Abstract
The reaction of berberine derivatives containing at the O-9 position N,N-disubstituted acetamide fragments with sodium borohydride in methanol at 0 °C leads to a mild reduction of the “C” cycle with the formation of corresponding tetrahydroberberine derivatives with moderate to good yields.
1. Introduction
The isoquinoline alkaloid berberine (berberine chloride, sulfate) is known to have a wide range of diverse biological activities. Currently, research on its hypolipidaemic [1,2,3], anti-inflammatory and antioxidant [4,5], and anti-cancer [6,7] activities is being actively conducted. Works on such types of berberine activity as anti-epileptic [8,9], antidepressant [10,11], and antiallergic [12] are being developed. Berberine chloride contains in its structure an aromatic positively charged nitrogen atom; such a salt has low solubility and, as a result, low bioavailability. In order to increase the bioavailability of berberine, its water-soluble compositions are being developed [13], and complexes of berberine with Ag or Au nanoparticles [14,15], natural polymers such as chitosan [16,17], peptides [18], or hyaluronic acid [19] are used. A number of berberine derivatives have been found with activities exceeding that of the initial alkaloid, such as hypolipidemic [20], hypoglycemic [21], antibacterial [22], and antiviral [23].
The most common modification of berberine chloride involves its demethylation at the O-9 position to form the alkaloid berberubine 1, and further obtaining derivatives at this position by means of alkylation or acylation [20,24], O-9-arylation [25], or C-9 arylation [26]. Thus, according to this scheme, we previously synthesized aromatic acetamides 2 by reacting berberubine 1 with bromoacetic acid amides in the presence of a base (Scheme 1) [27,28]. A separate direction of berberine modification is the synthetic production of berberine-like molecules [29]. Another popular branch of the chemical modification of berberine is the reduction of its isoquinolinium system. The resulting dihydro or tetrahydro derivatives are less stable than the original berberines, but have much greater solubility and often have good bioactivity. Tetrahydroberberine itself has a pronounced lipid-lowering effect [30], although there is some evidence of hepatotoxicity [31]. Among tetrahydroberberine derivatives, examples with good lipid-lowering [32,33,34], antiproliferative [35], and antibacterial (antifungal) activity [36] were found. Unusual examples of activity have also been found. For example, we have shown that tetrahydroberberrubine polyfluoroaromatic sulfonates are inhibitors of tyrosyl-DNA phosphodiesterase 1 (Tdp1), an important enzyme of the DNA repair system [37].
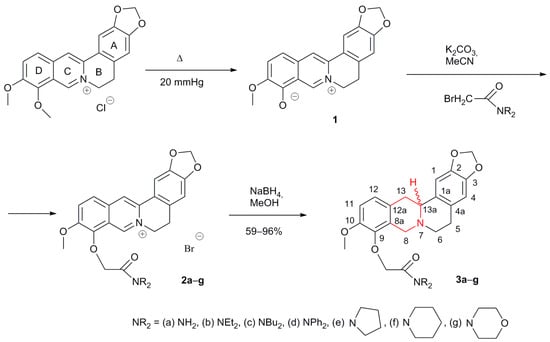
Scheme 1.
Synthesis of tetrahydroberberine N,N-derived O-acetamides 3.
The aim of the present work was to synthesize new N,N-disubstituted O-acetamide derivatives of tetrahydroberberine.
2. Results and Discussion
2.1. Synthesis of 3
The synthesis of acetamides 2 by the substitution of berberubine 1 with seven bromoacetamides in the presence of potassium carbonate was performed by our group (Scheme 1) [27,28]. Compound 2 contains in its structure an aromatic heterocyclic ring (cycle “C”) in the isoquinolinium system, which is reduced by the action of various reducing agents to form dihydro or tetrahydro derivatives. We have shown that the reaction of compounds 2a–g with 4 mol equivalents of sodium borohydride in methanol at 0 °C resulted in a mild reduction of the “C” cycle to produce tetrahydroberberine derivatives of 3a–g (Scheme 1). The reaction conditions used are similar to those in which our group previously carried out the reduction of compound 1 and some of its derivatives [32]. The reduction proceeds non-stereoselectively, and the products are a mixture of diastereoisomers according to position 13a. The products were purified using column chromatography, followed by hexane re-precipitation from isopropyl alcohol. The best yields were achieved for compounds containing a dibutylamide fragment (3c, 96%), a diethylamide fragment (3b, 87%), and a piperidinamide fragment (3f, 80%). The yields of the remaining compounds 3a,d,e were 59–66%, which is probably due to their greater solubility in the hexane–isopropyl alcohol system. To the best of our knowledge, compounds 3a–g have not been previously described in the literature.
2.2. Spectral Data of 3
The structures of amides 3 were characterized using spectral data. IR spectra exhibited vibrations in the range 1645–1698 cm−1 that corresponded to the vibrational frequency of tertiary amides. Mass spectra contained peaks (m/z) corresponding to the [M-H]+ positively charged molecular fragment. Among the fragmentation peaks, there is a fragment with m/z 324.1, which corresponds to the tetrahydroberberrubine cation-radical C19H18NO4, [M-H]+.
