydantoin and its thioanalogue. J. Mol. Struct. 2016, 1108, 602–610. [Google Scholar] [CrossRef]
- Marinova, P.; Marinov, M.; Kazakova, M.; Feodorova, Y.; Blazheva, D.; Slavchev, A.; Sbirkova-Dimitrova, H.; Sarafian, V.; Stoyanov, N. Crystal Structure of 5′-Oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione and Biological Activity of Its Derivatives. Russ. J. Gen. Chem. 2021, 91, 939–946. [Google Scholar] [CrossRef]
- Breitmaier, E. Structure Elucidation by NMR in Organic Chemistry: A Practical Guide, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2002. [Google Scholar]
- Kleinpeter, E. Quantification and Visualization of the Anisotropy Effect in NMR Spectroscopy by Through-Space NMR Shieldings. Annu. Rep. NMR Spectrosc. 2014, 82, 115–166. [Google Scholar]
- Nguyen, T.K.P.; Nguyen, K.P.P.; Kamounah, F.S.; Zhang, W.; Hansen, P.E. NMR of a series of novel hydroxyflavothiones. Magn. Reson. Chem. 2009, 47, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).