The 1H NMR and 13C NMR spectra of compound 3 showed characteristic resonances for the tetrahydroberberine skeleton. In order to analyze these resonances, we used the example of compound 3b for which standard one-dimensional and two-dimensional NMR experiments (COSY, NOESY, HSQC, HMBC) were recorded. When considering the 1H–13C heteronuclear correlation (HSQC) spectrum (Figure S8 in Supplementary Materials), we determined the correspondence of signals from carbon atoms and protons. Thus, a multiplet signal from the proton at δH 3.46–3.52 ppm corresponds to the signal at δC 59.32 ppm (C13a), multiplet signals at δH 2.73–2.81 and 3.12–3.20 ppm (H13) correspond to the signal at δC 36.09 ppm (C13), and doublet signals at δH 3.55 and 4.29 ppm (H8) correspond to the signal at δC 53.62 ppm (C8), which is typical for the signals of ring C in tetrahydroberberine systems.
The 1H NMR spectra of 3 exhibited resonances for methylene protons of OCH2CON as an AB system with chemical shifts δH 4.20–4.80 ppm. The resonances of chemically identical protons of the alkyl substituents in the amide were nonequivalent. This was indicative of the hindered rotation that is characteristic of tertiary amides. The resonances of the carbon atoms of alkyl substituents in the amide, e.g., dibutylamide 3c, were also nonequivalent in the 13C NMR spectra (Figure S12 in Supplementary Materials). The corresponding chemical shifts were δC 45.38 and 46.76 ppm (NCH2), 29.57 and 31.03 ppm (NCH2CH2CH2CH3), 19.92 and 20.09 ppm (NCH2CH2CH2CH3), and 13.67 and 13.74 ppm (NCH2CH2CH2CH3). This was consistent with data in the literature for analogous amides [28,38].
3. Materials and Methods
3.1. General
Berberine chloride hydrate was purchased from TCI company (Tokyo, Japan), and the basic substance content was 81%. Commercially available organic and inorganic chemicals (reagent grade) from Khimservis Company (Staraya Kupavna, Moscow Oblast, Russia) were used without additional purification. Solvents from Khimservis Company (Staraya Kupavna, Moscow Oblast, Russia) were distilled prior to use. Column chromatography was performed on silica gel manufactured by Macherey-Nagel, fraction 63–200 μm. Berberrubine 1 was synthesized as the solvate with one EtOH molecule according to the procedure in the literature [27]. Berberrubine acetamides bromides 2a–g were prepared following a previously reported procedure [27,28].
3.2. Instrumentation and Analysis
The spectral and analytical studies of the products were carried out at the Multi-access Chemical Service Center of the Siberian Branch of the Russian Academy of Sciences. The UV spectra were recorded on a HP 8453 UV–Vis spectrophotometer in EtOH (c = 10−4 mol/L). The IR spectra were measured on a Vector 22 FTIR spectrometer in KBr pellets. Melting points (mp) were obtained with a Metler Toledo FP 900 instrument and Kofler stage. Elemental analyses were from Carlo Erba 1106. High-resolution mass spectra were obtained on a DFS-Thermo-Scientific spectrometer in a full scan mode (15–500 m/z, 70 eV electron-impact ionization, direct sample introduction). HPLC analyses were carried out on a Econova (Novosibirsk, Russia) “Milichrome A-02” HPLC system using ProntoSIL-120-5-C18AQ reversed-phase sorbent (particle size 5 μm, column 75 × 2 mm) at 35 °C, 3.0–3.6 MPa, and a flow rate of 150 μL/min with elution by a linear gradient of solvents from 100% A to 100% B over 25 min (solvent A, 0.1% TFA in H2O; solvent B, MeOH) and simultaneous multiwave detection at six wavelengths (220, 240, 260, 280, 320, and 360 nm). The 1H and 13C NMR spectra of 5–10% solutions of compounds in CDCl3 or DMSO-d6 were recorded on Bruker (Billerica, MA, USA) AV-400, DRX-500, and AV-600 spectrometers. Solvent signals (δH 7.24 and δC 76.90 ppm for CDCl3 or δH 2.50 and δC 39.52 ppm for DMSO-d6) were used as internal references. The numbering of carbon and hydrogen atoms in the spectra of compounds is shown on Scheme 1 and Figure S2. The assignments of the signals in the 1H and 13C NMR spectra marked with an asterisk (or a double asterisk) can be interchanged.
3.3. General Procedure for Tetrahydroderivative 3a–g Synthesis
At a temperature of 0 °C and stirring on a magnetic stirrer, 4 equivalents of sodium borohydride were added portion by portion to a suspension of 0.6–1.5 mmol (1 eq.) of a derivative of berberine bromide 2 in 10 mL of methanol. The mixture was stirred for 30 min while cooling and then for 4 h at room temperature until the starting substance disappeared (TLC, SiO2 plates, methylene chloride–methanol 10:1). The reaction mixture was evaporated and purified using column chromatography on silica gel or aluminum oxide for 3a. The eluent is methylene chloride–methanol, 100:2, 100:4. The fractions containing product 3 were combined and dissolved by heating in 5 mL of isopropyl alcohol, and the precipitate was deposited with the addition of 10 mL of hexane.
- 2-[(9-Demethoxy-7,8,13,13a-tetrahydroberberine-9-yl)oxy]-acetamide 3a
According to the general procedure, 372 mg of compound 3a was obtained from 700 mg of compound 2a after chromatography on aluminum oxide (3rd degree of activity) in the form of a light-yellow crystal solid; yield 64%.
M.p. 230.9 °C. IR (neat, υmax, cm−1): 3435, 3161, 2910, 1683, 1494, 1483, 1280, 1246, 1225. UV (EtOH, λmax, nm): 284, 352. MS (m/z): 381.1451, calculated for C21H21O5N2+: 381.1445. EA (%): C 65.20, H 5.64, N 7.23, calculated for C21H22O5N2: C 65.96, H 5.80, N 7.33. 1H NMR (400 MHz, CDCl3, δ, ppm, J (Hz)): 2.40–2.64 m (3H, H5, H6, H13), 2.82–2.95 m (1H, H5), 3.03–3.11 m (1H, H13), 3.27–3.45 m (3H, H6, H8, H13a), 3.78 s (3H, OCH3), 4.19 d (1H, H8, 16.0), 4.25 d (1H, OCH2CO, 14.8), 4.32 d (1H, OCH2CO, 14.8), 5.92–5.96 m (2H, OCH2O), 6.66 s (1H, H4), 6.87–6.93 m (3H, H1, H11, H12), 7.44 d (2H, NH2, 12.4). 13C NMR (100 MHz, DMSO-d6, δ, ppm): 29.00 (C5), 35.69 (C13), 50.67 (C6), 53.16 (C8), 55.80 (OCH3), 58.99 (C13a), 70.65 (OCH2CO), 100.53 (OCH2O), 105.72 (C1), 108.06 (C4), 111.07 (C11), 124.10 (C12), 127.49 * (C4a), 127.67 * (C12a), 128.31 (C8a), 130.86 (C1a), 142.67 (C9), 145.42 ** (C2), 145.71 ** (C3), 149.35 (C10), 170.58 (CO).
- N,N-Diethyl-2-[(9-demethoxy-7,8,13,13a-tetrahydroberberine-9-yl)oxy]-acetamide 3b
According to the general procedure, 460 mg of compound 3b was obtained from 618 mg of compound 2b in the form of a low-melting amorphous beige powder; yield 87%.
IR (neat, υmax, cm−1): 2934, 1645, 1487, 1276, 1248, 1222. UV (EtOH, λmax, nm): 285, 341. MS (m/z): 437.2070, calculated for C25H29O5N2+: 437.2071. EA (%): C 65.98, H 6.79, N 5.81, calculated for C25H30O5N2+H2O: C 65.77, H 7.07, N 6.14. 1H NMR (600 MHz, CDCl3, δ, ppm, J (Hz)): 1.10–1.19 m (6H, N(CH2CH3)2), 2.54–2.63 m (2H, H5, H6), 2.73–2.81 m (1H, H13), 3.00–3.10 m (1H, H5), 3.12–3.20 m (2H, H6, H13), 3.36–3.42 m (4H, NCH2), 3.46–3.52 m (1H, H13a), 3.55 d (1H, H8, 16.2), 3.78 s (3H, OCH3), 4.29 d (1H, H8, 15.6), 4.56 d (1H, OCH2CO, 12.6), 4.66 d (1H, OCH2CO, 12.6), 5.8–5.88 m (2H, OCH2O), 6.53 s (1H, H4), 6.67 s (1H, H1), 6.73 d (1H, H11, 8.4), 6.82 d (1H, H12, 8.4). 13C NMR (150 MHz, CDCl3, δ, ppm): 12.75 q (NCH2CH3), 14.17 q (NCH2CH3), 29.24 t (C5), 36.09 t (C13), 39.87 t (NCH2), 41.02 t (NCH2), 51.05 t (C6), 53.62 t (C8), 55.74 q (OCH3), 59.32 d (C13a), 70.62 t (OCH2CO), 100.56 t (OCH2O), 105.28 d (C1), 108.20 d (C4), 110.82 d (C11), 124.01 d (C12), 127.55 s (C4a, C12a), 128.37 s (C8a), 130.39 s (C1a), 143.37 s (C9), 145.72 * s (C2), 145.92 * s (C3), 149.58 s (C10), 167.29 s (CO).
- N,N-Dibutyl-2-[(9-demethoxy-7,8,13,13a-tetrahydroberberine-9-yl)oxy]-acetamide 3c
According to the general procedure, 359 mg of compound 3c was obtained from 435 mg of compound 2c in a beige oil form; yield 96%.
IR (neat, υmax, cm−1): 3452, 2957, 1647, 1485, 1279, 1248, 1223. UV (EtOH, λmax, nm): 285, 341. MS (m/z): 493.2680, calculated for C29H37O5N2+: 493.2700. EA (%): C 68.22, H 7.64, N 5.21, calculated for C29H38O5N2+H2O: C 67.94, H 7.86, N 5.46. 1H NMR (400 MHz, CDCl3, δ, ppm, J (Hz)): 0.90 t (6H, (N(CH2CH2CH2CH3)2), 7.2), 1.25–1.35 m (4H, NCH2CH2CH2), 1.47–1.58 m (4H, NCH2CH2), 2.57–2.67 m (2H, H5, H6), 2.75–2.88 m (1H, H13), 3.05–3.22 m (3H, H5, H6, H13), 3.23–3.37 m (4H, NCH2), 3.49–3.64 m (2H, H8, H13a), 3.78 s (3H, OCH3), 4.32 d (1H, H8, 16.0), 4.58 d (1H, OCH2CO, 12.8), 4.69 d (1H, OCH2CO, 12.8), 5.87 s (2H, OCH2O), 6.54 s (1H, H4), 6.67 s (1H, H1), 6.74 d (1H, H11, 8.3), 6.83 d (1H, H12, 8.3). 13C NMR (100 MHz, CDCl3, δ, ppm): 13.67 (NCH2CH2CH2CH3), 13.74 (NCH2CH2CH2CH3), 19.92 (NCH2CH2CH2CH3), 20.09 (NCH2CH2CH2CH3), 29.22 (C5), 29.57 (NCH2CH2CH2CH3), 31.03 (NCH2CH2CH2CH3), 36.08 (C13), 45.38 (NCH2) 46.76 (NCH2) 51.07 (C6), 53.65 (C8), 55.78 (OCH3), 59.37 (C13a), 70.59 (OCH2CO), 100.61 (OCH2O), 105.33 (C1), 108.26 (C4), 111.00 (C11), 123.97 (C12), 127.58 (C4a, C12a), 127.82 (C8a), 129.89 (C1a), 143.55 (C9), 145.85 * (C2), 146.02 * (C3), 149.66 (C10), 167.67 (CO).
- N,N-Diphenyl-2-[(9-demethoxy-7,8,13,13a-tetrahydroberberine-9-yl)oxy]-acetamide 3d
According to the general procedure, 228 mg of compound 3d was obtained from 228 mg of compound 2d in the form of an amorphous beige powder; yield 66%.
M.p. 86.5 °C (with decomposition). IR (neat, υmax, cm−1): 2900, 1695, 1493, 1280, 1248, 1221. UV (EtOH, λmax, nm): 284, 345. MS (m/z): 533.2067, calculated for C33H29O5N2+: 533.2071. EA (%): C 73.51, H 5.56, N 5.08, calculated for C33H30O5N2: C 74.14, H 5.66, N 5.24. 1H NMR (400 MHz, CDCl3, δ, ppm, J (Hz)): 2.54–2.66 m (2H, H5, H6) 2.71–2.82 m (1H, H13), 3.10–3.22 m (3H, H5, H6, H13), 3.55–3.60 m (2H, H8, H13a), 3.70 s (3H, OCH3), 4.32 d (1H, H8, 16.4), 4.49 d (1H, OCH2CO, 14.7), 4.61 d (1H, OCH2CO, 14.7), 5.88 s (2H, OCH2O), 6.55 s (1H, H4), 6.68 s (1H, H1), 6.69 d (1H, H11, 8.3), 6.80 d (1H, H12, 8.3), 7.20–7.31 m (6H, Ph), 7.20–7.31 m (6H, Ph), 7.31–7.40 m (4H, Ph). 13C NMR (100 MHz, CDCl3, δ, ppm): 29.41 (C5), 36.24 (C13), 51.12 (C6), 53.79 (C8), 55.90 (OCH3), 59.37 (C13a), 70.38 (OCH2CO), 100.59 (OCH2O), 105.34 (C1), 108.25 (C4), 111.07 (C11), 123.79 (C12), 127.73 (C4a, C12a), 128.51 (C8a), 129.26 (C2′,C3′,C4′,C5′,C6′), 130.70 (C1a), 143.60 (C9), 145.71 (C2), 145.92 * (C3), 149.28 * (C10), 168.07 (CO).
- 2-[(9-Demethoxy-7,8,13,13a-tetrahydroberberine-9-yl)oxy]-1-(pyrrolidin-1-yl)ethan-1-one 3e
According to the general procedure, 2.00 g of compound 3e was obtained from 3.18 g of compound 2e in the form of an amorphous light-yellow powder; yield 63%.
M.p. 145.1 °C (with decomposition). IR (neat, υmax, cm−1): 2922, 1649, 1491, 1248, 1222. UV (EtOH, λmax, nm): 285. MS (m/z): 435.1911, calculated for C25H27O5N2+: 435.1914. EA (%): C 68.98, H 6.58, N 6.25, calculated for C25H28O5N2: C 68.79, H 6.47, N 6.42. 1H NMR (400 MHz, DMSO-d6, δ, ppm, J (Hz)): 1.78–2.00 m (4H, N(CH2CH2)2), 2.53–2.70 m (2H, H5, H6), 2.70–2.88 m (1H, H13), 3.00–3.25 m (3H, H5, H6, H13), 3.40–3.65 m (6H, NCH2, H13a, H8), 3.79 s (3H, OCH3), 4.32 d (1H, H8, 16.0), 4.53 d (1H, OCH2CO, 13.6), 4.66 d (1H, OCH2CO, 13.6), 5.88 s (2H, OCH2O), 6.55 s (1H, H4), 6.69 s (1H, H1), 6.75 d (1H, H11, 8.5), 6.84 d (1H, H12, 8.5). 13C NMR (75 MHz, DMSO-d6, δ, ppm): 23.52 (NCH2CH2) 25.45 (C5), 25.72 (NCH2CH2) 32.05 (C13), 44.63 (NCH2) 45.58 (NCH2) 50.08 (C6), 51.43 (C8), 56.06 (OCH3), 58.84 (C13a), 69.82 (OCH2CO), 101.20 (OCH2O), 105.63 (C1), 108.20 (C4), 112.84 (C11), 122.79 * (C4a), 123.79 (C12), 125.02 * (C12a), 125.20 * (C8a), 125.63 * (C1a), 142.97 (C9), 146.61 ** (C2), 146.77 ** (C3), 149.62 (C10), 166.23 (CO).
- 2-[(9-Demethoxy-7,8,13,13a-tetrahydroberberine-9-yl)oxy]-1-(piperidin-1-yl)ethan-1-one 3f
According to the general procedure, 497 mg of compound 3f was obtained from 730 mg of compound 2f in the form of a low-melting amorphous light-yellow powder; yield 80%.
IR (neat, υmax, cm−1): 3458, 2935, 1643, 1487, 1276, 1248, 1221. UV (EtOH, λmax, nm): 285, 343. MS (m/z): 449.2068, calculated for C26H29O5N2+: 449.2071. EA (%): C 66.71, H 6.30, N 5.81, calculated for C26H30O5N2+H2O: C 66.65, H 6.88, N 5.98. 1H NMR (400 MHz, CDCl3, δ, ppm, J (Hz)): 1.51–1.68 m (6H, NCH2CH2CH2), 2.59–2.72 m (2H, H5, H6), 2.77–2.92 m (1H, H13), 3.06–3.25 m (3H, H5, H6, H13), 3.45–3.70 m (6H, NCH2, H13a, H8), 3.79 s (3H, OCH3), 4.33 d (1H, H8, 15.9), 4.60 d (1H, OCH2CO, 12.8), 4.69 d (1H, OCH2CO, 12.8), 5.88 s (2H, OCH2O), 6.55 s (1H, H4), 6.68 s (1H, H1), 6.75 d (1H, H11, 8.4), 6.84 d (1H, H12, 8.4). 13C NMR (100 MHz, CDCl3, δ, ppm): 24.54 (NCH2CH2CH2), 25.57 (NCH2CH2), 26.51 (NCH2CH2), 29.36 (C5), 36.19 (C13), 42.97 (NCH2), 46.17 (NCH2), 51.23 (C6), 53.79 (C8), 55.97 (OCH3), 59.50 (C13a), 71.02 (OCH2CO), 100.78 (OCH2O), 105.49 (C1), 108.42 (C4), 111.08 (C11), 124.28 (C12), 127.69 (C4a, C12a), 128.40 (C8a), 130.46 (C1a), 143.56 (C9), 145.98 * (C2), 146.16 * (C3), 149.86 (C10), 166.67 (CO).
- 2-[(9-Demethoxy-7,8,13,13a-tetrahydroberberine-9-yl)oxy]-1-morpholinoethan-1-one 3g
According to the general procedure, 380 mg of compound 3g was obtained from 750 mg of compound 2g in the form of a light-yellow crystal solid; yield 59%.
M.p. 163.1 °C. IR (neat, υmax, cm−1): 2914, 1653, 1498, 1278, 1228. UV (EtOH, λmax, nm): 285. MS (m/z): 451.1860, calculated for C25H27O6N2+: 451.1864. EA (%): C 66.44, H 6.16, N 6.13, calculated for C25H28O6N2: C 66.36, H 6.24, N 6.19. 1H NMR (400 MHz, CDCl3, δ, ppm, J (Hz)): 2.56–2.68 m (2H, H5, H6), 2.74–2.86 m (1H, H13), 3.03–3.23 m (3H, H5, H6, H13), 3.48–3.74 m (10H, NCH2CH2O, H13a, H8), 3.78 s (3H, OCH3), 4.26 d (1H, H8, 15.9), 4.58 d (1H, OCH2CO, 12.6), 4.66 d (1H, OCH2CO, 12.6), 5.87 s (2H, OCH2O), 6.55 s (1H, H4), 6.68 s (1H, H1), 6.74 d (1H, H11, 8.4), 6.84 d (1H, H12, 8.4). 13C NMR (100 MHz, CDCl3, δ, ppm): 29.37 (C5), 36.19 (C13), 42.26 (NCH2), 45.79 (NCH2), 51.29 (C6), 53.77 (C8), 55.86 (OCH3), 59.52 (C13a), 66.88 (NCH2CH2O) 71.03 (OCH2CO), 100.80 (OCH2O), 105.48 (C1), 108.43 (C4), 110.90 (C11), 124.54 (C12), 127.67 * (C4a), 127.79 * (C12a), 128.37 (C8a), 130.39 (C1a), 143.20 (C9), 146.01 ** (C2), 146.18 ** (C3), 149.79 (C10), 167.06 (CO).
4. Conclusions
New N,N-disubstituted O-acetamide derivatives of tetrahydroberberine were synthesized by reducing the corresponding derivatives of berberubine by the action of sodium borohydride. The structure of the compounds was determined using NMR and HRMS methods.
Supplementary Materials
Figures S1–S25: 1H NMR, 13C NMR, and MS spectra of compounds 3a–g.
Author Contributions
Conceptualization and methodology: I.V.N. and N.F.S.; synthesis: I.V.N.; HPLC chromatograms: N.I.K.; original draft preparation: I.V.N.; review and editing: I.V.N., N.I.K. and N.F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation, under the governmental assignment project number FWUE-2022-0007.
Data Availability Statement
Data are contained within the article and the Supplementary Materials.
Acknowledgments
The authors would like to acknowledge the Multi-Access Chemical Research Center SB RAS for spectral and analytical measurements.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wang, Q.; Shen, W.; Shao, W.; Hu, H. Berberine alleviates cholesterol and bile acid metabolism disorders induced by high cholesterol diet in mice. Biochem. Biophys. Res. Commun. 2024, 719, 150088. [Google Scholar] [CrossRef]
- Shams, G.; Allah, S.A.; Ezzat, R.; Said, M.A. Ameliorative effects of berberine and selenium against paracetamol-induced hepatic toxicity in rats. Open Vet. J. 2024, 14, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiao, Y.; Zhou, J.; Mo, H.; Li, X.; Li, Y.; Wang, Y.; Zhong, M. Effects of Berberine on glucolipid metabolism among dehydroepiandrosterone-induced rats of polycystic ovary syndrome with insulin-resistance. Heliyon 2024, 10, e24338. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, F.; Kiani, S.; Rahimi, G.; Boskabady, M.H. Anti-inflammatory, antioxidant, and immunomodulatory effects of Berberis vulgaris and its constituent berberine, experimental and clinical, a review. Phytother. Res. 2024, 38, 1882–1902. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yin, J.; Chen, J.; Ma, J.; Si, H.; Xia, D. Inhibition of inflammation by berberine: Molecular mechanism and network pharmacology analysis. Phytomedicine 2024, 128, 155258. [Google Scholar] [CrossRef]
- Ali, M.; Mishra, D.; Singh, R.P. Cancer Pathways Targeted by Berberine: Role of microRNAs. Curr. Med. Chem. 2024, 31, 5178–5198. [Google Scholar] [CrossRef] [PubMed]
- Khezri, M.R.; Mohammadipanah, S.; Ghasemnejad-Berenji, M. The pharmacological effects of Berberine and its therapeutic potential in different diseases: Role of the phosphatidylinositol 3-kinase/AKT signaling pathway. Phytother. Res. 2024, 38, 349–367. [Google Scholar] [CrossRef]
- Jivad, N.; Heidari-Soureshjani, S.; Bagheri, H.; Sherwin, C.M.; Rostamian, S. Anti-seizure Effects and Mechanisms of Berberine: A Systematic Review. Curr. Pharm. Biotechnol. 2024, 25, 2253–2265. [Google Scholar] [CrossRef]
- El-Nahas, A.E.; Elbedaiwy, H.M.; Helmy, M.W.; El-Kamel, A.H. Simultaneous Estimation of Berberine and Piperine in a Novel Nanoformulation for Epilepsy Control via HPLC. J. Chromatogr. Sci. 2024, 62, 120–126. [Google Scholar] [CrossRef]
- Tang, Y.; Su, H.; Nie, K.; Wang, H.; Gao, Y.; Chen, S.; Lu, F.; Dong, H. Berberine exerts antidepressant effects in vivo and in vitro through the PI3K/AKT/CREB/BDNF signaling pathway. Biomed. Pharmacother. 2024, 170, 116012. [Google Scholar] [CrossRef]
- Gao, Y.; Nie, K.; Wang, H.; Dong, H.; Tang, Y. Research progress on antidepressant effects and mechanisms of berberine. Front. Pharmacol. 2024, 15, 1331440. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Gao, M.; Lin, Q. Integration of bioinformatics analysis, molecular docking and animal experiments to study the therapeutic mechanisms of berberine against allergic rhinitis. Sci. Rep. 2024, 14, 11999. [Google Scholar] [CrossRef]
- Kozlov, S.V.; Staroverov, S.A.; Skvortsova, N.I.; Soldatov, D.A.; Chekunov, M.A.; Kozlov, E.S.; Artemev, D.A.; Chekunova, E.D.; Klyukina, A.D.; Rakhkho, V.; et al. Method of Preparing Water-Soluble Pharmaceutical Composition Based on Berberine. Patent RU2814497C1, 29 February 2024. [Google Scholar]
- Chen, C.; Xie, M.; Yan, Y.; Li, Y.; Li, Z.; Zhang, T.; Gao, Z.; Deng, L.; Wang, H. Preparation of berberine hydrochloride-Ag nanoparticle composite antibacterial dressing based on 3D printing technology. J. Biomater. Appl. 2024, 38, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Agharazi, F.; Hosseinzadeh, S.A.; Mashayekhi, M.; Saffari, Z.; Shafiei, M.; Nader, S.; Ebrahimi-Rad, M.; Sadeghi, M. Gold nanoparticle conjugation enhances berberine’s antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Talanta 2024, 268 Pt 1, 125358. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.; Sheorain, J.; Bakshi, J.; Thakur, R.; Grewal, S.; Dhingra, D.; Kumari, S. Synthesis and evaluation of berberine loaded chitosan nanocarrier for enhanced in-vitro antioxidant and anti-inflammatory potential. Carbohydr. Polym. Technol. Appl. 2024, 7, 100474. [Google Scholar] [CrossRef]
- Guo, S.; Shen, C.; Chen, T.; Zhao, L.; Qiao, R.; Li, C. A stimuli-responsive demethyleneberberine-conjugated carboxylmethyl chitosan prodrug for treatment of inflammatory bowel diseases. Mater. Lett. 2024, 357, 135730. [Google Scholar] [CrossRef]
- Saleh, S.R.; Abd-Elmegied, A.; Madhy, S.A.; Khattab, S.N.; Sheta, E.; Elnozahy, F.Y.; Mehanna, R.A.; Ghareeb, D.A.; Abd-Elmonem, N.M. Brain-targeted Tet-1 peptide-PLGA nanoparticles for berberine delivery against STZ-induced Alzheimer′s disease in a rat model: Alleviation of hippocampal synaptic dysfunction, Tau pathology, and amyloidogenesis. Int. J. Pharm. 2024, 658, 124218. [Google Scholar] [CrossRef]
- Sun, J.; Ye, T.; Chen, X.; Li, B.; Wei, Y.; Zheng, H.; Piao, J.-G.; Li, F. A self-assembly active nanomodulator based on berberine for photothermal immunotherapy of breast cancer via dual regulation of immune suppression. Int. J. Pharm. 2024, 653, 123898. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Z.; Zhang, W.; Liu, Y.; Wang, X.; Sun, M.; Fang, X.; Han, W. The study on synthesis and vitro hypolipidemic activity of novel berberine derivatives nitric oxide donors. Fitoterapia 2024, 176, 105964. [Google Scholar] [CrossRef]
- Khvostov, M.V.; Gladkova, E.D.; Borisov, S.A.; Zhukova, N.A.; Marenina, M.K.; Meshkova, Y.V.; Luzina, O.A.; Tolstikova, T.G.; Salakhutdinov, N.F. Discovery of the First in Class 9-N-Berberine Derivative as Hypoglycemic Agent with Extra-Strong Action. Pharmaceutics 2021, 13, 2138. [Google Scholar] [CrossRef]
- Teng, Q.; Meng, Q.; Zhu, X.; Jiang, W.; Miao, C.; Yang, H. Synthesis and Use of 9-O-Aryl Substituted Berberine Derivatives and Its Application in Antibacterial Drugs. Patent CN109232557, 18 January 2019. [Google Scholar]
- Valipour, M.; Khatir, Z.Z.; Abdollahi, E.; Ayati, A. Recent Applications of Protoberberines as Privileged Starting Materials for the Development of Novel Broad-Spectrum Antiviral Agents: A Concise Review (2017–2023). ACS Pharmacol. Transl. Sci. 2024, 7, 48–71. [Google Scholar] [CrossRef] [PubMed]
- Afroozandeh, Z.; Ranjbar, P.R.; Khoobi, M.; Forootanfar, H.; Ameri, A.; Foroumadi, A. New Berberine Conjugates with Self-Assembly and Improved Antioxidant/Neuroprotection Properties: Effect of the Anchored Part on CMC, Shape and Size of the Nanomicelles. J. Clust. Sci. 2024, 35, 1305–1315. [Google Scholar] [CrossRef]
- Teng, Q.; Zhu, X.; Guo, Q.; Jiang, W.; Liu, J.; Meng, Q. Synthesis of 9-O-arylated berberines via copper-catalyzed CAr–O coupling reactions. Beilstein J. Org. Chem. 2019, 15, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Groß, P.; Hoffmann, R.S.; Müller, M.; Schönherr, H.; Ihmels, H. Fluorimetric Cell Analysis with 9-Aryl-Substituted Berberine Derivatives as DNA-Targeting Fluorescent Probes. ChemBioChem 2024, 25, e202300761. [Google Scholar] [CrossRef]
- Nechepurenko, I.V.; Komarova, N.I.; Vasil’ev, V.G.; Salakhutdinov, N.F. Synthesis of berberine bromide analogs containing tertiary amides of acetic acid in the 9-O-position. Chem. Nat. Compd. 2013, 48, 1047–1053. [Google Scholar] [CrossRef]
- Nechepurenko, I.V.; Komarova, N.I.; Shernyukov, A.V.; Vasil’ev, V.G.; Salakhutdinov, N.F. Smiles rearrangements in a series of berberine analogues containing a secondary acetamide fragment. Tetrahedron Lett. 2014, 55, 6125–6127. [Google Scholar] [CrossRef]
- Lai, R.; Lin, Z.; Yang, C.; Hai, L.; Yang, Z.; Guo, L.; Nie, R.; Wu, Y. Novel berberine derivatives as p300 histone acetyltransferase inhibitors in combination treatment for breast cancer. Eur. J. Med. Chem. 2024, 266, 116116. [Google Scholar] [CrossRef]
- Wei, G.; Huang, N.; Li, M.; Guan, F.; Chen, L.; Liao, Y.; Xie, X.; Li, Y.; Su, Z.; Chen, J.; et al. Tetrahydroberberine alleviates high-fat diet-induced hyperlipidemia in mice via augmenting lipoprotein assembly-induced clearance of low-density lipoprotein and intermediate-density lipoprotein. Eur. J. Pharmacol. 2024, 968, 176433. [Google Scholar] [CrossRef]
- Wang, D.; Wang, K.; Sui, D.; Ouyang, Z.; Xu, H.; Wei, Y. Effects of tetrahydroberberine and tetrahydropalmatine on hepatic cytochrome P450 expression and their toxicity in mice. Chem. Biol. Interact. 2017, 268, 47–52. [Google Scholar] [CrossRef]
- Nechepurenko, I.V.; Shirokova, E.D.; Khvostov, M.V.; Frolova, T.S.; Sinitsyna, O.I.; Maksimov, A.M.; Bredikhin, R.A.; Komarova, N.I.; Fadeev, D.S.; Luzina, O.A.; et al. Synthesis, hypolipidemic and antifungal activity of tetrahydroberberrubine sulfonates. Russ. Chem. Bull. 2019, 68, 1052–1060. [Google Scholar] [CrossRef]
- Kong, Y.; Yi, Y.; Liu, X.-Q.; Yu, P.; Zhao, L.-G.; Li, D.-D. Discovery and structural optimization of 9-O-phenylsulfonyl-berberines as new lipid-lowering agents. Bioorg. Chem. 2022, 121, 105665. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Y.; Zhang, H.; Han, W. Review of synthesis and activity of tetrahydroberberine derivatives. Zhongguo Linchuang Yaolixue Zazhi 2021, 37, 138–140. [Google Scholar] [CrossRef]
- Mari, G.; De Crescentini, L.; Benedetti, S.; Palma, F.; Santeusanio, S.; Mantellini, F. Synthesis of new dihydroberberine and tetrahydroberberine analogues and evaluation of their antiproliferative activity on NCI-H1975 cells. Beilstein J. Org. Chem. 2020, 16, 1606–1616. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, H. Preparation of Tetrahydroberberine Thiazolidinedione Compound and Application as Antibacterial and/or Antifungal Agents. Patent CN108658971, 16 October 2018. [Google Scholar]
- Gladkova, E.D.; Nechepurenko, I.V.; Bredikhin, R.A.; Chepanova, A.A.; Zakharenko, A.L.; Luzina, O.A.; Ilina, E.S.; Dyrkheeva, N.S.; Mamontova, E.M.; Anarbaev, R.O.; et al. The First Berberine-Based Inhibitors of Tyrosyl-DNA Phosphodiesterase 1 (Tdp1), an Important DNA Repair Enzyme. Int. J. Mol. Sci. 2020, 21, 7162. [Google Scholar] [CrossRef] [PubMed]
- Kalinowsky, H.-O.; Berger, S.; Brawn, S. 13-C NMR Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 1988; pp. 198–219. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).